Abstract
Fructose is a simple sugar present in honey and fruit, but can also exist as a polymer (fructans) in pasture grasses. Mammals are unable to metabolize fructans, but certain gram positive bacteria contain fructanases and can convert fructans to fructose in the gut. Recent studies suggest that fructose generated from bacteria, or directly obtained from the diet, can induce both increased intestinal permeability and features of metabolic syndrome, especially the development of insulin resistance. The development of insulin resistance is driven in part by the metabolism of fructose by fructokinase C in the liver, which results in oxidative stress in the hepatocyte. Similarly, the metabolism of fructose in the small bowel by intestinal fructokinase may lead to increased intestinal permeability and endotoxemia. While speculative, these observations raise the possibility that the mechanism by which fructans induce laminitis could involve intestinal and hepatic fructokinase. Further studies are indicated to determine the role of fructanases, fructose and fructokinase in equine metabolic syndrome and laminitis.
Keywords: Fructose, Fructans, Fructokinase, Laminitis, Equine Metabolic Syndrome
Fructose is a monosaccharide present in fruit and honey, but is also a component of sugar (sucrose) and high fructose corn syrup (HFCS). Fructose can also be generated by the breakdown of fructans by bacteria in the gut. Recent studies suggest that the unique metabolism of fructose by fructokinase C can lead to increased intestinal permeability and the development of insulin resistance and features of metabolic syndrome. The potential involvement of fructose and fructokinase as mediator systems involved in equine related diseases are briefly discussed.
Fructose and Fructokinase as Mediators of Insulin Resistance and Metabolic Syndrome
Fructose is unique among foods in its remarkable ability to induce metabolic syndrome in animals. The administration of fructose to laboratory rats, for example, can induce insulin resistance, elevated blood pressure, increased serum triglycerides, low HDL cholesterol, fatty liver (hepatic steatosis), and increased visceral fat stores [1]. Fructose administration also induces leptin resistance in rats, resulting in increased energy intake and weight gain [2]. While the intake of fructose (or sucrose, which contains fructose) can lead to increased energy intake, the ability of fructose to induce insulin resistance and other features of metabolic syndrome does not require excessive energy intake. For example, we have reported that when rats are fed the same total number of calories, that the rats fed a high fructose (or sucrose) diet develop features of metabolic syndrome whereas glucose (or starch) fed animals do not [3-5]. Laboratory rats will even develop fatty liver and insulin resistance when placed on dietary restriction provided that the diet is enriched in sucrose [6] .
There is also evidence that fructose, especially when present in added sugars such as sucrose or HFCS, may have a role in metabolic syndrome in humans. The ingestion of sugary soft drinks is strongly associated with the development of insulin resistance and obesity [7]. Experimental studies also show a unique ability of fructose as compared to glucose to induce insulin resistance and visceral fat accumulation in humans [8]. In one study, the administration of fructose (200 g/d) resulted in the de novo development of metabolic syndrome in 25 percent of healthy men in only two weeks [9]. This has led to interest into the role of fructose in the epidemic of obesity and diabetes [10].
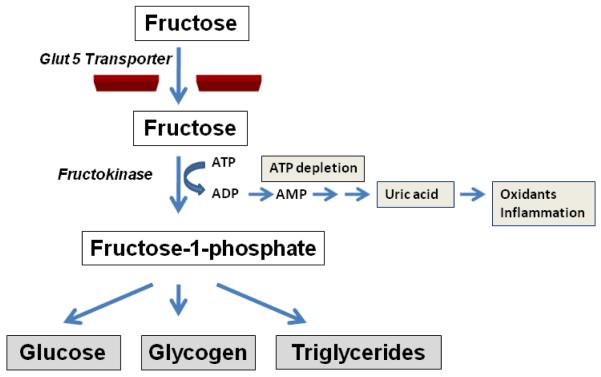
The observation that fructose induces features of metabolic syndrome independent of energy intake suggests there is something unique about the metabolism of fructose that may be responsible for its metabolic effects. Fructose is distinct from glucose only in its initial steps of metabolism. Fructose is taken up by the fructose-specific transporter, Glut5, in the intestinal epithelium, and then is transported to the liver. Here much of the fructose is metabolized by the enzyme, fructokinase C (also known as ketohexokinase C, or KHK-C) to generate fructose-1-phosphate (Figure 1). Unlike glucokinase, which has a negative feedback system to prevent excessive phosphorylation of glucose, KHK-C will rapidly phosphorylate the fructose, resulting in intracellular phosphate and ATP depletion [11]. Hepatic ATP depletion occurs in response to relatively small doses of fructose in both laboratory animals and humans [12-13].
Figure 1. Metabolism of Fructose.
