Abstract
Objective
The apolipoprotein E (APOE) ε4 allele enhances cerebral accumulation of β-amyloid (Aβ) and is a major risk factor for sporadic Alzheimer’s disease (AD). We hypothesized that HIV-associated neurocognitive disorders (HAND) would be associated with the APOE ε4 genotype and cerebral Aβ deposition.
Design
Clinico-pathological study of HIV-infected adults from four prospective cohorts in the U.S. National NeuroAIDS Tissue Consortium.
Methods
We used multivariable logistic regressions to model outcomes (Aβ plaques [immunohistochemistry] and HAND [standard criteria]) on predictors (APOE ε4 [allelic discrimination assay], older age [≥ 50 years], Aβ plaques, and their two-way interactions) and co-morbid factors.
Results
Isocortical Aβ deposits generally occurred as diffuse plaques and mild to moderate amyloid angiopathy. Isocortical phospho-Tau-immunoreactive neurofibrillary lesions were sparse. The APOE ε4 and older age were independently associated with the presence of Aβ plaques (adjusted odds ratio [OR] 10.16 and 5.77 [95% confidence interval (CI) 2.89–35.76 and 1.91–17.48], P=0.0003 and 0.0019, respectively, n=96). The probability of HAND was increased in the presence of Aβ plaques among APOE ε4 carriers (adjusted OR 30.00 [95% CI 1.41–638.63], P=0.029, n=15), but not in non-ε4 carriers (n=57).
Conclusion
The APOE ε4 and older age increased the likelihood of cerebral Aβ plaque deposition in HIV-infected adults. Generally Aβ plaques in HIV brains were immunohistologically different from those in symptomatic AD brains. Nonetheless, Aβ plaques were associated with HAND among APOE ε4 carriers. The detection of APOE ε4 genotype and cerebral Aβ deposition biomarkers may be useful in identifying living HAND subjects who could benefit from Aβ-targeted therapies.
Keywords: Apolipoprotein E, β-amyloid, HIV dementia, neurofibrillary pathology, phospho-Tau
Introduction
In the current era of highly active antiretroviral therapy (HAART), HIV-associated neurocognitive disorders (HAND) continue to affect the clinical outcome of HIV infection [1, 2]. Specifically, the milder forms of HAND, asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND), are more common than HIV-1-associated dementia (HAD). The differential susceptibility to HAND may be explained by individual differences in HIV variants, host genetic polymorphisms, and co-morbid factors (e.g., aging, substance use, and adverse effects of HAART), which may interact with each other in contributing to neural injury [3]. Some of these factors may trigger or promote a cascade of metabolic disturbances, leading to neurodegeneration and thereby neurocognitive impairment. For instance, postmortem studies showed extracellular β-amyloid (Aβ) deposition (as plaques) in the isocortex [4–8] and hippocampus [9] in subsets of HIV-infected adults.
The disturbance in cerebral Aβ metabolism may be one of the potential pathophysiologic pathways leading to HAND. While no systematic correlative analyses between cerebral Aβ deposition and neurocognitive impairment were available in previous autopsy studies [5, 8, 9], a clinical study by Clifford et al. [10], in agreement with a report by Brew et al. [11], showed that Aβ42 levels in the cerebrospinal fluid (CSF) were decreased in HAND patients similar to the levels in patients with mild Alzheimer-type dementia, when compared to those in cognitively normal subjects. The decrease in CSF Aβ42 levels reflects generally the presence of cerebral Aβ deposition detected by [11C] Pittsburgh compound B (PiB) positron emission tomography (PET) [12, 13]. However, the findings in those CSF studies were not confirmed in a similar study by Gisslen et al. [14], which might be explained by between-study differences in patients’ age and antiretroviral therapy.
The apolipoprotein E (APOE) ε4 allele correlates with the earlier onset and greater extent of cerebral Aβ accumulation [15, 16] and is a major risk factor for sporadic Alzheimer’s disease (AD) and cerebral amyloid angiopathy (CAA) [17]. The major co-dominant alleles (i.e., ε2, ε3, and ε4) in the human APOE gene are associated with differential biological activities of their protein products [18]. The APOE is an Aβ-binding molecule that may influence the clearance of soluble Aβ at the blood-brain barrier and affect Aβ seeding and aggregation [18–20]. Across several studies in HIV-infected adults, it remains controversial as to whether the APOE ε4 increases the susceptibility to HAND [21–26].
The present study was aimed at exploring the influence of APOE ε4 on cerebral Aβ deposition in HIV-infected adults and studying their significance in contributing to HAND. We followed a clinico-pathological correlative approach in studying HIV-infected adults who received detailed clinical, neuropsychological, and laboratory assessments as part of the National NeuroAIDS Tissue Consortium (NNTC). We hypothesized that HAND would be associated with the APOE ε4 genotype and cerebral Aβ deposition. If so, the detection of APOE ε4 and brain Aβ deposition may be useful in identifying HAND patients who could benefit from Aβ-targeted therapies.
