Abstract
Background/Aims
Hypercholesterolemia in midlife increases risk for Alzheimer’s disease (AD) and contributes to cerebrovascular dysregulation - an early finding in preclinical AD pathology. Statins improve vascular reactivity, but it is unknown if they increase regional cerebral blood flow (CBF) in individuals at risk for AD.
Methods
In a randomized, controlled, double-blind pilot study, 16 asymptomatic middle-aged adults with parental history of AD were randomized to atorvastatin or placebo daily for 4 months. At baseline and month 4, regional CBF was measured using arterial spin-labeling magnetic resonance imaging and endothelial function was measured using brachial artery ultrasound.
Results
At baseline, participants with low HDL-cholesterol, higher global vascular risk, and greater endothelial dysfunction had reduced regional CBF in areas of the brain related to memory and learning (all p<0.03). Using voxel-based analysis, 4 months of atorvastatin increased CBF in bilateral hippocampi, fusiform gyrus, putamen and insular cortices compared to placebo.
Conclusion
In this pilot study, atorvastatin increased regional CBF in persons at risk for AD. Further research is warranted to confirm whether statins increase CBF in areas of the brain related to memory and learning and whether such perfusion changes are associated with a delay in the onset of AD.
Keywords: Alzheimer’s disease, cerebral blood flow, dementia, MRI perfusion, prevention, statins
INTRODUCTION
Alzheimer’s disease is a devastating illness that will affect a rapidly increasing number of older adults in the coming decades, unless effective preventive strategies are developed. Lack of effective preventive therapies coupled with the incipient projected dramatic increase in the number of persons with AD in the coming decades has created an imperative to quickly find effective treatment strategies to prevent or delay the disease. A delay in onset of just 5 years has been projected to reduce the prevalence of AD by half. (1)
Midlife atherosclerotic vascular disease risk factors, including hypercholesterolemia, are risk factors for AD and potential targets for AD prevention strategies. (2–4) Hypercholesterolemia is associated with oxidative stress and endothelial dysfunction in cerebral arterioles, even in the absence of atherosclerotic lesions. (5) Cerebrovascular dysregulation is an early finding in preclinical AD. (6) Persons with AD and at risk for the disease have regional cerebral hypoperfusion in areas of the brain related to memory and learning. (7, 8) Although these reductions in cerebral blood flow (CBF) are not sufficient to produce acute ischemic injury, chronically reduced CBF may lead to neuronal dysfunction and alter cognitive function.
Statins improve CBF in animals (9) and are associated with a reduced prevalence of AD in adults, (10, 11) although not all studies support that statin use is related to protection against dementia. (12) It is unknown, however, whether statins increase regional CBF in asymptomatic persons at risk for AD or whether such increases protect against cognitive decline.
The primary aim of this 4-month randomized, placebo-controlled, double-blind pilot study was to evaluate the effects of atorvastatin vs. placebo on regional CBF using arterial spin-labeling magnetic resonance imaging (ASL-MRI) in middle-aged adults at increased risk for AD. (13, 14) In order to investigate mechanisms through which statins may modify cerebral perfusion, a secondary aim was to assess the association of regional CBF with brachial artery reactivity, a measure of endothelial function.
METHODS
Participants
Seventeen asymptomatic adults (ages 38–66 years) with parental history of AD were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) or from the community following the screening of 24 individuals. (13) Sixteen subjects completed the baseline assessment and were included in this analysis. Prior to enrollment, a diagnosis of definite or probable AD in one or both parents was confirmed using the NINCDS-ADRDA criteria (15) through clinical evaluation and/or chart or autopsy review by physicians and neuropsychologists with expertise in the diagnosis of dementia. Persons with contraindications to MRI or statins were excluded from participation. The University of Wisconsin Human Subjects Committee approved this study which was conducted in accordance with the standards within the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from each participant.