Fructose enters intestinal epithelial cells via the Glut5 transporter, and is then metabolized by fructokinase C, resulting in the generation of fructose-1-phosphate that is further metabolized to generate glucose, glycogen and triglycerides. During the initial phosphorylation of fructose, ATP depletion commonly occurs, resulting in the turnover of adenine nucleotides with the generation of uric acid, oxidants, and inflammatory mediators (such as chemokines).
There is increasing evidence that the intracellular ATP depletion may have a role in inducing the metabolic phenotype. Evidence for this came from studies investigating the role of KHK-C and a different KHK isoform, KHK-A, in laboratory mice. Unlike KHK-C, the metabolism of fructose by KHK-A is slow with only minimal ATP consumption. Interestingly, the obesity, fatty liver and insulin resistance that occurs in fructose-fed mice is prevented in mice lacking both KHK-C and KHK-A, but was exacerbated in KHK-A knockout despite similar overall energy intake [14]. The mechanism appeared to be due to the fact that a lack of KHK-A resulted in more delivery of fructose to the liver where it was metabolized by KHK-C. These studies suggest a key role for hepatic KHK-C in the induction of insulin resistance.
Fructose, Fructokinase, and Intestinal Permeability
As mentioned, the metabolism of fructose by KHK-C results in transient intracellular phosphate and ATP depletion. This process is not benign, and leads to transient interruption of protein synthesis [15]. The intracellular phosphate depletion also stimulates AMP deaminase, which leads to the stepwise degradation of adenine nucleotides to generate uric acid inside the cell [11, 16-18]. In kidney epithelial cells we have found that fructose stimulates the production of oxidants and monocye chemoattractant protein-1 (MCP-1) via a KHK-dependent pathway [18]. We also have found that fructose can increase oxidative stress in hepatocytes (HepG2 cells) and this can be prevented in cells in which KHK has been silenced (Lanaspa MA, unpublished).
The major sites of KHK-C expression in laboratory animals and humans is the liver, small intestine, and kidney [19]. We have found that KHK-C is expressed in both the small bowel and cecum of the mouse, with the highest expression in the duodenum and jejunum (Figure 2). This raises the possibility that the metabolism of fructose in the intestinal wall might lead to local inflammation and increased intestinal permeability. Indeed, the administration of fructose to rats has been reported to cause transient endotoxemia in the rat, documenting an increase in intestinal permeability [20]. We have also found that fructose reduces claudin 4 expression in cultured intestinal epithelial (Caco-2) cells, consistent with alterations in tight junctions that would increase intestinal permeability (Lanaspa MA, unpublished). Moreover, wild type mice administered fructose (15% in water) show both an increased expression in fructokinase mRNA in the duodenum, but also a decrease in expression of tight junction genes including occludin and ZO-1. These alterations are not observed in mice in which both fructokinase A and C isoforms have been deleted (Figure 3). Thus, the metabolism of fructose might be expected to result in intestinal wall permeability and the development of metabolic syndrome. In addition, if a large amount of fructose is administered, the fructose that is not absorbed is rapidly broken down by gut bacteria to generate a lactic acidosis [21].
Figure 2. Fructokinase C is expressed throughout the intestines in mice.
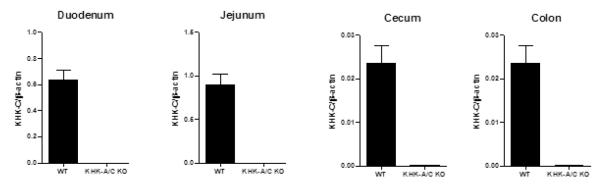
Four-month-old male wild type mice (WT) or fructokinase A/C knockout mice (KHK-A/C KO), which were C57BL6 background, were sacrificed after 20 hours of fasting and the intestines (duodenum, jejunum, cecum and colon) removed and the lining scraped, and analyzed for fructokinase C (KHK-C) mRNA expression by quantitative real time PCR using β-actin as an internal control. Fructokinase C is expressed in both the small and large bowel in the wild type mouse. N = 3-4 per group. All data are presented as the mean ± s.e.m.
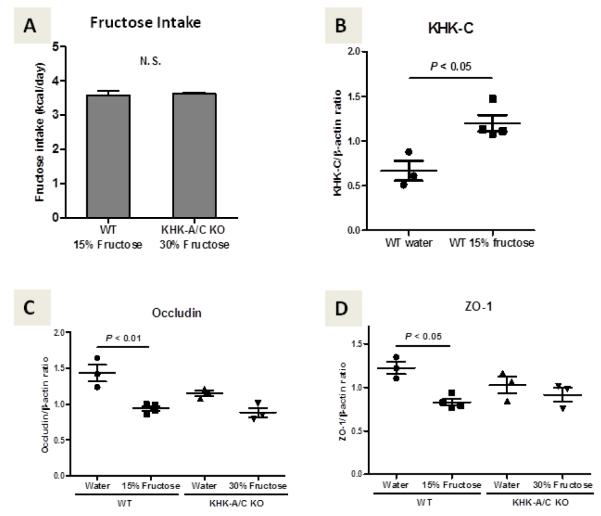
Figure 3. Fructokinase mediates intestinal permeability in mice.
