Abstract
Object
Traumatic brain injury (TBI), the third most common central nervous system (CNS) pathology, plagues 5.3 million Americans with permanent TBI-related disabilities. To evaluate injury severity and prognosis, physicians rely on clinical variables. Here we seek objective, biochemical markers reflecting molecular injury mechanisms specific to the CNS as more accurate measurements of injury severity and outcome. One such secondary injury mechanism, the innate immune response, is regulated by the inflammasome, a molecular platform that activates caspase-1 and interleukin-1β.
Methods
We investigated whether inflammasome components are present in the cerebrospinal fluid (CSF) of 23 TBI patients, and whether levels of inflammasome components correlate with outcome. We performed immunoblot analysis of CSF samples from TBI patients and non-trauma controls and assessed outcome five months post-injury by the Glasgow Outcome Scale (GOS). Data were analyzed by Mann-Whitney U tests and linear regression analysis.
Results
Patients with severe or moderate cranial trauma exhibited significantly higher CSF levels of the inflammasome proteins apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, and NAcht leucine-rich-repeat protein-1 (NALP-1) compared to non-trauma controls (P < 0.0001; P = 0.0029; P = 0.0202, respectively). Expression of each protein correlated significantly with GOS at five months post-injury (P < 0.05). ASC, caspase-1, and NALP-1 were significantly higher in the CSF of patients with unfavorable outcomes, including death and severe disability (P < 0.0001).
Conclusions
NALP-1 inflammasome proteins are potential biomarkers to assess TBI severity, outcome, and the secondary injury mechanisms impeding recovery, serving as adjuncts to clinical predictors.
Keywords: biomarkers, traumatic brain injury, inflammation, innate immunity, inflammasome
Introduction
TBI affects an estimated 1.5 million people each year and causes one third of injury-related deaths. Approximately 5.3 million Americans are living today with a permanent TBI-related disability. Predicting the severity and outcome of TBI is difficult given the lack of objective, laboratory-based biomarkers. Currently the Glasgow Coma Score (GCS) is the best available clinical predictor of injury severity; however its value is limited in patients undergoing pharmacological paralysis for intubation, as a motor score cannot be obtained1. Predicting outcome is further complicated by the heterogeneity of pathology in patients with a similar GCS. Therefore, the identification of diagnostic and prognostic biomarkers that directly reflect injury to CNS cells is imperative. Such biomarkers of TBI will enable clinicians to assess the degree of damage to the brain, relay prognostic information to the patient's family members, and target acute and chronic treatments to specific brain damage mechanisms. For the research scientist and clinician— these biomarker discoveries will advance our understanding of the pathophysiological mechanisms of TBI and allow new therapies to be developed.
Morbidity and mortality remain high after TBI due to secondary cell injury and the progressive loss of CNS tissue. The initial injury is exacerbated by a robust and poorly controlled inflammatory response, which contributes to secondary cell death in areas of the brain distant to the initial trauma. The innate immune response is activated by endogenous danger signals, termed damage-associated molecular patterns (DAMPs). DAMPs are molecules derived from injured host tissue that alert the body to impending danger by initiating an inflammatory response in the absence of microbial infection20.. Primary cell death, which occurs at the location of the initial force of injury due to immediate mechanical tissue damage, results in the release of DAMPs, including heat shock proteins, uric acid, adenosine triphosphate, and nucleic acids. DAMPs permeate throughout the extracellular space and are found in the CSF of patients following TBI5,16. Since CSF directly reflects the extracellular milieu bathing the neurons, it is likely that activation of the innate immune response by DAMPs contributes to inflammation after TBI.
DAMPs are recognized by NOD-like receptors (NLRs), a group of cytosolic pattern recognition receptors that initiate the innate immune response6. NAcht leucine-rich-repeat protein-1 (NALP-1) is a NLR that interacts with caspase-1, an inflammatory cysteine-aspartic protease, and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), an adaptor protein, to form a multi-protein complex termed the inflammasome19,25. Activation of the inflammasome leads to cleavage of pro-caspase-1 into proteolytically active subunits, which in turn cleave prointerluekins-1β and -18 (IL-1β and IL-18) into mature, active forms.
