Abstract
Approximately half of EGFR mutant non-small cell lung cancer (NSCLC) patients treated with small molecule EGFR kinase inhibitors develop drug resistance associated with the EGFR T790M “gatekeeper” substitution, prompting efforts to develop covalent EGFR inhibitors, which can effectively suppress EGFR T790M in pre-clinical models. However, these inhibitors have yet to prove clinically efficacious, and their toxicity in skin, reflecting activity against wild-type EGFR, may limit dosing required to effectively suppress EGFR T790M in vivo. While profiling sensitivity to various kinase inhibitors across a large cancer cell line panel, we identified indolocarbazole compounds, including a clinically well-tolerated FLT3 inhibitor, as potent and reversible inhibitors of EGFR T790M, which spare wild-type EGFR. These findings demonstrate the utility of broad cancer cell profiling of kinase inhibitor efficacy to identify unanticipated novel applications, and they identify indolocarbazole compounds as potentially effective EGFR inhibitors in the context of T790M-mediated drug resistance in NSCLC.
INTRODUCTION
“Oncogene addicted” cancers define a clinical context in which rationally-targeted drug therapies have been somewhat successful. In many cases, these cancers are defined by the presence of mutationally activated tyrosine kinases, such as BCR-ABL, c-KIT, HER2, and EGFR (1). Consequently, intense efforts have been focused on tyrosine kinase inhibitors (TKIs) as promising molecularly-targeted therapies for genotype-defined subsets of cancers (1-4). In ~10-20% of NSCLCs, somatic activating alleles affecting the catalytic kinase domain of EGFR have been well correlated with the clinical response to the small molecule EGFR kinase inhibitors, gefitinib and erlotinib (5, 6). In the adenocarcinoma subtype of NSCLC, nearly 90% of these mutations are either in-frame small deletions affecting exon 19 or the missense mutation L858R. Both of these mutations have been shown to promote the activation of EGFR signaling and a state of EGFR dependency (7, 8).
Despite the dramatic clinical responses to gefitinib and erlotinib that have been observed in some advanced NSCLCs, treated patients invariably develop acquired resistance to these drugs, typically around 1 year following the initiation of treatment (9). Approximately 50% of patients who initially responded to EGFR TKI therapy and subsequently develop drug resistance have acquired within their tumors a secondary mutation within the EGFR kinase domain, a substitution of methionine for threonine at position 790 (T790M) (10, 11). In vitro studies have demonstrated that this mutation renders EGFR TKI-refractory, while preserving catalytic function in the presence of gefitinib or erlotinib.
Two potential mechanisms by which the EGFR T790M mutation confers drug resistance have been proposed. Several groups have focused on the “gatekeeper model”, which was originally described in the context of the analogous T315I mutation of the BCR-ABL fusion kinase associated with acquired drug resistance in chronic myelogenous leukemia patients treated with the ABL TKIs imatinib and dasatinib (12). Similarly, substitution with the bulkier methionine in EGFR T790M mutants causes a steric hindrance, thus preventing drug binding by EGFR inhibitors (10, 11, 13). A more recent report proposed another mechanism in which the T790M substitution increases the binding affinity of EGFR for ATP, resulting in reduced cellular potency of reversible EGFR TKIs (14). Although the specific resistance mechanisms associated with the T790M substitution remain controversial, relapsed NSCLCs with acquired T790M mutations appear to remain dependent on EGFR signaling for their growth, prompting substantial efforts to discover second-generation EGFR inhibitors that can overcome the effects of the T790M substitution.
Several second-generation EGFR kinase inhibitors that covalently bind to a cysteine residue within the EGFR catalytic domain (Cys 797) have demonstrated pre-clinical therapeutic potential for overcoming EGFR T790M through increased occupancy of the ATP binding site (13, 15, 16). However, all of these irreversible inhibitors currently undergoing clinical testing, such as BIBW2992, PF00299804, and HKI-272, have thus far shown limited clinical efficacy, possibly because of their potency against wild-type EGFR, leading to skin rash and GI toxicity, which has limited their maximal dosing to levels less than those that may be required to achieve drug exposure sufficient to overcome the EGFR T790M mutation (17, 18). An encouraging recent study, however, demonstrated a preclinical irreversible pyrimidine-based mutant-selective EGFR inhibitor with greater potency against EGFR T790M than current clinical pyrimidine-based irreversible inhibitors (19).
Using a high-throughput cancer cell line screening platform to profile 705 tumor-derived cancer cell lines for sensitivity to a variety of validated and investigational anti-cancer small compounds (20), we unexpectedly identified a bis-indole-based tool compound that inhibits EGFR T790M resistance-associated mutants, and was largely inactive against wild-type EGFR. A structurally related reversible kinase inhibitor, PKC412, that is currently undergoing Phase III clinical testing as a FLT3 kinase inhibitor, was found to exhibit potent inhibition of EGFR T790M, while completely sparing wild-type EGFR. These findings indicate that it should be possible to develop reversible EGFR T790M inhibitors for which dosing is not limited by on-target toxicities, and may therefore be advantageous relative to currently available irreversible EGFR inhibitors.
RESULTS
The PKC Inhibitor Gö6976 Promotes Apoptosis in EGFR Mutant NSCLC Cells Independently of PKC Inhibition
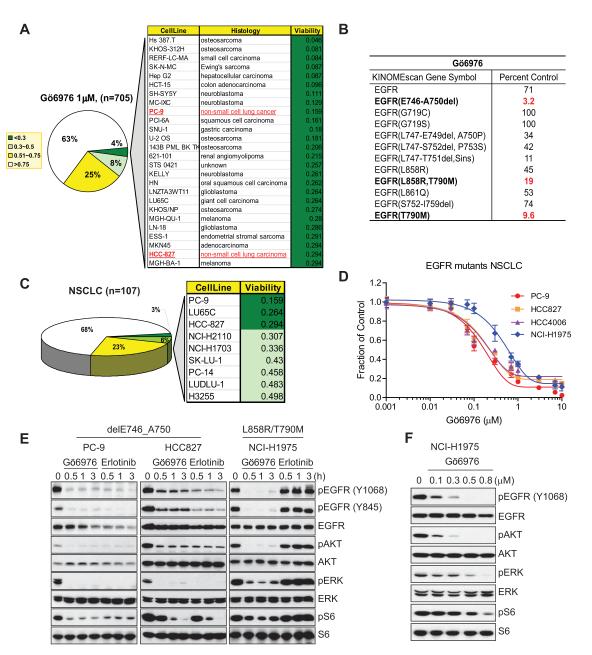
Among a variety of kinase inhibitors profiled for growth inhibitory activity against a panel of 705 human cancer cell lines derived from various solid tumor types, we tested Gö6976, a widely used staurosporine-related inhibitor of “classical” PKCs (Protein Kinase C-α, β, and γ), which have been implicated in oncogenesis (21). Less than 4% of tested cell lines exhibited strong sensitivity to this compound, as defined by greater than 70% growth suppression at 1 micromolar (Fig. 1A; Supplementary Dataset 1). Notably, among the identified Gö6976-sensitive cell lines, two EGFR mutant NSCLC cell lines, PC-9 and HCC827, were unexpectedly strongly growth inhibited by Gö6976.
