Abstract
The objectives of the present study were to characterize γ -ray, 1 GeV/n proton, and 1 GeV/n iron ion radiation-induced adverse biological effects in terms of toxicity and transformation of HTori-3 human thyroid epithelial cells; to evaluate the ability of L-selenomethionine (SeM) to protect against radiation-induced transformation when present at different times during the assay period; and to evaluate the tumorigenicity of HTori-3 cells derived from anchorage-independent colonies following iron ion radiation exposure. Cell survival was determined by a clonogenic assay, transformation was measured by a soft agar colony formation assay, and the tumorigenic potential of the cells was determined by injecting them subcutaneously into athymic nude mice and monitoring tumor formation. The results demonstrate that exposure of HTori-3 cells to γ -ray, proton, or iron ion radiation resulted in decreased clonogenic survival, which persisted for weeks after the radiation exposure. Treatment with SeM initiated up to 7 days after the radiation exposure conferred significant protection against radiation-induced anchorage-independent growth. HTori-3 cells derived from all evaluated anchorage-independent colonies formed tumors when injected into athymic nude mice, indicating that these cells are tumorigenic and that anchorage-independent colony growth is a reliable surrogate endpoint biomarker for the radiation-induced malignant transformation of HTori-3 cells.
INTRODUCTION
As reviewed by Hellweg and Baumstark-Khan (1), the main components of radiation in interplanetary space are galactic cosmic rays (GCR) and solar cosmic radiation (SCR). GCR originates from outside of the solar system and consists of 98% baryons and 2% electrons. The baryonic component consists of 87% protons (hydrogen nuclei), 12% alpha particles (helium nuclei), and approximately 1% of heavier nuclei with atomic numbers (Z) up to 92 (uranium). These heavier nuclei include highly energetic, heavy, and charged particles known as HZE particles. Although iron ions, as a specific type of HZE particle, account for less than 1% of the GCR particle fluxes, iron ions contribute significantly to the total radiation dose received by individual cells exposed to GCR due to the fact that the dose to an individual cell is proportional to the square of the particle’s energy-dependent effective charge (2). Thus, iron ion radiation is of a special interest in space radiation research. As for people on earth, the use of protons has become increasingly common in cancer radiotherapy due to the physical characteristics of proton beams that can be designed to yield a uniform dose across the target and then virtually zero dose deep to the target for nonsuperficial lesions (3). The characteristics of proton radiotherapy are thought to result in an improved tumor control probability and lower tissue complication probability (3). Some heavy charged particle beams, such as carbon ion beams, have also become an accepted part of radiation therapy because of their increased biological effectiveness as compared to proton beams (4,5).
Exposure to space radiation may place astronauts at significant risk of developing both acute and long-term radiation-induced adverse biological effects. Acute effects arising from exposure to a solar particle event (SPE) radiation can include radiation sickness (nausea and/or vomiting), skin injury, changes in hematopoietic and immune system functions, and fatigue. Exposure to either SPE or GCR radiation can result in long-term effects such as the induction of cancer. It is known that exposures of several human populations to radiation have resulted in an increased incidence of cancer, with some types of human cancer having measurable dose-response relationships down to relatively low doses (e.g., 10 cGy, received as a total body dose) (6–8). While avoidance of the radiation risk is the best protective strategy for astronauts, it is nearly impossible to avoid the radiation risk completely. In therapeutic settings, radiation damage to healthy tissues surrounding tumors and radiation-induced secondary malignancies are the major challenges for the optimal prognosis of cancer survivors after radiotherapy. Thus, countermeasures capable of mitigating proton and HZE particle radiation-induced adverse biological effects are likely to be important for successful future exploration class missions involving higher radiation doses than are currently received by astronauts and might also be beneficial for cancer survivors after radiotherapy with proton or HZE particle beams.
In previous studies performed in our laboratory, exposure to iron ion radiation significantly decreased the clonogenic survival of MCF10 human breast epithelial cells and treatment with SeM protected MCF10 human breast epithelial cells from iron ion radiation-induced cytotoxicity (9). Exposure to iron ion radiation also significantly increased the yield of anchorage-independent colonies of HTori-3 human thyroid epithelial cells, which was prevented by treatment with 5-µM SeM in the medium (9). Exposure to 0.25 GeV proton radiation at 600 cGy also increased the yield of anchorage-independent colonies of the irradiated HTori-3 cells (10). The present study extended the previous studies with the main aims being to determine the dose-response relationships for cell survival and anchorage-independent growth of HTori-3 cells exposed to γ-ray, 1-GeV proton, and 1-GeV/n iron ion radiation, the temporal effect of the SeM treatment on radiation-induced cytotoxicity and anchorage-independent growth of HTori-3 cells, and the tumorigenicity of HTori-3 cells derived from the anchorage-independent colonies.
MATERIALS AND METHODS
Chemicals
L-Selenomethionine (SeM) used in the experiments was purchased from Sigma Chemical Company (St. Louis, MO).
Cells and Cell Culture
HTori-3 cells are a human thyroid epithelial cell line immortalized by transfecting primary cultures of human thyroid epithelial cells with an origin-defective SV40 genome. This cell line is not tumorigenic and unirradiated HTori-3 cells form colonies in soft agar with a relatively low efficiency of approximately 0.3% (11).
In these studies, HTori-3 cells were normally cultured in DMEM supplemented with 10% fetal bovine serum before experiments were performed. The cells were dissociated by trypsin-EDTA treatment and sub-cultured as needed prior to being used for the experiments described here.
Radiation and SeM Treatment
HTori-3 cell transformation experiments were performed with 0.662-MeV γ -rays emitted from a 137Cesium source or 1-GeV protons (LET of approximately 0.24 keV/µm) and iron ions (LET of 150 keV/µm) generated by the Alternating Gradient Synchrotron (AGS) at the Brookhaven National Laboratory (BNL). To perform the radiation experiments, HTori-3 cells cultured in T-25 tissue culture flasks were irradiated with γ -rays and protons at doses of 0.4, 1, 2, 4, and 6 Gy or iron ions at doses of 0.1, 0.2, 0.4, 1 and 2 Gy. Sham irradiated cells were included in each experiment as controls. After the radiation exposures, the cells were cultured in control medium for 2 wk before the clonogenic survival and soft agar colony formation assays were performed.