IL-1β is a critical mediator of the inflammatory response following TBI4,10. In rodents, neutralization of inflammasome components decreases contusion volume after fluid-percussion brain injury, results in tissue sparing and functional improvement after spinal cord injury, and significantly reduces the levels of proinflammatory cytokines in a mouse model of stroke2,7,8. Although IL-1β is elevated in the CSF of TBI patients21, the levels of inflammasome components in the CSF have not been studied.
Upon activation of the inflammasome by DAMPs, ASC and caspase-1 are secreted22. We thus sought to identify whether the CSF of TBI patients contained components of the inflammasome that may contribute to the pathophysiology of TBI. Our a priori hypotheses were: (a) inflammasome proteins ASC, caspase-1, and NALP-1 are acutely elevated in the CSF of TBI patients compared to non-trauma controls, and (b) there is a difference in inflammasome protein levels between functional outcomes.
Here, we report quantitative analysis of inflammasome components in the CSF from TBI patients and non-trauma controls. We correlate levels of these biomarkers with disability five months after injury, assessed by the GOS. As inflammation is a key mediator of secondary damage after TBI, analysis of inflammasome proteins may be used to assess the extent of inflammation-induced CNS damage, predict the functional outcome of patients, and direct therapeutic interventions.
Methods
Participants
TBI patients recruited for this single-center prospective, observational study from Jackson Memorial Hospital (Miami, Florida) presented with the following inclusion criteria: severe or moderate head trauma (GCS ≤ 12); age 1 month to 65 years; and ventriculostomy. Control patients required a ventriculostomy for non-traumatic pathology. Patients with acute meningitis, cerebral vasculitis, or other recent CNS infection were excluded. Written informed consent was obtained from all patients. The University of Miami’s Institutional Review Board approved the study.
Patient demographics are shown in Table 1. Nine patients, five males and four females, with a mean age of 66.3 years (range 29–91) served as controls. A total of 45 CSF samples were collected from 23 TBI patients on the day of injury and up to three days post-injury. Of the 23 TBI patients, 18 were males and five were females. The mean age was 29.78 years (range 16 to 65). The mechanisms of injury included: 18 motor vehicle accidents (MVA) (including one ATV and four motorcycle occupants); two gunshot wounds; one fall; one sports injury; and one assault. 22 of the TBI patients suffered severe brain trauma (GCS ≤ 8) and one suffered moderate brain trauma (GCS 9–12). At five months post-injury, three patients had a GOS of 1 (death), 11 patients had a GOS of 3 (severe disability), six patients had a GOS of 4 (moderate disability), and three patients had a GOS of 5 (good recovery). Within the sample of TBI patients, no patient remained with a GOS of 2 (persistent vegetative state).
Table 1.
Summary of TBI patient demographic data. GCS was obtained on admission. GOS was assessed five months post-injury. MVA= motor vehicle accident.