Figure 1.
Gö6976, a classical PKC inhibitor, inhibits the viability of EGFR mutant NSCLC cell lines. A, Pie chart representing the Gö6976 sensitivity distribution (1 μM) across 705 tested tumor cell lines treated for 72 hr. The legend indicates the sensitivity as measured by the fraction of viable cells relative to untreated controls. Details for 4% of the most sensitive cell lines are shown in the chart and the cell lines are listed in order of decreasing sensitivity. B, Ambit kinome screening results for Gö6976. Kinome profiling was performed using a panel of 442 human kinases on Gö6976 (500 nM). The potential targets are indicated in bold and corresponding inhibitory scores (the percent of DMSO control) are in red. C, Pie chart representation of the Gö6976 sensitivity of 107 tested NSCLC lines. Among the 10 NSCLC lines most sensitive to Gö6976, 3 cell lines, PC-9, HCC-827, and PC-14, harbor activating EGFR mutants. D, Confirmation of Gö6976 efficacy in EGFR mutant-driven NSCLCs. PC-9, HCC827, HCC4006, or NCI-H1975 cells were treated with Gö6976 at the indicated concentration for 72 hr. Error bars represent mean ± SEM. E, Comparison of potency between Gö6976 and erlotinib on EGFR signaling in EGFR mutant-driven NSCLCs. del E746_A750-driven PC-9 or HCC827 cells or L858R/T790M-driven NCI-H1975 cells were treated with either 1 μM Gö6976 or erlotinib for the indicated times followed by immunoblotting with antibodies to detect effects on EGFR signaling. Erlotinib did not affect EGFR signaling in NCI-H1975 cells. F, Potency of Gö6976 in NCI-H1975 cells. Gö6976 treatment in NCI-H1975 at various concentrations for 3 hr. IC50 concentration for suppression of pEGFR is ~100 nM.
We initially hypothesized that PKC might positively regulate the EGFR pathway and that disruption of this regulation by Gö6976 would lead to EGFR inhibition and growth suppression in these cells. To determine whether the classical PKC pathway drives proliferation and survival in PC-9 and HCC827 cells, we treated cells with bis-indolylmaleimide I (Bis) and sotrastaurin, two other classical PKC inhibitors. However, these inhibitors failed to detectably affect growth of either PC-9 or HCC827 cells, even at high concentrations (Supplementary Fig. S1A). While all three inhibitors potently suppressed the PKC pathway, only Gö6976 induced apoptosis, associated with PARP cleavage (Supplementary Fig. S1B). Moreover, inhibition of PKC-α and/or -β by RNAi caused no measurable effects on EGFR signaling or cell viability, (Supplementary Fig. S1C and D), whereas RNAi-mediated knock-down of EGFR, as a positive control, induced apoptosis in both cell lines (Supplementary Fig. S1D). Together, these results suggest that Gö6976 promotes apoptosis in EGFR mutant PC-9 and HCC827 cells by targeting a PKC-independent cell survival pathway.
Gö6976 Inhibits TKI-Sensitizing and -Resistant Mutants of EGFR
To explore the molecular basis for Gö6976 sensitivity in PC-9 and HCC827 cells, we profiled this compound against a panel of 442 human kinases using the Ambit kinome profiling platform (Supplementary Dataset 2). Significantly, Gö6976 (at 500 nM) exhibited substantial binding affinity for EGFR delE746_A750, T790M, or L858R/T790M mutants, while it showed significantly less affinity for wild-type EGFR (Fig. 1B).
By sorting the original cell line screening data on the basis of tissue origin, we found that, among 107 tested NSCLC-derived cell lines, 3 out of 9 of the most sensitive cell lines harbor activating EGFR mutations, consistent with the observation that Gö6976 may be especially efficacious in EGFR mutant cell lines (Fig. 1C). In light of the kinome profiling data, we selected 3 NSCLC lines harboring the EGFR delE746_A750 mutation and one NSCLC line with the L858R/T790M mutation for further evaluation. Gö6976 effectively reduced cell viability in each of these lines, with IC50s of approximately 100-200 nM for EGFR delE746_A750 mutant lines and 800 nM for the L858R/T790M mutant line (Fig. 1D). It has been previously reported that the EGFR mutant cell lines PC-9 and HCC827 are very sensitive to erlotinib, whereas cells harboring an EGFR T790M mutant (NCI-H1975) are relatively erlotinib resistant (11). We compared the ability of Gö6976 and erlotinib to suppress EGFR signaling in these cells. Gö6976 suppressed EGFR signaling as efficiently as erlotinib in both PC-9 and HCC827 cells. Significantly, the EGFR pathway signaling was effectively suppressed by Gö6976 in the erlotinib-resistant NCI-H1975 cells, with an IC50 of ~100 nM (Fig. 1E and F). Taken together with the Ambit profiling results, these findings suggested that Gö6976 can directly inhibit the activity of the EGFR T790M mutant.
Investigational Indolocarbazole Derivatives Directly Inhibit EGFR T790M
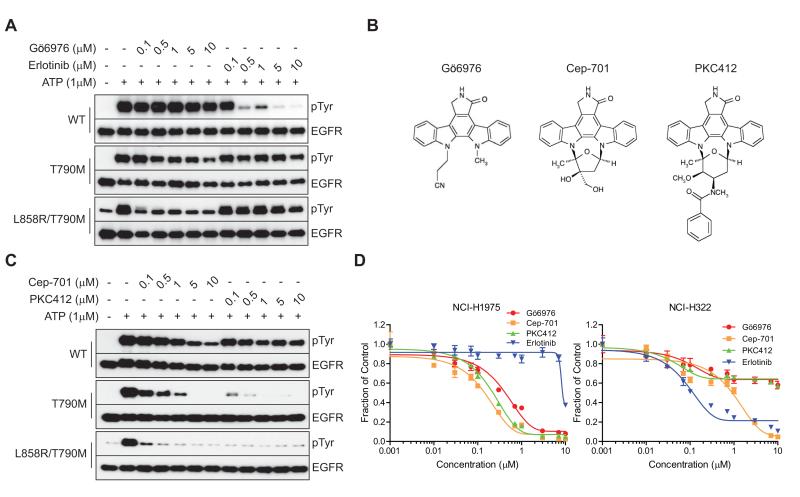
To further explore the ability of Gö6976 to directly inhibit wild-type and mutant forms of EGFR, we employed an in vitro autophosphorylation assay using the recombinant cytoplasmic domain of EGFR. We initially compared the ability of erlotinib and Gö6976 to inhibit ATP-dependent autophosphorylation of wild-type, T790M, and L858R/T790M forms of EGFR. As expected, erlotinib effectively inhibited autophosphorylation of wild-type EGFR, and was ineffective against T790M or L858R/T790M mutant forms. In contrast, Gö6976 was ineffective against wild-type EGFR, even at 10 micromolar, exhibited weak inhibitory activity against the isolated T790M mutant EGFR, and very potently inhibited the autophosphorylation of the L858R/T790M double mutant EGFR-IC50 <100 nM (Fig. 2A). It is worth noting that the purified L858R/T790M mutant EGFR protein is associated with a baseline level of phosphorylation that can be detected even in the absence of added ATP; consequently, even complete inhibition of ATP-dependent autophosphorylating activity would be expected to yield protein product associated with low level phosphorylation in this assay. These findings demonstrate that Gö6976 is a wild-type sparing potent inhibitor of the erlotinib-resistant L858R/T790M mutant.