A separate experiment was performed with γ -rays to evaluate the effects of SeM on radiation-induced cell transformation. In this experiment, HTori-3 cells cultured in T-25 flasks were divided into 8 treatment groups and incubated in control medium (Groups 1 and 3) or medium containing 5 µM SeM for 16 days starting 2 days prior to the radiation exposure (Groups 2 and 4), for 2 days prior to the radiation exposure (Group 5), for 14 days starting immediately after the radiation exposure (Group 6), for 12 days starting 2 days after the radiation exposure (Group 7), or for 7 days starting 1 wk after the radiation exposure (Group 8). The cells in Groups 1 and 2 were sham-irradiated and included as controls whereas the cells in Groups 3 through 8 were irradiated with γ -rays at a single dose of 6 Gy. After the radiation exposure and SeM treatment for the periods indicated as above, the cells were cultured in control medium for an additional 6 wk before being used for the clonogenic survival and soft agar colony formation assays initiated 8 wk after the radiation exposure.
Clonogenic Survival and Soft Agar Colony Formation Assays
The effects of radiation on cell survival were evaluated by a clonogenic survival assay. To determine the cell survival level, the cells were dissociated by treatment with trypsin-EDTA, resuspended in medium, plated in T-25 tissue culture flasks at 200 to 2,000 cells per flask and cultured for 6 days. At the end of the incubation period, the cell colonies were fixed and stained with crystal violet and methylene blue dissolved in 90% ethanol and counted under a dissection microscope.
The transformation of irradiated HTori-3 cells (11) was quantitated by a soft agar colony formation assay that measures the ability of the cells to grow under anchorage-independent conditions. To carry out the assay, sham-irradiated and irradiated HTori-3 cells were treated with trypsin and suspended in growth medium containing 0.8% methyl cellulose and plated in 24 multi-well polystyrene plates at a density of 4,000 surviving cells/well. The bottoms of the wells were precoated with an agar layer prepared by adding 1.8% agar to 2× DMEM medium to give a final agar concentration of 0.9%. The plates were incubated at 37°C and the medium was changed twice a week. Four wells per treatment group were stained with Neutral Red at 3–4 wk after plating. After 3 wk of incubation, discrete colonies were observable. Anchorage-independent clones were removed from the soft agar and isolated, expanded in standard adherent tissue culture conditions, and stored frozen in liquid nitrogen.
Tumorigenicity Determination in Nude Mice
HTori-3 cells have previously been adapted for studies of radiation transformation (12,13). Anchorage-independent growth is a phenotypic change associated with the ability of cells to form tumors in animals; and tumor formation has previously been reported within 7–20 wk after irradiated HTori-3 cells were transplanted into athymic nude mice (12). To verify the tumorigenicity of anchorage-independent colonies derived from HTori-3 cells exposed to iron ion radiation, cell cultures derived from 8 anchorage-independent colonies of HTori-3 cells irradiated with 200 cGy of 1-GeV/n iron ions were expanded in T-75 flasks. The cells were dissociated by treatment with trypsin, centrifuged, resuspended in sterile PBS at a concentration of 108 cells/ml and injected subcutaneously into athymic nude mice (Harlan). Each mouse was given bilateral injections of 0.1 ml (107 cells) per site. One group of 3 mice was injected with cells derived from each anchorage-independent clone. Two groups of 3 mice were injected with cell-free PBS and used as controls. After the injection, mice were observed daily and those with tumor masses of over 5-mm diameter were killed, and the tumor tissues were excised. At the end of 8 mo, the remaining animals were killed and the observable tumors excised. The human origin of the tumors was determined by Southern Analysis.
Southern Analysis
Genomic DNA extraction from the excised tumor tissues and Southern Analysis were performed using standard procedures (14). Briefly, 10 µg sample of tumor-extracted DNA was digested with 30 units of AluI (Invitrogen, Carlsbad, CA) overnight at 37°C. Digested DNA was run on a 1% agarose gel buffered in Tris-borate-EDTA, pH 8.2. Southern transfer was performed onto Immobilion-NY+ membranes (Millipore, Bedford, MA). Hybridizations were performed using a 6.9 kb cDNA human plasminogen activator inhibitor probe (ATCC, Rockville, MD) that includes part of intron 1 through intron 7 with multiple Alu repeat sequences (15). The probe was labeled and detected using DIG high prime DNA labeling and detection kit (Roche, Mannheim, Germany).
Data and Statistical Analyses
The plating efficiency of cells in each flask was calculated by dividing the number of cell colonies by the number of cells plated in the flask. The plating efficiency of each flask was divided by the mean plating efficiency of the control group to calculate the surviving fraction. The surviving fraction data were plotted against the radiation doses to calculate radiation sensitivity constants according to the multitarget theory (16) using the equation S = ne−kD, where S is the surviving fraction, n represents the number of targets, −k is the radiation sensitivity constant, and D is the dose of radiation (cGy).
The anchorage-independent colony formation efficiency in each well on the soft agar plates was calculated by dividing the number of colonies formed in each well by the number of viable cells plated onto the soft agar in the well. At the time, the cells were plated into soft agar; the same cell suspension used for the anchorage-independent growth assay was used in a plating efficiency by plating a determined number of cells on conventional tissue culture plastic. The number of viable cells plated onto each well for the anchorage independence assay is equal to the number of cells plated in the well multiplied by the plating efficiency of that cell suspension.
The results of the nude mice tumorigenicity experiment are expressed as the fraction of injection sites with tumor, which was calculated by dividing the injection sites with palpable tumor(s) by the total number of injection sites in each group.
The cell-surviving fraction and anchorage-independent colony formation efficiency results were compared among different treatment groups by 1-way analysis of variance (ANOVA) followed by Tukey’s test. The fractions of injection sites with tumors were compared by Fisher’s exact test between the control group of mice injected with PBS and each group of mice injected with cells of anchorage-independent clones derived from iron ion irradiated HTori-3 cells. The nonlinear regression was performed using SigmaPlot 2001 software (SPSS, Inc.). One-way ANOVA, Tukey’s test, and Fisher’s exact test were performed using GraphPad InStat statistical software (GraphPad Software, San Diego, CA).