Three-month-old male wild type (WT) mice or fructokinase A/C knockout mice (KHK-A/C KO) (obtained from David Bonthron, Leeds , UK)[72] were administered fructose with normal chow for 3 weeks. Two additional groups of WT mice and KHK-A/C KO mice were given normal chow with tap water that did not contain fructose as a control. Due to the different preference for fructose water between WT mice and KHK-A/C KO mice, mice were given 15% or 30% of fructose water, respectively. Both WT mice and KHK-A/C KO mice drank the same amount of fructose (Figure A). For each group, the duodenum was removed after 20 hours of fasting of normal chow and the lining scraped and RNA extracted. Quantitative real time PCR was performed for fructokinase C (KHK-C, Figure B), and tight junction genes occludin (Figure C) and ZO-1 (Figure D) using β actin as an internal control. As shown, WT mice fed fructose show an upregulation of KHK mRNA expression in association with a significant decrease in occludin and ZO-1 mRNA. These studies suggest fructose is increasing intestinal permeability. The observation that this does not occur in fructokinase KO mice suggests that the intestinal permeability is mediated by fructokinase. N = 3-4 per group. All data are presented as the mean ± s.e.m. Data graphics and statistical analysis was performed using Prism 5 (GraphPad). Data was analyzed by one-way ANOVA with the Tukey post hoc test. P < 0.05 was regarded as statistically significant. N.S., not significant.
Fructans: A Storage Form of Fructose Critical for Temperate Grasses
Plants use photosynthesis to generate carbohydrates as the primary fuels for energy. In the tropics the two major carbohydrates generated are starch and sucrose. Warm season (C4) grasses typically produce starch which is concentrated in chloroplasts and has limited storage capacity since it can be rapidly saturated; hence it is stored in the leaves and is utilized relatively soon after production [22]. Certain C4 grasses, such as sugarcane and sorghum, produce sucrose [23]. In contrast, grasses living in temperate zones (cool season, C3 grasses), as well as perennial flowering plants, store energy as polymers of fructose (fructans) which they use a major nutritional source [24].
The production of fructans begins with the generation of triose phosphate (a 3-carbon sugar) in the chloroplast which is transferred to the cytoplasm, converted to sucrose and moved to the vacuole where it is converted to fructans with the release of glucose via a series of enzymatic steps [25]. Two major types of fructans exist, consisting of levans, which are polymers consisting of β(2®6)-linked fructosyl units with branching at the 2-1 position, and inulins, which have β(2®1) linked fructosyl units with a terminal glucose unit [26]. The number of polymers in any fructan molecule can vary from 3 to 200[26], with most plants having fructans with polymers in the range of 3 to 60 [25]. The fructans are then transported via the plant circulation where they concentrate in vacuoles in the stems , tubers, and roots [25]. Large amounts of fructans can be generated and stored, accounting for up to 70% of the dry weight of the storage organ of the plant [22, 25]. This contrasts with starch- and sucrose-storing plants, where the amount stored is 5 to 10-fold less [24].
Fructans are important for the survival of plants, especially under conditions of drought and cold temperatures [24, 27-28]. In addition to being a nutrition source when food production is limited, fructans may act as a cryoprotectant during cold temperatures, allowing the plant to survive osmotic-induced stress [24, 27-28]. When plants are genetically modified to produce fructans, the plants show increased survival during drought [27]. Fructan content in C3 grasses tends to be highest at the end of the day, and lowest in the morning, consistent with utilization of fructans during the night [29]. In the spring, activation of β-fructofruranosidase (fructan hydrolases) allows the degradation of fructans to fructose which then becomes an important nutrition source [25].
Fructans are present in approximately 15% of flowering plants (approximately 45,000 plants) [25, 27, 30]. The primary plants producing fructans are in the Gramineae family (grasses), which produces primarily inulins, and the Compositae family (aster and daisy family), which preferentially produces levans [26]. Examples of fructan-containing plants include wheat and barley (Poacea family), onions and tulips (Liliacea family) and the Jerusalem chicory and artichoke (Asteraceae family) . Production of fructans by fructan producing species occurs in both early spring and winter [24]. Nevertheless, total fructan content in C3 grasses tends to be highest in the spring, followed by the fall and summer [22]. Fructan production is greatest when photosynthesis is high but growth is limited such as when there is a drought or cold temperatures (<12 C°)[27]. Fructan content in underground storage organs such as tubers of perennial plants appears to be maximal at the end of the growing season, and likely help keep the plant alive under conditions of low rainfall and seasonality [25, 30].