Figure 2.
Discovery of Gö6976-related potent reversible EGFR T790M inhibitors. A, In vitro inhibition of recombinant EGFR autophosphorylation by Gö6976 or erlotinib. Recombinant EGFR wild-type, T790M, or L858R/T790M was phosphorylated by addition of 1 μM ATP for 15 min in the presence of the indicated concentrations of Gö6976 or erlotinib. Immunoblots show autophosphorylated tyrosine residues in EGFR and total EGFR. B, Chemical structures of Gö6976 and the structurally related Cep-701 and PKC412 inhibitors. C, Comparison of Cep-701 and PKC412 for their inhibitory activities on EGFR T790M. Each recombinant EGFR protein was subjected to an autophosphorylation assay in the presence of ATP with Cep-701 or PKC412 for 15 min. D, Viability assay of NCI-H1975 or NCI-H322 cells treated with Gö6976, Cep-701, PKC412 or erlotinib. NCI-H1975 or NCI-H322 cells were treated with various concentrations of the indicated inhibitors for 72 hr. Error bars represent mean ± SEM.
Gö6976 is derived from staurosporine, an indolocarbazole alkaloid natural product, and is a relatively non-selective kinase inhibitor. Thus, while Gö6976 was originally developed to target classical PKCs, recent studies revealed inhibitory activities for other cancer-associated kinases, including Chk1, FLT3 and JAK2 (22, 23). Notably, some indolocarbazole derivatives have demonstrated greater kinase selectivity, and have been developed for clinical use. Therefore, we extended these findings to examine potential EGFR inhibitory activity for other structurally-related indolocarbazole derivatives that are currently undergoing clinical development. Two such compounds, PKC412 and CEP-701, are small molecule inhibitors that were developed to target the FLT3 kinase, which is frequently activated in acute myeloid leukemia (24). Gö6976, CEP-701, and PKC412 share the indolocarbazole backbone (Fig. 2B).
To examine the potential activity of these investigational compounds as inhibitors of NSCLC-associated EGFR mutants, we again utilized the in vitro EGFR autophosphorylation assay, as well as cell survival assays in the context of NSCLC cell lines expressing either wild-type or the T790M mutant EGFR. In the in vitro biochemical assays, both PKC412 and CEP-701 were found to potently inhibit EGFR T790M and EGFR L858R/T790M autophosphorylating activity. While CEP-701 displayed some weak activity against wild-type EGFR, PKC412 did not cause detectable inhibition of wild-type EGFR at a concentration as high as 10 micromolar. Moreover, PKC412 exhibited the greatest potency among the three indolocarbazole inhibitors tested against T790M mutant EGFR, and was more than 100-fold more potent than Gö6976, despite their structural similarity (Fig. 2C).
This activity was similarly reflected in cell viability assays. Thus, NCI-H1975 NSCLC cells, which harbor EGFR L858R in cis with the T790M substitution, are refractory to erlotinib, but exhibit striking sensitivity to all three of the tested indolocarbazole compounds. In contrast, erlotinib displayed some growth inhibitory activity on treated NCI-H322 NSCLC cells, which express wild-type EGFR (IC50 ~100 nM), whereas these cells were largely refractory to the indolocarbazole compounds (Fig. 2D). Collectively, these data indicate that some indolocarbazole analogues, including two compounds currently undergoing clinical investigation, display potent inhibition of the EGFR T790M mutant in vitro and in NSCLC cell line models.
PKC412 Is More Selective for EGFR T790M than Irreversible EGFR Inhibitors
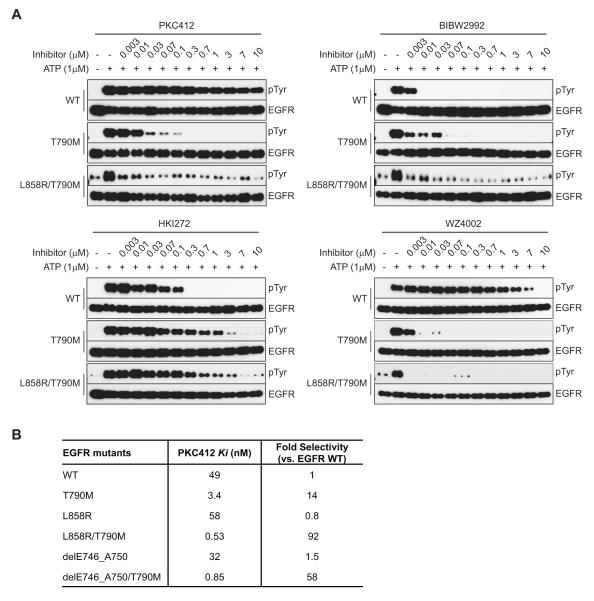
The dosing limitation associated with the irreversible EGFR inhibitors currently undergoing clinical evaluation, such as BIBW2992, PF00299804, and HKI-272, is likely to reflect, at least in part, their lack of selective inhibitory activity for T790M EGFR mutants versus wild-type EGFR (17, 18). Since we observed potent activity of PKC412 on EGFR T790M mutants, we directly compared the potency and selectivity of PKC412, BIBW2992, and HKI-272 against wild-type and mutant forms of EGFR. As reported previously, both of the irreversible inhibitors suppressed the autophosphorylating activity of wild-type EGFR at least as effectively as they inhibited T790M EGFR or L858R/T790M EGFR, indicating that these agents would be unable to reach a sufficient plasma concentration in patients to cause EGFR T790M inhibition without the adverse effects caused by inhibition of wild-type EGFR. Of these two inhibitors, HKI-272 displayed significantly less potency on EGFR T790M than wild-type EGFR (Fig. 3A). This is consistent with previous pre-clinical findings suggesting that a relatively high concentration of HKI-272 would be required to overcome EGFR T790M-medated erlotinib resistance in NSCLC cell lines (25). In contrast, EGFR T790M mutants were inhibited at concentrations of PKC412 as low as 3-30 nM, whereas wild-type EGFR was not detectably affected by PKC412 concentrations as high as 10 micromolar (Fig. 3A).
Figure 3.
In vitro potency of PKC412 against EGFR T790M. A, Comparison of potency between PKC412 and irreversible EGFR inhibitors on phosphorylation of EGFR T790M. Recombinant EGFR proteins were subjected to autophosphorylation reactions in the presence of the indicated concentrations of PKC412, BIBW2992, HKI-272, or WZ4002. Note that the potency of BIBW2992 and HKI-272 against EGFR wild-type and T790M is similar. B, Assessment of inhibition constant (Ki) values of PKC412 for EGFR T790M. Ki values for recombinant EGFR wild-type, del E746_A750/T790M, or L858R/T790M and fold selectivity over EGFR wild-type were calculated for PKC412. Ki values were calculated as described in Methods.