Study Design
Data were collected at the University of Wisconsin Hospital and General Clinical Research Center. Participants completed three visits during the 4-month study (baseline, month 1, and month 4). At baseline and month 4, all participants had fasting blood samples, MRI perfusion images, and brachial arterial function measured. Framingham 10-year cardiovascular disease (CVD) risk, a commonly used clinical measure of overall vascular risk, was calculated using age, total cholesterol, smoking status, high-density lipoprotein cholesterol (HDL-C), and systolic blood pressure (treated vs. untreated). (16)
At baseline, participants were randomized in a 1:1 ratio to receive atorvastatin 40 mg nightly (n=8) or matching placebo (n=8). Randomization was conducted by the University of Wisconsin Pharmaceutical Research Center and was stratified based on gender and apolipoprotein E ε4 (APOE4) allele status (positive or negative), a key genetic risk factor for AD. Participants and investigators were blinded to study medication identity and cholesterol-lowering effects during the study.
Laboratory Evaluation
Blood was collected for assay following a 12-hour fast. Total cholesterol, triglycerides, HDL-C, creatine kinase (CK), aspartate aminotransferase (AST) and alanine aminotranferease (ALT) levels were measured using enzymatic precipitation techniques. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. (17) APOE4 allele was measured using standard PCR and DNA sequencing techniques.
MRI Acquisition
At baseline and month 4, participants had an MR imaging protocol on a 3T scanner (GE Signa, Wakesha, WI), including a three plane localizer for slice selection; a sagittal T1 fast spoiled gradient localizer; an axial T2 fluid attenuation inversion recovery; and a pseudo-continuous ASL scan using a 3D fast spin echo sequence. Continuous labeling was performed with a published method for multi-slice spin labeling with a single coil. (18) Imaging parameters and post-processing methods have been described previously. (19) Three ASL scans were averaged to generate final perfusion-weighted images. CBF maps were normalized to a standard PET MNI template and then smoothed with an 8 mm full width at half maximum Gaussian kernel in SPM5.
Non-Invasive Brachial Arterial Function Testing
At baseline and month 4, B-mode and Doppler ultrasound were used to measure flow-mediated vasodilation (FMD) of the brachial artery. Brachial artery diameters and blood flows were imaged with a Siemens Acuson Sequoia C512 ultrasound machine and an 8 MHz linear array vascular transducer. A standardized protocol was used to induce reactive hyperemia. In a temperature-controlled room, participants were placed in the supine position for 10 minutes prior to imaging. A small forearm pressure cuff was placed on the widest part of proximal right forearm. Optimal images of the anterior and posterior walls were identified. Image location was documented for future image comparison. Video loops of B-mode images of the brachial artery were acquired and stored digitally using AccessPoint from Kardia-Freeland (Westfield, IN). The forearm pressure cuff was inflated to 250 mmHg for 5 minutes. At 60 seconds after cuff deflation, repeat imaging was obtained. FMD was calculated as the ratio of the brachial artery diameter after reactive hyperemia to the baseline diameter, expressed as a percent change. Intraobserver agreement in brachial artery measurements, as measured by an intraclass correlation coefficient from a nested two-way ANOVA, was 0.987 demonstrating a high level of reproducibility. (20)
Statistical Analysis
Non-parametric methods were employed to limit the impact of outliers in this pilot clinical trial. Pearson chi-square statistics (for categorical variables) and Wilcoxon statistics (for continuous variables) were used to compare baseline characteristics between treatment groups and the effects of treatment on changes in cholesterol levels. Mean global and regional CBF values were generated for the following standardized regions of interest in MNI space (21): hippocampus, parahippocampus, posterior cingulate, parietal, thalamus, and cingulate. Correlations between variables were computed using the Spearman rank correlation coefficients method. The effects of statin therapy on FMD measurements were adjusted for baseline vessel diameter using nonparametric Wilcoxon score general linear models. (22)
Treatment effect was evaluated using voxel-based analyses with the Mann-Whitney test assessing the ratio of ASL CBF at month 4/baseline. Between subjects multiple comparisons were only performed within gray matter (GM) tissue using the standardized GM ROI described in Maldjian et al. (21) A voxel level threshold of pvoxel <0.05 with extent threshold for cluster size > 1200 voxels was applied (AlphaSim, AFNI) with a false discovery rate (FDR) threshold of p<0.05.