From an evolutionary standpoint, plant species producing fructans increased approximately 30 to 15 million years ago (Ma) in association with climatological changes associated with seasonal drought and cooling [24, 28]. The expansion of fructan-producing Gramineae and Compositae families increased primarily during the early to mid Miocene [28]. The greatest expansion of fructan producing species occurred in latitudes of 20-60° such as in Europe [28]. The period of 15 to 5 Ma is also known for a continued increased in seasonality with global cooling and droughts, in which as many as 30% of mammalian species became extinct [31]. Thus, the ability of mammalian species to harness fructans as a food source could have been critical for survival of species during this period.
The Metabolism of Fructans to Fructose by Gut Bacteria
Fructans can be degraded by fructanases (inulinases and levanases) to generate fructose [26, 32-33]. These enzymes are quite effective at generating fructose; indeed, inulinases convert almost all (95%) of inulin to D-fructose in a single step process [32]. However, mammals do not produce fructanases, and hence fructans are considered indigestible polysaccharides. In contrast, fructanases are produced by some plants, fungi, yeast and bacteria [32, 34]. The primary bacteria producing fructanases are in the Firmicute phyla, and consist primarily of gram positive bacteria including Streptococcus salivarus, Strep mutans, Bacillus sp, Clostridia sp, Bifidobacterium, and others [32]. Some Pseudomonas [32] and Bacteroides fragilis [33] may also produce fructanases.
Interestingly, many of the bacteria that degrade fructans also produce fructans and use them for food storage, similar to plants. One of the best examples is Streptocococcus mutans. Strep mutans lives in the human mouth where it uses sucrose as a major food source, and converts it to fructans that are stored in the dental plaque [35-36]. These bacteria then use fructanases (such as fructanase A, or fruA) to release the fructans as a food source during the time when humans are not eating and hence food is not available. This process turns out to be critical for caries formation [35-36]. Interestingly, exposure of these bacteria to fructans or sucrose results in the increased expression of fructanase A, whereas glucose represses the expression [37]. Studies in broiler chickens have also shown that fructans stimulate the growth of fructanase-producing bacteria in the gut, such as Lactobacillus and Bifidobacteria [38]. Thus the bacterial flora is highly responsive to diet, and the ingestion of fructans will alter the gut flora favoring the growth of fructanase-expressing bacteria.
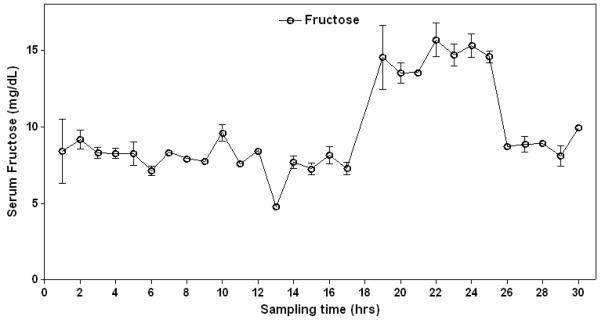
Studies of bacterial flora in multiple species have documented that the largest numbers of bacteria are in the large intestine, but bacteria are also present in the ileum and to a lesser degree in the proximal small intestine [39]. The horse is distinct in having large numbers of anaerobic bacteria in the small intestine (which amounts to 70 feet in length), with equal numbers of bacteria in both the small and large intestine [40]. When fructans (such as from Jersualem artichokes) are administered to horses, there is the rapid degradation of fructans in the small bowel [41]. Thus, the administration of fructans is expected to result in fructose generation in the gut of horses that have fructan-degrading bacteria. Consistent with this proposal, we have found that fructose levels increase in the blood of some horses eating fructan-rich pasture grasses (Figure 4).
Figure 4. Serum Fructose levels increase in a Horse eating Pasture-rich Fructans.
In a study performed in 2005 in a horse pasture in Virginia, serial blood samples were taken from horses throughout the day (provide by the Equine Studies Group, Waltham Centre for Pet Nutrition, UK Equine). Fructan content of the pastural grasses showed a peak level in April, with concentrations of fructose reaching 5-6% of the dry weight by the end of the day. This is significantly less than what has been reported in some studies (in which fructans can increase to 40-50% content of grasses) [60]. Nevertheless, in some horses a rise in serum fructose could be observed at the end of the day, consistent with the hypothesis that some fructans are being converted to fructose.