It has been reported that pyrimidine-based irreversible EGFR inhibitors exhibit greater selectivity against EGFR T790M than clinical quinazoline-related inhibitors in tested cell lines and mouse models (19). To compare the properties of one of these irreversible inhibitors, WZ4002, with the reversible PKC412 inhibitor, we performed an in vitro EGFR autophosphorylation assay. As previously reported, WZ4002 exhibited potent suppression of ATP-dependent autophosphorylation of EGFR T790M or EGFR L858R/T790M. At most tested concentrations, WZ4002 was ineffective against wild-type EGFR; however, unlike PKC412, at concentrations above 1 micromolar, WZ4002 demonstrated detectable inhibition of wild-type EGFR (Fig. 3A).
To extend these findings, we next conducted enzyme inhibition studies with purified recombinant wild-type and mutant EGFR proteins to compare the activities of PKC412, BIBW2992, and WZ4002. All three compounds demonstrated similar Ki values (<10 nM) for the L858R/T790M and del E746_A750/T790M mutant proteins, within 2-fold of each other, indicating that they are all very potent T790M inhibitors. Notably, only PKC412 and WZ4002 were more potent against the T790M mutants than wild-type EGFR (Supplementary Fig. S2A). Interestingly, when PKC412 was pre-incubated with enzyme prior to ATP addition, Ki values of PKC412 for both EGFR L858R/T790M and del E746_A750/T790M significantly shifted the selectivity ratio for wild-type EGFR to 92- and 58-fold, respectively (Fig. 3B). Moreover, when we compared off-rates of PKC412 for L858R/T790M and wild-type EGFR in this experimental setting, PKC412 showed a very slow off-rate (residence time of 133 minutes, data not shown) for the mutant only. This suggests that one mechanism contributing to the selectivity and potency of PKC412 against EGFR T790M may be the longer occupancy of the ATP binding site once it binds. Overall, PKC412 exhibited the greatest selectivity for EGFR T790M versus wild-type EGFR. (Fig. 3B; Supplementary Fig. S2A and B). These results provide further evidence that PKC412 is a very potent inhibitor of T790M mutant forms of EGFR and does not significantly inhibit wild-type EGFR.
PKC412 Potently Inhibits Ligand-Mediated EGFR T790M Activation in Cell Lines
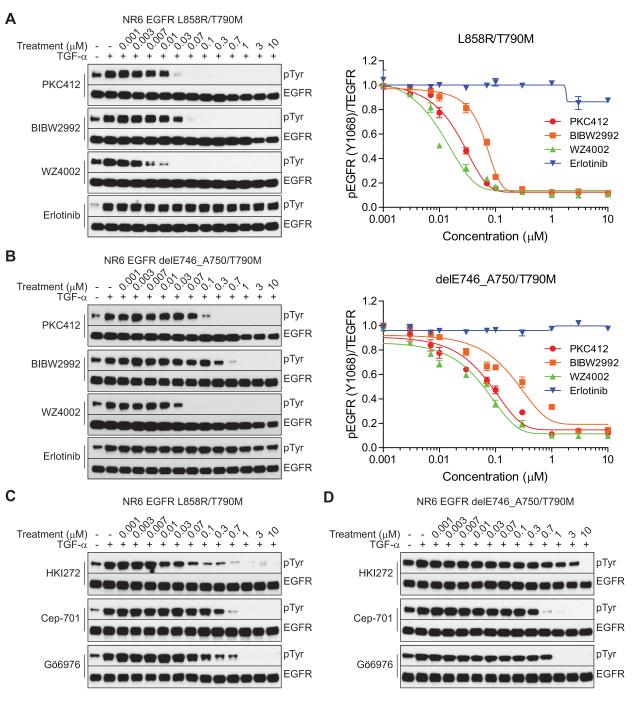
To extend the comparative in vitro enzyme inhibition studies to cell line models, we next assessed the ability of the various EGFR T790M inhibitors to block ligand-induced (TGF-α) EGFR phosphorylation in NR6 cells (immortalized murine fibroblasts) engineered to stably express wild-type, L858R/T790M, or del E746_A750/T790M mutant forms of EGFR. As shown, PKC412, BIBW2992, and WZ4002 displayed similarly potent ability to inhibit L858R/T790M, and WZ4002 was the most potent inhibitor of ligand-induced phosphorylation of del E746_A750/T790M (Fig. 4A and B). The immunoblot results were confirmed using a quantitative immunocytochemical approach to detect pEGFR and total EGFR (Fig. 4A and B). In contrast, at sub-micromolar concentrations, HKI-272 only inhibited the L858R/T790M mutant. CEP-701 and Gö6976 effectively inhibited both of the T790M-containing mutants (Fig. 4C and D). Phosphorylation of wild-type EGFR in ligand-stimulated NR6 cells was largely suppressed by BIBW2992, and was modestly affected by WZ4002, only at high concentrations. Notably, activation of wild-type EGFR was not significantly inhibited by PKC412, consistent with the findings that PKC412 selectively inhibits T790M-containing forms of EGFR (Supplementary Fig. S3). Moreover, we confirmed the reversible nature of PKC412’s activity in PC-9 cells that had been selected in vitro for erlotinib resistance and were found to have acquired a del E746_A750/T790M EGFR mutation (Supplementary Fig. S4) by examining the recovery of pEGFR after treatment in a drug “wash-out” assay (Supplementary Fig. S5A and B).
Figure 4.
Comparison of EGFR T790M inhibitors in EGFR-expressing NR6 cell lines. A and B, NR6 cells stably expressing EGFR L858R/T790M or del E746_A750/T790M were treated with the indicated concentrations of PKC412, BIBW2992, WZ4002, or erlotinib for 16 hr and stimulated by TGF-α for 15 min. The EGFR phosphorylation change was determined by immunoblotting with phospho-tyrosine antibody (left) or using an in-cell immunodetection assay using phospho-EGFR antibody (right). Error bars represent mean ± SEM. C and D, NR6 EGFR L858R/T790M or del E746_A750/T790M cells were treated with HKI-272, Cep-701, or Gö6976 followed by TGF-α stimulation as described in A and B. Ligand-induced EGFR phosphorylation was detected by anti-phospho-tyrosine antibody.