| Patient | Age | Gender | Race | Mechanism of Injury | GCS | GOS | Intracranial Pathology |
|---|---|---|---|---|---|---|---|
| 1 | 26 | M | White | MVA | 3 | 4 | Bilateral temporal cortical contusions, subarachnoid hemorrhage, subdural hemorrhage |
| 2 | 22 | M | Hispanic | Motorcycle Accident | 3 | 4 | Subdural hemorrhage, diffuse subarachnoid hemorrhage |
| 3 | 19 | F | Hispanic | MVA | 7 | 3 | Fronto, temporoparietal, and basal ganglia hemorrhagic contusions, frontoparietal subarachnoid hemorrhages |
| 4 | 30 | F | Hispanic | MVA | 8 | 4 | Scattered subarachnoid hemorrhage and diffuse cerebral edema |
| 5 | 21 | M | Hispanic | Motorcycle Accident | 5 | 3 | Frontal subdural hematoma and subarachnoid hemorrhage |
| 6 | 17 | F | Hispanic | MVA | 4 | 3 | Diffuse extra-axial and intraparenchymal hemorrhage |
| 7 | 26 | M | Hispanic | MVA | 8 | 3 | Diffuse subarachnoid hemorrhage, intraventricular hemorrhage, and parenchymal hemorrhagic contusions |
| 8 | 36 | M | White | ATV Accident | 3 | 5 | Subdural hemorrhage, subarachnoid hemorrhage, and diffuse cerebral edema |
| 9 | 16 | M | Hispanic | MVA | 7 | 5 | Frontotemporal subdural hematoma, frontal lobe contusion, and cerebral edema |
| 10 | 22 | M | Hispanic | Motor Vehicle Crash | 3 | 3 | Subdural hematoma |
| 11 | 36 | M | Black | Gunshot Wound | 3 | 1 | Subdural hematoma and bullet fragments in frontal lobe |
| 12 | 54 | M | Hispanic | Fall | 3 | 3 | Frontal hemorrhagic contusions, parietoocciptal hemorrhagic contusion, subdural hematoma, and brain edema |
| 13 | 62 | M | Black | MVA | 8 | 3 | Diffuse frontoparietal hemorrhage, frontal and parietal lobe hemorrhagic contusions |
| 14 | 49 | F | White | Assault | 8 | 3 | Temporal and frontal lobe hemorrhagic contusions, frontotemporal subarachnoid hemorrhage, frontal dural hematoma |
| 15 | 20 | M | Hispanic | Motorcycle Accident | 6 | 4 | Bone fragments in the frontoparietal brain parenchyma, scattered frontoparietal subarachnoid hemorrhage and hemorrhagic contusions |
| 16 | 28 | M | Hispanic | Motorcycle Accident | 4 | 3 | Subdural hemorrhage |
| 17 | 21 | M | Black | MVA | 5 | 4 | Mild cerebral edema |
| 18 | 45 | M | Hispanic | Gunshot Wound | 8 | 3 | Bullet fragments in occipital lobe, occipital subdural hemorrhage, and minimal parietal and occipital pneumocephalus |
| 19 | 21 | M | White | MVA | 3 | 3 | Diffuse axonal injury, scattered subarachnoid hemorrhage, parietal subdural hematoma, and mild hydrocephalus |
| 20 | 17 | M | Hispanic | Sports Injury | 4 | 4 | Subdural hemorrhage, subarachnoid hemorrhage, and diffuse cerebral edema |
| 21 | 19 | F | Hispanic | MVA | 11 | 5 | Bone fragments in frontal lobe parenchyma, frontal lobe contusion, edema, and pneumocephalus |
| 22 | 65 | M | White | MVA | 7 | 1 | Parietal subdural hematoma, subarachnoid hemorrhage, parietal hemorrhagic contusion, and uncal herniation |
| 23 | 18 | M | White | MVA | 3 | 1 | Diffuse axonal injury, scattered hemorrhagic contusions in frontal, temporal, parietal lobes and corpus callosum, intraventricular hemorrhage |
Sample Collection and Analysis
CSF samples were collected within 12 hours of injury and up to 72 hours after injury. Samples were centrifuged at 2000 × g for 10 min at 4°C to pellet cellular bodies and debris. Supernatants were resolved by gel electrophoresis and immunoblotted as described8. Quantification of band density was performed with UN-SCAN-IT gel digitizing software (Silk Scientific, Orem, Utah). Due to the low volume of sample available, NALP-1 was analyzed in six of the nine controls, caspase-1 was analyzed in 43 of the 45 TBI samples, and NALP-1 was analyzed in 42 of the 45 TBI samples.
Antibodies
Rabbit anti-ASC serum was prepared by Bethyl Laboratories (Montgomery, TX) as described8. Anti-caspase-1 (Epitomics, Burlingame, CA) and anti-NALP-1 (Abcam, Cambridge, MA) were purchased commercially.
Outcome Evaluation
Observers blind to biomarker concentrations assessed global outcomes 5 months post-injury with the Glasgow Outcome Score (GOS)13, assigned retrospectively using medical records.
