Abstract
Objectives
Pancreatic adenocarcinoma (PDAC) has a dismal 5-year survival rate of 5%. There is an urgent need for early detection while the tumors are small and surgically resectable. We assessed serum osteopontin (OPN) and tissue inhibitor of metalloproteinase 1 (TIMP-1) as possible diagnostic and prognostic biomarkers in a novel cohort of pancreatic cancer patients.
Methods
OPN and TIMP-1 levels were determined in serum from 86 PDAC patients, 86 healthy control subjects, and 48 chronic pancreatitis patients. Regression models were used to relate OPN and TIMP-1 to gender, age, stage, class, and treatment. Survival analyses were performed using univariate and multivariate Cox models.
Results
Serum levels of both OPN and TIMP-1 distinguished PDAC from chronic pancreatitis (P ≤ 0.0001) and healthy control subjects (P <0.0001). Serum levels of both OPN and TIMP-1 also distinguished early stage, resectable PDAC cases from chronic pancreatitis (P < 0.04) and healthy control subjects (P <0.01). High serum levels of OPN were significantly correlated with reduced patient survival.
Conclusions
Serum OPN and TIMP-1 have utility as diagnostic biomarkers in PDAC. Our data suggests a potential benefit of utilizing OPN, TIMP-1 and CA 19-9 in a panel to improve diagnostic accuracy in PDAC.
Keywords: secreted phosphoprotein 1, SPP1, TIMP metallopeptidase inhibitor 1
Introduction
Pancreatic cancer is the fourth most common cause of cancer mortality in the United States despite comprising only 3% of estimated new cancer cases each year. It is anticipated that there will be 44,030 cases of pancreatic cancer with 37,660 deaths due to the disease in 2011.1 Pancreatic cancer often presents with non-specific symptoms such as abdominal pain, back pain, jaundice, and weight loss, leading to a delay in diagnosis in many patients.2 This delay often prevents surgical resection, currently the best and only treatment for possible cure, which contributes to a dismal 5 year survival of 5%.3 As such, there is a need to identify this disease early while it is still surgically treatable.
Recent research has focused on identifying biomarkers in the serum of patients with PDAC in order to detect cancer earlier. The current best serum biomarker for pancreatic cancer is CA19-9. However, its sensitivity and specificity is 79% and 82%, respectively, for detecting pancreatic cancer, indicating a need for diagnostic biomarkers with higher accuracy.4 Osteopontin (OPN) and tissue inhibitor of metalloproteinase 1 (TIMP-1) are secreted proteins that are upregulated in PDAC tumors5, and both have been analyzed as possible diagnostic serum biomarkers for PDAC. Prior research has demonstrated that OPN may contribute to the invasiveness of pancreatic cancer cells as well as the growth of metastases6, 7, while studies on TIMP-1 have shown a relationship between TIMP-1 overexpression and reduced tumor growth, implantation, and metastasis.8 These results suggest that serum OPN and TIMP-1 levels may have prognostic utility in PDAC cases.
In this study, we validated the utility of serum OPN and TIMP-1 to distinguish between PDAC, healthy controls, and chronic pancreatitis in a novel patient cohort with emphasis on the ability of the biomarkers to identify early stage disease. We evaluated the diagnostic accuracy of OPN and TIMP-1 in comparison to CA19-9, and assessed the possible improvement in accuracy with a diagnostic panel including OPN, TIMP-1, and CA19-9. We further extended prior research by assessing serum OPN and TIMP-1 as prognostic biomarkers to predict survival in pancreatic cancer.
Materials and Methods
Serum Samples
Serum from 220 subjects was analyzed in this study (Table 1). Pretreatment serum samples were obtained from 86 patients with histologically or cytologically confirmed PDAC. Eighty-six healthy control subjects (CON) were selected to age-approximate and gender-match the PDAC cases. These control serum samples were obtained from both healthy adults accompanying index patients to clinic visits and excess sera from de-identified healthy controls obtained from the large reference laboratory (ARUP Laboratories) managed by the University of Utah Department of Pathology. Serum samples from 48 patients with chronic pancreatitis (ChPT) were also analyzed.
Table 1.