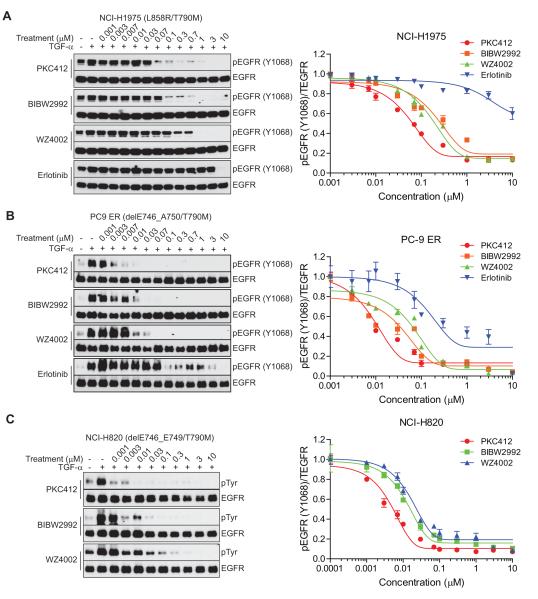
We next examined the activity of the three most potent EGFR T790M inhibitors in NSCLC-derived cell lines, including NCI-H1975, which harbors an EGFR L858R/T790M mutation, and NCI-H820, which harbors an EGFR del E746_A750/T790M mutation. Significantly, PKC412 and BIBW2992 exhibited ~ 2-fold less potency, while WZ4002 demonstrated ~ 40-fold less potency in NCI-H1975 cells (EGFR L858R/T790M) than in NR6 cells expressing L858R/T790M EGFR (Fig. 5A). Unexpectedly, the potency of PKC412 against del E746_A750/T790M EGFR in NCI-H820 was increased by ~ 100-fold relative to NR6 cells expressing the same EGFR mutant. However, WZ4002 potency against del E746_A750/T790M remained the same in both cell lines (Fig. 5B). A similar observation was made in PC-9 cells harboring a del E746_A750/T790M EGFR mutation (Fig. 5C). This cell line also displayed significant sensitivity to PKC412, ~ 30-fold greater than that seen in NR6 cells expressing del E746_A750/T790M EGFR.
Figure 5.
Comparison of EGFR T790M inhibitors in human NSCLC-derived cell lines. A, NCI-H1975 (EGFR L858R/T790M), B, NCI-H820, or C, PC-9 ER (del E746_A750/T790M) cells were treated with the indicated inhibitors for 16 hr and stimulated by TGF-α for 15 min. The drug effects on EGFR phosphorylation were observed by immunoblotting and an in-cell immunodetection assay as described in Fig. 4.
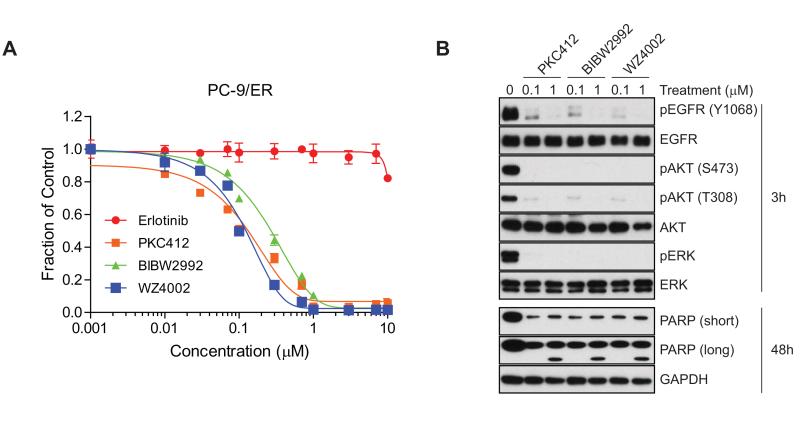
We then directly compared the efficacy of these various inhibitors in assays of proliferation and apoptosis. Notably, the growth inhibition assay revealed smaller IC50 variations between inhibitors than the ligand-induced EGFR activation assay (Figs. 6A and 5A-C). EGFR signaling was invariably suppressed at early time points and apoptosis was induced at later time points by all of the tested inhibitors (Fig. 6B). These findings suggest that the mechanism of ligand-induced EGFR activation may differ from that of ligand-independent activation, and that PKC412 has greater potency against ligand-induced EGFR activation than other tested compounds in EGFR T790M mutant NSCLC cells.
Figure 6.
PKC412 is a potent and selective EGFR T790M inhibitor in EGFR T790M NSCLC cells. A, Viability assay of PC-9/ER cells treated with erlotinib, PCK412, BIBW2992, or WZ4002. PC-9/ER cells were treated with various concentrations of the indicated inhibitors for 72 hr. Error bars represent mean ± SEM. B, Comparison of potency between PKC412, BIBW2992, and WZ4002 on EGFR signaling. PC-9/ER cells were treated with either 0.1 or 1 μM of indicated inhibitors for 3 or 48 hr followed by immunoblotting with antibodies on EGFR signaling.
PKC412 Suppresses EGFR T790M-Promoted Tumor Growth in Vivo
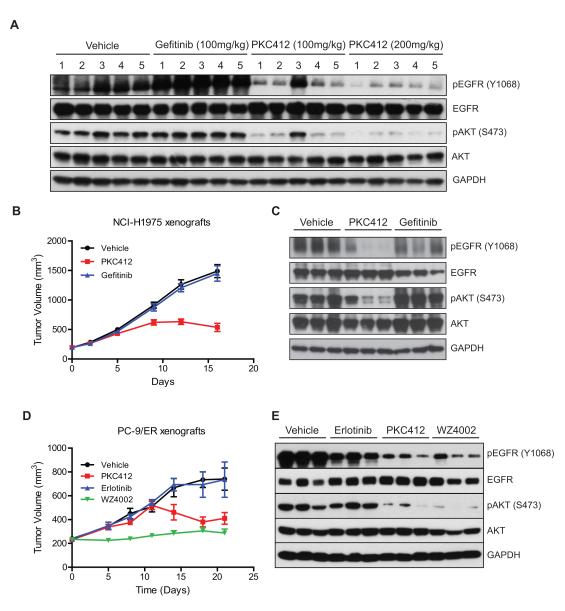
To confirm the observed PKC412 efficacy in an in vivo tumor model, we performed xenograft studies using NCI-H1975 NSCLC and PC-9/ER lines, which harbor EGFR L858R/T790M and del E746_A750/T790M, respectively. We first determined whether PKC412 could suppress EGFR signaling in vivo. Established NCI-H1975 tumor xenografts were treated daily for 3 days with 100 mg/kg, 200 mg/kg of PKC412, vehicle or 100 mg/kg of gefitinib. Significant suppression of phospho-EGFR and phospho-AKT was observed following PKC412 treatment (Fig. 7A), whereas vehicle control or gefitinib (100 mg/kg) failed to inhibit EGFR signaling, as previously reported (11). We then assessed the ability of these treatments to retard tumor growth. Mice bearing established NCI-H1975-derived tumors were treated daily with orally administered PKC412, gefitinib, or vehicle control for the duration of the study (Fig. 7B and C). PKC412 significantly suppressed tumor growth by 50% compared to control-treated animals at day 12.
Figure 7.
PKC412 inhibits EGFR signaling and tumor growth in xenograft models of EGFR T790M NSCLC. A, NCI-H1975 tumor cells were implanted (s.c.) into right flanks of Balb/c nude mice as described in Methods. Dosing was started on Day 1 after tumors reached a required volume range (350-400 mm3). Tumor samples were collected at 2 hr after the 3rd dosing of the indicated drugs. Drug efficacy was assessed by immumoblotting using pEGFR and pAKT antibodies. B, Antitumor activity of PKC412 on NCI-H1975 xenografts. Tumor growth assays of vehicle-, PKC412-, or gefitinib-treated NCI-H1975 xenografts were performed as described in Methods. C, PKC412 efficacy on EGFR suppression. EGFR signaling in tumor samples collected from each group at day 16 was examined as in A. D, Antitumor activity of PKC412 on PC-9/ER xenografts. Tumor growth assays of vehicle-, PKC412-, erlotinib- or WZ4002-treated xenografts were performed as described in Methods. E, PKC412 efficacy on EGFR suppression in PC-9/ER xenografts. EGFR signaling in tumor samples of each indicated group at Day 21 at 4 hr after the last dosing was examined as in A.