Statistics
Statistical comparisons were made using nonparametric tests, considering the ordinal nature of the GOS variable, a non-Gaussian data set, and a small sample size. Two-tailed p values are reported for all statistical tests. CSF expression levels of ASC, caspase-1, and NALP-1 were compared between TBI patients and non-trauma controls using the Mann-Whitney U Test.
GOS were dichotomized into favorable (GOS-4 or 5) and unfavorable (GOS-3 or 1) outcome for ease of presentation and interpretation of results. Expression levels of ASC, caspase-1, and NALP-1 were compared between the outcome dichotomies using the Mann-Whitney U Test.
To control for multiple comparisons, target p values for data presented in Figures 1 and 2 were adjusted according to the sequentially selective Bonferroni method12. The adjusted target p values to reject the null hypothesis are p≤0.017 (ASC), p≤0.025 (caspase-1), and p≤0.05 (NALP-1).
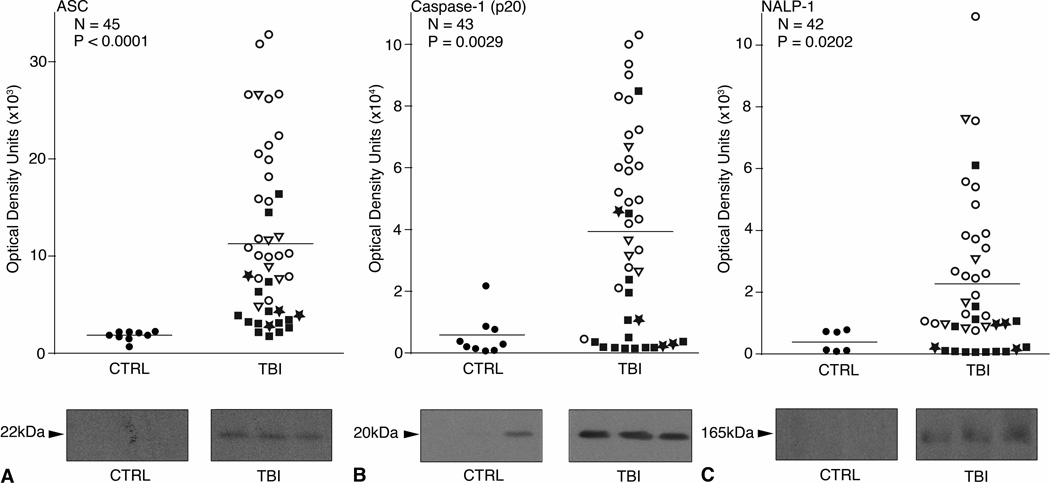
Fig. 1.
Scatter plots of expression of inflammasome proteins in control and TBI patients. N = the number of TBI samples analyzed. Samples were immuoblotted for (A) ASC, (B) Caspase-1, and (C) NALP-1. P values in the upper left corner represent results of a Mann-Whitney U test. Densitometric analysis revealed a significant increase in expression of ASC, Caspase-1 (p20), and NALP-1 in the CSF of TBI patients compared to non-trauma controls. Solid lines denote mean values for each group. Different shapes correspond to patient outcomes at 5 months post injury. ★ GOS-5.■ GOS-4.○ GOS-3. ▽ GOS-1. Representative immunoblots are shown. Samples were run on the same gel but were noncontiguous.
