Abstract
Implantable drug-delivery systems provide new means for achieving therapeutic drug concentrations over entire treatment durations in order to optimize drug action. This article focuses on new drug administration modalities achieved using implantable drug-delivery systems that are enabled by micro- and nano-fabrication technologies, and microfluidics. Recent advances in drug administration technologies are discussed and remaining challenges are highlighted.
Recent discoveries of new molecular and cellular targets coupled with advancements in genomics, proteomics and other biotechnologies have accelerated the discovery and development of novel pharmaceutical compounds. This emerging class of substances includes biologics, gene therapies, siRNAs, small molecules and other nanoparticle-based therapeutics. However, it is typically not compatible with standard oral formulations or the enteral route of administration because the drugs are not amenable to the absorptive and metabolic processes of the digestive tract and liver, respectively. Instead, these drugs are well suited for parenteral administration, for example, intravenous, intramuscular, intra ocular, subcutaneous and intrathecal, to attain either the desired systemic or local effect. While many of these novel compounds have high specificity and potency, they possess limited bioactivity, relatively short half-life and stability, and other pharmacokinetic properties that require specialized delivery modalities [1,2]. To achieve the highest bioavailability and efficacy, many drugs require continuous or repeated administration to achieve and maintain therapeutic concentration for the desired treatment duration. In some instances, physiological barriers such as the blood-brain barrier must be bypassed. The vascularization and compartmentalization of the targeted tissue and/or organ may also determine selection of the drug-delivery modalities. For conditions that have drug targets distributed to several body regions, such as cancer and viral infections, systemic administration with repeated or continuous dosing is needed to achieve adequate biodistribution. In cases where focal treatment is needed, such as intrathecal chronic pain management, delivery directly to the site of action offers distinct advantages including localized area of drug exposure, minimized drug quantity required for treatment, and limited unwanted systemic side effects [3,4].
Implantable drug-delivery devices are often relied upon to meet these specialized dosing challenges and provide a means to achieve the drug delivery required by these novel pharmaceutical agents and their atypical drug regimen. Implantable drug pumps are routinely utilized in pharmacokinetic and pharmacodynamics studies, which are now an integral process in drug discovery and preclinical and clinical evaluation of efficacy [5]. These drug-delivery methods offer a convenient and cost-effective tool to validate the targets in vivo [6]. They are also used for comparative effectiveness animal studies of different dosing regimens such as continuous versus bolus administration. In the clinical setting, several implantable dosing methods have become the last line of treatment for patients suffering from uncontrollable spasticity or severe chronic pain.
Micro- and nano-fabrication technologies and microfluidics have enabled implantable drug-delivery systems that achieve the desired drug-dosing profile in a miniaturized form factor suitable for surgical placement and operation in vivo. Although there are many drug-delivery technologies that utilize micro- and nano-fabrication technologies such as microneedles and capsules, the focus of this article is specifically on devices and systems that are surgically implanted and best suited for site-specific delivery. Such delivery technologies are each associated with a range of performance and limitations. In turn, each drug treatment should be matched to the appropriate drug-delivery approach based on the method’s particular strengths and weaknesses. The selection determinants such as drug formulations and stability, routes of administration, and dosing profiles for a given body region will not be discussed in detail, and is beyond the scope of this article. These topics for laboratory animals are reviewed in more detail elsewhere [7,8]. The focus of this article is to review recent developments in implantable drug-delivery systems that are enabled by micro- and nano-fabrication technologies.
Implantable drug-delivery devices
Implantable drug infusion pumps have been in use for many years and for both preclinical and clinical applications. Typical implantable systems possess three fundamental components: drug container, drug release mechanism and an implantable packaging. Certain systems may include a catheter for directing the drug, either systemically or to localized therapeutic targets. The differentiating factors include the selection of materials that define the drug release mechanism aswell as the driving force for the drug release. Two classifications will be reviewed: reservoir-based drug-delivery devices and drug-infusion micropumps.
Compared with oral or other external drug infusion modalities such as external drug-infusion pumps, syringe needles and needle-free injection devices, implantable drug-delivery systems are typically associated with three main drawbacks, which are higher cost, surgical placement and specialized training to ensure safety and proper implementation. However, some pharma ceutical agents (e.g., drugs with short half life or rapid metabolic/degradation rates) are not effective or available for oral or external infusion methods. In addition, physiological barriers may limit the accessibility of target treatment sites within the body by these routes. When chronic administration is necessary to these remote targets such as in intra thecal delivery of medications for intractable chronic pain, implantable infusion pumps are the only practical option. The many benefits of implantable drug-delivery devices include minimization of side effects, better titration of drug administration, predictable duration of action, automation in dosing operation, ambulatory use and improved compliance to the prescribed drug regimen. These features enable controlled, continuous release of therapeutics over extended periods (zero order release profile), period release following circadian rhythms or in response to therapeutic measures (nonzero order release profile). Implantables also achieve and sustain higher drug concentrations within localized areas, which are not easily accessible with peripheral drug administration. In addition to the ‘set-and-forget’ benefits, implantables also avoid the constant discomfort and infection risks associated with chronic transcutaneous catheter drug-delivery techniques.
Conventional technologies for research & preclinical use
For laboratory animals and preclinical research, conventional technology-based implantables have become invaluable tools. The Alzet® pump (ALZA/Durect Corp., Cupertino, CA) has been the gold standard in implantable, controlled-release drug delivery in animal research (i.e., mice and rats) since their introduction in 1977 for a wide range of experimental agents (featured in over 13,500 references) [101]. This pump operates on the basis of osmotic principles, utilizing the osmotic gradient generated following subcutaneous implantation. The gradient draws extracellular water across a semipermeable membrane into an expandable chamber containing an osmotically active agent (e.g., NaCl). This in turn applies pressure to a collapsible drug reservoir, and induces drug release at a constant rate through an orifice, which may be connected to a catheter to allow proper physiological placement of the delivered drug. The iPrecio® microinfusion pump (Primetech) has integrated electronics for preprogramming of complex drug regimen prior to implantation [9]. This microinfusion pump utilizes a battery-powered motor to drive a set of pins, generating a peristaltic actuation to controllably pump the soluble drug out of a reservoir. These implantable delivery pumps provide minimal animal handling, stress-free dosing and reduction in needle-injury risks.