Methods
Study cohort
We assembled 160 HIV cases in total (age range 27–67 years) autopsied during 1999–2010. Frozen tissues were available for APOE genotyping in 151 cases and formalin-fixed middle-frontal sections for immunohistochemistry in 105 cases. These brains were obtained from HIV subjects who participated in neuropsychological testing at a median of 20.7 weeks before death (interquartile range [IQR] 37.7 weeks). Seven domains of neurocognitive functioning were assessed: information processing speed, attention/working memory, learning, recall memory, verbal fluency, abstraction/executive functioning, and motor/psychomotor speed, with statistical correction for demographic variables (i.e., age, sex, ethnicity, and education), as described previously [27]. Based on standard criteria [28], HIV-associated neurocognitive diagnoses were made, including normal cognition (n=32), ANI (n=19), MND (n=37), and HAD (n=22). There were 47 subjects affected by neuropsychological impairment due to other or undetermined causes, and 3 subjects whose diagnoses were inconclusive; these cases were excluded from the statistical analysis regarding HAND.
Histories of antiretroviral treatment available in 101 HIV subjects were recorded within a median of 17.6 weeks (IQR 32.3 weeks) before death and grouped into ‘no treatment’ (n=31), ‘non-HAART regimens’ (n=6), and ‘HAART regimens’ (n=64). The antiretroviral regimens and their durations varied markedly among HIV subjects. Hepatitis C virus (HCV) infection was present in 47 (37.6%) of 125 HIV subjects having serological testing. We used either Psychiatric Research Interview for Substance and Mental Disorders [29]or Composite International Diagnostic Interview [30] to ascertain lifetime substance use disorders based on the Diagnostic and Statistical Manual of Mental Disorders (fourth edition). Of 122 HIV subjects evaluated for methamphetamine use, 46 (37.7%) were recorded as having ‘lifetime’ methamphetamine use (combining ‘abuse’ and ‘dependence’ categories); at the final premortem visits, only 2 of these 46 had current dependence and none had current abuse. Of 121 HIV subjects evaluated for major depressive disorder (MDD), 56 (46.3%) were recorded as having ‘lifetime’ MDD; 12 of these 56 had ‘current’ MDD at the final premortem visits. Because of the high prevalence of co-morbid factors described above, we included them as covariates in the statistical analysis.
Systemic autopsy findings were commonly diagnostic of AIDS; other frequent findings included hepatic cirrhosis and bronchopneumonia. Of 160 HIV brains, 44 were normal, 24 had minimal histopathologic changes, 18 with Alzheimer type II gliosis, 29 with vascular pathology (e.g., hypoxic-ischemic changes, infarcts, and hemorrhages), 16 with HIV encephalitis, 8 with leukoencephalopathy, 10 with microglial nodules, 19 with one or more opportunistic infections (e.g., cytomegalovirus encephalitis, toxoplasmosis, cryptococcosis, and progressive multifocal leukoencephalopathy), and 10 focally involved by primary or secondary non-Hodgkin’s lymphoma. Of 105 HIV brains available for immunohistochemistry, only 1 showed HIV encephalitis.
Non-HIV controls (n=22, age range 24–90 years) with no clinical history of neurological diseases were autopsied during 1992–2009. The systemic autopsy findings included organ transplantation, hepatic cirrhosis, lymphomas, and cardiovascular diseases. The neuropathologic diagnosis was either normal or minimal histopathologic changes. The formalin-fixed isocortex sections were available for immunohistochemistry.
APOE genotyping
Tissue samples obtained at autopsy were stored at −80°C until the time of total DNA extraction using DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD, USA). The amount of genomic DNA was quantified by using NanoDrop® Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). For APOE genotyping, we used the allelic discrimination assay (Taqman® SNP Genotyping Assays, Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s instructions. The allele calls and genotypes of samples were determined by using the Taqman® Genotyper software.