Subject Characteristics
| Diagnosis | Subject Group | Number of Cases | Median Age in Years (Range) |
|---|---|---|---|
| Pancreatic adenocarcinoma | Total | 86 | 66 (44–87) |
| Female | 40 | 69 (44–87) | |
| Male | 46 | 62 (47–83) | |
| Stage IA | 1 | ||
| Stage IB | 6 | ||
| Stage IIA | 11 | ||
| Stage IIB | 24 | ||
| Stage III | 17 | ||
| Stage IV | 27 | ||
| Class N0 | 37 | ||
| Class N1 | 22 | ||
| Class M1 | 27 | ||
| Healthy Control | Total | 86 | 63.5 (34–94) |
| Female | 40 | 63 (50–94) | |
| Male | 46 | 64 (34–85) | |
| Chronic Pancreatitis | Total | 48 | 48 (29–80) |
| Female | 25 | 46 (30–70) | |
| Male | 23 | 50 (29–80) |
PDAC cases were stratified by stage, extent of metastatic dissemination (“class”, defined here as N0, N1, or M1 disease), and whether the patients received treatment expected to affect survival (either resection or at least one full course of chemotherapy and/or radiation). Due to the paucity of early-stage cases in the cohort, stage IA and IB cases were combined into a single group in statistical models. Here, stratification based on class refers to node negative (N0), node positive (N1), or presence of distant metastases (M1) independent of node status. Blood was collected prior to the patient receiving treatment, other than placement of a stent in jaundiced cases. Blood was separated into the serum component, aliquoted and stored frozen until used for analyses.
OPN, TIMP-1 and CA 19-9 ELISA Assays
Serologic OPN and TIMP-1 measurements were performed using ELISA kits from R&D Systems, Inc. (Minneapolis, MN, USA) and CA 19-9 determinations using ELISA from Diagnostic Automation, Inc. (Calabasas, CA) according to the manufacturer’s recommendations. Samples for OPN were diluted 1:25 for OPN and 1:100 for TIMP-1 analyses. A calibration curve was generated using known concentrations of analyte. Samples were further diluted and re-assayed if readings were above the linear range of the calibration curve. Data is reported as mean ± standard deviation.
Data Analysis
Stratified Kaplan-Meier curves were used to plot survival and compute median survival whereas Cox models and logrank tests were used for formal survival analysis. Linear regression models were used to relate OPN and TIMP-1 to class, gender, age, stage, treatment, and diagnosis. A priori significance levels were set at P values < 0.05. Statistical analyses and classification tree analyses were carried out using the R version 2.10.0 statistical software package.9 Discrete cutoffs for survival models were identified by visual inspection of martingale residual plots where residuals are the “observed–expected” events.
All studies were carried out with the approval of the University of Utah Institutional Review Board.
Results
OPN and TIMP-1 as Diagnostic Biomarkers
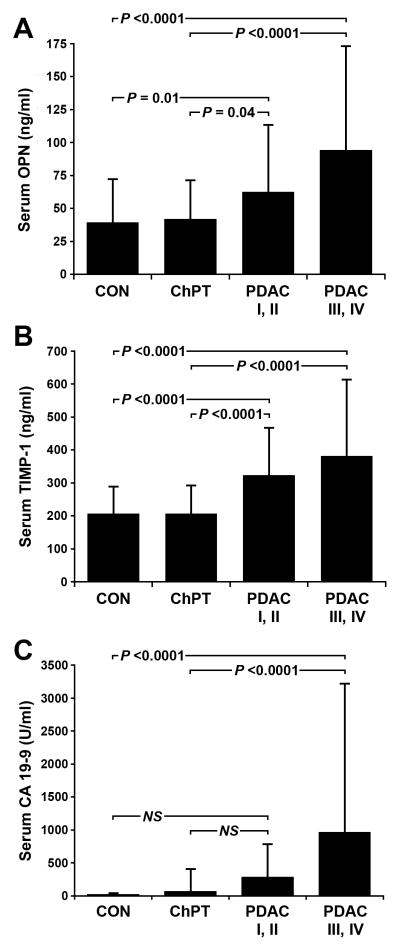
We assessed the utility of OPN and TIMP-1 as diagnostic biomarkers by comparing serum levels of each protein in PDAC patients to serum levels of healthy control subjects and chronic pancreatitis patients. Serum OPN levels were 39.5 ± 32.8 ng/ml (mean ± standard deviation) in healthy control subjects, 41.8 ± 29.8 ng/ml in chronic pancreatitis patients, and 77.6 ± 67.3 ng/ml in PDAC patients. There was a statistically significant difference between OPN serum levels in patients with PDAC and both control groups (P ≤ 0.0001) and serum OPN levels were not elevated in chronic pancreatitis patients relative to healthy control subjects (P = 0.774). Similar differences were seen for TIMP-1 where healthy control subjects had TIMP-1 serum levels of 207.2 ± 82.3 ng/ml, chronic pancreatitis patients had TIMP-1 serum levels of 206.6 ± 12.5 ng/ml, and PDAC patients had serum TIMP-1 levels of 350.7 ± 191.9 ng/ml. Mean serum TIMP-1 levels were significantly elevated in PDAC relative to both healthy controls (P < 0.0001) and chronic pancreatitis patients (P < 0.0001), but the difference between healthy controls and chronic pancreatitis was not statistically significant (P = 0.980). Age, but not gender, was significantly related to diagnosis (P < 0.0001) likely due to the lower age of ChPT group (Table 1). Age was also significantly related to OPN levels (P < 0.0001) with OPN levels increased in older patients. However, OPN remained a significant predictor of PDAC after adjusting for age in multivariate models (P < 0.0001).