RESULTS
Baseline participant characteristics were similar between treatment groups (Table 1). On average, participants were middle-aged, overweight, prehypertensive, and had lipid values similar to mean levels noted in adults in the United States. (23) Given their parental history of AD, participants had higher APOE4 allele prevalence (25%), a risk factor for both AD and vascular dysfunction, than is seen in the general population (~15%). Framingham risk scores were low, (16) yet decreased mean FMD values suggested that participants had impaired endothelial function and increased vascular risk (Table 1). (24)
Table 1.
Baseline Participant Characteristics
| Characteristics | Atorvastatin (N=8) | Placebo (N=8) | p Value |
|---|---|---|---|
| Age, mean (SD), y | 51.9 (10.0) | 54.0 (5.9) | 0.527 |
| Women, No. (%) | 5 (62.5) | 5 (62.5) | 1.000 |
| Education, mean (SD), y | 15.4 (2.2) | 16.0 (2.8) | 0.745 |
| MMSE score, mean (SD) | 29.1 (0.8) | 29.6 (0.5) | 0.203 |
| Caucasian, No. (%) | 7 (87.5) | 7 (87.5) | 1.000 |
| APOE4 carrier, No (%) | 2 (25.0) | 2 (25.0) | 1.000 |
| Current smoker, No. (%) | 2 (25.0) | 1 (12.5) | 0.522 |
| Body mass index, mean (SD), kg/m2 | 28.6 (6.9) | 27.6 (3.7) | 1.000 |
| Systolic blood pressure, mean (SD), mm Hg | 127.4 (22.3) | 124.8 (10.0) | 0.753 |
| Current use of antihypertensive medications, No. (%) | 1 (12.5) | 1 (12.5) | 1.000 |
| Physical exercise per week (30+min), median | 4 | 4 | 1.000 |
| Total cholesterol, mean (SD), mg/dL [mmol/L] |
200.0 (29.4) [5.2 (0.8)] |
205.5 (38.0) [5.3 (1.0)] |
0.674 |
| Triglycerides, mean (SD), mg/dL [mmol/L] |
133.3 (68.5) [1.5 (0.8)] |
85.5 (36.6) [1.0 (0.4)] |
0.142 |
| High-density lipoprotein cholesterol, mean (SD), mg/dL [mmol/L] |
63.3 (23.3) [1.6 (0.6)] |
69.3 (15.5) [1.8 (0.4)] |
0.636 |
| Low-density lipoprotein cholesterol, mean (SD), mg/dL [mmol/L] |
110.6 (22.2) [2.9 (0.6)] |
119.1 (37.2) [3.1 (1.0)] |
1.000 |
| Framingham 10-Year CVD Risk, mean (SD), % | 3.6 (3.8) | 3.3 (3.7) | 0.537 |
| Flow-mediated vasodilation (60 sec), mean (SD), % | 3.3 (2.6) | 4.6 (2.2) | 0.248 |
| Global CBF, mean (SD), ml/100g/min (n=14) | 66.0 (17.3) | 61.5 (9.4) | 0.606 |
Values are means (SD) unless otherwise indicated.
MMSE=Mini Mental State Examination; APOE4=apolipoprotein E ε4 allele; HDL-C=high density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; CVD=cardiovascular disease; CBF=cerebral blood flow.
All 16 participants completed the study. Complete ASL-MRI data (baseline and month 4) were available on 13 participants. Technical software problems and poor image quality led to the loss of two baseline images (both in atorvastatin arm) and one at month 4 (placebo arm).