Fructans and Bacteria as a General Mechanism for Obesity
Recent studies suggest that fructan-degrading bacteria may have a role in obesity. Jeffrey Gordon and colleagues demonstrated that obese humans and laboratory animals have a typical gut flora consisting primarily of Firmicutes as opposed to Bacteroidetes [42-43]. Firmicutes are the primary bacterial phyla producing fructanases [32], and consistent with this observation, the bacteria associated with human obesity were found to have a unique ability to metabolize indigestible polysachharides [44] (such as fructans) and to express fructose metabolic activity [45] Evidence that these bacteria contribute to obesity was shown by experiments in which the colonic bacteria from obese (ob/ob) mice were transferred to lean mice which resulted in the latter gaining more fat as determined by dual-energy X-ray absorptiometry.[45]. Moreover, if western diet is given to mice lacking gut bacteria (germ-free mice), obesity does not develop [46].
Antibiotics and Obesity
Alteration of gut flora by antibiotics has been shown to enhance growth of livestock, and especially chickens. The introduction of antibiotics in animal feed in the 1950s resulted in increased “broiler performance”, as noted by a 50% increase in average market weight of chickens between 1955 and 1995 [47]. The increase in weight has also been associated with a progressive increase in fat content, with a doubling in fat since 1970 [48]. According to some estimates, between 3 and 25 million pounds of growth-promoting antibiotics are used each year in the USA, which accounts for 13 to 70% of all antibiotic use [47]. This has resulted in increased numbers of antibiotic-resistant bacteria with potential public health consequences [39, 49].
The mechanism by which antibiotics promote growth and fat content is due to the effects of the antibiotics on gut flora. It is known, for example, that oral antibiotics do not promote growth in germ-free chickens [50-51]. Furthermore, the effects of antibiotics on fat content in chickens varies. Some antibiotics, such as penicillin, reduce fat, whereas others, such as streptomycin, increase abdominal fat in broilers [52]. This is interesting as penicillin is effective against fructanase-expressing Streptococci whereas streptomycin primarily acts on gram negative organisms that do not express fructanases.
While fructanase-expressing bacteria may have a role in driving obesity, a paradox is that some studies have reported that the administration of fructans or fructanase expressing bacteria may have beneficial effects on obesity. Both probiotics and prebiotics are now commonly used to stimulate growth of chickens and livestock. Probiotics are living organisms such as Bacillus subtilis and have been reported to reduce abdominal fat [53]. Bacillus subtilis produces fructanase. Some prebiotics are fructans, and have been reported to stimulate good bacteria growth, such as Lactobacillus, and are thought to aid weight loss [54-55]. In chickens fructans are thought to increase growth but reduce fat production [56]. Furthermore, short chain fructo-oligosaccharides (45 g/d for 6 weeks) actually improve insulin sensitivity in obese horses compared to horses fed the same amount of maltodextrin [57]. Fructans may also reduce serum lipids [58] and weight [59] in humans, although most trials were short and often the placebo group consisted of sucrose [58].
The reason for the paradox remains unclear. We postulate that if the amount of fructan is large or prolonged, virulent fructanase-producing Streptococci could generate sufficient fructose that induce a marked inflammatory response in the bowel. In contrast, the administration of lower doses of fructans might not generate the required amount of fructose to cause ATP depletion within the intestinal epithelial cells, and hence may act more like fiber that inhibits intestinal absorption of sterols and lipids [54].
Relevance to Equine Research
One of the more serious diseases in horses is laminitis, in which the epidermal lamellae of the inner hoof separate from the dermal lamellae of the distal phalanx resulting in lameness [22, 60-61]. The laminitis is thought to be initiated by the ingestion of grasses rich in fructans. [60-61] The typical horse may ingest as much as 10-15 kg of pasture grass per day[62], resulting in a wide range of fructan ingestion from as little as 0.75 kg to as much as 7 kg [22]. Thus, a 500 kg horse could ingest as much as 10 to 15 g fructan/kg body wt per day if the grasses were rich in fructans [60]. In addition, pasture grasses also contain simple sugars (sucrose, fructose and glucose) that can vary from 0.2 to 2.7 kg per day [22].
Evidence that grasses rich in fructans may have a role in laminitis has been supported by experimental studies in which laminitis can be induced within 48 hours by the administration of oligofructose (10 g/kg body wt). The fructans are thought to cause laminitis due to their degradation in the cecum by gram positive bacteria, resulting in the development of lactic acidosis and endotoxemia that activates local metalloproteinases that break down the basement membrane, causing a detachment of the distal phalanx from the inner hoof wall [60, 63-64]. Fecal flora is altered following administration of oligofructans, with a shift to gram positive organisms, especially Streptococci [65]. Horses with spontaneous laminitis also show a predominance of Streptococcus bovis/equines in the cecum [66].
Laminitis is also associated with and predicted by the presence of equine metabolic syndrome. [67-69]. The latter is characterized primarily by the presence of insulin resistance, often with hypertriglyceridemia, hypertension, and obesity (with preferential fat deposition in the neck and tailhead) [67, 70-71].