Immunohistochemistry
Five-μm-thick paraffin-embedded isocortex sections with no significant histopathologic changes were immunostained with primary antibodies to Aβ-4G8 (mouse monoclonal, clone 4G8, #SIG-39220, Covance, Princeton, NJ, USA, 1:20,000 dilution), Aβ40 (rabbit polyclonal, #AB-5074P, Millipore, Billerica, MA, USA, 1:500), Aβ42 (rabbit polyclonal, #AB-5078P, Millipore, 1:500), and phospho-Tau (p-Tau, mouse monoclonal, clone AT8, #MN1020, Pierce Biotechnology, Rockford, IL, USA, 1:1,000). The sections were incubated with 90% formic acid (5 min for Aβ staining) or 10 mM sodium citrate/0.05% Tween 20 buffer (pH 6) in 121°C autoclave (20 min for p-Tau staining). Immunohistochemical signals were developed using species-appropriate ImmPRESS™ anti-IgG (peroxidase) polymer detection kits (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine (ImmPACT™ DAB peroxidase substrate, Vector Laboratories), as previously described [31]. The sections were counterstained with hematoxylin. Isocortex sections from AD were used as positive tissue controls. For negative reagent controls, the primary antibodies were omitted.
Light microscopy
The presence of Aβ plaques or CAA was confirmed when these lesions were found in any of Aβ-4G8, Aβ40, and Aβ42 immunostained slides due to the fact that cerebral Aβ deposits characteristically exhibit an uneven multifocal distribution [32]. The density of Aβ plaques was graded as ‘focal’ and ‘widespread.’ CAA was qualitatively graded according to Vonsattel criteria [33] as ‘mild,’ ‘moderate,’ and ‘severe.’ The density of p-Tau-immunoreactive neurites was graded as ‘1’ (barely present at x100 magnification), ‘2’ (easily noted at x100 magnification), and ‘3’ (notable with naked eye inspection), a scoring system adapted from a BrainNet Europe Consortium study [34].
Statistical analysis
We used multivariable logistic regressions to model outcomes (i.e., cerebral Aβ plaques and HAND [vs. normal cognition]) on predictors (i.e., APOE ε4, older age [≥50 years], Aβ plaques, and their two-way interactions) and each of four covariates (i.e., antiretroviral treatment, HCV infection, methamphetamine use, and MDD). The statistical analyses were performed using R (version 2.10.0, 2009, http://www.r-project.org). All two-tailed P values were considered significant at a threshold of P<0.05.
Results
Cohort characteristics
Between HIV and non-HIV control groups, there was no significant difference in age (median 46 and 50 years, IQR 14 and 22.3 years, n=160 and 22, respectively; P=0.42, Mann-Whitney U test) or postmortem interval (median 12 and 15 h, IQR 13.4 and 13 h, n=158 and 21, respectively; P=0.33, U test). The proportion of women in the HIV group (n=19 of 160) was lower than in the control group (n=10 of 22; P=0.0004, Fisher’s exact test).
APOE genotyping
Among 151 HIV cases, the prevalence of APOE ε2/ε2 was 0.7%, ε2/ε3 11.3%, ε3/ε3 62.3%, ε2/ε4 2.6%, ε3/ε4 20.5%, and ε4/ε4 2.6%. This genotypic distribution in HIV cases was not significantly different from that in the general population [18] (χ2=4.87, df=5, P>0.25, chi-square goodness-of-fit test). APOE ε4 carriers (having 1 or 2 ε4 alleles) composed 28.0% of 93 young and 22.4% of 58 older HIV cases (P=0.57, Fisher’s exact test).
Cerebral Aβ deposition
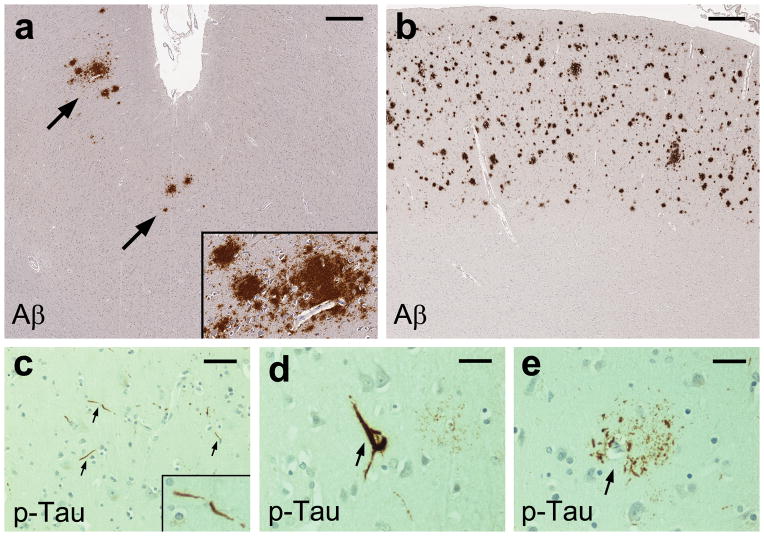
In both HIV and control groups, parenchymal Aβ deposits were found in most instances as diffuse plaques in the cortical gray matter, often exhibiting perivascular and perineuronal accumulation (Fig. 1a and b). Cored Aβ plaques were seen in only 1 HIV case and 2 controls, in concurrence with diffuse Aβ plaques. The prevalence of Aβ plaques was 29.5% of 105 HIV cases (focal 24, widespread 7) and 22.7% of 22 controls (focal 2, widespread 3). CAA was found in 6.7% (mild 3, moderate 3, severe 1) of 105 HIV cases and in 4.5% (moderate 1) of 22 controls, always together with Aβ plaques. Intracellular Aβ immunoreactivity in neuronal soma was focally observed (more often on Aβ-4G8 than Aβ40 or Aβ42 staining) in subsets of HIV cases and controls, without regard to the presence of Aβ plaques in the same section.