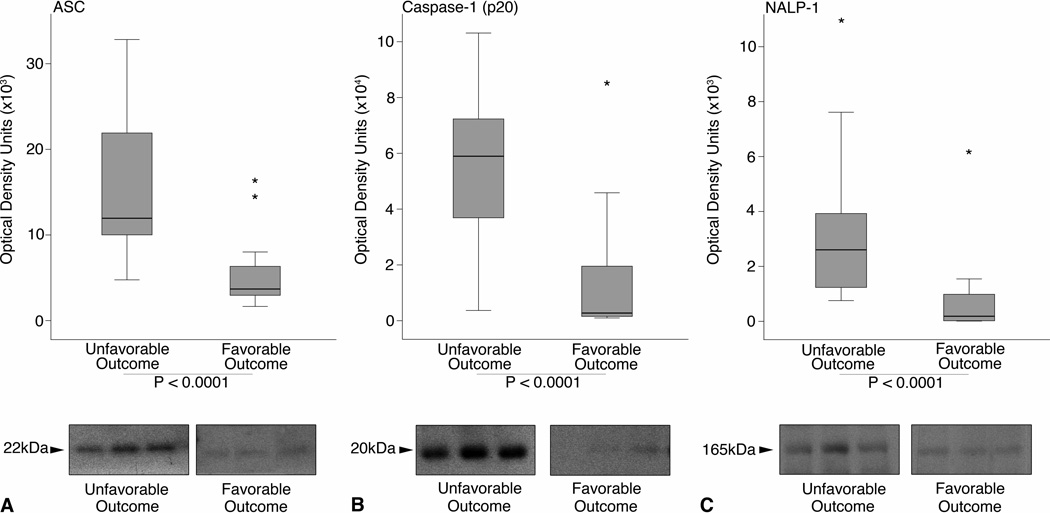
Fig. 2.
Boxplots of expression of inflammasome proteins sorted by outcome category. The ends of the whiskers represent the lowest datum within 1.5 interquartile range (IQR) of the lower quartile and the highest datum within 1.5 IQR of the upper quartile. Outliers are represented as *. Mann-Whitney U tests indicate higher expression of (A) ASC, (B) Caspase-1 (p20), (C) and NALP-1 are significantly associated with an unfavorable outcome 5 months after injury (P < 0.0001). Representative immunoblots of each protein are shown. Samples were run on the same gel but were noncontiguous.
Correlations between GOS and CSF expression levels of ASC, caspase-1, and NALP-1 were analyzed by linear regression, although GOS is an ordinal variable. A Kruskal-Wallis test with post-hoc comparisons was utilized to analyze expression levels of ASC, caspase-1, and NALP-1 for each GOS category. Dunn’s post-hoc tests controlled for multiple comparisons.
Results
Immunoblot analysis shows the inflammasome proteins ASC, caspase-1 (p20), and NALP-1 are present in the CSF of TBI patients and non-trauma controls. Quantitative data from a densitometric analysis are shown in Figure 1. Expression of the 22kDa isoform of ASC (Figure 1A), the p20 subunit of cleaved caspase-1 (Figure 1B), and NALP-1 (Figure 1C) is significantly elevated in the CSF of TBI patients compared to non-trauma controls (P<0.0001; P=0.0029; P=0.0202, respectively).
To determine if the levels of inflammasome components correlate with outcome, we grouped study subjects by outcome category (GOS-1 and 3=unfavorable outcome; GOS-4 and 5=favorable outcome). We detected significantly higher levels of ASC (Figure 2A), caspase-1 (p20) (Figure 2B), and NALP-1 (Figure 2C) in the CSF of TBI patients with unfavorable outcomes, including death and severe disability with complete dependence on others for activities of daily living (P < 0.0001).