Conventional technologies for clinical use
Several US FDA-approved implantable drug-delivery systems are currently in use for a select number of indications. Most clinical applications address chronic drug therapy for palliative care. Insulin, steroids, chemotherapeutics, antibiotics, analgesics, contraceptives, heparin, vaccines and hormones are examples of drugs that may benefit from the use of implantable pumps [10]. Duros® (ALZA/Durect Corp.) is a nonbiodegradable osmotic pump for clinical use. It has been paired with Viadur® (leuprolide acetate, Bayer, FDA approved in March 2000) for the palliative treatment of prostate cancer for a 1 year duration, offering an alternative to frequent leuprolide injections; however, this product was discontinued. Duros® was explored for the delivery of sufentanil, an analgesic, using the Chronogesic® Pain Therapy System for chronic pain management [11] and for exenatide (type 2 diabetes) and ω-interferon (Hepatitis C) [102]. ALZAmer® depot system (ALZA/Durect Corp.) is an injectable biodegradable polymer containing lyophilized proteins and small-molecule compounds. Similar biodegradable systems for sustained delivery include SABER™ Delivery System (Durect Corp. Cupertino, CA), Atrigel® (QLT, Inc., Vancouver, Canada), Durasert™ (pSivida) and Gliadel® wafer system (MGI Pharmaceutical, Eisai, Woodcliff Lake, NJ, USA).
Actively driven drug-infusion systems are also in clinical use for long-term chronic administration, relatively large drug-volume dosing, and aqueous drug formulation. Approved for severe spasticity (2003) and chronic pain (2004), Synchromed® II (Medtronic,, Minneapolis, MN, USA) utilizes a hybrid pumping mechanism, pressurized gas expansion combined with a battery-operated peristaltic action. The Codman® 3000 (Codman and Shurtleff/Johnson and Johnson, New Brunswick, NJ, USA) utilizes a pressurized gas expansion mechanism. Prometra® (Flowonix, Medical, Mt. Olive, NJ, USA; FDA approved in 2012) and Medstream™ (Codman and Shurtleff/Johnson and Johnson; FDA approved in 2011) utilize a similar pressurized gas expansion actuation but regulates drug flow with battery-powered valves. Limited telemetric capabilities have been incorporated into most pumps for in-clinic programming by the care provider. A number of pumps with integrated sensors, for example, AMF Medallion (Alfred Mann Foundation, Valencia, CA, USA), which are currently under development or clinical testing will not be reviewed. Implantable pumps with conventional dosing mechanisms offer a suite of desirable features. These implantable infusion systems offer ease of use by the clinician and ambulatory use for the patient to improve comfort, mobility and quality of life. By contrast, conventional catheters driven by external pumps may require significant assistance for operation by healthcare specialists and in the clinical environment, limiting practical ambulatory use in home environments. Remaining challenges include dosing accuracy, MRI-compatibility, pump size and infection risks associated with the surgical procedure.
Micro- & nano-technology enabled implantable drug-delivery systems
Micro- and nano-technology has enhanced the design features of implantable systems. The size of individual components as well as whole systems has been miniaturized to a minimally invasive implantable form factor. Surface modification techniques and biocompatible material selection have been utilized to provide a stable, chemically inert interface between the implant and the body. These technologies have enabled tunable drug-release mechanisms to achieve great control of drug-release profiles including programmable, cyclic, pulsatile or continuous.
Reservoir-based drug-delivery devices
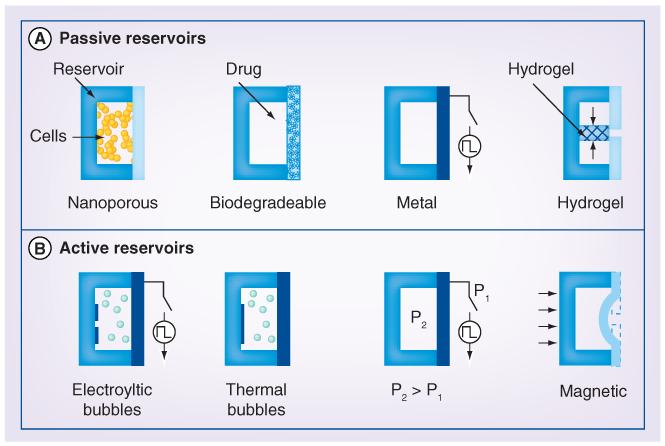
Microfabricated reservoirs (microreservoirs) are simple drug containers that allow temporary storage of drug payloads prior to their release in the body. The drug may be released either slowly over time through nanoporous membranes, orifices located in the reservoir wall or at precise times when an impermeable seal is removed, to allow drug release by diffusion or expulsion through other transport processes. Reservoir-based delivery devices are advantageous for their simple format and often simple implementation. However, in current embodiments, drug payloads cannot be replenished limiting the therapeutic lifetime of such drug-releasing implants. Illustrations describing the drug-release mechanism for passive diffusion-based and actively driven reservoir devices are shown in Figure 1.
Figure 1. Reservoir-based drug-delivery approaches.
(A) Four embodiments of passive-diffusion-based reservoir drug-delivery devices. The release rate and/or onset of delivery of drug stored or produced in the reservoir is regulated by a membrane that may be modified to be nanoporous, biodegradable, electrochemically or electrically removable (metal thin film), or shrunken by application of thermal energy (hydrogel block valve). (B) Four embodiments of actively driven reservoir drug-delivery devices. The mechanism by which drug is actively displaced from the reservoir is depicted including electrolytically generated bubbles, thermally generated bubbles, preloaded pressure difference, or magnetic modulation of membrane displacement and delivery orifice dimensions.
Passive diffusion-based reservoir devices
The earliest micro- and nano-fabricated reservoir delivery systems relied on simple diffusion-mediated transport of drugs stored or produced within. The precise control of feature dimension was exploited in in silico-based immunoisolating capsules, consisting of a nanoporous membrane integrated into a reservoir. Pancreatic islet cells contained within these reservoirs achieved glucose-responsive insulin delivery through the membrane, while immunologic cells and antibodies were excluded by virtue of size from interacting with the transplanted cells [12-14]. As an alternative to joining multiple micro fabricated substrates to form reservoirs, recently, a method to form self-folding polymer polyhedral containers to house particles and cells for drug delivery was described [15]. Containers were initially fabricated as a flat structured sheet that folded into the final polyhedral shape following heat application.
Drug reservoirs microfabricated by etching into silicon substrates can alternatively be capped with temporary thin metallic membranes. The lyophilized drug contained in the reservoirs was selectively exposed for diffusive release by electrochemical dissolution [16,17] or electrothermal degradation of the membrane cap [18]. Later, the drug-reservoir array chip was integrated with wireless electronics to allow pulsatile release of leuprolide in canines for several months [19]. After more than a decade of development, MicroCHIPS™ (MA, USA) recently reported first-in-human results using an investigational device consisting of two chips with ten reservoirs each for the delivery of the human parathyroid hormone fragment in the treatment of osteoporosis [20]. This line of work demonstrated the possibility of pulsatile release of discrete drug amounts at distinct time points.