RESULTS
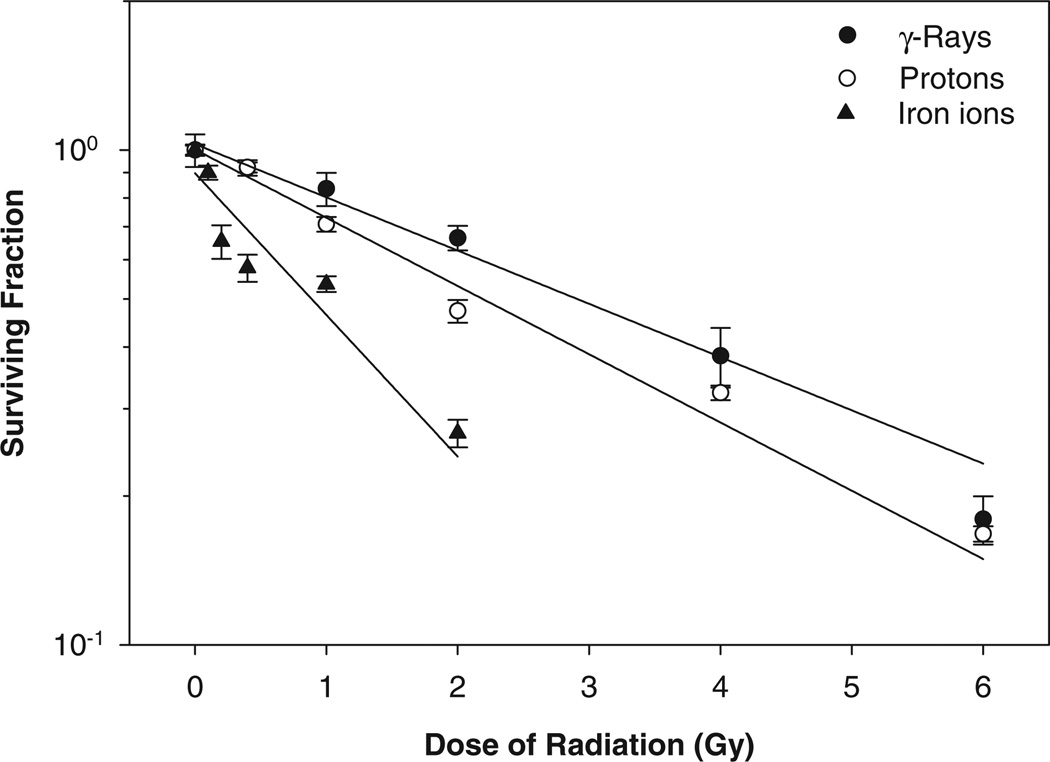
The present studies were performed to compare the capability of γ -ray, proton, and iron ion irradiation to induce HTori-3 cell transformation as indicated by anchorage-independent colony formation efficiency and verify the tumorigenicity of HTori-3 cells derived from cells growing under anchorage-independent conditions. In addition, this study also determined the effect of SeM, applied at different time points relative to the radiation exposure, on anchorage-independent colony formation efficiency of irradiated HTori-3 cells. In the experiments performed to compare the capability of γ -ray, proton, and iron ion irradiation to induce HTori-3 cell transformation, the anchorage-independent colony formation assay was performed 2 wk after the radiation exposure to allow sufficient time for the cells to recover from the acute lethal effects of the radiation exposure. The results demonstrated dose-dependent decreases in the survival of irradiated HTori-3 cells seeded in plastic tissue culture flasks 2 wk after the radiation exposure (Fig. 1). The fact that the dose-dependent decrease in the cell survival was observed 2 wk after the radiation exposure indicated delayed lethal effects of γ -ray, proton, and iron ion radiation on HTori-3 cells. The D0 values calculated from the γ -ray, proton, and iron ion radiation survival results were 4.0, 3.2, and 1.5 Gy, respectively. Based on the D0 ratio between γ -rays and protons or iron ions, the relative biological effectiveness (RBE) value for the proton and iron ion radiation was estimated to be 1.3 and 2.7, respectively, using the cell survival levels measured at 2 wk after the radiation exposure as the biological endpoint.
FIG. 1.
Survival curve of HTori-3 cells irradiated with γ -rays, protons or iron ions. HTori-3 cells were irradiated with 0.662-MeV γ -rays, 1-GeV protons or 1-GeV/n iron ions at the radiation doses indicated. Each data point represents a mean of 4 replicate flasks. The standard deviation is indicated by the error bars.