In order to improve patient outcomes, it is desirable that biomarkers have the ability to diagnose PDAC at an earlier, treatable stage. We therefore examined the ability of OPN and TIMP-1 to distinguish PDAC patients with resectable disease from healthy controls and chronic pancreatitis cases (Figure 1). Of the 86 PDAC cases examined, 45 cases were stage I or II and considered resectable. In this subset, OPN serum levels were 62.4 ± 50.9 ng/ml and TIMP-1 serum levels were 323.1 ± 144.3 ng/ml. Both OPN and TIMP-1 remained significantly elevated in serum from resectable cases relative to serum from healthy controls (OPN P = 0.01, TIMP-1 P < 0.0001) and chronic pancreatitis patients (OPN P = 0.04, TIMP-1 P < 0.0001). In contrast, serum CA 19-9 levels in resectable cases (281.6 ± 504.7 U/ml) did not reach the level of significance compared to healthy controls (17.2 ± 25.2 U/ml, P = 0.16) or chronic pancreatitis patients (65.8 ± 344.5 U/ml, P = 0.31). Mean serum levels of OPN, TIMP-1, and CA 19-9 were further elevated in advanced PDAC (stage III and IV) compared to earlier stages (Figure 1), consistent with a direct relationship between serum levels of all three biomarkers and tumor burden.
Figure 1.
Serum marker levels. OPN (A), TIMP-1 (B), and CA 19-9 levels were determined in serum from healthy control subjects (CON), patients with chronic pancreatitis (ChPT), and pretreatment samples from patients with cytologically confirmed pancreatic ductal adenocarcinoma (PDAC). Statistical comparisons were made by ANOVA and Fisher's PLSD post-hoc tests. Comparisons between CON, ChPT, and PDAC split into early stage resectable (PDAC I, II) and late stage (PDAC III, IV) groups are shown. Statistical comparison of CON, ChPT, and the combined PDAC group is reported in the text. Data is reported as mean ± standard deviation. NS = not significant.
Receiver operating characteristic (ROC) curves and area under the curves (AUC) were calculated for serum OPN, TIMP-1 and CA 19-9. Neither OPN (AUC = 0.721) nor TIMP1 (AUC = 0.769) had improved diagnostic capability over CA 19-9 (AUC = 0.918) when comparing PDAC patients to a combined control group consisting of healthy controls and chronic pancreatitis patients. However, by using iterative classification tree analyses, a diagnostic algorithm could be identified that improved diagnostic accuracy (Figure 2). This algorithm splits patients at a CA19-9 level of 37 U/ml into two groups. Those patients with CA 19-9 levels above 37 U/ml are further split by an OPN level of 25 ng/ml, and those with CA 19-9 levels below 37 U/ml are further split by a TIMP-1 level of 411 ng/ml. This algorithm improved sensitivity (0.87), specificity (0.91), and overall accuracy (0.895) over the sensitivity (0.84), specificity (0.88), and overall accuracy (0.86) of CA 19-9 alone.
Figure 2.