While admittedly speculative, it is possible that the chronic ingestion of fructans may induce the expression of fructanase-producing Streptococci, and that the sudden ingestion of a massive amount of fructans can lead to the rapid generation of fructose in the small intestine and hindgut. Some of the fructose generated would be metabolized in the intestinal wall leading to local inflammation with increased intestinal permeability and endotoxemia, some fructose might enter the liver to stimulate insulin resistance, and the rest would be degraded by local bacteria resulting in a local acidosis. We recommend studies examining the expression and activity of fructokinase in equine metabolic syndrome and laminitis.
In conclusion, the preferential expression of fructans in temperate grasses appears to have had evolutionary benefit for the plants to survive under drought or colder temperatures, but may have also provided a survival pathway for mammals in which intestinal and colonic fructanase-expressing bacteria were present. However, in the setting where excessive amounts of fructans are ingested, it may provide a stealth source of fructose that could be playing a role in both equine associated metabolic syndrome and laminitis.
Acknowledgements
We thank Pat Harris at the Equine Studies Group, Waltham Centre for Pet Nutrition, UK Equine for providing the blood samples for the horse on pasture grass shown in Figure 4.
Supported by NIH grants HL-68607 and startup funds from the Department of Medicine to Dr Richard J Johnson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. American journal of physiology. 2008;295:R1370–5. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. American journal of physiology. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- [4].Reungjui S, Roncal CA, Mu W, Srinivas TR, Sirivongs D, Johnson RJ, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. 2007;18:2724–31. doi: 10.1681/ASN.2007040416. [DOI] [PubMed] [Google Scholar]
- [5].Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. American journal of physiology. 2007;293:F1256–61. doi: 10.1152/ajprenal.00181.2007. [DOI] [PubMed] [Google Scholar]
- [6].Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism: clinical and experimental. 2011;60:1259–70. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. The Journal of clinical investigation. 2009;119:1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–61. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- [10].Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- [11].van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. The Biochemical journal. 1977;162:601–9. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bode JC, Zelder O, Rumpelt HJ, Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973;3:436–41. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- [13].Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. Jama. 1999;282:1659–64. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- [14].Ishimoto T, Lanaspa M, Le M, Diggle CP, MacLean PS, Jackman MR, et al. Opposing Effects of Fructokinase C and A Isoforms on Fructose-Induced Metabolic Syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1119908109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maenpaa PH, Raivio KO, Kekomaki MP. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science (New York, NY. 1968;161:1253–4. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- [16].Kim KM, Henderson GN, Ouyang X, Frye RF, Sautin YY, Feig DI, et al. A sensitive and specific liquid chromatography-tandem mass spectrometry method for the determination of intracellular and extracellular uric acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2032–8. doi: 10.1016/j.jchromb.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stirpe F, Della Corte E, Bonetti E, Abbondanza A, Abbati A, De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- [18].Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–53. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, Markham AF, et al. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem. 2009;57:763–74. doi: 10.1369/jhc.2009.953190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–92. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- [21].Davids MR, Segal AS, Brunengraber H, Halperin ML. An unusual cause for ketoacidosis. Qjm. 2004;97:365–76. doi: 10.1093/qjmed/hch064. [DOI] [PubMed] [Google Scholar]
- [22].Longland AC, Byrd BM. Pasture nonstructural carbohydrates and equine laminitis. The Journal of nutrition. 2006;136:2099S–102S. doi: 10.1093/jn/136.7.2099S. [DOI] [PubMed] [Google Scholar]