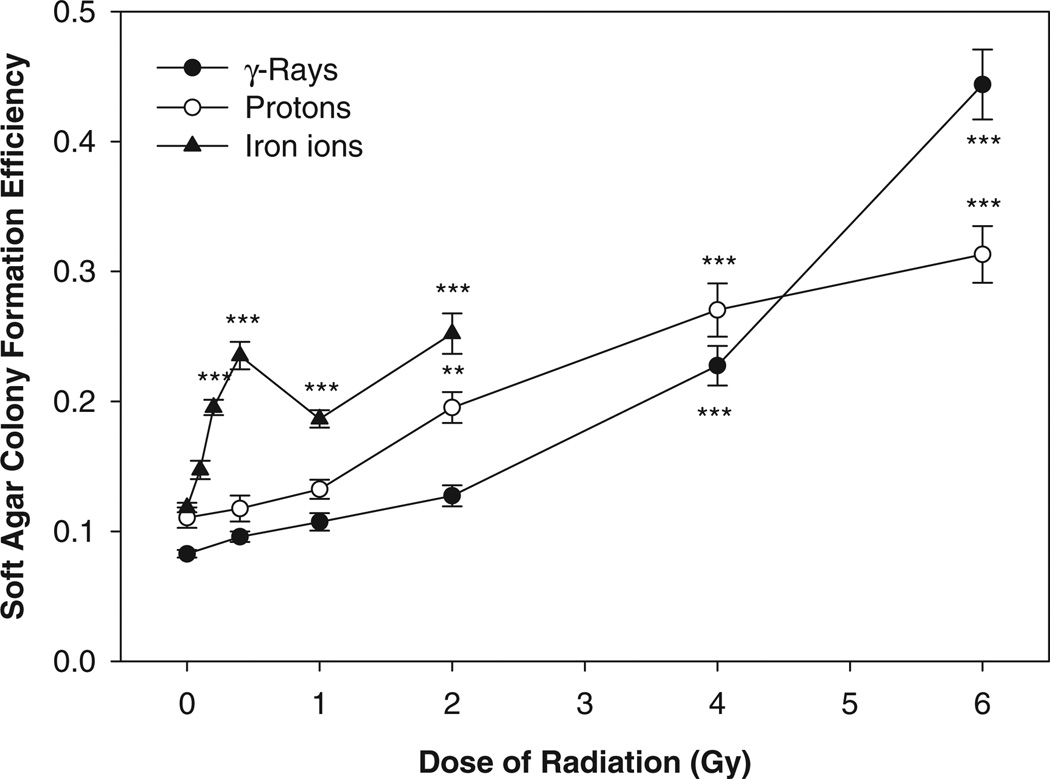
In the soft agar colony formation assay performed with cells seeded in soft agar 2 wk after the radiation exposure, the results showed that the anchorage-independent colony formation efficiency increased significantly for HTori-3 cells irradiated with γ -rays at a radiation dose of 4 Gy or higher, protons at a radiation dose of 2 Gy or higher, or iron ions at a radiation dose of 0.2 Gy or higher (Fig. 2).
FIG. 2.
Soft agar colony formation efficiency of HTori-3 cells irradiated with γ -rays, protons or iron ions. HTori-3 cells were irradiated with 0.662-MeV γ -rays, 1-GeV protons or 1-GeV/n iron ions at the radiation doses indicated. Each data point represents a mean of 12 replicate flasks.
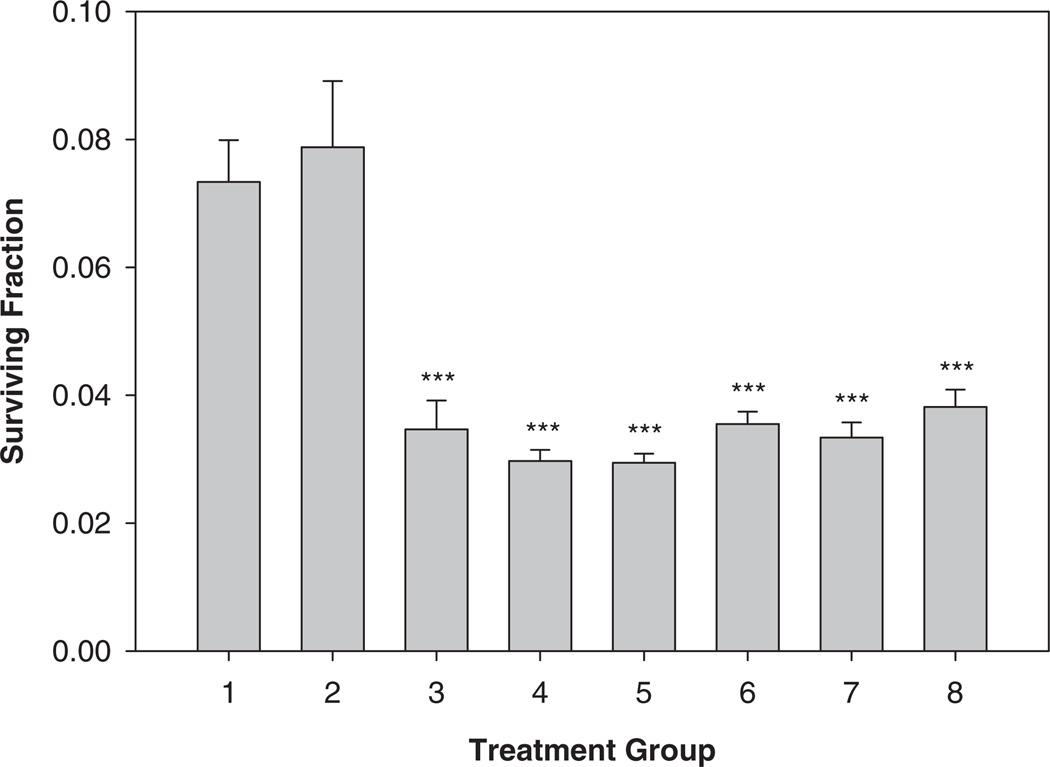
In separate experiments carried out to determine the effect of SeM on the anchorage-independent colony formation efficiency of irradiated HTori-3 cells, the anchorage-independent colony formation assay was performed 8 wk after exposure to γ -ray irradiation at a single dose of 6 Gy. The cells were also plated into plastic tissue culture flasks during the anchorage-independent colony formation assay, so that the cell survival levels could be determined. The results show that the survival of the irradiated HTori-3 cells (Groups 3–8) were significantly below that of the sham irradiated cells cultured in control medium (Group 1) or medium containing 5 µM SeM (Fig. 3), again indicating the presence of a delayed radiation effect on cell survival 8 wk after the γ -ray radiation exposure. Treatment with SeM before, during, and/or after the radiation exposure had no significant effect on the survival of irradiated cells (Groups 4–8 vs. Group 3) or sham irradiated cells (Group 2 vs. Group 1) when assayed 8 wk post-irradiation.
FIG. 3.
Survival of HTori-3 cells irradiated with γ -rays and treated with SeM. HTori-3 cells were treated with 5 µM SeM for 16 days starting 2 days prior to the radiation exposure (Groups 2 and 4), 2 days prior to the radiation exposure (Group 5), 14 days starting immediately after the radiation exposure (Group 6), 12 days starting 2 days after the radiation exposure (Group 7), or 7 days starting 1 wk after the radiation exposure (Group 8). The cells in Groups 1 and 2 were sham-irradiated and included as controls whereas the cells in Groups 3 through 8 were irradiated with γ -rays at a single dose of 600 cGy. After the radiation exposure and SeM treatment, the cells were cultured in control medium for an additional 6 wk and then used for the clonogenic survival assay. Each data point represents a mean of 4 replicate flasks. The standard deviation is indicated by the error bars.