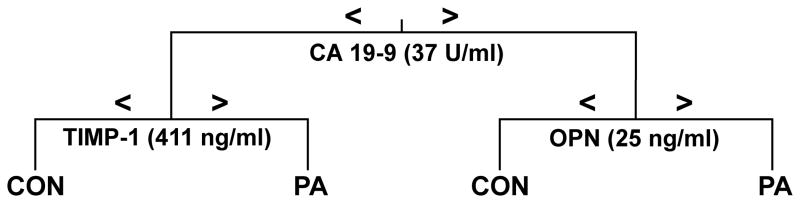
Diagnostic algorithm utilizing OPN, TIMP-1 and CA19-9. A classification tree was generated for discrimination of pancreatic ductal adenocarcinoma (PDAC) from a combined control group (CON) consisting of both healthy control cases and chronic pancreatitis patients. The tree depicts an algorithm derived to minimize misclassification when all three markers were considered. The specific threshold cutoffs used in the algorithm for the individual markers are given in parentheses.
OPN and TIMP-1 as Prognostic Biomarkers
We analyzed the relationship of serum OPN and TIMP-1 to survival in patients with PDAC. Univariate analyses indicated that serum OPN levels, serum TIMP-1 levels, age, gender, treatment with resection and/or chemoradiation, and the presence of distant metastasis (stage IV/M1 class) were all significant predictors of survival (Table 2). Serum OPN levels used as a continuous variable did not reach the level of significance (P = 0.055), but using a discrete cutoff of 150 ng/ml, serum OPN levels were a significant predictor of survival (P = 0.007). Using the discrete cutoff, OPN remained a significant predictor of survival after adjusting for age, class, and treatment (Table 3). An inverse relationship existed between serum OPN levels and survival, with higher levels corresponding to poorer prognosis. In our data set of 86 PDAC patients, those with OPN serum levels below the threshold of 150 ng/ml had a median survival of 337 days whereas those with levels above the threshold had a median survival of 179 days, a difference of approximately 5 months. Serum TIMP-1 levels were a significant predictor of survival when TIMP-1 was used as both a continuous variable (P = 0.046) and when using a discrete cutoff of 454 ng/ml, (P = 0.017). As with OPN, there was an inverse relationship between serum TIMP-1 levels and survival, with higher TIMP-1 levels corresponding to poorer prognosis. However, after adjusting for extent of metastatic dissemination (Class) and whether the patient received treatment (Treatment) in multivariate models, TIMP-1 levels were no longer significant for prognosis (Table 3).
Table 2.
Univariate Survival Analyses
| Predictor | Level | Median Survival (days) | 95% CI | Hazard Ratio | P-value |
|---|---|---|---|---|---|
| OPN | Continuous (N = 86) | 316 | 253–385 | 1.0036* | 0.055 |
| OPN | ≤150 ng/ml (N = 79) | 337 | 269–435 | 1.00 | Reference |
| >150 ng /ml (N = 7) | 179 | 63 - ∞ | 3.01 | 0.0072 | |
| TIMP-1 | Continuous (N = 86) | 316 | 253–385 | 1.0013* | 0.046 |
| TIMP-1 | ≤454 ng /ml (N = 64) | 345 | 306–448 | 1.00 | Reference |
| >454 ng /ml (N = 22) | 231 | 130–385 | 1.91 | 0.017 | |
| Age | Continuous (N = 86) | 316 | 253–485 | 1.028** | 0.014 |
| Class | N0 (N = 21) | 616 | 331-∞ | 1.00 | Reference |
| N1 (N =38) | 381 | 316–480 | 1.73 | 0.098 | |
| M1 (N =26) | 120 | 82–264 | 7.67 | 3.65e-08 | |
| Gender | Female (N = 40) | 229 | 135–345 | 1.00 | Reference |
| Male (N = 46) | 430 | 306–617 | 0.51 | 0.0045 | |
| Stage | IA, IB (N =8) | 466 | 166 - ∞ | 1.00 | Reference |
| IIA (N =11) | 703 | 435-∞ | 0.78 | 1.00 | |
| IIB (N =24 ) | 428 | 345–749 | 1.40 | 0.29 | |
| III (N = 17) | 306 | 253–480 | 1.90 | 0.21 | |
| IV (N = 26) | 120 | 82–204 | 6.35 | 0.00015 | |
| Treatment | No (N = 23) | 122 | 82 –264 | 1.00 | Reference |
| Yes (N =63 ) | 381 | 326–480 | 0.28 | 2.5e-06 |
Per 1 ng/ml increase in biomarker level
Per 1 year increase in age
Table 3.