Temporal control of drug release was also achieved from reservoirs gated with thermo-responsive hydrogel valves. Valves were activated wirelessly by inductive coupling between metallic coil pairs. Heat dissipation following wireless activation from the planar microfabricated coils integrated with the reservoirs resulted in reversible shrinking of the hydrogel valves to allow drug release [21].
In addition to electronically controlled release, biodegradable membrane seals have also been explored, in which physicochemical properties control membrane dissolution rate and the onset of drug delivery [22-24]. The use of resorbable polymer membranes such as poly (l-lactic acid) or poly(lactide-co-glycolide) eliminates the need for electronics. Delivery of pulses of dextran, heparin, human growth hormone, glucose and glycerol was demonstrated using arrays of reservoirs capped with membranes having differing molecular masses to control release time [23,24].
Actively driven reservoir devices
Diffusion-based drug-releasing reservoirs are limited by small dose volumes, the slow release rate and dependence on favorable physiological conditions. Therefore, active mechanisms to expel drugs following removal of the membrane seal were proposed. To improve upon the concept of exposing the drug loaded into silicon wells following electrochemical dissolution of a metal capping film, electrodes were integrated into the interior of the well to enable the drug solution to be expelled by electrolysis. Delivery of vasopressin intended for emergency treatment of hemorrhagic shock was achieved, but only 37% of the drug solution was recovered from reservoirs (15 μl) and chemical degradation of the drug during electrolysis of the solution was observed [25]. Another group proposed an alternate concept, using thermal energy to generate bubbles to both mechanically rupture the sealing membrane and rapidly expel drug from the reservoir (20 μl). The thermal energy was provided by resistive heating of a metallic resistor at the bottom of the reservoir and the mechanism could recover 85% active drug with minimal thermal degradation [26].
Rapid release of reservoir contents can also be achieved by prepressurizing the reservoir. Temporary valves formed from polymer membranes with metallic resistor structures were robust enough to securely contain reservoir contents. A short current pulse applied to the metallic resistor was used to thermally degrade the polymer membrane and allow release of reservoir contents [27,28].
An alternative to removal of membrane capping of the drug reservoir is to control the release of drug by modulating the size of a tiny aperture through which the drug is released. Such a device was described for intraocular delivery of low solubility docetaxel, intended for drug management of diabetic retinopathy. The drug was contained in a polymer reservoir sealed on one side with an elastic, magnetically responsive membrane containing the release aperture. Wireless activation with an external magnetic field deformed the membrane, resulting in release of discrete drug volumes (171 ± 16.7 ng) [29].
Drug-infusion micropumps
Micropumps can be classified as either passive or active, as distinguished by the mechanism employed to displace and deliver the drug. Active pumps require electrical power for operation whereas passive pumps utilize alternative sources of energy (e.g., chemical or mechanical) to supply the pressure differential to drive drug pumping. Infusion pumps, although more complicated than simple reservoirs may, in some cases, be refilled to allow chronic dosing. Recent work on drug-infusion micropumps, with an emphasis on implantable pumps is reviewed.
Passive micropumps
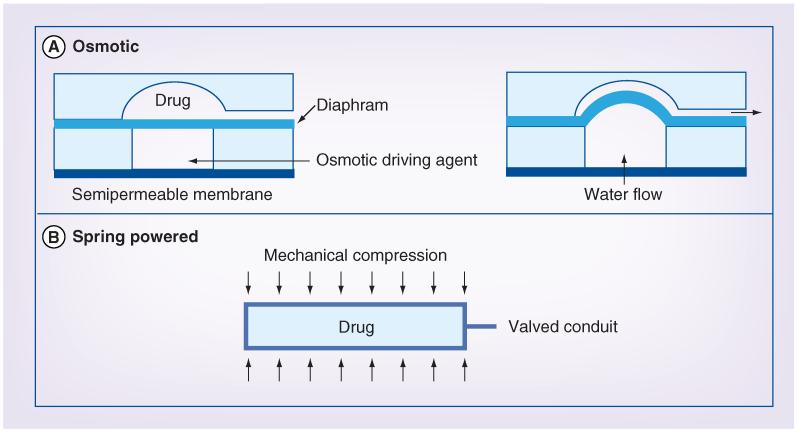
Passive micropumps broadly include mechanisms utilizing osmosis or prepressurized reservoirs. As these do not require a separate electrical power source, the overall size of the pump can be minimized. Both osmotic and spring-powered passive micropump concepts are illustrated in Figure 2.
Figure 2. Passive micropumping approaches.
(A) The operation of an osmotically powered pump. A semipermeable membrane regulates water uptake by the osmotic driving agent, which results in a pressure increase that deflects a flexible diaphragm. The diaphragm in turn displaces the drug stored in the adjacent reservoir. (B) A spring-powered pump concept. External mechanical compression of a compliant drug reservoir provides the pressure differential required to displace drug from the reservoir. Drug delivery is regulated by a valve in line with the pump outlet.
Osmotic micropumps
The osmotic mechanism, as described previously, allows continuous delivery of agents independent of their specific molecular conformation, physical properties and chemical properties. These pumps do not require electrical power. However, its simplicity also imposes limits on the control of dosing profile and timing; once the osmotic gradient is initiated, the osmotic pumps operate continuously with constant rate release and cannot be turned off. The osmotically active agent cannot easily be restored to its original state and therefore, osmotic pumps are typically single use.
A microfabricated osmotic pump was reported containing a 2 ml drug charge capable of slow release (< 2 μl/h) release. While similar in operation to an Alzet pump, this micropump was produced using microfabrication methods resulting in a stacked and planar arrangement of the osmotic chamber, drug reservoir and delivery port [30,31]. Subsequent work carried out by one of the authors resulted in an larger cylindrical osmotic piston pump for release of bone morphogenetic proteins to facilitate bone growth in maxillofacial distraction osteogenesis [32].
A biodegradable osmotic implant for localized delivery of bFGF with application in tissue regeneration was reported (40 ng/day) [33]. Among osmotic micropumps, this particular device is unique for its synthetic biodegradable polymer construction. However, difficulties in controlling the material properties, especially its controllable degradation, still remain.