The anchorage-independent colony formation efficiency results of the irradiated HTori-3 cells demonstrated a statistically significant increase in the anchorage-independent colony formation efficiency of the cells exposed to 6 Gy γ -ray irradiation as compared to the sham irradiated cells (Fig. 4; Group 3 vs. Group 1). Supplementing the medium with 5 µM SeM for 16 days starting 2 days prior to the radiation exposure (Group 4), for 2 days prior to the radiation exposure (Group 5), for 14 days immediately after the radiation exposure (Group 6), for 12 days starting 2 days after the radiation exposure (Group 7) or for 7 days starting a week after the radiation exposure (Group 8) brought the cell survival adjusted anchorage-independent colony formation efficiency down to levels that were not significantly different from those of the sham irradiated cells cultured in control medium (Group 1) or medium supplemented with SeM (Group 2). Treatment with SeM alone (without radiation exposure) for 16 days (Group 2) had no significant effect on the adjusted anchorage-independent colony formation efficiency.
FIG. 4.
Soft agar colony formation efficiency of HTori-3 cells irradiated with γ -rays and treated with SeM. HTori-3 cells were treated as described in the legend for Fig. 3. After the radiation exposure and SeM treatment, the cells were cultured in control medium for an additional 6 wk and were then used for the soft agar colony formation assay. Each data point represents a mean of 6 replicate flasks. The standard deviation is indicated by the error bars.
To verify the tumorigenicity of the anchorage-independent colonies, the cells derived from anchorage-independent colonies established with HTori-3 cells irradiated with iron ions were inoculated into 24 athymic nude mice (3 mice per cell colony). Of the 8 anchorage-independent colonies evaluated, all produced palpable tumors in at least 1 athymic nude mouse within 8 mo after injection (Table 1). A portion of 13 of the 40 tumors excised were placed in 10% neutral buffered formalin and processed for histopathological examination using standard methods. Twelve of the 13 were scored as undifferentiated anaplastic carcinoma, which in humans would likely result in death within 12 mo. The 13th was scored as epithelial growth. The fractions of injection sites with established tumors ranged from 2 out of 6 (33%) to 6 out of 6 (100%). No palpable mass was observed at any of the 12 injection sites on 6 mice injected with cell-free PBS. The fractions of injection sites with tumors for animals injected with HTori-3 cells of anchorage-independent colonies were significantly higher than the control (P ≤ 0.005 by Fisher’s exact test) for all but 1 clone (clone-4, P = 0.098) evaluated. For all 8 clones combined, the fractions of injection sites with tumors were 30 out of 40 (67%), which was also significantly above control levels (P < 0.0001 by Fisher’s exact test). The human origin of the tumors was confirmed by Southern analysis.
TABLE 1.
Results of tumorigenicity determination in athymic nude mice
| Anchorage- Independent Clone |
Cage-Mouse ID |
Tumor at Injection Sites |
Fraction of injection sites with tumor |
Fisher’s exact test P valuea |
|
|---|---|---|---|---|---|
| Left | Right | ||||
| 1 | 1–1 | Yes | Yes | 6/6 (100%) | < 0.0001 |
| 1–2 | Yes | Yes | |||
| 1–3 | Yes | Yes | |||
| 2 | 2–1 | Yes | Yes | 2/2 (100%) | 0.0110 |
| 2–2 | Animals diedb | ||||
| 2–3 | |||||
| 3 | 3–1 | Yes | Yes | 2/2 (100%) | 0.0110 |
| 3–2 | Animals diedb | ||||
| 3–3 | |||||
| 4 | 4–1 | Yes | Yes | 2/6 (33%) | 0.0980 |
| 4–2 | No | No | |||
| 4–3 | No | No | |||
| 5 | 6–1 | Yes | Yes | 4/6 (67%) | 0.0049 |
| 6–2 | Yes | Yes | |||
| 6–3 | No | No | |||
| 6 | 8–1 | Yes | Yes | 4/6 (67%) | 0.0049 |
| 8–2 | Yes | Yes | |||
| 8–3 | No | No | |||
| 7 | 9–1 | Yes | Yes | 4/6 (67%) | 0.0049 |
| 9–2 | Yes | Yes | |||
| 9–3 | No | No | |||
| 8 | 10–1 | Yes | Yes | 6/6 (100%) | < 0.0001 |
| 10–2 | Yes | Yes | |||
| 10–3 | Yes | Yes | |||
| Saline (control) | 5–1 | No | No | 0/12 (0%) | N/A |
| 5–2 | No | No | |||
| 5–3 | No | No | |||
| 7–1 | No | No | |||
| 7–2 | No | No | |||
| 7–3 | No | No | |||
Fisher’s exact test was performed to compare the fractions of injection sites with tumors between the control group (injected with saline) and each group of mice injected with cells of anchorage-independent clones derived from HZE particle irradiated HTori-3 cells.
Animals died before any tumors became palpable.