Multivariate Survival Analysis
| Biomarker | Adjusted Co-variates | Level | Hazard Ratio | P-value |
|---|---|---|---|---|
| OPN | Class, Treatment, Age | (continuous) | 1.003* | 0.16 |
| OPN | Class, Treatment, Age | ≤150 ng/ml | 1.00 | Reference |
| >150 ng/ml | 2.71 | 0.025 | ||
| TIMP-1 | Class, Treatment | (continuous) | 1.0002* | 0.66 |
| TIMP-1 | Class, Treatment | ≤454 ng /ml | 1.00 | Reference |
| >454 ng /ml | 1.45 | 0.20 |
Per 1 ng/ml increase in biomarker level
Discussion
The genes for OPN and TIMP-1 are routinely overexpressed in PDAC tumors5. As secreted proteins, they are logical choices for diagnostic biomarkers and several studies have shown that OPN and TIMP-1 are significantly elevated in peripheral blood of PDAC cases compared to both healthy controls and patients with benign disease6, 10–15. Our study is consistent with these previous studies showing a significant elevation of OPN and TIMP-1 in patients with PDAC. Importantly, serum levels of OPN and TIMP-1 appear to differentiate patients with PDAC from chronic pancreatitis cases, a persistent diagnostic problem for patients presenting with periampullary disease. As diagnostic biomarkers, neither protein was superior to CA 19-9, however, in combination, OPN, TIMP-1 and CA 19-9 provides a panel that improves diagnostic accuracy over any of the three biomarkers individually. OPN and TIMP-1 have been shown to improve diagnostic accuracy in other panels10–15, but this is the first demonstration using the three biomarkers in combination. A recent study examined serum levels in a large number of cases and identified a panel including TIMP-1 and CA 19-9 as being most accurate in discriminating PDAC patients from patients with benign pancreatic disease, but failed to identify a panel consisting of CA 19-9, OPN, and TIMP-1.10 Since no individual biomarker or biomarker panel has been identified with sensitivity and specificity sufficient for application in the general population or in high-risk populations without unacceptable levels of false positive results, further work is necessary. Taken together, the body of evidence suggests that serum OPN and TIMP-1 will be useful biomarkers in larger diagnostic panels. Furthermore, our study demonstrates that both OPN and TIMP-1, but not CA 19-9, were able to distinguish early stage, resectable PDAC from healthy controls and chronic pancreatitis patients, highlighting their utility for early detection. This finding is consistent with a previous study that demonstrated a significant elevation of serum TIMP-1 levels in early stage PDAC relative to healthy controls and chronic pancreatitis cases15, but it is a novel finding for OPN.
Upregulation of OPN and TIMP-1 in PDAC tumors is suggestive of their participation in PDAC progression. Both proteins appear to contribute to tumorigenesis via multiple mechanisms16–18 suggesting that increased serum levels may predict decreased survival. In our patient cohort, higher serum levels of OPN and TIMP-1 corresponded to poorer prognosis when examined in univariate analysis, however, only OPN maintained a significant relationship to survival after adjusting for treatment and class in multivariate models. Since PDAC patients with distant metastases (M1 disease) are not candidates for resection, treatment and class are correlated. Thus, the relationship between serum TIMP-1 levels and survival is likely explained by its correlation with late-stage disease. Our results for the prognostic capacity of TIMP-1 exactly recapitulate a prior study13, but to our knowledge, this is the first demonstration of the relationship between serum OPN levels and survival in PDAC subjects.
In summary, our study demonstrated serum OPN and TIMP-1 are significant diagnostic biomarkers and can distinguish PDAC from healthy controls or chronic pancreatitis patients. These findings verify prior research examining the diagnostic capabilities of both biomarkers. In addition, serum OPN and TIMP-1 can distinguish both early stage, resectable PDAC from both healthy control subjects and chronic pancreatitis patients, increasing its clinical utility. While neither OPN nor TIMP-1 improved diagnostic accuracy over CA 19-9 alone, a diagnostic panel including OPN, TIMP-1 and CA19-9 may increase diagnostic accuracy for PDAC. Finally, high serum levels of OPN may be a useful prognostic indicator.