Spring-powered micropumps
Few have investigated mechanical spring-loaded mechanisms to drive drug displacement, but such pumps offer distinct advantages over osmotic and other actively driven pumps. The Spring Zone® insulin pump uses a spring-based mechanism, Intellispring™, which allowed elimination of the motor found in other pumps to achieve the smallest size and low weight [103]. An alternative to conventional implantable intrathecal drug-delivery pumps is the spring pressurized dual-reservoir-pump system, which is enabled using microfabricated parts [34-36]. This system was introduced to allow multidrug capability for polyanalgesia, which offers significant benefits over monotherapy in the management of chronic pain [37,38]. The use of springs simplifies the system design. Polymer drug reservoirs were pressurized using external springs and the drug was accurately metered using smart, electronically activated microvalves and delivered through a single catheter [35]. The delivery rate of pain medications could be monitored using pressure sensors integrated on the same piece of silicon as the microvalves; such integration affords great advantages in overall system miniaturization. Measurement of drug flow allowed compensation against changes in spinal fluid pressure.
Active micropumps
A major advantage of microelectromechanical systems technologies is the ability to miniaturize electronically controlled pumping mechanisms into tiny form factors suitable for implementation in implantable or external transdermal patch pumps. Various methods have been explored for pumping at the microscale including nonmechanical mechanisms, such as electrohydrodynamic, electroosmotic, ultrasounic and thermo-capillary mechanisms [39], as well as mechanical mechanisms such as electrostatic [40-44], piezo-electric [45-55], electrochemical [56-64], thermal [65-69], shape memory alloy [70,71], bimetallic and electromagnetic mechanisms. These and other drug-delivery mechanisms are explored in more depth in several engineering-focused reviews [72-75].
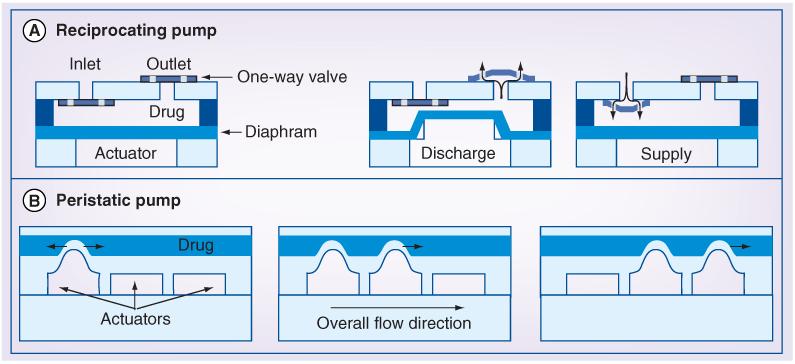
Herein, the focus will be on the more commonly employed mechanical pumps in which a flexible membrane is actuated using one of the aforementioned mechanical driving mechanisms (electrostatic, piezoelectric, electrochemical or thermal) to displace liquid drug from a reservoir. These driving mechanisms are distinctly different from those used in meso- and milli-scale devices and are favored for specific advantages gained as a result of miniaturization [76]. These electronically controlled micropumps allow great control over the onset, profile and termination of drug administration. However, moving components, such as valves and pumping diaphragms are required, which complicates their manufacture. The reduction in power consumption achieved with miniaturization introduces the possibility of wireless-powered and -operated pumps, while eliminating the need for implantable batteries. The two common micropump formats, reciprocating and peristaltic, are generically depicted in Figure 3.
Figure 3. Active pumping approaches.
(A) The fundamental configuration of a reciprocating diaphragm pump showing the major components. Pumping is achieved when the reciprocating actuator (pumping mechanism) alternates between the supply and discharge states. During these stages, the drug chamber above the diaphragm is filled and emptied, respectively, as regulated by two opposing one-way valves. (B) Peristaltic pump consisting of three pumping chambers. Each chamber is separately controlled and activated in a prescribed sequence, such as the one illustrated, to squeeze drug in one direction.
Electrostatic micropumps
A significant mechanical force results from the coulombic attraction between closely spaced (at the micron scale) but oppositely charged metallic plates. If one or more of the plates is movable, then this electrostatic attraction phenomenon can be controlled by modulating the charge on one or more of the plates, as to sequentially squeeze out and resupply fluid between the plates sourced from a reservoir. Therefore, packets of fluid are delivered in a pulsatile manner. Both reciprocating diaphragm [40,41] and peristaltic (multiple-reciprocating diaphragms) [42] type electrostatic micropump configurations have been explored. In peristaltic pumps, the reciprocating diaphragms are linearly arranged, controlled individually and triggered in a prescribed sequence to coordinate the action of the diaphragms, so as to achieve net pumping in one direction. As opposed to reciprocating pumps that require mechanical valves to regulate fluid flow direction, each diaphragm acts as an electronically controlled valve that can shut off the fluid flow path to prevent reverse back flow into the reservoir. Electrostatic mechanisms enjoy fast mechanical response and low power consumption but act over short distances, which limits the fluid volume that can be displaced with each stroke. Moreover, high voltages are required for operation.
Although electrostatic pumping has been widely explored for microfluidic applications, the aforementioned limitations impede their use in drug-delivery applications. To improve the linearity of reciprocating diaphragm electrostatic micropumps, a circular disk was attached to the diaphragm to better define the drug volume displaced [41]. A promising peristaltic electrostatic pump was designed and simulated, but not implemented [42].
Piezoelectric micropumps
Piezoelectric materials exhibit the property of mechanical deformation that can be controlled by an applied electric field. By attaching a piezoelectric thin film or sheet to a flexible diaphragm and applying the appropriate electronic control signal, the diaphragm can be reciprocated to pump fluid in a pulsatile manner as in the previously described electrostatic micropumps. Piezoelectric pumps can also be configured for peristaltic action. Overall, piezoelectric mechanisms provide high force and fast mechanical response, but require high voltages.
Debiotech, a Swiss company, has pioneered piezoelectric micropumps for medical use including the nonimplantable JewelPump™, insulin Nanopump™ for diabetes treatment and the MIP implantable micropump. The JewelPump is designed to be worn on the skin as a patch and comes with a 4.5 ml reservoir [104]. The piezoelectric pump contains a pair of mechanical oneway (check) valves to control flow direction and also a double limiter feature that provides accurate and repeatable control of fluid packets delivered by the pump with each reciprocation cycle [45,47]. An active microport system concept was proposed by an academic group using a piezoelectric pump for patient-specific, continuous release of antiangiogenic drugs targeting metronomic and chronotherapies against cancer [54,55]. Piezoelectrically-actuated micropumps were also described in recent work to address the challenge of delivering suspensions containing aggregates or particles [50,51].
Electrochemical micropumps
The liquid–gas phase change resulting from electrolysis of water offers intriguing possibilities for implantable pump applications. A small current or voltage applied through a pair of electrodes to water drives the generation of oxygen and hydrogen gas bubbles, which produce an increase in pressure that can be used to drive a flexible diaphragm. The reaction is reversible and the gases recombine into water when power is removed to allow a reciprocating action. In contrast with other mechanisms, electrochemical micropumps utilize a slightly different configuration that offers smooth continuous delivery. The flexible diaphragms displace the drug directly from the reservoir. The low power consumption coupled with large achievable mechanical displacement are unique advantages of this method.