DISCUSSION
In a previous study performed in our laboratory, exposure to 5-GeV/n iron radiation at doses up to 40 cGy significantly decreased the clonogenic survival of MCF10 human breast epithelial cells and treatment with 5-µM SeM in the medium protected MCF10 cells from 5-GeV/n iron radiation-induced cell killing with a dose-modifying factor of 2.24 (9). Exposure to 5-GeV/n iron radiation at a single dose of 125 cGy also significantly increased the yield of anchorage-independent colonies of HTori-3 human thyroid epithelial cells, which was prevented by treatment with 5-µM SeM in the medium (9). In a separate study, the effects of 1-GeV/n and 5-GeV/n iron radiation on the clonogenic survival of HTori-3 cells were compared with that of the γ -ray radiation, and the RBE of the 1-GeV/n and 5-GeV/n iron radiation were estimated to be 2.5 and 2.6, respectively (10). In addition, exposure to 0.25 GeV proton radiation at 600 cGy was also shown to increase the yield of anchorage-independent colonies of the irradiated HTori-3 cells (10). The present study extended the previous studies in 4 aspects: 1) a dose response of HTori-3 cells exposed to 1-GeV proton radiation was determined and compared to that of the HTori-3 cells exposed to γ -ray and 1-GeV/n iron radiation for determinations of the RBE values; 2) the yields of anchorage-independent colonies of HTori-3 cells exposed to γ -ray, 1-GeV proton, and 1-GeV/n iron radiation were determined at multiple radiation doses to establish the radiation dose responses; 3) the temporal effect of the SeM treatment was evaluated to determine the time window during which the SeM was effective in preventing radiation-induced HTori-3 cell transformation; and 4) the tumorigenicity of the HTori-3 cells derived from the anchorage-independent colonies was verified by inoculating the cells into athymic nude mice. In the present study, the RBE values determined based on the HTori-3 cell clonogenic survival results were 1.3 and 2.7 for the proton and iron ion radiation, respectively, indicating that 1-GeV/n iron ion radiation is more effective than 1-GeV proton radiation at killing HTori-3 cells. The RBE value determined for 1-GeV/n iron ion radiation is similar to that determined in the previous study (10).
In the soft agar colony formation assay experiments, the yield of anchorage-independent colonies of HTori-3 cells increased in a dose-dependent manner after exposure to γ -ray and proton radiation at doses up to 600 cGy. For the cells exposed to the iron ion radiation, the yield of anchorage-independent colonies increased in a dose-dependent manner up to a radiation dose of 40 cGy. The anchorage-independent colony yield in the treatment groups receiving iron ion radiation at a dose of 100 or 200 cGy was not significantly higher than that of the 40 cGy dose group, suggesting a plateau at 40 cGy. The dose-response relationships of HZE particle radiation-induced transformation have also been reported previously for Syrian hamster embryo cells exposed to carbon or silicon ion radiation (17) and 10T1/2 mouse fibroblasts exposed to helium or iron ion radiation (18). Plateaus of transformation frequency were observed in the dose-response curves for the carbon or silicon ion irradiated Syrian hamster embryo cells between doses of 5 and 10 cGy (17). In contrast, the transformation frequency increased linearly for the 10T1/2 cells irradiated with helium or iron ions with no plateau in the dose-response curves (18). The cause for the discrepancies in the shape of the dose-response (with or without a plateau) remains to be determined.
SeM was identified more than 5 decades ago in plant proteins and proteins of bacteria and yeasts grown in selenium-containing media (19). When administered orally, 95.5–97.3% of 75Se-labeled SeM was absorbed intestinally (20) via the Na+-dependent neutral amino acid transportation system (21). The maximum plasma level of the SeM is reached 3 to 4 hours after oral dosing, and urinary excretion accounted for 6–9% of the absorbed dose in the first 2 weeks (20). The absorbed SeM that is not metabolized immediately is incorporated into organs with high rates of protein synthesis (22). SeM is incorporated into proteins in place of methionine because tRNAmet does not discriminate against SeM over methionine for transmethylation (23), and the incorporation of SeM into proteins allows selenium to be stored in the body and reversibly released by the normal metabolic process. In a study performed with 4 women with each receiving an oral dose of 20 µCi 75Se-SeM, the 75Se counts in plasma and erythrocytes declined in 3 phases with half-lives ranging from 0.8 to 1.4 day for the first phase, 12 to 19 days for the second phase, and 130 to 328 days for the third phase (20). In a separate study performed with 3 men and 3 women with each receiving an oral dose of 200 µg 74S-SeM, the half-life for SeM turnover varied from 0.01 to 1.1, 1.6 to 3.1, and 61 to 86 days in plasma, liver-pancreas, and peripheral tissues, respectively (24). The much longer half-lives observed in the whole-body or in peripheral tissues indicated that SeM was used and reused extensively (20,24).
Selenium is known to have many effects relevant to carcinogenesis. SeM has been shown to be capable of regulating the cellular antioxidant defense systems and DNA chain break control (25). Selenium is particularly effective in limiting the action of chemical carcinogens during the initiation phase of liver and colorectal in vivo carcinogenesis (25,26). In the Ames’s test, which is commonly used to test chemical mutagenic activity, selenium was shown to depress the mutagenic activity of the tested chemicals in a dose-dependent manner (27). In prior in vitro experiments performed in our laboratory, treatment with SeM alone (9) or in combination with other agents having antioxidant activities (10) was shown to prevent HTori-3 cell transformation induced by iron ion radiation. In the previous study performed with HTori-3 cells irradiated with 5-GeV/n iron ion radiation, SeM treatment was initiated 18 h prior to the radiation exposure and maintained during and after the radiation exposure for the clonogenic survival and soft agar colony formation assays (9). To determine when SeM must be present in the cell culture medium to exert its protective effect on radiation-induced cytotoxicity and anchorage-independent growth, the temporal effect of the SeM treatment was determined in the present study by supplementing the medium with SeM at different time periods relative to the radiation exposure. The results indicate that treatment with SeM was effective when applied as late as a week after the radiation exposure. This observation is important in evaluating SeM as a preventive agent to protect against radiation-induced adverse biological effects, because it is not always possible to initiate SeM treatment prior to a radiation exposure, such as in circumstances of a radiation accident or a terrorist attack. Initiating SeM treatment after radiation exposure in cancer radiotherapy would allow SeM to exert its protective effects without interfering with the acute cell killing effects of radiation require for effective cancer treatment.