Baseline Perfusion Measures
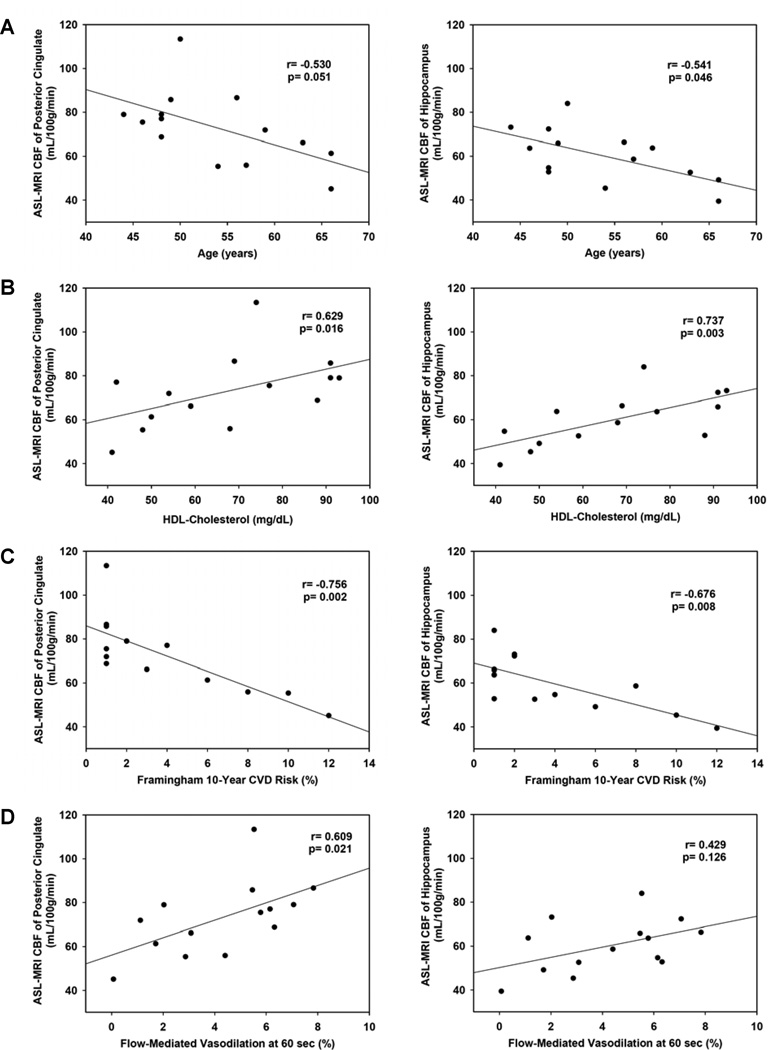
Evaluation of the baseline relationships between participant characteristics and mean regional CBF measures showed that increasing age, low HDL-C, higher Framingham 10-year CVD risk, and worse endothelial function, as measured by FMD, correlated with reduced regional CBF (Figure 1). LDL-C was not significantly related to CBF.
Figure 1.
A. Baseline age correlated inversely with baseline CBF in the posterior cingulate (shown, r=−0.530, p=0.051), hippocampus (shown, r=−0.541, p=0.046), parahippocampus (r=−0.577, p=0.031), thalamus (−0.546, p=0.043), and the cingulate gyrus (r=−0.566, p=0.035). B. Baseline HDL-C correlated directly with baseline CBF in the posterior cingulate (shown, r=0.629, p=0.016), hippocampus (shown, r=0.737, p=0.003), parahippocampus (r=0.722, p=0.004), and thalamus (r=0.607, p=0.021), as well as with global perfusion (r=0.651, p=0.012). C. Higher baseline Framingham 10-year CVD risk correlated with worse baseline CBF in the posterior cingulate (shown, r=−0.756, p=0.002), hippocampus (shown, r=−0.676, p=0.008), parahippocampus (r=−0.712, p=0.004), parietal lobe (r=−0.751, p=0.002), thalamus (r=−0.770, p=0.001), cingulate gyrus (r=−0.712, p=0.004), superior parietal lobe (r=−0.551, p=0.041), and inferior parietal lobe (r=−0.616, p=0.019), as well as with global perfusion (r=−0.719, p=0.004). D. At baseline, FMD at 60 seconds was directly correlated with baseline CBF in the posterior cingulate (shown, r=0.609, p=0.021), thalamus (r=0.701, p=0.005) and cingulate gyrus (r=0.675, p=0.008) and showed a trend toward a positive correlation in the parahippocamus (r=0.512, p=0.061), parietal lobe (r=0.512, p=0.061) and on global perfusion (r=0.516, p=0.059). FMD at baseline did not correlate with baseline CBF in the hippocampus (shown, r=0.429, p=0.126).