Fig. 1. β-Amyloid (Aβ) and phospho-Tau (p-Tau) pathology in the middle frontal cortex of HIV-infected adults.
Immunohistochemical staining with anti-Aβ antibody (clone 4G8) shows diffuse plaques of focal (a, arrows) or widespread (b) density in the cortex; scale bars 500 μm. Immunohistochemical staining with anti-p-Tau antibody (clone AT8) shows scattered neurites (c, arrows), an intraneuronal neurofibrillary tangle (d, arrow), and a cluster of dystrophic neurites, consistent with a neuritic plaque, (e, arrow); scale bars 30 μm.
p-Tau-immunoreactive neurofibrillary pathology
Among 105 HIV cases, scattered p-Tau-immunoreactive neurites (Fig. 1c) were present in 34.3% (grade-1 density 34, grade-2 density 2, grade-3 density 0). Of these 36 cases with neurites, 8 had rare neurons with diffuse soma labeling, and 3 showed rare neurons with neurofibrillary tangles (Fig. 1d). Rare clusters of p-Tau-immunoreactive dystrophic neurites, consistent with neuritic plaques (Fig. 1e), were found in 5 HIV cases (4.8%). Of 22 controls, 8 (36.4%) showed p-Tau-immunoreactive neurites of grade-1 density, 2 of which concurrently had rare neurons with diffuse soma labeling.
APOE ε4 and older age independently predicted cerebral Aβ plaque deposition
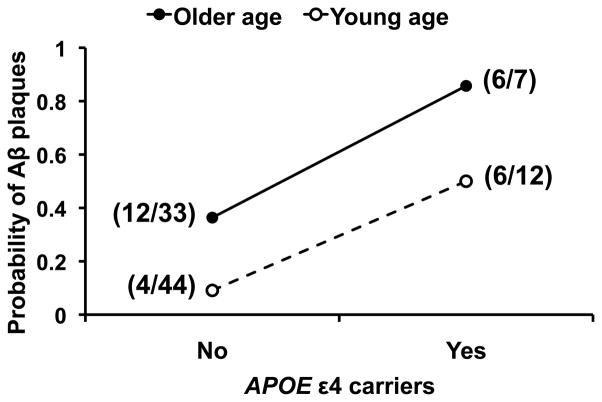
In univariable logistic regression, the presence of Aβ plaques was significantly associated with the APOE ε4 and older age (P<0.001 and =0.016, respectively), but not with each of the four covariates (i.e., antiretroviral treatment, HCV infection, methamphetamine use, and MDD) [P>0.15]. In multivariable logistic regression (Model: Aβ = APOE ε4 + older age + covariate), the APOE ε4 and older age remained significant independent predictors for Aβ plaques after adjusting for each of the four covariates (P<0.05). In contrast, none of these covariates showed significant association with Aβ plaques after adjusting for the APOE ε4 and older age (P>0.16). Accordingly, all the covariates were excluded. In Model (n=96): Aβ = APOE ε4 + older age, the APOE ε4 predicted Aβ plaques (adjusted odds ratio [OR] 10.16 [95% confidence interval (CI) 2.89–35.76], P=0.0003), as did older age (adjusted OR 5.77 [95% CI 1.91–17.48], P=0.0019). The interaction effect of APOE ε4 and older age on the presence of Aβ plaques was not statistically significant (P=0.97) [Fig. 2].
Fig. 2. The apolipoprotein E (APOE)ε4 genotype and older age (≥50 years) independently predicts cerebral β-amyloid (Aβ) plaque deposition in HIV-infected adults.
There is no significant interaction effect of the APOE ε4 and older age on the presence of Aβ plaques. The probability of Aβ plaques is increased either with the APOE ε4 (adjusted odds ratio [OR] 10.16 [95% confidence interval (CI) 2.89–35.76], P=0.0003) or older age (adjusted OR 5.77 [95% CI 1.91–17.48], P=0.0019). Shown in parentheses is the number of cases with Aβ plaques out of the total number of cases with and without Aβ plaques in each of the four APOE ε4–age subgroups.