In the PC-9/ER xenograft study, we included a WZ4002 treatment group for comparison (Fig. 7D and E). Notably, PKC412 did not suppress tumor growth until 12 days on treatment, after which time tumors began to regress. These kinetics of response were similarly observed in the NCI-H1975 xenograft study (Fig. 7B). In both studies, no significant body weight loss was detected (Supplementary Fig. S6A and B), suggesting that the observed tumor regression is not due to accumulated toxicity of PKC412. In addition, immunoblotting of PKC412-treated xenografts (4 hours after the last dosing), confirmed effective suppression of EGFR signaling in tumors, indicating that the inhibitory effect of PKC412 against EGFR T790M was maintained during the course of treatment (Fig. 7C and E). Tumor growth in mice treated with WZ4002 was efficiently inhibited for 21 days of the study. Pharmacokinetic analysis was performed from plasma and tumor samples immediately following the final dosing, and revealed a Cmax of 2.91 μM at 30 min post-treatment, and a plasma concentration of 0.659 μM by 24 hours post-treatment (Supplementary Fig. S7A-C).
We extended the in vivo analysis to examine the efficacy of PKC412 in a genetically engineered NSCLC model that develops lung adenocarcinomas driven by transgenic expression of EGFR L858R/T790M. In this study, PKC412-treated tumors were growth inhibited for the first two weeks of treatment, and then began to increase in size at three and four weeks of treatment in three mice, albeit at a slower rate than vehicle-treated tumors (#4212, 3208, 4220), while steady tumor growth suppression during the overall time period of treatment was observed in the other three treated mice (Supplementary Fig. S8A). No significant weight loss was observed during 4 weeks of PKC412 treatment (Supplementary Fig. S8B). Biochemical analysis demonstrated that the anti-tumor activity of PKC412 is well correlated with a significant decrease in phosphorylation of EGFR L858R/T790M of tumors treated for 5 days, indicating that PKC412 can effectively suppress EGFR T790M activity in vivo in this model (Supplementary Fig. S8C).
DISCUSSION
The successful development of gefitinib and erlotinib for the treatment of EGFR mutant NSCLCs has been a very significant advancement in the clinical management of metastatic cancer. Moreover, the observed association between the clinical activity of these agents and the presence of activated alleles of EGFR within a subset of tumors has helped to advance the paradigm of “personalized medicine” for cancer patients. Although these drugs were initially developed as inhibitors of wild-type EGFR, the fact that they exhibit ~10-fold increased potency against the mutationally activated forms of EGFR, which has been attributed to the reduced affinity of these mutants for ATP (26, 27), has probably also contributed to their clinical efficacy. Despite their impressive clinical activity in a subset of treated patients, the inevitable acquisition of drug resistance has prompted significant efforts to develop second-generation inhibitors, especially those that can overcome the frequently observed EGFR T790M gatekeeper mutation. While the first-generation EGFR inhibitors effectively compete for ATP in the context of the clinically-observed activating mutants, the T790M substitution at the gatekeeper position restores ATP affinity, consequently imposing a more formidable challenge for competitive inhibition (14).
The need to overcome EGFR T790M-mediated resistance to gefitinib or erlotinib has prompted substantial efforts to discover inhibitors that exhibit increased potency against EGFR T790M or limit access of ATP to the binding pocket. Thus far, all of the reported investigational agents that have been developed to target EGFR T790M are irreversible EGFR inhibitors which covalently occupy the ATP binding site as a means of reducing ATP binding and thereby inhibiting catalysis. Indeed, these second-generation irreversible inhibitors, including BIBW2992, PF00299804, CI-1033, and HKI-272 exhibit substantially greater potency against EGFR T790M than gefitinib or erlotinib in in vitro studies. However, like the first-generation inhibitors, the irreversible inhibitors are also very active against wild-type EGFR, leading to an on-target dose-limiting toxicity associated with severe skin rash in some patients. Our studies have confirmed that BIBW2992 and HKI-272 are in fact more potent against wild-type EGFR than the T790M-containing mutants, and due to dose-limiting toxicity, such inhibitors may not reach sufficient plasma concentrations to effectively inhibit EGFR T790M in patients (17).
WZ4002 is a recently reported irreversible inhibitor that displays much improved selectivity against EGFR T790M over wild-type EGFR in preclinical studies (19). Indeed, at concentrations as low as 10 nM, WZ4002 inhibits ATP-dependent autophosphorylation of EGFR T709M without any observed effects on wild-type EGFR. However, at higher concentrations, we found that this compound also inhibits wild-type EGFR in vitro and in cell line studies. Due to the irreversible nature of its inhibitory mechanism, prolonged administration of this agent could potentially affect wild-type EGFR, particularly in tissues where it accumulates.
The non-covalent indolocarbazole compounds described here, including Gö6976 and PKC412, appear to function as more selective inhibitors of EGFR T790M, which is consistent with a recent study implicating Gö6976 as an EGFR T790M inhibitor (28). Importantly, we demonstrated that PKC412 is at least 100-fold more potent against EGFR T790M than Gö6976 and is comparably potent with the irreversible EGFR inhibitors, but with 500-fold less activity against wild-type EGFR in drug-treated cells. Consistent with these findings, the in vitro binding specificity of indolocarbazole compounds for EGFR T790M has been previously reported (29). PKC412 is currently undergoing Phase III clinical testing in AML patients with activating FLT3 mutations, and its safety has been demonstrated in a Phase I trial of patients with solid tumors. Although PKC412 is a potent inhibitor of PKC, FLT3, KIT, KDR, PDGFRα and β in vivo (30-33), inhibition of these signaling kinases may not be detrimental to normal cell physiology in most tissues. Similarly, it has been somewhat unexpectedly observed that potent multi-kinase inhibitors, including dasatanib and sunitinib, can be safely administered to cancer patients.
Recently, a concern regarding the potential clinical efficacy of PKC412 was raised with respect to its relatively low free concentration in plasma due to its rapid metabolization rate, and substantial binding affinity for the serum protein alpha-1-acid glycoprotein (AAG) (34). However, despite these pharmacokinetic limitations, the clinical findings with FLT3 mutant AML patients treated with PKC412 have been encouraging. Moreover, clinical responses were well correlated with FLT3 inhibition, and despite the rapid metabolization of PKC412, concentrations of its major metabolite, CGP52421, remain high (20 to 25 μM) in plasma and may be active against the target (24, 34-36). Furthermore, the direct measurement of PKC412 and its metabolites in solid tumor tissues of metastatic melanoma patients treated with PKC412 at 225 mg/d for 28 days, demonstrated that the tissue concentration of PKC412 was relatively high (median 593 nM)--8-200 times the IC50 required for inhibition of EGFR T790M mutants in NCI-H1975 and PC-9/ER cells. Considering that the in vitro binding affinity of CGP52421 (median tissue concentration, 1.5 μM) for EGFR T790M mutants is similar to that of PKC412, the cumulative plasma concentration of CGP52421 and PKC412 would be expected to be sufficient to inhibit EGFR T790M (37, 38). Notably, while our xenograft studies did not demonstrate tumor regression in the relatively short treatment window, the tumor inhibition curve was trending to regression at the time when animals needed to be taken down due to vehicle-associated ulceration in the NCI-H1975 model, and significant tumor regression was observed in the PC-9/ER model, raising the possibility that the slow accumulation of the CGP52421 metabolite could yield increased efficacy after a period of time during which an active drug metabolite accumulates.