- [23].Vu JC, Allen LH., Jr Stem juice production of the C4 sugarcane (Saccharum officinarum) is enhanced by growth at double-ambient CO2 and high temperature. J Plant Physiol. 2009;166:1141–51. doi: 10.1016/j.jplph.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [24].Brocklebank KJ, Hendry GAF. Characteristics of plant species which store different types of reserve carbohydratres. New Phytol. 1989;112:255–60. [Google Scholar]
- [25].Pollock CJ. Fructans and the Metabolism of Sucrose in Vascular Plants New Phytol. 1986;104:1–24. doi: 10.1111/j.1469-8137.1986.tb00629.x. [DOI] [PubMed] [Google Scholar]
- [26].Andersen R, Sorensen A. An enzymatic method for the determination of fructans in foods and food products. Eur Food Res Tech. 1999;210 [Google Scholar]
- [27].Pilon-Smits E, Ebskamp M, Paul MJ, Jeuken M, Weisbeek PJ, Smeekens S. Improved Performance of Transgenic Fructan-Accumulating Tobacco under Drought Stress. Plant Physiol. 1995;107:125–30. doi: 10.1104/pp.107.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hendry GAF. Evolutionary origins and natural functions of fructans-- a climatological, biogeographic and mechanistic appraisal. New Phytol. 1993;123:3–14. [Google Scholar]
- [29].Garner HE, Hutcheson DP, Coffman JR, Hahn AW, Salem C. Lactic acidosis: a factor associated with equine laminitis. J Anim Sci. 1977;45:1037–41. doi: 10.2527/jas1977.4551037x. [DOI] [PubMed] [Google Scholar]
- [30].Polllock CJ, Cairns AJ. Fructan metabolism in grasses and cereals. Ann Rev Plant Physio,l and Plant Mol Biol. 1991;42:77–101. [Google Scholar]
- [31].Johnson RJ, Andrews P. Fructose, Uricase and the Back to Africa Hypothesis. Evolutionary Anthropol. 2010;19:250–7. [Google Scholar]
- [32].Singh P, Gill PK. Production of inulinases: Recent advances. Food Technol Biotechnol. 2006;44:151–62. [Google Scholar]
- [33].Blatch GL, Woods DR. Molecular characterization of a fructanase produced by Bacteroides fragilis BF-1. J Bacteriol. 1993;175:3058–66. doi: 10.1128/jb.175.10.3058-3066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Legaz ME, Martin L, Pedrosa MM, Vicente C, de Armas R, Martinez M, et al. Purification and Partial Characterization of a Fructanase which Hydrolyzes Natural Polysaccharides from Sugarcane Juice. Plant Physiol. 1990;92:679–83. doi: 10.1104/pp.92.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wexler DL, Penders JE, Bowen WH, Burne RA. Characteristics and cariogenicity of a fructanase-defective Streptococcus mutants strain. Infect Immun. 1992;60:3673–81. doi: 10.1128/iai.60.9.3673-3681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bergeron LJ, Burne RA. Roles of fructosyltransferase and levanase-sucrase of Actinomyces naeslundii in fructan and sucrose metabolism. Infect Immun. 2001;69:5395–402. doi: 10.1128/IAI.69.9.5395-5402.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wen ZT, Burne RA. Analysis of cis- and trans-acting factors involved in regulation of the Streptococcus mutans fructanase gene (fruA) J Bacteriol. 2002;184:126–33. doi: 10.1128/JB.184.1.126-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li X, Qiang L, Xu C. Effects of supplementation of fructooligosaccharide and/or Bacillus subtilis to diets on performance and on intestinal microflora in broilers. Arch Tierz Dummerstorf. 2008;51:64–70. [Google Scholar]
- [39].Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 2005;84:634–43. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- [40].De Fombelle A, Varloud M, Goachet A-G, Jacotot E, Phillippeau C, Drogul C, et al. Characterization of the microbial and biochemical profile of the different segments of the digestive tract in horses given two distinct diets. J Animal Sci. 2003;77:293–304. [Google Scholar]
- [41].Coenen M, Mosseler A, Vervuert I. Fermentative gases in breath indicate that inulin and starch start to be degraded by microbial fermentation in the stomach and small intestine of the horse in contrast to pectin and cellulose. The Journal of nutrition. 2006;136:2108S–10S. doi: 10.1093/jn/136.7.2108S. [DOI] [PubMed] [Google Scholar]
- [42].Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- [43].Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- [45].Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Graham JP, Boland JJ, Silbergeld E. Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep. 2007;122:79–87. doi: 10.1177/003335490712200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Y, Lehane C, Ghebremeskel K, Crawford MA. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public Health Nutr. 2010;13:400–8. doi: 10.1017/S1368980009991157. [DOI] [PubMed] [Google Scholar]
- [49].Diarra MS, Silversides FG, Diarrassouba F, Pritchard J, Masson L, Brousseau R, et al. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl Environ Microbiol. 2007;73:6566–76. doi: 10.1128/AEM.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Coates ME, Davies MK, Kon SK. The effect of antibiotics on the intestine of the chick. The British journal of nutrition. 1955;9:110–9. doi: 10.1079/bjn19550016. [DOI] [PubMed] [Google Scholar]