Furthermore, the APOE ε4 was significantly associated with the abundance of Aβ plaques (none, focal, widespread) on multinomial logistic regression (overall P=0.002, n=96). That is, the odds of having focal Aβ plaques (relative to none) was higher among APOE ε4 carriers compared to non-ε4 carriers (OR 3.35 [95% CI 1.22–9.19], P=0.019), as was the odds of having widespread Aβ plaques [relative to none] (OR 11.73 [95% CI 2.05–67.20], P=0.006).
Interaction effect of APOE ε4 and cerebral Aβ plaque deposition on HAND
Univariable logistic regression showed no significant association between HAND and each of demographic and biologically relevant variables (P>0.09) [Table 1]. We used multivariable logistic regression to explore the effects of APOE ε4, Aβ plaques, older age, and their two-way interactions on HAND. The model selection process was pursued according to the Akaike Information Criteria (a measure of the relative goodness of fit of a statistical model) to include only those variables and interactions that provided the most accurate prediction of HAND. The interaction effects of older age and APOE ε4 or Aβ plaques, as well as the main effect of older age, on HAND were not statistically significant.
Table 1.
Demographic and biologically relevant factors in regard to HAND.
| Factors | HAND | Normal cognition | % HAND |
|---|---|---|---|
| Overall | 78 | 32 | 70.9 |
| Age | |||
| Median [IQR] (y) | 44 [14.5] | 45.5 [12.3] | |
| Young [<50 y] | 53 | 22 | 70.7 |
| Older [≥ 50 y] | 25 | 10 | 71.4 |
| Gender | |||
| Female | 7 | 5 | 58.3 |
| Male | 71 | 27 | 72.4 |
| Ethnicity | |||
| White | 48 | 22 | 68.6 |
| Hispanic | 15 | 4 | 78.9 |
| Black | 11 | 4 | 73.3 |
| Asian | 2 | 1 | 66.7 |
| Others | 2 | 1 | 66.7 |
| Education | |||
| Median [IQR] (y), n | 12 [2], 73 | 13 [1.5], 31 | |
| Antiretroviral treatment | |||
| None | 15 | 8 | 65.2 |
| Non-HAART | 2 | 1 | 66.7 |
| HAART | 22 | 18 | 55 |
| Hepatitis C virus infection | |||
| (+) | 17 | 9 | 65.4 |
| (−) | 41 | 16 | 71.9 |
| Methamphetamine use (lifetime) | |||
| (+) | 23 | 14 | 62.2 |
| (−) | 40 | 11 | 78.4 |
| Major depressive disorder (lifetime) | |||
| (+) | 25 | 14 | 64.1 |
| (−) | 38 | 11 | 77.6 |
| HIV encephalitis | |||
| (+) | 8 | 3 | 72.7 |
| (−) | 69 | 29 | 70.4 |
HAND, HIV-associated neurocognitive disorders; IQR, interquartile range; y, years; n, number of subjects; HAART, highly active antiretroviral therapy; (+), present; (−), absent.
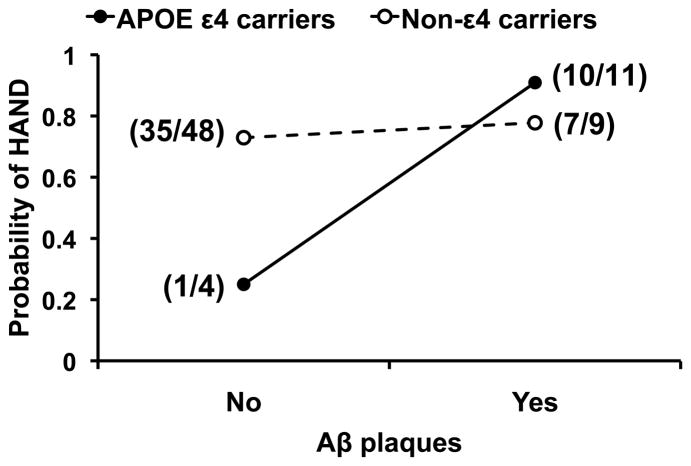
In Model (n=72): HAND = APOE ε4 + Aβ + [APOE ε4 × Aβ], the interaction effect of APOE ε4 and Aβ plaques on HAND approached statistical significance (P=0.078). The probability of HAND was increased in the presence of Aβ plaques among APOE ε4 carriers (adjusted OR 30.00 [95% CI 1.41–638.63], n=15, P=0.029), but not in non-ε4 carriers (adjusted OR 1.30 [95% CI 0.24–7.09], n=57, P=0.76) [Table 2] (Fig. 3).