In summary, these findings demonstrate the utility of broad cancer cell line sensitivity profiling to identify unanticipated and potentially useful applications for small molecule kinase inhibitors. The follow-up studies have provided a proof-of-principle demonstration that some selective indolocarbazole derivatives can function as potent inhibitors of EGFR T790M in a reversible manner in vitro and in vivo, largely sparing wild-type EGFR. This suggests that such inhibitors may be effective without the adverse effects associated with the irreversible EGFR T790M inhibitors currently undergoing clinical evaluation, which are also potent inhibitors of wild-type EGFR. It is also possible that these reversible inhibitors could be used in combination with first-generation EGFR TKIs, since the first-generation inhibitors appear to be somewhat more active against the classical EGFR activating mutations, and might therefore be most effective once a secondary T790M mutation has been acquired. Since some NSCLC tumors harbor multiple EGFR alleles, with or without the presence of T790M mutations (8), a combination strategy might be required to optimally suppress signaling from more than one form of mutant EGFR within a single tumor. Lastly, it will certainly be of interest to determine whether structurally-related alternative inhibitors can be generated that maintain these properties while demonstrating even greater selectivity for EGFR over other kinases.
METHODS
Human Cancer Cell Lines and High-Throughput Tumor Cell Line Screening
Human cancer cell lines were tested to assess treatment effects on viability using an automated platform as previously described (20). Cells were treated with 1 μM Gö6976 for 72 hr and then assayed for cell viability. Cell lines were either maintained in RPMI 1640 or in DMEM/F12 (GIBCO) supplemented with 10% FBS (GIBCO), 50 units/mL penicillin, 50 units/mL streptomycin and 2mM L-glutamine (GIBCO). PC-9 cells were kindly provided by Dr. Kazuto Nishio (National Cancer Center Hospital, Tokyo). HCC827, NCI-1975, and NCI-H820 cells were obtained from the American Type Culture Collection (ATCC). Cells were tested and authenticated by SNP genotyping. NR6 EGFR lines and PC-9/ER cells were authenticated by immunoblotting for EGFR and sequencing EGFR from PCR-amplified genomic DNA.
Cell Viability Assays
Cell viability was assessed using the fluorescent DNA-staining dye SYTO60 (Invitrogen). 5×103 cells were plated in 96 well plates in triplicate, and the following day cells were treated with a variety of drug concentrations. After 72 hr, cells were fixed in 4% formaldehyde and stained with SYTO60 followed by fluorescent measurement using the Odyssey Imaging system (absorption at 700 nm; LI-COR). Drug sensitivity was calculated as the fraction of drug-treated cells relative to untreated cells. Data were subjected to a non-linear regression model and drug-response curves were obtained using GraphPad Prism version 5.3 (GraphPad Software, Inc.).
Kinase Inhibitors
Gö6976 was obtained from EMD Chemicals Inc. Bisindolylmaleimide I was purchased from Tocris Bioscience and sotrastaurin was from Axon Medchem. Cep-701 and PKC412 were obtained from LC Laboratories. BIBW-2992 and HKI-272 were purchased from Selleckchem. Gefitinib was obtained from Astrazeneca. WZ4002 and erlotinib were synthesized at Genentech.
EGFR Autophosphorylation Assay
All EGFR recombinant proteins were purchased from Millipore. 100 ng protein was used for autophosphorylation reactions. Reactions were carried out in 8 mM MOPS-NaOH pH 7.0, 1 mM EDTA, 10 mM MnCl2, 10 mM MgCl2, 0.8 M (NH4)2SO4, and 1 mM ATP with or without various concentration of inhibitors. Reactions were at 34°C for 15 min and stopped by adding SDS-sample buffer. Samples were electrophoresed on SDS-PAGE, and phosphorylated EGFR was detected by phospho-Tyrosine antibody.
Ki Value Assessment (In Vitro Inhibitory Enzyme Kinetic Assays)
EGFR proteins were purchased from Invitrogen or Carna Biosciences. The protein constructs used in enzymatic assays were EGFR WT (catalytic domain aa668-1210), T790M (catalytic domain aa668-1210); L858R (catalytic domain aa668-1210), L858R/T790M (catalytic domain aa668-1210), delE746_A750 (catalytic domain aa669-745, 751-1210), delE746_A750/T790M (catalytic domain aa669-745, 751-1210). PKC412 was pre-incubated with EGFR kinase (WT 0.006 γM; T790M 0.017 γM; L858R 0.016 γM; L858R/T790M 0.0092 γM; delE746_A750 0.004 γM; delE746_A750/T790M 0.0046 γM) in 50 mM HEPES, pH 7.5, 10 mM MgCl2, 4 mM MnCl2, 0.01% Brij-35, 1 mM DTT for 30 minutes followed by the addition of 5 γM ATP (Sigma) and 1 γM Fl-EEPLYWSFPAKKK-CONH2 peptide substrate (Caliper Life Sciences). After an additional 30 minutes (60 minutes for EGFR T790M), reactions were terminated by addition of EDTA to a final concentration of 80 mM. Concentrations of substrate and product were quantified using a mobility shift chip (Caliper Life Sciences) on a LabChip 3000 instrument. Dose response curves were fit to % inhibition data using the Morrison Equation. Ki values are subsequently calculated using the equation Ki = Morrison Ki / (1 + [ATP]/Km) for competitive inhibitors (39). ATP Km values for enzymes were as follows: WT 1.7 γM; T790M 2.0 γM; L858R 5.3 γM; L858R/T790M 1.3 γM; delE746_A750 2.8 γM; delE746_A750/T790M 2.1 γM.
Immunoblotting
Cells were lysed in NP-40 lysis buffer containing protease inhibitor cocktail (Roche). Lysates were prepared by taking supernatants from centrifugation for 15 min at 12,000 g. Equivalent amounts of proteins were loaded and separated by SDS-PAGE followed by transfer to membranes. Antibodies used for immune-detection of proteins were; phospho-EGFR (Y845; #6963), EGFR (#2232), AKT (#9272), phospho-ERK (#9101), ERK (#9102), phospho-S6 (#2211), S6 (#2217), PKCα (#2056), PKC substrates (#2261), cleaved PARP (#9541), phospho-tyrosine (#9411), and GAPDH (#2118) (Cell Signaling). Phospho-EGFR (Y1068; Abcam, ab40815), and phospho-AKT (Invitrogen, 44621G).