Electrochemical micropumps were described first for ocular drug-delivery applications [56-59] followed by gene delivery to treat cancer [60,61] and experimental drug-delivery paradigms in small animals [62-64]. These electrolysis pumps share a similar format: a flexible bellows diaphragm is adjacent to a drug reservoir with an attached delivery catheter. The bellows diaphragm houses a pair of electrodes and water (serving as the electrolyte in the electrochemical cell). The catheter may also be connected to a flow regulating valve that prevents backflow of fluids into the reservoir. The magnitude of the electrical power source, usually current, is linearly correlated to the resulting flow rate and so a wide range of rates are accessible. In ocular drug-delivery applications, small concentrated doses of drug (approximately in the nanoliter to microliter range) are instilled by the pump directly into the intraocular space to avoid blood retina and corneal barriers that limit penetration of oral or topical drugs while avoiding trauma sustained under repeat intraocular injections [77,78]. Pumps were refillable to allow practical use in the treatment of chronic, incurable eye disease without requiring repeat surgical procedures.
The ability of electrochemical micropumps to deliver larger drug doses was demonstrated in two applications. To sensitize cancerous cells to radiation, small interfering RNA targeting SPHK1 was electrostatically conjugated with gold nanorods and locally delivered to tumors (daily dose of 50 μl over 30 min) in the hind flanks of nude mice [60,61]. Greater tumor regression was demonstrated over delivery of conjugates by needle injection and diffusion. An ongoing challenge in small-animal drug delivery is the ability to perform administration while still enabling normal animal behavior and interaction with the environment. The use of implantable pumps overcomes the challenges faced by external pumps with catheter tethers, but only offers low infusion rates. High flow rate intravenous delivery (~100 μl/min) or small boluses (in the microliter range) with wireless-operated electrolysis-based pumps was investigated for use in a tetherless self-administration paragdigm in freely behaving rats and mice for the study of drug addiction [62-64].
Thermal micropumps
Thermal principles such as thermally induced material expansion or phase change (e.g., from liquid to gas) can be used to drive the flexible pump diaphragm [65,66,68,69]. Expansion that can be harnessed for pumping, for example, may result when a heater is used to apply heat to and expand working fluid that acts on a flexible diaphragm. A thermopneumatic pump using a two-phase liquid-vapor perfluoro carbon propellant was reported, which included demonstration of pulsatile flow [68]. However, in reciprocating-type thermal pumps, the long thermal time constant, especially during cooling, limits the mechanical response time. High power consumption is also of concern especially for in vivo applications in which the pump is housed entirely within the body.
Thermal micropumps were investigated for intracerebral delivery to freely moving rodents, also with the motivation of enabling natural behavior which are not possible with current tethered infusion systems. These skull-mounted micropumps featured micromachined silicon probes (8 mm long) with etched orifices (25 μm diameter) to allow intracerebral delivery of an NMDA-receptor antagonist to the prefrontal cortex of rats for studies on visual attention and impulsivity. Drug was delivered as quantized packets (0.3 μl) from individual reservoirs using the thermally-induced expansion of microspheres (expansion up to 60-times [79]) and modulation of impulsivity was demonstrated [69].
Future perspective
As implantable drug systems are miniaturized using micro- and nano-fabricated technologies, challenges such as the limitation in drug payload within the small footprint and the availability of appropriate drug formulations that are stable for extended storage at body temperature will become more prominent. Future efforts require joint collaborations with chemists, pharmacologists and pharmaceutical manufacturers at the earliest design phase of device development. Another challenge for actively driven implantables will be the power supply with appropriate weight and size parameters. Future emphasis will be on high density implantable batteries, wireless powering and technologies that scavenge power from natural sources such as body temperature, body motion or tissue micromotion. New material selection and packaging approaches will be developed that integrate form and function into compact implants with advance dosing control beyond passive diffusion. Potential refinements may include flexible or dissolvable microelectronic sensors, actuators and integrated implants. As more dynamic biological processes and biorhythms are identified, advance temporal control of repeated dosing, for example, metronomic dosing, will become increasing in demand by patients and care providers for chronotherapy and drug therapy synchronized to biological cycles, for example, insulin production.
With future advancements in affordable and personalized genomic and proteomic profiling, innovative drug-delivery systems will be needed to realize the full potential of personalized medicine and drug therapy. These future systems must be capable of more complex drug regimens and improved time-resolved chronotherapies to provide better clinical outcomes with minimized unintended side effects. Micro- and nano-fabricated technologies will continue to drive innovation towards greater functionality, packaged in a miniature form factor. Integrated electronics and sensors for diagnostics will provide feedback-controlled drug delivery – drug release that is modulated or triggered in response to an acute marker detection or biological event. This will require major advances in in vivo sensor technology as well as greater knowledge of which markers are appropriate to track. Two-way telemetry will enable drug-delivery systems to log dosing events coupled with other relevant physiological events and real-time connectivity with electronic medical health systems to facilitate access for instantaneous review and analysis by the patient, care giver and clinician. Emergence of wireless/telehealth will allow the migration of drug administration by implantable, portable and wearable systems from the clinical setting to the home-care setting. Wearable and implantable hybrid dosing systems will be industrial designed for both aesthetics and mobility. Next generation drug-delivery systems will provide improved capability to capture, share and act upon actionable medical information to mediate improved clinical outcomes in a potential therapeutically effective care.
Executive summary.
Implantable drug-delivery devices
-
□
Peripheral drug administration methodologies and associated technologies offer limited control of dosing profiles.
-
□
Implantable systems offer long-term access with repeated dosing of remote therapeutic targets.
-
□
Implantable systems with conventional technologies suffer from many challenges, which limit their application.
-
□
The implementation of micro- and nano-technologies for implantable drug-delivery systems offer new possibilities in achieving control of in vivo drug release.
Reservoir-based devices
-
□
The simplest miniaturized implantable drug-delivery systems feature a reservoir with a membrane that controls the onset or rate of drug administration.
-
□
Reservoir-based drug-delivery systems may require additional energy to overcome the diffusion limited transport of drug in the immediate vicinity of the implant.
Drug-infusion micropumps
-
□
Drug infusion micropumps utilize a wide variety of mechanisms to propel drugs to in vivo targets.
-
□
Each mechanism must be carefully selected based on the dosing profile desired and other system-level constraints.
-
□
Chronic-controlled drug administration is possible with drug infusion pumps having refill capability.