Effects of Atorvastatin on Lipoprotein Levels and Arterial Function
Atorvastatin significantly reduced mean serum total cholesterol (−30%), triglycerides (−37%), and LDL-C levels (−49%) (all p<0.03), compared to placebo (Table 2). Four months of atorvastatin use did not significantly affect FMD compared to placebo in this pilot study (Table 2).
Table 2.
Effects of Atorvastatin vs. Placebo on Changes in Outcome Measures Compared to Baselin
| Characteristics | Atorvastatin Group | Placebo Group |
p Value (difference between groups) |
|---|---|---|---|
| N=8 | N=8 | ||
| Serum Cholesterol Levels | |||
| Serum total cholesterol, mean change (SD), mg/dL [mmol/L] |
−59.1 (28.3) [−1.5 (0.7)] |
−1.8 (13.6) [0.0 (0.4)] |
0.002 |
| Serum triglycerides, mean change (SD), mg/dL [mmol/L] |
−49.1 (36.7) [−0.6 (0.4)] |
−12.9 (19.4) [−0.1 (0.2)] |
0.024 |
| Serum HDL-C, mean change (SD), mg/dL [mmol/L] |
3.9 (9.3) [0.1 (0.2)] |
−1.0 (7.7) [0.0 (0.2)] |
0.226 |
| Serum LDL-C, mean change (SD), mg/dL [mmol/L] |
−53.8 (21.5) [−1.4 (0.6)] |
2.0 (11.6) [0.0 (0.3)] |
0.001 |
| Peripheral Vasoreactivity Measures | |||
| Flow-mediated vasodilation (60 sec), mean change (SD), % | −0.4 (2.1) | 0.3 (2.3) | 0.462 |
| Global MRI Cerebral Perfusion Measures | |||
| Global CBF, mean change (SD), ml/100g/min (n=13) | −5.0 (18.8) | −14.0 (16.7) | 0.199 |
HDL-C=high density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; CBF=cerebral blood flow; ASL-MRI=arterial spin-labeling magnetic resonance imaging.