In the present study, the observed effect of radiation on cell survival persisted for up to at least 2 wk after proton and iron ion radiation exposure and at least 8 wk after the γ -ray radiation exposure. It is possible that the effect of γ -ray, proton, and iron ion radiation on cell survival may last longer than 2 to 8 wk, which was the last time point in the studies reported here when the irradiated cells were evaluated in the cell survival and soft agar colony formation assays. These results clearly demonstrated delayed lethal effects of radiation inHTori-3 cells. Delayed lethal effects of radiation exposure, also termed lethal sectoring (28–31), delayed cell death (32), delayed toxicity (33), or delayed lethal mutation (33–35) have also been reported previously in other experimental models with the common phenomenon of decreased plating efficiency that persists many cell generations after the radiation exposure. The delayed lethal effects of radiation exposure were observed 13–15 cell doublings after X-ray irradiation in mouse 3T3 fibroblast cells (36), and more than 30 cell population doublings after X-ray irradiation in Chinese hamster ovary cells (37). The delayed lethal effects after radiation exposure were reported to be largely due to delayed apoptosis, which occurred up to 45 cell doublings after radiation exposure (38), although a loss of cell adhesion ability to cell culture dishes might also be a contributing factor (37). Given the delayed lethal effects of the radiation exposure on cell survival, it is important to determine the plating efficiencies at the same time when irradiated cells are plated for the soft agar colony formation assay so that the yields of anchorage-independent colonies are not overestimated due to the reduced plating efficiencies, which persist for long periods after the radiation exposure.
Treatment with SeM was previously shown to improve the survival of MCF10 cells irradiated with 5-GeV/n iron ions (9). However, treatment with SeM in the present study had no significant effect on the survival of irradiated HTori-3 cells determined 8 wk after γ -ray irradiation. The cause for the discrepancy is difficult to ascertain due to multiple differences in cell lines and radiation sources used in these studies. However, it can be speculated that SeM may only protect cells against the acute effects of radiation on cell survival and have no significant protective effects against the delayed lethal effects of radiation on cell survival.
Anchorage-independent growth is a phenotypic change of cells in culture that is associated with tumorigenicity of the cells in vivo, and HTori-3 cells isolated from anchorage-independent colonies were shown to produce tumors when transplanted into athymic nude mice (12,13). In our previous studies, the yield of anchorage-independent colonies of HTori-3 cells increased significantly after irradiation with a single dose of 125 cGy 5-GeV/n iron ions (9) or 600 cGy of 250 MeV protons (10), and the increase in the 5-GeV/n iron ion radiation experiment was prevented by treatment with SeM (9). The tumorigenicity of the anchorage-independent colonies derived from irradiated HTori-3 cells was assumed in our previous studies but not verified experimentally. In the present study, HTori-3 cells derived from all 8 anchorage-independent colonies established after exposure to iron ion radiation were tumorigenic and produced palpable tumors when injected into athymic nude mice, which confirmed the tumorigenicity of the HTori-3 cells growing in the anchorage-independent colonies and indicated that the yield of anchorage-independent colonies is a reliable surrogate endpoint marker of radiation-induced malignant transformation of HTori-3 cells. Since the ability of irradiated HTori-3 cells to form anchorage-independent colonies in soft agar was effectively prevented by treatment with SeM applied as late as 7 days after the radiation exposure, the results of the present study support the use of SeM as a potential countermeasure to prevent the development of radiation-induced malignant transformation, the acquisition of the ability to form tumors in animals, and tumorigenic potential.
ACKNOWLEDGMENTS
This research was supported by a grant from NASA (NAG9–1517). We thank the staff members of the Brookhaven National Laboratory for help in the performance of these studies. In particular, we would like to acknowledge the contributions of Drs. Adam Rusek and I Hung Chiang to the research investigations described here.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Jeffrey H. Ware, Department of Radiation Oncology, the University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA
Zhaozong Zhou, Department of Radiation Oncology, the University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA.
Ana L. Romero-Weaver, Department of Radiation Oncology, the University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA
X. Steven Wan, Department of Radiation Oncology, the University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA.
Paul M. Newberne, Department of Pathology, Boston University School of Medicine, Boston, Massachusetts, USA
Ann R. Kennedy, Department of Radiation Oncology, the University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA
REFERENCES
- 1.Hellweg CE, Baumstark-Khan C. Getting ready for the manned mission to Mars: the astronauts’ risk from space radiation. Naturwissenschaften. 2007;94:517–526. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- 2.Katz R, Ackerson B, Homayoonfar M, Sharma SC. Inactivation of cells by heavy ion bombardment. Radiation Res. 1971;47:402–425. [PubMed] [Google Scholar]
- 3.Suit HD. Protons to replace photons in external beam radiation therapy? Clin Oncol. 2003;15:S29–S31. doi: 10.1053/clon.2002.0171. [DOI] [PubMed] [Google Scholar]
- 4.Kramer M, Weyrather WK, Scholz M. The increased biological effectiveness of heavy charged particles: from radiobiology to treatment planning. Tech Cancer Res Treat. 2003;2:427–436. doi: 10.1177/153303460300200507. [DOI] [PubMed] [Google Scholar]
- 5.Jongen Y. Radiotherapy systems using proton and carbon beams. Academie Royale de Medecine de Belgique. 2008;163:471–478. [PubMed] [Google Scholar]
- 6.Upton AC. Board of Radiation Effects Research, Committee on Life Sciences, National Research Council. Washington, DC: National Academy Press; 1990. Health effects of exposure to low levels of ionizing radiation. BEIR V Committee on the Biological Effects of Ionizing Radiations. [Google Scholar]
- 7.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. New York: United Nations; 1988. [Google Scholar]