Acknowledgments
This work was supported in part by research grants from the National Institutes of Health (R03CA115225, U01CA151650, R33CA155586 to SJM and P30CA042014 to the Huntsman Cancer Institute for support of core facilities), grants from the Huntsman Cancer Institute Pancreas Cancer Research Program and through support from the Huntsman Cancer Foundation. KEP was supported in part by a Ruth L. Kirschstein National Research Service Award (T35HL007744).
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
Contributor Information
Katherine E. Poruk, Department of Surgery, University of Utah School of Medicine, Salt Lake City, UT.
Matthew A. Firpo, Department of Surgery and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT.
Courtney L. Scaife, Department of Surgery and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT.
Douglas G. Adler, Department of Internal Medicine, Division of Gastroenterology and Hepatology and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT.
Lyska L. Emerson, Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT.
Kenneth M. Boucher, Department of Oncological Sciences and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT.
Sean J. Mulvihill, Department of Surgery and Huntsman Cancer Institute, University of Utah School of Medicine, Salt Lake City, UT.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Murr MM, Sarr MG, Oishi AJ, et al. Pancreatic cancer. CA Cancer J Clin. 1994;44:304–318. doi: 10.3322/canjclin.44.5.304. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Harsha HC, Kandasamy K, Ranganathan P, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb A, Kleeff J, Guweidhi A, et al. Osteopontin influences the invasiveness of pancreatic cancer cells and is increased in neoplastic and inflammatory conditions. Cancer Biol Ther. 2005;4:740–746. doi: 10.4161/cbt.4.7.1821. [DOI] [PubMed] [Google Scholar]
- 7.Zhivkova-Galunska M, Adwan H, Eyol E, et al. Osteopontin but not osteonectin favors the metastatic growth of pancreatic cancer cell lines. Cancer Biol Ther. 2010;10:54–64. doi: 10.4161/cbt.10.1.12161. [DOI] [PubMed] [Google Scholar]
- 8.Bloomston M, Shafii A, Zervos EE, et al. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J Surg Res. 2002;102:39–44. doi: 10.1006/jsre.2001.6318. [DOI] [PubMed] [Google Scholar]
- 9.The R Project for Statistical Computing [R-project Web site] 2010 May 31; Available at: http://www.r-project.org.
- 10.Brand RE, Nolen BM, Zeh HJ, et al. Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res. 2011;17:805–816. doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R, Crispin DA, Pan S, et al. Pilot study of blood biomarker candidates for detection of pancreatic cancer. Pancreas. 2010;39:981–988. doi: 10.1097/MPA.0b013e3181dac920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koopmann J, Fedarko NS, Jain A, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- 13.Mroczko B, Lukaszewicz-Zajac M, Wereszczynska-Siemiatkowska U, et al. Clinical significance of the measurements of serum matrix metalloproteinase-9 and its inhibitor (tissue inhibitor of metalloproteinase-1) in patients with pancreatic cancer: metalloproteinase-9 as an independent prognostic factor. Pancreas. 2009;38:613–618. doi: 10.1097/MPA.0b013e3181a488a0. [DOI] [PubMed] [Google Scholar]
- 14.Pan S, Chen R, Crispin DA, et al. Protein alterations associated with pancreatic cancer and chronic pancreatitis found in human plasma using global quantitative proteomics profiling. J Proteome Res. 2011;10:2359–2376. doi: 10.1021/pr101148r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Sokoll LJ, Bruzek DJ, et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev. 1998;7:109–112. [PubMed] [Google Scholar]
- 16.Ahmed M, Behera R, Chakraborty G, et al. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets. 2011;15:1113–1126. doi: 10.1517/14728222.2011.594438. [DOI] [PubMed] [Google Scholar]
- 17.Bigelow RL, Williams BJ, Carroll JL, et al. TIMP-1 overexpression promotes tumorigenesis of MDA-MB-231 breast cancer cells and alters expression of a subset of cancer promoting genes in vivo distinct from those observed in vitro. Breast Cancer Res Treat. 2009;117:31–44. doi: 10.1007/s10549-008-0170-7. [DOI] [PubMed] [Google Scholar]
- 18.Davidsen ML, Wurtz SO, Romer MU, et al. TIMP-1 gene deficiency increases tumour cell sensitivity to chemotherapy-induced apoptosis. Br J Cancer. 2006;95:1114–1120. doi: 10.1038/sj.bjc.6603378. [DOI] [PMC free article] [PubMed] [Google Scholar]