Acknowledgments
The authors acknowledge the NIH (R21EY018490, R21DA026970 and R01NS050171), Qualcomm/TATRC Wireless Health Innovation Challenge and Wallace H Coulter Foundation for financial support. The authors have a financial interest in Fluid Synchrony LLC (Alhambra, CA, USA).
No writing assistance was utilized in the production of this manuscript.
Key Terms
- Microfluidics
Interdisciplinary field focuses on the manipulation of fluids at small scales and exists at the intersection of engineering, physics, chemistry, biology and medicine. ‘Micro’ typically refers to length scale or volume. Microreservoirs and micropumps are considered microfluidic devices.
- Micropump
Miniaturized device that produces mechanical work in order to move fluids. Drug pumps are used only to move liquids (as opposed to gases, which are also considered fluids).
- Microreservoir
Miniaturized storage container. The contents include cells, liquid formulations and lyophilized drug. The release of container contents is typically controlled by the properties or responsiveness of a membrane structure integrated into the container.
- Microelectromechanical systems
Systems consist of components having nano- to milli-meter scale features and are produced using specialized micro- and nano-fabrication methods, typically performed in batch processes that originate from the integrated circuit industry. These tiny devices benefit from interesting physical phenomena that dominate at small size scales.
- Diaphragm
Thin mechanical element anchored at its periphery and constructed of a semi-flexible material that can be used to displace fluid in microreservoirs and micropumps.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
□ of interest
□□ of considerable interest
- 1.Frost and Sullivan . Growth Opportunities in the Drug Discovery and Diagnostic Technologies Market. 2006. [Google Scholar]
- 2.Kalorama Research . Drug Delivery Markets. 2009. [Google Scholar]
- 3.Lawson EF, Wallace MS. Advances in intrathecal drug delivery. Curr. Opin Anaesthesiol. 2012;25(5):572–576. doi: 10.1097/ACO.0b013e3283572319. [DOI] [PubMed] [Google Scholar]
- 4.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat. Rev. Clin. Oncol. 2010;7(8):455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 5.Maas J, Kamm W, Hauck G. An integrated early formulation strategy-from hit evaluation to preclinical candidate profiling. Eur. J. Pharm. Biopharm. 2007;66(1):1–10. doi: 10.1016/j.ejpb.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Neervannan S. Preclinical formulations for discovery and toxicology: physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2006;2(5):715–731. doi: 10.1517/17425255.2.5.715. [DOI] [PubMed] [Google Scholar]
- 7.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011;50(5):600–613. □ □□ Comprehensive discussion of experimental considerations relevant to administration of substances to laboratory animals including formulation and equipment selection.
- 8.Turner PV, Pekow C, Vasbinder MA, Brabb T. Administration of substances to laboratory animals: equipment considerations, vehicle selection, and solute preparation. J. Am. Assoc. Lab. Anim. Sci. 2011;50(5):614–627. □ □□ Comprehensive discussion of experimental considerations relevant to administration of substances to laboratory animals including formulation and equipment selection.
- 9.Tan T, Watts SW, Davis RP. Drug delivery: enabling technology for drug discovery and development. iPRECIO® micro infusion pump: programmable, refillable and implantable. Front. Pharmacol. 2011;2(44) doi: 10.3389/fphar.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paolino D, Sinha P, Fresta M, Ferrari M. Drug delivery systems. In: Webster JG, editor. Encyclopedia of Medical Devices and Instrumentation. 2nd Edition John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2006. □ □□ Comprehensive review of different controlled micro- and nano-drug-delivery systems including other technologies beyond the scope of this article.
- 11.Fisher DM, Kellet N, Lenhardt R. Pharmacokinetics of an implanted osmotic pump delivering sufentanil for the treatment of chronic pain. Anesthesiology. 2003;99(4):929–937. doi: 10.1097/00000542-200310000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Desai TA, Hansford D, Ferrari M. Characterization of micromachined silicon membranes for immunoisolation and bioseparation applications. J. Memb. Sci. 1999;159(1-2):221–231. [Google Scholar]
- 13.Desai TA, Hansford DJ, Kulinsky L, et al. Nanopore technology for biomedical applications. Biomed. Microdevices. 1999;2(1):11–40. [Google Scholar]
- 14.Leoni L, Boiarski A, Desai TA. Characterization of nanoporous membranes for immunoisolation: diffusion properties and tissue effects. Biomed. Microdevices. 2002;4(2):131–139. [Google Scholar]
- 15.Azam A, Laflin KE, Jamal M, Fernandes R, Gracias DH. Self-folding micropatterned polymeric containers. Biomed. Microdevices. 2011;13(1):51–58. doi: 10.1007/s10544-010-9470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santini JT, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 17.Santini JT, Jr, Richards AC, Scheidt RA, Cima MJ, Langer RS. Microchip technology in drug delivery. Ann. Med. 2000;32(6):377–379. doi: 10.3109/07853890008995941. [DOI] [PubMed] [Google Scholar]
- 18.Maloney JM, Uhland SA, Polito BF, Sheppard NF, Jr, Pelta CM, Santini JT., Jr Electrothermally activated microchips for implantable drug delivery and biosensing. J. Control. Release. 2005;109(1-3):244–255. doi: 10.1016/j.jconrel.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Prescott JH, Lipka S, Baldwin S, et al. Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat. Biotechnol. 2006;24(4):437–438. doi: 10.1038/nbt1199. □□ First-in-human demonstration of a microreservoir-based drug-delivery system.