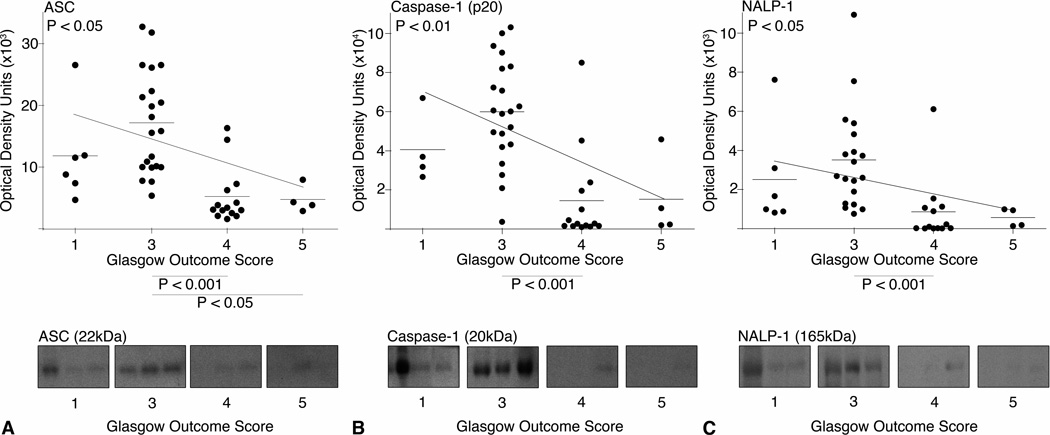
To further understand the relationship between outcome and inflammasome proteins, we constructed modified scatter plots of expression levels of ASC, caspase-1 (p20), and NALP-1 and GOS (Figure 3). A calculated linear regression line is shown for each plot. Linear regression analysis shows that expression of ASC (Figure 3A; P<0.05), caspase-1 (p20) (Figure 3B; P<0.01), and NALP-1 (Figure 3C; P<0.05) correlate significantly with outcome at five months. Post-hoc, Dunn’s multiple comparison tests following a Kruskal-Wallis test showed that the levels of ASC are significantly higher in patients with severe disability (GOS = 3) compared to patients with moderate disability (GOS = 4) (P<0.001) and patients with mild to no disability (GOS = 5) (P<0.05). Similarly, expression levels of caspase-1 (p20) and NALP-1 are significantly higher in patients with severe disability (GOS = 3) compared to patients with moderate disability (GOS = 4) (P<0.001).
Fig. 3.
Scatter plots and estimated linear regression of (A) ASC, (B) Caspase-1 (p20), and (C) NALP-1 expression in the CSF with GOS. P values of the linear regression are shown in the top left of each graph. Expression of each protein correlated significantly with GOS at 5 months post-injury. The P values on the X-axis represent post-hoc comparisons of a Kruskal-Wallis test. Representative immunoblots are shown. Samples were run on the same gel but were noncontiguous.
Discussion
Inflammasome Proteins are Acutely Elevated in the CSF of TBI Patients
Inflammation contributes strongly to chronic neuronal cell death after TBI17. We therefore analyzed protein components of the innate immune response to tissue injury in the CSF of TBI patients and non-trauma controls. ASC is an adaptor molecule that contributes to inflammation in neurons and astrocytes by recruiting pro-caspase-1 to the inflammasome24. Upon inflammasome activation, ASC oligomerizes into a supramolecular complex that serves as a platform for caspase-1 activation9. ASC is elevated in cortical homogenates of rats after spinal cord injury and TBI, and immunocytochemistry reveals ASC expression is increased specifically in neurons following CNS traumatic injury7,8. Our finding that ASC is elevated in the CSF of TBI patients within 72 hours of injury, coupled with the existing data, suggests ASC plays a role in the acute phase of TBI.
The p20 subunit is a component of active caspase-1, which cleaves pro-IL-1β into mature, active IL-1β. IL-1β is a mediator of the inflammatory response to TBI, contributing to post-traumatic hyperthermia, blood-brain-barrier permeability, leukocyte recruitment, and further cytokine production23. Thus, levels of caspase-1 (p20) in the CSF reflect the robust activation of inflammation in the CNS following TBI.
NALP-1 is a pattern recognition receptor and a member of the NOD-like receptor family, critical in innate immune responses to tissue injury. NALP-1 forms an inflammasome with ASC and caspase-1, and antibody-mediated neutralization of the NALP-1 protein reduces the production of inflammatory cytokines in a mouse model of stroke2. While ASC and caspase-1, studied predominantly in macrophages, are secreted upon activation of the inflammasome22, NALP-1 has not been identified in the supernatant of stimulated cells. Qu and colleagues (2007) propose exosomes containing assembled inflammasome complexes are packaged into multivesicular bodies that fuse with the plasma membrane and release inflammasome proteins into the extracellular space. This is one potential mechanism for releasing proteins into the CSF, though further studies are needed. In sum, elevation of ASC, active caspase-1, and NALP-1 in the CSF following TBI may reflect the extent of neuroinflammation, suggesting inflammasome components are acute biomarkers of CNS injury.