- [51].Coates ME, Fuller R, Harrison GF, Lev M, Suffolk SF. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. The British journal of nutrition. 1963;17:141–50. doi: 10.1079/bjn19630015. [DOI] [PubMed] [Google Scholar]
- [52].Onifade AA. Growth performance, carcass characteristics, organs measurement and haematology of broiler chickens fed a high fibre diet supplemented with antibiotics or dried yeast. Nahrung. 1997;41:370–4. [Google Scholar]
- [53].Santoso U, Tanaka K, Ohtani S. Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. The British journal of nutrition. 1995;74:523–9. doi: 10.1079/bjn19950155. [DOI] [PubMed] [Google Scholar]
- [54].Yamamoto Y, Takahashi Y, Kawano M, Iizuka M, Matsumoto T, Saeki S, et al. In vitro digestibility and fermentability of levan and its hypocholesterolemic effects in rats. J Nutr Biochem. 1999;10:13–8. doi: 10.1016/s0955-2863(98)00077-1. [DOI] [PubMed] [Google Scholar]
- [55].Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. The Journal of nutrition. 1999;129:1438S–41S. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- [56].Yusrizal, Chen TC. Effect of adding chicory fructans in feed on broiler growth performance, serum cholesterol and intestinal length. Int J Poult Sci. 2003;2:214–9. [Google Scholar]
- [57].Respondek F, Myers K, Smith TL, Wagner A, Geor RJ. Dietary supplementation with short-chain fructo-oligosaccharides improves insulin sensitivity in obese horses. J Anim Sci. 2011;89:77–83. doi: 10.2527/jas.2010-3108. [DOI] [PubMed] [Google Scholar]
- [58].Williams CM, Jackson KG. Inulin and oligofructose: effects on lipid metabolism from human studies. The British journal of nutrition. 2002;87(Suppl 2):S261–4. doi: 10.1079/BJNBJN/2002546. [DOI] [PubMed] [Google Scholar]
- [59].Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. The American journal of clinical nutrition. 2009;89:1751–9. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].van Eps AW, Pollitt CC. Equine laminitis induced with oligofructose. Equine Vet J. 2006;38:203–8. doi: 10.2746/042516406776866327. [DOI] [PubMed] [Google Scholar]
- [61].Pollitt CC. Equine Laminitis. Australia: 2001. [Google Scholar]
- [62].McMeniman NP. Nutrition of Grazing Broodmares, Their Foals and Young horses. Rural Industries Research and Development Corporation; Barton Australia: 2000. [Google Scholar]
- [63].Nourian AR, Asplin KE, McGowan CM, Sillence MN, Pollitt CC. Equine laminitis: ultrastructural lesions detected in ponies following hyperinsulinaemia. Equine Vet J. 2009;41:671–7. doi: 10.2746/042516409x407648. [DOI] [PubMed] [Google Scholar]
- [64].Pollitt CC. Equine laminits. Clin Tech Equine Pract. 2004;3:34–44. [Google Scholar]
- [65].Milinovich GJ, Burrell PC, Pollitt CC, Klieve AV, Blackall LL, Ouwerkerk D, et al. Microbial ecology of the equine hindgut during oligofructose-induced laminitis. ISME J. 2008;2:1089–100. doi: 10.1038/ismej.2008.67. [DOI] [PubMed] [Google Scholar]
- [66].Milinovich GJ, Trott DJ, Burrell PC, Croser EL, Al Jassim RA, Morton JM, et al. Fluorescence in situ hybridization analysis of hindgut bacteria associated with the development of equine laminitis. Environ Microbiol. 2007;9:2090–100. doi: 10.1111/j.1462-2920.2007.01327.x. [DOI] [PubMed] [Google Scholar]
- [67].Carter RA, Treiber KH, Geor RJ, Douglass L, Harris PA. Prediction of incipient pasture-associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalised and localised obesity in a cohort of ponies. Equine Vet J. 2009;41:171–8. doi: 10.2746/042516408x342975. [DOI] [PubMed] [Google Scholar]
- [68].Frank N, Elliott SB, Brandt LE, Keisler DH. Physical characteristics, blood hormone concentrations, and plasma lipid concentrations in obese horses with insulin resistance. J Am Vet Med Assoc. 2006;228:1383–90. doi: 10.2460/javma.228.9.1383. [DOI] [PubMed] [Google Scholar]
- [69].Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J Am Vet Med Assoc. 2006;228:1538–45. doi: 10.2460/javma.228.10.1538. [DOI] [PubMed] [Google Scholar]
- [70].Bailey SR, Habershon-Butcher JL, Ransom KJ, Elliott J, Menzies-Gow NJ. Hypertension and insulin resistance in a mixed-breed population of ponies predisposed to laminitis. Am J Vet Res. 2008;69:122–9. doi: 10.2460/ajvr.69.1.122. [DOI] [PubMed] [Google Scholar]
- [71].Frank N, Geor RJ, Bailey SR, Durham AE, Johnson PJ. Equine metabolic syndrome. J Vet Intern Med. 2010;24:467–75. doi: 10.1111/j.1939-1676.2010.0503.x. [DOI] [PubMed] [Google Scholar]
- [72].Diggle CP, Shires M, McRae C, Crellin D, Fisher J, Carr IM, et al. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiol Genomics. 2010;42A:235–43. doi: 10.1152/physiolgenomics.00128.2010. [DOI] [PubMed] [Google Scholar]