- 20.Farra R, Sheppard NF, Jr, Mccabe L, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci. Transl. Med. 2012;4(122):122ra121. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi S, Sarraf EH, Wong GK, Takahata K. Implantable drug delivery device using frequency-controlled wireless hydrogel microvalves. Biomed. Microdevices. 2011;13(2):267–277. doi: 10.1007/s10544-010-9491-5. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Bonner C, Desai TA. Bioadhesive microdevices with multiple reservoirs: a new platform for oral drug delivery. J. Control. Release. 2002;81(3):291–306. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 23.Richards Grayson AC, Choi IS, Tyler BM, et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat. Mater. 2003;2(11):767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 24.Richards Grayson AC, Cima MJ, Langer R. Molecular release from a polymeric microreservoir device: influence of chemistry, polymer swelling, and loading on device performance. J. Biomed. Mater. Res. A. 2004;69(3):502–512. doi: 10.1002/jbm.a.30019. [DOI] [PubMed] [Google Scholar]
- 25.Chung AJ, Huh YS, Erickson D. A robust, electrochemically driven microwell drug delivery system for controlled vasopressin release. Biomed. Microdevices. 2009;11(4):861–867. doi: 10.1007/s10544-009-9303-y. [DOI] [PubMed] [Google Scholar]
- 26.Elman NM, Ho Duc HL, Cima MJ. An implantable MEMS drug delivery device for rapid delivery in ambulatory emergency care. Biomed. Microdevices. 2009;11(3):625–631. doi: 10.1007/s10544-008-9272-6. [DOI] [PubMed] [Google Scholar]
- 27.Li PY, Givrad TK, Holschneider DP, Maarek JM, Meng E. A Parylene MEMS electrothermal valve. J. Microelectromech. Syst. 2009;18(6):1184–1197. doi: 10.1109/JMEMS.2009.2031689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li PY, Givrad TK, Sheybani R, Holschneider DP, Maarek JMI, Meng E. A low power, on demand electrothermal valve for wireless drug delivery applications. Lab Chip. 2010;10(1):101–110. doi: 10.1039/b910248e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirmoradi FN, Jackson JK, Burt HM, Chiao M. On-demand controlled release of docetaxel from a battery-less MEMS drug delivery device. Lab Chip. 2011;11(16):2744–2752. doi: 10.1039/c1lc20134d. [DOI] [PubMed] [Google Scholar]
- 30.Su YC, Lin LW. A water-powered micro drug delivery system. J. Microelectromech. Syst. 2004;13(1):75–82. [Google Scholar]
- 31.Su YC, Lin LW, Pisano AP. A water-powered osmotic microactuator. J. Microelectromech. Syst. 2002;11(6):736–742. [Google Scholar]
- 32.Li YH, Su YC. Miniature osmotic actuators for controlled maxillofacial distraction osteogenesis. J. Micromech. Microeng. 2010;20(6) [Google Scholar]
- 33.Ryu WH, Huang ZN, Prinz FB, Goodman SB, Fasching R. Biodegradable micro-osmotic pump for long-term and controlled release of basic fibroblast growth factor. J. Control. Release. 2007;124(1-2):98–105. doi: 10.1016/j.jconrel.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Evans AT, Chiravuri S, Gianchandani YB. Transdermal power transfer for recharging implanted drug delivery devices via the refill port. Biomed. Microdevices. 2010;12(2):179–185. doi: 10.1007/s10544-009-9371-z. [DOI] [PubMed] [Google Scholar]
- 35.Evans AT, Chiravuri S, Gianchandani YB. A multidrug delivery system using a piezoelectrically actuated silicon valve manifold with embedded sensors. J. Microelectromech. Syst. 2011;20(1):231–238. [Google Scholar]
- 36.Evans AT, Park JM, Chiravuri S, Gianchandani YB. A low power, microvalve regulated architecture for drug delivery systems. Biomed. Microdevices. 2010;12(1):159–168. doi: 10.1007/s10544-009-9372-y. [DOI] [PubMed] [Google Scholar]
- 37.Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J. Pain Symptom Manage. 2003;26(1):668–677. doi: 10.1016/s0885-3924(03)00144-1. [DOI] [PubMed] [Google Scholar]
- 38.Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J. Pain Symptom Manage. 2001;22(4):862–871. doi: 10.1016/s0885-3924(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 39.Yun KS, Cho IJ, Bu JU, Kim CJ, Yoon E. A surface-tension driven micropump for low-voltage and low-power operations. J. Microelectromech. Syst. 2002;11(5):454–461. [Google Scholar]
- 40.Bourouina T, Bosseboeuf A, Grandchamp JP. Design and simulation of an electrostatic micropump for drug-delivery applications. J. Micromech. Microeng. 1997;7(3):186–188. [Google Scholar]
- 41.Yih TC, Wei C, Hammad B. Modeling and characterization of a nanoliter drug-delivery MEMS micropump with circular bossed membrane. Nanomedicine. 2005;1(2):164–175. doi: 10.1016/j.nano.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Teymoori MM, Abbaspour-Sani E. Design and simulation of a novel electrostatic peristaltic micromachined pump for drug delivery applications. Sens. Actuators. A Phys. 2005;117(2):222–229. [Google Scholar]
- 43.Lin Q, Yang BZ, Xie J, Tai YC. Dynamic simulation of a peristaltic micropump considering coupled fluid flow and structural motion. J. Micromech. Microeng. 2007;17(2):220–228. [Google Scholar]
- 44.Xie J, Shih J, Lin QA, Yang BZ, Tai YC. Surface micromachined electrostatically actuated micro peristaltic pump. Lab Chip. 2004;4(5):495–501. doi: 10.1039/b403906h. [DOI] [PubMed] [Google Scholar]
- 45.Schneeberger N, Allendes R, Bianchi F, et al. Drug delivery micropump with built-in monitoring. Procedia Chemistry. 2009;1(1):1339–1342. [Google Scholar]
- 46.Esashi M, Shoji S, Nakano A. Normally closed microvalve and micropump fabricated on a silicon wafer. Sens. Actuators. 1989;20(1-2):163–169. [Google Scholar]
- 47.Maillefer D, Van Lintel H, Rey-Mermet G, Hirschi R. A high-performance silicon micropump for an implantable drug delivery system; Presented at: Micro Electro Mechanical Systems 1999; Orlando, FL, USA. 1999.Jan 17–21, [Google Scholar]
- 48.Mescher M, Abe T, Brunett B, Metla H, Schlesinger TE, Reed M. Piezoelectric lead-zirconate-titanate actuator films for microelectromechanical system applications; Presented at: Micro Electro Mechanical Systems 1995; Amsterdam, The Netherlands. 1995; 29 January – 2 February. [Google Scholar]
- 49.Cao L, Mantell S, Polla D. Design and simulation of an implantable medical drug delivery system using microelectromechanical systems technology. Sens. Actuators. A Phys. 2001;94(1-2):117–125. [Google Scholar]
- 50.Su GG, Pidaparti RM. Drug Particle delivery investigation through a valveless micropump. J. Microelectromech. Syst. 2010;19(6):1390–1399. [Google Scholar]