Lower Levels of Inflammasome Proteins are Associated with Favorable Outcomes after Moderate and Severe TBI
We found that inflammasome proteins are significantly higher in the CSF of patients with death and severe disability compared to patients with moderate to no disability. The data suggest inflammasome activation produces chronic neuroinflammation, contributing to secondary injury and poor outcome five months after TBI. The extent of acute elevation of these proteins potentially predicts an unfavorable vs. favorable outcome. Such markers could also direct treatment and rehabilitation efforts. The clinician would target therapies to patients identified has having a greater risk of inflammation-mediated secondary injury. Response to treatment could be monitored by following the levels of ASC, active caspase-1, and NALP-1 in the CSF. One such treatment, therapeutic hypothermia, attenuates the endogenous inflammatory response of the CNS to TBI by decreasing cytokine production and reducing activation of astrocytes and microglia3,11,15,28; and, cortical neurons exposed to moderate hypothermia in culture show a decrease in activation of the inflammasome27. Looking forward, ASC, active caspase-1, and NALP-1 could serve as objective, biochemical indicators of treatment efficacy. Additional studies that employ a larger sample size and extended post-injury time points are needed to explore this potential.
There is a significant, positive correlation between the acute levels of inflammasome proteins in the CSF and the long-term functional outcome of TBI patients. However, post-hoc comparisons did not identify a significant difference between GOS-3 and GOS-5 for caspase-1 or NALP-1. Note that samples were limited— few patients had mild to no disability at follow-up. No significant differences were found between GOS-3, 4, or 5 and GOS-1 (death). TBI is a highly heterogeneous disease, and the pathology of the injury may be clinically devastating while causing only a modest release of inflammatory mediators. For example, a relatively small lesion affecting the brainstem and not triggering massive release of inflammatory mediators into the CSF could still be incompatible with life. Indeed, one subject represented near the lowest datum point in GOS-1 for each protein in Figure 3 suffered an uncal herniation that compressed the brainstem. The area of lesioned brain may have been small and the levels of biomarkers relatively low, but the neurological and functional impact was significant. These findings highlight the need for multiple clinical, radiological, and biochemical assessments to determine prognosis. Another subject, also near the lowest datum point in GOS-1 in Figure 3, suffered from diffuse axonal injury. This patient had only a modest increase in ASC, caspase-1, and NALP-1 in his CSF. His poor outcome was likely determined by chronic Wallerian degeneration rather than the acute release of inflammatory mediators. Note this patient received therapeutic hypothermia prior to the collection of CSF, possibly attenuating the inflammatory response to injury and release of biomarkers. No other subjects were treated with hypothermia.
While our findings illustrate a diagnostic potential for these proteins in distinguishing patients with and without a TBI, it remains to be seen if the same proteins distinguish a TBI patient from one experiencing an acute cerebrovascular accident (CVA). The inflammasome mediates the acute inflammatory response to a variety of insults, not just trauma26, and may be elevated in the CSF of patients with other severe disruptions to the brain parenchyma. This unknown should be explored in future studies that include non-trauma control patients whose brain parenchyma was still violated (i.e. CVA, tumor resection, abscess, etc.).
Conclusion
Inflammasome proteins are acutely elevated in the CSF of patients suffering from a TBI compared to non-trauma controls, and the degree of elevation correlates significantly with long-term functional outcome. Our findings are clinically relevant, as CSF biomarkers are more specific indicators of neuropathology than serum biomarkers. CSF directly bathes the brain, closely reflecting the extracellular milieu and biochemical changes that are specific to the CNS. Sampling the CSF eliminates influences of multi-organ trauma or other systemic pathology represented in the serum— significant as TBI patients often present with trauma to other organ systems.
Diagnosis of TBI severity and prediction of recovery currently require neurological evaluation. If developed, biomarkers could also predict the initial damage to the brain and recovery potential. In the acute phase of TBI, the physician could rely on the predictive value of these biomarkers in choosing a neuroprotective treatment. Therefore, an early, accurate test designed to measure inflammasome proteins as biomarkers of TBI should provide a most desirable prognostic tool.