Table 2.
| Predictors | HAND | Normal cognition | % HAND |
|---|---|---|---|
| APOE ε4 carriers | |||
| (+) Aβ plaques | 10 | 1 | 90.9 |
| (−) Aβ plaques | 1 | 3 | 25 |
| Non-ε4 carriers | |||
| (+) Aβ plaques | 7 | 2 | 77.8 |
| (−) Aβ plaques | 35 | 13 | 72.9 |
APOE, apolipoprotein E gene; Aβ, β-amyloid protein; HAND, HIV-associated neurocognitive disorders; (+), present; (−), absent.
With 1 or 2 APOE ε4 alleles.
Aβ plaques detected by immunohistochemistry in the middle frontal cortex.
Fig. 3. The interaction effect of apolipoprotein E (APOE)ε4 genotype and cerebral β-amyloid (Aβ) plaque deposition on HIV-associated neurocognitive disorders (HAND).
The probability of HAND is increased in the presence of Aβ plaques among APOE ε4 carriers (adjusted odds ratio [OR] 30.00 [95% confidence interval (CI) 1.41–638.63], P=0.029), but not in non-ε4 carriers (adjusted OR 1.30 [95% CI 0.24–7.09], P=0.76). Shown in parentheses is the number of HAND cases out of the total number of HAND cases and cases with normal cognition in each of the four APOE ε4–Aβ plaque subgroups.
Potential effects of co-morbid factors on HAND
We further investigated whether the interaction effect of APOE ε4 and Aβ plaques on HAND remained after adjusting for older age and each of the four covariates. Age remained irrelevant in all of these models. In Model: HAND = APOE ε4 + Aβ + [APOE ε4 × Aβ] + covariate, neither antiretroviral treatment nor HCV infection was a significant predictor of HAND (P=0.67 and 0.91, respectively).
Among 66 HIV cases (with complete data on HAND, APOE ε4, Aβ plaques, methamphetamine use, and MDD), methamphetamine use was significantly associated with the lower probability of HAND (adjusted OR 0.27 [95% CI 0.08–0.97], P=0.045), as was MDD (adjusted OR 0.24 [95% CI 0.07–0.89], P=0.032). Neither the interaction effect of methamphetamine use and APOE ε4 nor Aβ plaques on HAND was statistically significant (P=0.38 and 0.82, respectively), nor was that of MDD (P=0.28 and 0.95, respectively). The issue of multicollinearity was of trivial concern because there was no significant association between methamphetamine use (or MDD) and APOE ε4 (or Aβ plaques) [P>0.6, all chi-square tests].
In Model: HAND = APOE ε4 + Aβ + [APOE ε4 × Aβ] + methamphetamine use, the probability of HAND tended to increase in the presence of Aβ plaques among APOE ε4 carriers (adjusted OR 20.15 [95% CI 0.86–471.24], P=0.062), but not in non-ε4 carriers (adjusted OR 1.22 [95% CI 0.20–7.55], P=0.83).
In Model: HAND = APOE ε4 + Aβ + [APOE ε4 × Aβ] + MDD, the interaction effect of APOE ε4 and Aβ plaques on HAND was statistically significant (P=0.039). The probability of HAND was increased in the presence of Aβ plaques among APOE ε4 carriers (adjusted OR 39.13 [95% CI 1.59–962.24], P=0.025), but not in non-ε4 carriers (adjusted OR 0.78 [95% CI 0.12–4.94], P=0.80).
Discussion
Generally Aβ plaques first appear in the isocortex and then expand with increasing age hierarchically into further brain regions, representing different phases of Aβ deposition [35]. The middle frontal gyrus is one of the isocortex regions having relatively high Aβ plaque density [36]. Accordingly, we chose to examine this brain region for Aβ plaques. We found that cerebral Aβ deposits both in HIV cases and non-HIV controls occurred mostly as diffuse plaques and were rarely associated with p-Tau-immunoreactive neurofibrillary lesions. These findings agree with those in previous studies of HIV brains [4–6]. Diffuse Aβ plaques likely represent the earliest stage of temporal progression of Aβ plaques [37], in contrast to neuritic cored Aβ plaques characteristically present in symptomatic AD brains.
Regarding the APOE genotypic distribution, our HIV case series appeared to represent the general population. We found the APOE ε4 and older age were independently associated with the presence of cerebral Aβ plaques after adjusting for each co-morbid factor. Furthermore, the APOE ε4 was associated with the abundance of cerebral Aβ plaques. These findings in HIV subjects concur with those in the general population [15, 16, 38].
Notably, we found an interaction effect of the APOE ε4 and cerebral Aβ plaques on HAND. That is, the presence of Aβ plaques was associated with HAND among APOE ε4 carriers, but not in non-ε4 carriers. Our finding suggests APOE isoforms differentially modulate the association between cerebral Aβ plaques and HAND. Indeed this concurs with a clinical study in the older population by Kantarci et al. [39] showing that higher brain Aβ loads detected by PiB PET correlated with poorer cognitive performance among APOE ε4 carriers.