- 8.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes. Volume II: Effects. New York: United Nations; 2000. [Google Scholar]
- 9.Kennedy AR, Ware JH, Guan J, Donahue JJ, Biaglow JE, et al. Selenomethionine protects against adverse biological effects induced by space radiation. Free Rad Biol Med. 2004;36:259–266. doi: 10.1016/j.freeradbiomed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy AR, Zhou Z, Donahue JJ, Ware JH. Protection against space radiation induced adverse biological effects by the Bowman-Birk inhibitor and antioxidants. Radiation Res. 2006;166:327–332. doi: 10.1667/RR3599.1. [DOI] [PubMed] [Google Scholar]
- 11.Lemoine NR, Mayall ES, Jones T, Sheer D, McDermid S, et al. Characteristics of human thyroid epithelial cells immortalized in vitro by simian virus 40 DNA transfection. B J Cancer. 1989;60:897–903. doi: 10.1038/bjc.1989.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riches AC, Herceg Z, Bryant PE, Wynford-Thomas D. Radiation-induced transformation of SV40-immortalized human thyroid epithelial cells by single and fractionated exposure to γ-irradiation in vitro. Int J Radiat Biol. 1994;66:757–765. [PubMed] [Google Scholar]
- 13.Riches AC, Herceg Z, Bryant PE, Stevens DL, Goodhead DT. Radiation-induced transformation of SV40-immortalized human thyroid epithelial cells by single exposure to plutonium alpha-particles in vitro. Int J Radiat Biol. 1997;72:515–521. doi: 10.1080/095530097143013. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Bosma MJ, Berg EAVD, Koosstro T, Siemieniak DR, Slighton JL. Human plasiminogen activator inhibitor-1 gene. J Biol Chem. 1989;263:9129–9141. [PubMed] [Google Scholar]
- 16.Casarett AP. Radiation Biology. Englewood, NJ: Prentice-Hall; 1968. [Google Scholar]
- 17.Han Z, Suzuki H, Suzuki F, Suzuki M, Furusawa Y, et al. Neoplastic transformation of hamster embyro cells by heavy ions. Advance Space Res. 1998;22:1725–1732. doi: 10.1016/s0273-1177(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 18.Miller RC, Martin SG, Hanson WR, Marino SA, Hall EJ. Effect of track structure and radioprotectors on the induction of oncogenic transformation in murine fibroblasts by heavy ions. Advance Space Res. 1998;22:1719–1723. doi: 10.1016/s0273-1177(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 19.Shrift A. Metabolism of selenium by plants and microorganisms. In: Klayman DL, Gunther WHH, editors. Organic Selenium Compounds: Their Chemistry and Biology. New York: John Wiley & Sons; 1973. pp. 763–814. [Google Scholar]
- 20.Griffiths NM, Stewart RD, Robinson MF. The metabolism of [75Se]selenomethionine in four women. Br J Nutr. 1976;35:373–382. doi: 10.1079/bjn19760043. [DOI] [PubMed] [Google Scholar]
- 21.Vendeland SC, Deagen JT, Butler JA, Whanger PD. Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rat small intestine. BioMetals. 1994;7:305–312. doi: 10.1007/BF00144126. [DOI] [PubMed] [Google Scholar]
- 22.Hansson E, Jacobsson SO. Uptake of [75Se] selenomethionine in the tissues of the mouse studied by whole-body autoradiography. Biochimica et Biophysica Acta. 1966;115:285–293. doi: 10.1016/0304-4165(66)90427-2. [DOI] [PubMed] [Google Scholar]
- 23.Mudd SH, Cantoni GL. Selenomethionine in enzymatic transmethylations. Nature. 1957;180:1052. doi: 10.1038/1801052a0. [DOI] [PubMed] [Google Scholar]
- 24.Swanson CA, Patterson BH, Levander OA, Veillon C, Taylor PR, et al. Human [74Se]selenomethionine metabolism: a kinetic model. Amer J Clin Nutr. 1991;54:917–926. doi: 10.1093/ajcn/54.5.917. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee B, Basu M, Chatterjee M. Effect of selenomethionine on N-methylnitronitrosoguanidine-induced colonic aberrant crypt foci in rats. Eur J Cancer Prev. 2001;10:347–355. doi: 10.1097/00008469-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee B, Ghosh S, Chatterjee M. Chemopreventive efficacy of selenomethionine and its role in the antioxidant defense system in 2-acetylaminofluorene-induced hepatocarcinogenesis in rats. J Experimental Therapeutics Oncol. 1996;1:209–217. [PubMed] [Google Scholar]
- 27.Hocman G. Chemoprevention of cancer: selenium. Int J Biochem. 1988;20:123–132. doi: 10.1016/0020-711x(88)90475-2. [DOI] [PubMed] [Google Scholar]
- 28.James AP, Werner MM. Radiation-induced lethal sectoring in yeast. Rad Res. 1966;29:523–536. [PubMed] [Google Scholar]
- 29.James AP, Werner MM, Saunders AS, Harris MA. Persistence of x-ray-induced lethal sectoring in yeast. Rad Res. 1968;34:475–487. [PubMed] [Google Scholar]
- 30.James AP, Inhaber ER, Prefontaine GJ, et al. Lethal sectoring and the delayed induction of aneuploidy in yeast. Genetics. 1974;77:1–9. doi: 10.1093/genetics/77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki H. Lethal sectoring, genomic instability and delayed division delay in HeLa S3 cells surviving alpha- or X-irradiation. J. Radiation Res. 2004;45:497–508. doi: 10.1269/jrr.45.497. [DOI] [PubMed] [Google Scholar]
- 32.Roy K, Kodama S, Suzuki K, Watanabe M. Delayed cell death, giant cell formation and chromosome instability induced by X-irradiation in human embryo cells. J Radiation Res. 1999;40:311–322. doi: 10.1269/jrr.40.311. [DOI] [PubMed] [Google Scholar]
- 33.Mothersill C, Crean M, Lyons M, McSweeney J, Mooney R, et al. Expression of delayed toxicity and lethal mutations in the progeny of human cells surviving exposure to radiation and other environmental mutagens. Int J Radiation Biol. 1998;74:673–680. doi: 10.1080/095530098140934. [DOI] [PubMed] [Google Scholar]
- 34.Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiation Oncol Investigat. 1997;5:106–110. doi: 10.1002/(SICI)1520-6823(1997)5:3<106::AID-ROI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Smith LE, Nagar S, Kim GJ, Morgan WF. Radiation-induced genomic instability: radiation quality and dose response. Health Physics. 2003;85:23–29. doi: 10.1097/00004032-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Gorgojo L, Little JB. Expression of lethal mutations in progeny of irradiated mammalian cells. Int J Radiation Biol. 1989;55:619–630. doi: 10.1080/09553008914550661. [DOI] [PubMed] [Google Scholar]
- 37.Chang WP, Little JB. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiation Biol. 1991;60:483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- 38.Lyne JC, Melhem MF, Finley GG, Wen D, Liu N, et al. Tissue expression of neu differentiation factor/heregulin and its receptor complex in prostate cancer and its biologic effects on prostate cancer cells in vitro. Cancer J Sci Am. 1997;3:21–30. [PubMed] [Google Scholar]