Effects of Atorvastatin on Cerebral Perfusion
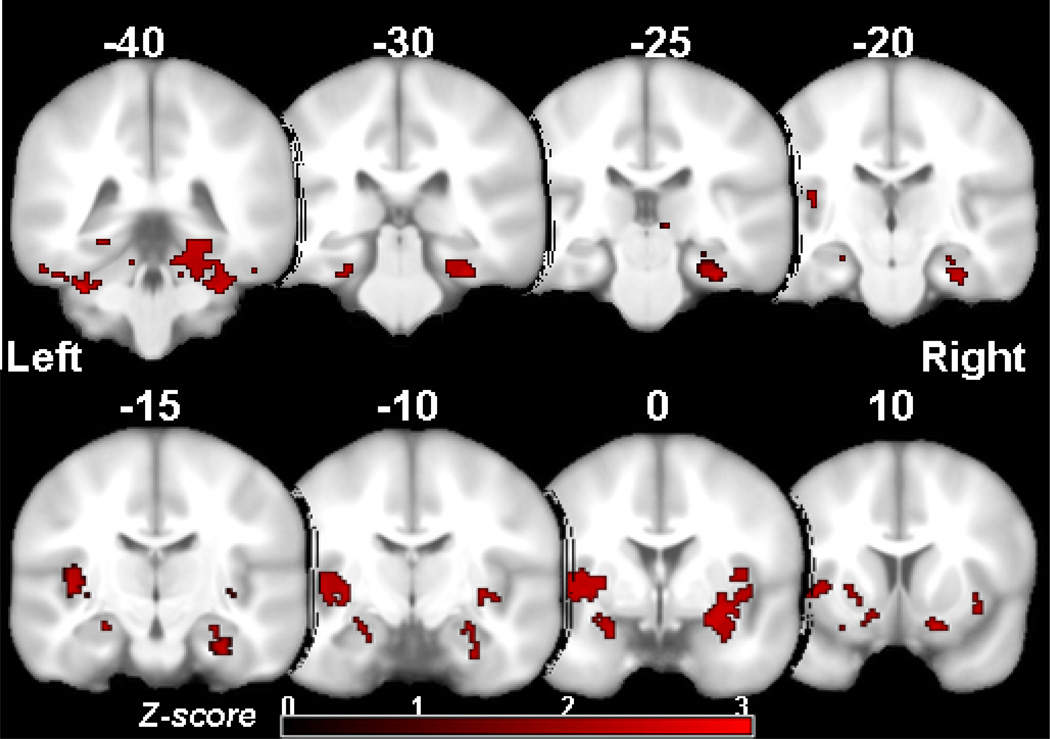
Using voxel-wise analyses, participants randomized to atorvastatin showed a greater increase in regional CBF in bilateral hippocampi, fusiform gyrus, putamen and insular cortices compared to participants on placebo (Figure 2). Mean global CBF changes at month 4 compared to baseline were not significantly different between the atorvastatin and placebo groups (Table 2).
Figure 2.
Participants randomized to atorvastatin treatment showed greater increase in regional CBF in bilateral hippocampus, fusiform gyrus, putamen and insular cortices compared to participants on placebo (pvoxel <0.05 with extent threshold for cluster size > 1200 voxels).
DISCUSSION
In this pilot clinical trial of asymptomatic middle-aged adults at risk for AD, participants randomized to four months of atorvastatin had greater increases in regional CBF in bilateral hippocampi, fusiform gyrus, putamen and insular cortices compared to placebo. Other studies have demonstrated relative hypoperfusion in the posterior cingulate, and the parietal and medial temporal lobes in adults with mild cognitive impairment (MCI) (7), cognitively healthy APOE4 allele carriers (8), and non-demented persons with memory complaints who later develop AD (25). Given that cerebrovascular dysregulation is one of the earliest changes in AD pathology, (6) targeting regional CBF may be a promising prevention strategy to delay or prevent AD. While findings from recent clinical trials suggest that statins have little effect in slowing cognitive decline in AD patients with advanced neurodegeneration, (26, 27) the role of these medications in preventing or delaying the onset of AD remains promising. Our findings of increased hippocampal perfusion with atorvastatin in asymptomatic adults at risk for AD suggest that further research is warranted in a larger more definitive trial to clarify if statins can not only increase CBF, but also whether such changes in perfusion are associated with cognitive benefits and reduced AD risk.
In addition to possibly improving cerebral perfusion, other studies support that statins have additional pleiotropic effects that could lead to neuroprotection and reduced AD risk, including beneficial changes on β-amyloid (28, 29), decreased inflammation (30), and potential direct cognitive benefits. (31, 32) Studies are underway to clarify the impact of statins on these AD-related processes and whether modifying these potential mechanisms of disease leads to long-term improved cognition and reduced risk for AD.