One challenge facing biomarker research in any field is the multitude of promising preliminary studies that upon further investigation are difficult to validate. However, the potential benefits to the patient provide sufficient justification to continue to explore this field. To illustrate, a key example of the power of validated biomarkers is the simple test of serum cholesterol, identified as a biomarker of cardiovascular disease by the Framingham Heart Study 13 years after the first patient was enrolled14. Here, we demonstrate a potential for inflammasome proteins as diagnostic and prognostic biomarkers in TBI, whereby better outcomes are associated with lower levels of ASC, caspase-1 (p20), and NALP-1 in the CSF. We hope our findings drive further investigation to validate inflammasome proteins as predictive markers of outcome and optimize an assay for clinical use.
Acknowledgements
The authors wish to thank Zsuzsanna Nemeth, Rebecca Safon, and Dr. Michael Wang for their assistance in enrolling patients in the study.
This study was supported by funds from the Miami Project to Cure Paralysis.
Footnotes
Portions of this work will be presented in poster form at the National Neurotrauma Symposium, National Neurotrauma Society, July 2012.
Disclosure
Stephanie Adamczak, Juan Pablo de Rivero Vaccari, M. Ross Bullock, W. Dalton Dietrich, and Robert Keane hold a patent on inflammasome proteins as diagnostic and prognostic indicators following central nervous system trauma.
References
- 1.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Initial management. J Neurotrauma. 2000;17:463–469. doi: 10.1089/neu.2000.17.463. [DOI] [PubMed] [Google Scholar]
- 2.Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 3.Aibiki M, Maekawa S, Ogura S, Kinoshita Y, Kawai N, Yokono S. Effect of moderate hypothermia on systemic and internal jugular plasma IL-6 levels after traumatic brain injury in humans. J Neurotrauma. 1999;16:225–232. doi: 10.1089/neu.1999.16.225. [DOI] [PubMed] [Google Scholar]
- 4.Clausen F, Hanell A, Israelsson C, Hedin J, Ebendal T, Mir AK, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2011;34:110–123. doi: 10.1111/j.1460-9568.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- 5.Cristofori L, Tavazzi B, Gambin R, Vagnozzi R, Signoretti S, Amorini AM, et al. Biochemical analysis of the cerebrospinal fluid: evidence for catastrophic energy failure and oxidative damage preceding brain death in severe head injury: a case report. Clin Biochem. 2005;38:97–100. doi: 10.1016/j.clinbiochem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frugier T, Morganti-Kossmann MC, O'Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27:497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- 11.Goss JR, Styren SD, Miller PD, Kochanek PM, Palmer AM, Marion DW, et al. Hypothermia attenuates the normal increase in interleukin 1 beta RNA and nerve growth factor following traumatic brain injury in the rat. J Neurotrauma. 1995;12:159–167. doi: 10.1089/neu.1995.12.159. [DOI] [PubMed] [Google Scholar]
- 12.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 13.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 15.Kumar K, Wu X, Evans AT. GFAP-immunoreactivity following hypothermic forebrain ischemia. Metab Brain Dis. 1997;12:21–27. doi: 10.1007/BF02676351. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Stange C, Wisniewski SR, Adelson PD, Janesko-Feldman KL, Brown DS, et al. Mitochondrial heat shock protein 60 is increased in cerebrospinal fluid following pediatric traumatic brain injury. Dev Neurosci. 2006;28:336–341. doi: 10.1159/000094159. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci. 2009;14:3795–3813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- 18.Marr ACV. Central Nervous System Injury Surveillance Data Submission Standards. Atlanta, GA: National Center for Injury Prevention and Control; 2002. [Google Scholar]
- 19.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 20.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 21.McClain CJ, Cohen D, Ott L, Dinarello CA, Young B. Ventricular fluid interleukin-1 activity in patients with head injury. J Lab Clin Med. 1987;110:48–54. [PubMed] [Google Scholar]
- 22.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 24.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 26.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 27.Tomura S, Vaccari JPdR, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism. 2012 doi: 10.1038/jcbfm.2012.99. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–134. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]