- 51.Su GG, Pidaparti RM. Transport of drug particles in micropumps through novel actuation. Microsyst. Technol. 2010;16(4):595–606. [Google Scholar]
- 52.Kan JW, Yang ZG, Peng TJ, Cheng GM, Wu B. Design and test of a high-performance piezoelectric micropump for drug delivery. Sens. Actuators. A Phys. 2005;121(1):156–161. [Google Scholar]
- 53.Cui QF, Liu CL, Zha XF. Study on a piezoelectric micropump for the controlled drug delivery system. Microfluid. Nanofluidics. 2007;3(4):377–390. [Google Scholar]
- 54.Geipel A, Goldschmidtboeing F, Jantscheff P, Esser N, Massing U, Woias P. Design of an implantable active microport system for patient specific drug release. Biomed. Microdevices. 2008;10(4):469–478. doi: 10.1007/s10544-007-9147-2. [DOI] [PubMed] [Google Scholar]
- 55.Geipel A, Goldschmidtoing F, Doll A, et al. An implantable active microport based on a self-priming high-performance two-stage micropump. Sens. Actuators. A. Phys. 2008;145:414–422. [Google Scholar]
- 56.Li PY, Sheybani R, Gutierrez C, Kuo JTW, Meng E. A parylene bellows electrochemical actuator. J. Microelectromech. Syst. 2010;19:215–228. doi: 10.1109/jmems.2009.2032670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li PY, Shih J, Lo R, et al. An electrochemical intraocular drug delivery device. Sensors and Actuators A Phys. 2008;143(1):41–48. [Google Scholar]
- 58.Saati S, Lo R, Li PY, Meng E, Varma R, Humayun MS. Mini drug pump for ophthalmic use. Trans. Am. Ophthalmol. Soc. 2009;107:60–71. [PMC free article] [PubMed] [Google Scholar]
- 59.Saati S, Lo R, Li PY, Meng E, Varma R, Humayun MS. Mini drug pump for ophthalmic use. Curr. Eye Res. 2010;35(3):192–201. doi: 10.3109/02713680903521936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gensler H, Sheybani R, Li PY, Lo Mann R, Meng E. An implantable MEMS micropump system for drug delivery in small animals. Biomed. Microdevices. 2012;14(3):483–496. doi: 10.1007/s10544-011-9625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gensler H, Sheybani R, Li Py, et al. Implantable mems drug delivery devices for cancer radiation reduction; Presented at: MEMS 2010; Hong Kong, China. 2010.Jan 24-28, [Google Scholar]
- 62.Sheybani R, Gensler H, Meng E. Rapid and repeatable bolus drug delivery enabled by high efficiency electrochemical bellows actuators; Presented at: Transducers 2011; Beijing, China. Jun 5-9, 2011. [Google Scholar]
- 63.Sheybani R, Meng E. High efficiency wireless electrochemical actuators: design, fabrication and characterization by electrochemical impedance spectroscopy; Presented at: Micro Electro Mechanical Systems 2011; Cancun, Mexico. Jan 23-27, 2011. [Google Scholar]
- 64.Sheybani R, Meng E. High Efficiency MEMS Electrochemical actuators and electrochemical impedance spectroscopy characterization. J. Microelectromech. Syst. 2012;21(5):1197–1208. [Google Scholar]
- 65.Jeong OC, Tang SS. Fabrication of a thermopneumatic microactuator with a corrugated p + silicon diaphragm. Sens. Actuators. 2000;80(1):62–67. [Google Scholar]
- 66.Ha S-M, Cho W, Ahn Y. Disposable thermo-pneumatic micropump for bio lab-on-a-chip application. Microelectron. Eng. 2009;86(4-6):1337–1339. [Google Scholar]
- 67.Mousoulis C, Ochoa M, Papageorgiou D, Ziaie B. A skin-contact-actuated micropump for transdermal drug delivery. Biomed. Eng. 2011;58(5):1492–1498. doi: 10.1109/TBME.2011.2113347. [DOI] [PubMed] [Google Scholar]
- 68.Cooney CG, Towe BC. A thermopneumatic dispensing micropump. Sens. Actuators. A Phys. 2004;116(3):519–524. [Google Scholar]
- 69.Spieth S, Schumacher A, Holtzman T, et al. An intra-cerebral drug delivery system for freely moving animals. Biomed. Microdevices. 2012;14(5):799–809. doi: 10.1007/s10544-012-9659-2. [DOI] [PubMed] [Google Scholar]
- 70.Benard WL, Kahn H, Heuer AH, Huff MA. Thin-film shape-memory alloy actuated micropumps. J. Microelectromech. Syst. 1998;7(2):245–251. [Google Scholar]
- 71.Reynaerts D, Peirs J, Vanbrussel H. An implantable drug-delivery system based on shape memory alloy micro-actuation. Sens. Actuators. A Phys. 1997;61(1-3):455–462. □□ Reviews detailing micropump and microvalve technologies for fluid control and manipulation.
- 72.Woias P. Micropumps - past, progress and future prospects. Sens. Actuators. B Chem. 2005;105(1):28–38. □□ Reviews detailing micropump and microvalve technologies for fluid control and manipulation.
- 73.Tsai NC, Sue CY. Review of MEMS-based drug delivery and dosing systems. Sens. Actuators. A Phys. 2007;134(2):555–564. □□ Reviews detailing micropump and microvalve technologies for fluid control and manipulation.
- 74.Amirouche F, Zhou Y, Johnson T. Current micropump technologies and their biomedical applications. Microsyst. Technol. 2009;15(5):647–666. □□ Reviews detailing micropump and microvalve technologies for fluid control and manipulation.
- 75.Laser DJ, Santiago JG. A review of micropumps. J. Micromech. Microeng. 2004;14(6):R35–R64. □□ Reviews detailing micropump and microvalve technologies for fluid control and manipulation.
- 76.Madou MJ. Solid-State Physics, Fluidics, and Analytical Techniques in Micro- and Nanotechnology. 3rd edition CRC Press; Boca Raton, FL, USA: 2011. Fundamentals of microfabrication and nanotechnology. □□ Comprehensive text covering all relevant aspects of microelectromechanical systems including the benefits attained as a result of scaling.
- 77.Ameri H, Chader GJ, Kim JG, Sadda SR, Rao NA, Humayun MS. The effects of intravitreous bevacizumab on retinal neovascular membrane and normal capillaries in rabbits. Invest. Ophthalmol. Vis. Sci. 2007;48(12):5708–5715. doi: 10.1167/iovs.07-0731. [DOI] [PubMed] [Google Scholar]
- 78.Saati S, Agrawal RN, Louie S, Chader GJ, Humayun MS. Effect of multiple injections of small divided doses vs single injection of intravitreal bevacizumab on retinal neovascular model in rabbits. Graefes. Arch. Clin. Exp. Ophthalmol. 2009;248(4):457–466. doi: 10.1007/s00417-009-1153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samel B, Griss P, Stemme G. A thermally responsive PDMS composite and its microfluidic applications. J. Microelectromech. Syst. 2007;16(1):50–57. [Google Scholar]
- 101.Alzet® Osmotic pumps. www.alzet.com
- 102.Intarcia therapeutic, Inc. www.intarcia.com
- 103.Spring: simple and clever diabetes solutions. www.springnow.com/insulin-pumps.html
- 104.Debiotech, Switzerland. www.debiotech.com