Generation of Erlotinib-Resistant PC-9 Clones
Erlotinib-resistant PC-9 clones were established by exposing parental cells to gradually increasing concentrations of erlotinib for 3 months. Clones capable of proliferating in the presence of drug were isolated and confirmed to be erlotinib resistant. A clone exhibiting the greatest level of EGFR T790M expression and no anti-proliferative response to 10 μM erlotinib was selected and designated as PC-9/ER.
Xenograft Studies
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Genentech and carried out in an AAALAC (Association for the Assessment and Accreditation of Laboratory Animal Care) accredited facility. NCI-H1975 cells were cultured in RPMI 1640 medium containing 10% serum with 2 mM L-glutamine, and 5×106 cells were implanted subcutaneously into right flanks of Balb/c nude mice. When tumor sizes reached ~200 mm3, mice were randomized into three groups of ten mice each. One group of mice was treated with PKC412 100 mg/kg, as described previously (32), another group was treated with gefitinib 100 mg/kg, and a third group of mice was treated with vehicle alone. All treatments were stopped at day 16 because of severe ulceration across the three treatment cohorts due to the unusual required formulation for PKC412 (32). The pharmacodynamic studies for each treatment were performed with an additional three mice after 2 days of treatment. For the PC-9/ER xenograft study, PC-9/ER cells were cultured in RPMI 1640 medium with 10% serum and 2 mM L-glutamine. Harlan athymic nude mice were inoculated subcutaneously into the right flank area with 5×106 cells suspended in HBSS/matrigel. When tumor sizes reached ~200-300 mm3, mice were randomized into four groups of 7 mice each. Each group of mice was dosed via daily oral gavage with PKC412 100 mg/kg, erlotinib 50 mg/kg, WZ4002 25 mg/kg, or vehicle alone for 21 days. Tumor volumes were determined using digital calipers (Fred V. Fowler Company, Inc.) using the formula (L×W×W)/2. The pharmacodynamics studies were performed 4 hours following the final treatment.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
EGFR mutant lung cancer patients who respond to currently used EGFR kinase inhibitors invariably develop drug resistance, which is associated with the EGFR T790M resistance mutation in about half these cases. We unexpectedly identified a class of reversible potent inhibitors of EGFR T790M that do not inhibit wild-type EGFR, revealing a promising therapeutic strategy to overcome T790M-associated drug-resistant lung cancers.
Acknowledgments
We are grateful for members of the Settleman laboratory for helpful discussions, to members of the MGH Cancer Center’s Center for Molecular Therapeutics for sensitivity profiling of cancer cell lines, and to Jose Imperio and the In Vivo Study Group for conducting the pharmacokinetic study. The authors acknowledge support from grants R00CA131488 and R01CA120247 (K. Politi). Additional support was received from Uniting Against Lung Cancer (K. Politi), the Labrecque Foundation (K. Politi) and the American Italian Cancer Foundation (V. Pirazzoli).
Footnotes
COI Disclosure: All authors except V.P. and K.P. are employees of Genentech and may be shareholders of Roche Pharmaceuticals. K.P. is an inventor on a patent for EGFR T790M testing that was licensed by Memorial Sloan-Kettering Cancer Center to MolecularMD.
Disclosure of Potential Conflicts of Interest All authors except for V.P. and K.P. are employees of Genentech, Inc., a member of the Roche group, and may have equity interest in Roche.
REFERENCES
- 1.Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–31. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 4.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Gazdar AF, Minna JD. Inhibition of EGFR signaling: all mutations are not created equal. PLoS Med. 2005;2:e377. doi: 10.1371/journal.pmed.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–99. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintás-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–48. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 13.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–70. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–32. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besse B, Eaton KD, Soria JC, Lynch TJ, Miller V, Wong KK, et al. Neratinib (HKI-272), an irreversible pan-ErbB receptor tyrosine kinase inhibitor: preliminary results of a phase 2 trial in patients with advanced non-small cell lung cancer. Presented at the 20th Annual EORTC-NCI-AACR Symposium; Geneva, Switzerland. 2008. abstr203. [Google Scholar]
- 18.Jänne PA, Schellens JH, Engelman JA, Eckhardt SG, Millham R, Denis LJ, et al. Preliminary activity and safety results from a phase I clinical trial of PF-00299804, an irreversible pan-HER inhibitor, in patients (pts) with NSCLC. J Clin Oncol. 2008;26(Suppl) abstr8027. [Google Scholar]
- 19.Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–4. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott U, Sharma SV, Dowell L, Greninger P, Montagut C, Lamb J, et al. Indentification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A. 2007;104:19936–41. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–94. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 22.Kohn EA, Yoo CJ, Eastman A. The protein kinase C inhibitor Gö6976 is a potent inhibitor of DNA damage-induced S and G2 cell cycle checkpoints. Cancer Res. 2003;63:31–5. [PubMed] [Google Scholar]
- 23.Grandage VL, Everington T, Linch DC, Khwaja A. Gö6976 is a potent inhibitor of the JAK 2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol. 2006;135:303–16. doi: 10.1111/j.1365-2141.2006.06291.x. [DOI] [PubMed] [Google Scholar]
- 24.Pratz K, Levis M. Incorporating FLT3 inhibitors into acute myeloid leukemia treatment regimens. Leuk Lymphoma. 2008;49:852–63. doi: 10.1080/10428190801895352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godin-Heymann N, Ulkus L, Brannigan BW, McDermott U, Lamb J, Maheswaran S, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–9. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- 26.Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–71. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 27.Mulloy R, Ferrand A, Kim Y, Sordella R, Bell DW, Haber DA, et al. Epidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinib. Cancer Res. 2007;67:2325–30. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- 28.Taube E, Jokinen E, Koivunen P, Koivunen JP. A novel treatment strategy for EGFR mutant NSCLC with T790M-mediated acquired resistance. Int J Cancer. 2011 doi: 10.1002/ijc.26461. doi: 10.1002/ijc.26461. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–51. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 30.Fabbro D, Buchdunger E, Wood J, Mestan J, Hofmann F, Ferrari S, et al. Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharmacol Ther. 1999;82:293–301. doi: 10.1016/s0163-7258(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 31.Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A, et al. PKC412-a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15:17–28. [PubMed] [Google Scholar]
- 32.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–43. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 33.Cools J, Stover EH, Boulton CL, Gotlib J, Legare RD, Amaral SM, et al. PKC412 overcomes resistance to imatinib in a murine model of FIP1L1-PDGFRα-induced myeloproliferative disease. Cancer Cell. 2003;3:459–69. doi: 10.1016/s1535-6108(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 34.Propper DJ, McDonald AC, Man A, Thavasu P, Balkwill F, Braybrooke JP, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–92. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 35.Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 36.Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–83. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–92. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millward MJ, House C, Bowtell D, Webster L, Olver IN, Gore M, et al. The multikinase inhibitor midostaurin (PKC412A) lacks activity in metastatic melanoma: a phase IIA clinical and biologic study. Br J Cancer. 2006;95:829–34. doi: 10.1038/sj.bjc.6603331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams JW, Morrison JF. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–67. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.