Other studies targeting different patient populations support our pilot study findings that short-term statin therapy is effective in improving CBF (9, 33–36). In two clinical studies, adults with underlying subcortical small vessel disease showed improved CBF in the middle cerebral artery after only 2–3 months of statin therapy (34, 35). In a trial of healthy adults, improvements in cerebral vasoreactivity were noted after 14 days of statin use (36). Our findings are consistent with these studies yet expand their findings to a unique population of asymptomatic middle-aged adults at increased risk for AD. The absence of an effect of statin therapy on the markers of endothelial function in our study is likely explained by the small sample size and by our subjects’ normal baseline cholesterol values. Larger studies of longer duration statin therapy are underway to evaluate the relationship between changes in FMD, CBF, and AD risk.
Asymptomatic adult children of persons with AD show early neuropathological changes consistent with AD even in the preclinical phase (14, 31) and, thus, are an ideal group to study for preventive therapies. Despite having fairly low vascular risk on their Framingham 10-year CVD risk assessment, our participants had reduced FMD suggesting increased risk for future vascular events. (24) Participants in our study who were older, had lower HDL-C, worse endothelial function, and higher CVD risk scores demonstrated reduced baseline regional CBF in areas of the brain related to memory and learning. These findings suggest that subclinical changes in vascular risk profiles in adult children of persons with AD may influence risk for regional cerebral hypoperfusion and, potentially, risk of clinical AD. Thus, targeting vascular risk factor modification in persons at risk for AD who have average vascular risk profiles may beneficially affect cerebral perfusion and cognitive function. Larger randomized controlled trials are underway in at-risk adults to address these questions. Since middle-aged adults with high cholesterol, diabetes mellitus, or increased CVD risk should already be taking statins for CVD prevention, these drugs will need to be tested in persons at risk for AD who otherwise would not be prescribed statins, such as our study population. Larger more definitive clinical trials are needed to clarify if modifying vascular risk in midlife beneficially alters not only brain perfusion and AD pathology, but also delays clinical onset of cognitive and functional loss.
CONCLUSIONS
In this pilot study of middle-aged adults at increased risk for AD, four months of atorvastatin therapy increased regional CBF in bilateral hippocampi, fusiform gyrus, putamen and insular cortices compared to placebo. These study findings suggest that further research is warranted to clarify if statins can not only improve regional CBF in at-risk adults, but also whether the improved perfusion is associated with cognitive benefits and reduced risk of AD. Larger, longer duration studies are currently underway to answer such questions.
ACKNOWLEDGMENTS
The work presented here was supported by a grant from the State of Wisconsin through the Wisconsin Comprehensive Memory Program Alzheimer’s Disease Pilot Grants; by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health (NIH); the Wisconsin Alzheimer’s Disease Research Center (NIH P50 AG033514) and by grant R01 AG027161 (PI: Sager). Dr. Carlsson was supported by a Paul B. Beeson Career Development Award, a grant jointly funded by the National Institute on Aging, the American Federation for Aging Research, the John A. Hartford Foundation, the Atlantic Philanthropies, and the Starr Foundation (NIA AG026752). Dr. Asthana was supported by NIH K07 AG021582 and R01 AG029624. This material is the result of work supported with resources and use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin. This is Madison Geriatric Research, Education and Clinical Center (GRECC) manuscript #2010-14. The authors would like to sincerely thank their dedicated research participants.