In a small study by Ances et al. [40] with the assessment of cortical PiB retention, none of 5 HAND and 11 cognitively normal HIV subjects had increased PiB retention in contrast to symptomatic AD subjects. On the other hand, CSF Aβ42 levels were decreased (<500 pg/ml cutoff value) in 2 of 4 HAND and 3 of 8 cognitively normal HIV subjects, but in only 1 of 8 non-HIV controls apparently matched for the APOE ε4 status and age. Previous clinical studies by Clifford et al. [10] and Brew et al. [11] also showed that CSF Aβ42 levels were reduced in HAND subjects compared to those in cognitively normal subjects. Decreases in CSF Aβ42 levels correlate generally with increases in cortical PiB retention indicating the presence of cerebral Aβ deposition [12, 13]; however, the CSF changes begin at earlier ages than changes in cortical PiB retention [16, 41]. As PiB (a derivative of thioflavin-T [42]) binds to β-pleated sheet aggregates of peptides (i.e., amyloid, including fibrillar Aβ), PiB readily marks cored Aβ plaques (whether or not they are neuritic) [41, 43]. Due to its higher affinity to fibrillar Aβ compared to the affinity of thioflavin-S [44], PiB also marks diffuse Aβ plaques [41, 43], characteristically composed of small amounts of fibrillar Aβ [45]. Taken together, it is likely that HIV subjects with reduced CSF Aβ42 levels have cerebral Aβ deposition, which (depending on the fibrillar Aβ load) may or may not be detected by PiB PET [16, 41]. Accordingly, measurement of CSF Aβ42 levels may be more sensitive than PiB PET for the detection of cerebral Aβ deposition in HIV-infected adults. PiB PET may be useful in the event that the cerebral Aβ load is high as is seen with the presence of widespread Aβ plaques (found in 6.7% of 105 HIV cases in our study).
Although APOE ε4 correlates with the earlier onset and greater extent of cerebral Aβ accumulation [15, 16], it is not an indispensable factor for cerebral Aβ deposition. Progressive Aβ accumulation may be caused by increased Aβ production by neurons, increased influx of Aβ from the circulation, decreased enzymatic degradation of Aβ, and defective efflux of soluble Aβ from the interstitial fluid (ISF) [46]. In addition to receptor-mediated transcytosis of Aβ across the blood-brain barrier, Aβ elimination may be mediated by perivascular macrophages [47], via bulk flow of ISF into the ventricles [48], and through perivascular ISF drainage along the basement membranes of capillaries and arteries [49, 50]. APOE isoforms may differentially regulate the clearance of soluble Aβ at the blood-brain barrier and the propensity for Aβ to aggregate [18–20]. In addition to its enhancing effect on cerebral Aβ accumulation, the APOE-4 isoform may potentiate the effect of Aβ plaques on the neurodegenerative process leading to HAND through other mechanisms yet to be determined.
Unexpectedly, we found that methamphetamine use and MDD were individually associated with the lower probability of HAND, after adjusting for the APOE ε4 and cerebral Aβ plaques. In our study, the majority (71.8%) of HAND cases were in milder forms (ANI and MND). Accordingly, neural injury in most HIV cases was probably not at irreversible stages, that is, the brain retained a degree of plasticity while exposed to methamphetamine. Previous studies showed that methamphetamine (low dose) enhanced cognitive performance [51], especially in tasks that required long periods of sustained attention in individuals with relatively low (prefrontal cortex-dependent) working memory capacity at baseline [52]. The combined effects of HIV and methamphetamine in this context are of particular interest and can be investigated in future studies. Regarding the association between MDD and HAND, the HIV-infected patients affected by MDD might be treated with selective serotonin reuptake inhibitors, which were shown to correlate with reductions in cerebral Aβ accumulation due to increased serotonin signaling [53]. However, we did not find any significant association between the presence of Aβ plaques and MDD.
In conclusion, we investigated the influence of APOE ε4 on cerebral Aβ deposition in HIV-infected adults and their significance in contributing to HAND, by using clinical, laboratory, and postmortem tissue resources available from the NNTC. We found that APOE ε4 and older age independently increased the likelihood of cerebral Aβ plaque deposition. Although Aβ plaques in HIV brains were immunohistologically similar to those in aging brains and different from those in symptomatic AD brains, cerebral Aβ deposition was associated with HAND among APOE ε4 carriers after adjusting for each co-morbid factor. Accordingly, the detection of APOE ε4 and biomarkers of cerebral Aβ deposition (e.g., decreases in CSF Aβ42 levels) may be useful in identifying HAND subjects who could benefit from Aβ-targeted therapies. Still, future studies in the HIV-infected population are warranted to confirm the inverse relationship between CSF Aβ42 levels and the abundance of cerebral Aβ plaques. Based on our finding that isocortical p-Tau-immunoreactive neurofibrillary pathology was sparse in HIV subjects, CSF p-Tau measurement may be useful in differentiating HAND from AD and other tauopathies in older patients [10, 54, 55].