LIST OF ABBREVIATIONS
- AFNI
Analysis of Functional NeuroImages
- AD
Alzheimer’s disease
- Aβ
β-amyloid
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- APOE4
apolipoprotein E ε4
- ASL
arterial spin-labeling
- AST
aspartate aminotransferase
- CBF
cerebral blood flow
- CK
creatine kinase
- CVD
cardiovascular disease
- DNA
deoxyribonucleic acid
- FMD
flow-mediated vasodilation
- GM
gray matter
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- MCI
mild cognitive impairment
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association
- PCR
polymerase chain reaction
- PET
positron emission tomography
- ROI
region of interest
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Breitner JC. Clinical genetics and genetic counseling in Alzheimer disease. Ann Intern Med. 1991 Oct 15;115(8):601–606. doi: 10.7326/0003-4819-601. [DOI] [PubMed] [Google Scholar]
- 2.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002 Aug 6;137(3):149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994 Mar;126(1):88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 4.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, et al. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000 Aug;7(4):321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 5.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007 Jul;38(7):2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004 May;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005 Mar;234(3):851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarmeas N, Habeck CG, Stern Y, Anderson KE. APOE genotype and cerebral blood flow in healthy young individuals. Jama. 2003 Oct 24;290(12):1581–1582. doi: 10.1001/jama.290.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001 May;32(4):980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 10.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000 Oct;57(10):1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 11.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000 Nov 11;356(9242):1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 12.Arvanitakis Z, Schneider JA, Wilson RS, Bienias JL, Kelly JF, Evans DA, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008 May 6;70(19 Pt 2):1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 13.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005 Dec;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, et al. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006 May 31;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–3421. [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 18.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008 Dec;60(6):1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR Biomed. Apr;23(3):286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999 Sep 7;100(10):1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 21.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003 Jul;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 22.Hettmansperger TP, McKean JW. Robust nonparametric statistical methods. London New York, NY: Arnold; John Wiley; 1998. [Google Scholar]
- 23.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, et al. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol. 2010 Oct 1;106(7):969–975. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005 Jun 21;45(12):1987–1993. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 25.El Fakhri G, Kijewski MF, Johnson KA, Syrkin G, Killiany RJ, Becker JA, et al. MRI-guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer disease. Arch Neurol. 2003 Aug;60(8):1066–1072. doi: 10.1001/archneur.60.8.1066. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010 Mar 23;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 27.Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011 Aug 9;77(6):556–563. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, et al. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons M, Schwarzler F, Lutjohann D, von Bergmann K, Beyreuther K, Dichgans J, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer's disease: A 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol. 2002 Sep;52(3):346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 30.Werner N, Nickenig G, Laufs U. Pleiotropic effects of HMG-CoA reductase inhibitors. Basic Res Cardiol. 2002 Mar;97(2):105–116. doi: 10.1007/s003950200000. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson CM, Gleason CE, Hess TM, Moreland KA, Blazel HM, Koscik RL, et al. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer's disease. J Alzheimers Dis. 2008 Apr;13(2):187–197. doi: 10.3233/jad-2008-13209. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Cao D, Kim H, Lester R, Fukuchi K. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006 Dec;60(6):729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]
- 33.Yamada M, Huang Z, Dalkara T, Endres M, Laufs U, Waeber C, et al. Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by L-arginine after chronic statin treatment. J Cereb Blood Flow Metab. 2000 May;20(4):709–717. doi: 10.1097/00004647-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Sterzer P, Meintzschel F, Rosler A, Lanfermann H, Steinmetz H, Sitzer M. Pravastatin improves cerebral vasomotor reactivity in patients with subcortical small-vessel disease. Stroke. 2001 Dec 1;32(12):2817–2820. doi: 10.1161/hs1201.099663. [DOI] [PubMed] [Google Scholar]
- 35.Pretnar-Oblak J, Sabovic M, Sebestjen M, Pogacnik T, Zaletel M. Influence of atorvastatin treatment on L-arginine cerebrovascular reactivity and flow-mediated dilatation in patients with lacunar infarctions. Stroke. 2006 Oct;37(10):2540–2545. doi: 10.1161/01.STR.0000239659.99112.fb. [DOI] [PubMed] [Google Scholar]
- 36.Sander K, Hof U, Poppert H, Conrad B, Sander D. Improved cerebral vasoreactivity after statin administration in healthy adults. J Neuroimaging. 2005 Jul;15(3):266–270. doi: 10.1177/1051228405277403. [DOI] [PubMed] [Google Scholar]