Acknowledgments
Source of funding
This work was supported in part by the Don & Marilyn Short Fellowship in Parkinson’s Disease (V.S.) and the U.S. National Institutes of Health grants R25 MH081482 (V.S., M.C.), P50 DA026306 (I.G., V.S., E.T.T., C.L.A.), R03 DA027513 (M.C., V.S., B.S., C.L.A.), P30 MH062512 (I.G., C.L.A.), U01 MH083506 and R24 MH059745 (I.G., C.L.A., D.J.M.), U01 MH008021 and R24 NS038841 (A.J.L. and E.J.S.), U01 MH083507 and R24 NS045491 (B.B.G.), U01 MH083501 and R24 MH059724 (S.M.), and P50 AG016570 and U01 AI035040 (H.V.V.). For the remaining authors none were declared.
We thank Wilmar Dumaop (University of California, San Diego), David A. Jones, Jr. and Spencer Tung (University of California, Los Angeles), Joshua G. Lisinicchia (University of Texas Medical Branch), and Ruijin Shi (Mount Sinai Medical Center) for their technical expertise in tissue preparation. We are grateful to PinYi Du (Center for AIDS Research, University of California, San Diego) for her technical expertise in running the Applied Biosystems 7900HT Real-Time PCR System.
Footnotes
V.S. reviewed the literature, designed the study, optimized immunohistochemistry protocols, examined immunohistopathology, interpreted the results, and wrote the first manuscript draft. B.S. and E.T.T. performed DNA extraction, APOE genotyping, and immunohistochemical experiments. A.U. performed statistical analyses. D.J.M., M.C., and E.M. (the California NeuroAIDS Tissue Network), A.J.L. and E.J.S. (the National Neurological AIDS Bank), B.B.G. (the Texas NeuroAIDS Research Center), and S.M. (the Manhattan HIV Brain Bank) provided diagnostic characterizations of HIV cases. B.G. managed the patients’ database in the California NeuroAIDS Tissue Network, and coordinated with the other 3 sites. I.G., H.V.V., and C.L.A. supervised the study design and result interpretation. All authors contributed to the manuscript and approved the final article.
References
- 1.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20:25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- 3.Jayadev S, Garden GA. Host and viral factors influencing the pathogenesis of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2009;4:175–189. doi: 10.1007/s11481-009-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 5.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izycka-Swieszewska E, Zółtowska A, Rzepko R, Gross M, Borowska-Lehman J. Vasculopathy and amyloid beta reactivity in brains of patients with acquired immune deficiency (AIDS) Folia Neuropathol. 2000;38:175–182. [PubMed] [Google Scholar]
- 7.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 9.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 10.Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 12.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 13.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gisslén M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 18.Mahley RW, Rall SCJ. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corder EH, Paganelli R, Giunta S, Franceschi C. Differential course of HIV-1 infection and APOE polymorphism. Proc Natl Acad Sci U S A. 2008;105:E87. doi: 10.1073/pnas.0808164105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Arrastia R, Gong Y, Kelly CJ, Gelman BB. Host genetic polymorphisms in human immunodeficiency virus-related neurologic disease. J Neurovirol. 2004;10 (Suppl 1):67–73. doi: 10.1080/753312755. [DOI] [PubMed] [Google Scholar]
- 24.Dunlop O, Goplen AK, Liestøl K, Myrvang B, Rootwelt H, Christophersen B, et al. HIV dementia and apolipoprotein E. Acta Neurol Scand. 1997;95:315–318. doi: 10.1111/j.1600-0404.1997.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 25.Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med. 2008;9:677–680. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 26.Pomara N, Belzer KD, Silva R, Cooper TB, Sidtis JJ. The apolipoprotein E epsilon4 allele and memory performance in HIV-1 seropositive subjects: differences at baseline but not after acute oral lorazepam challenge. Psychopharmacology (Berl) 2008;201:125–135. doi: 10.1007/s00213-008-1253-1. [DOI] [PubMed] [Google Scholar]
- 27.Levine AJ, Hinkin CH, Ando K, Santangelo G, Martinez M, Valdes-Sueiras M, et al. An exploratory study of long-term neurocognitive outcomes following recovery from opportunistic brain infections in HIV+ adults. J Clin Exp Neuropsychol. 2008;30:836–843. doi: 10.1080/13803390701819036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 30.Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 31.Soontornniyomkij V, Everall IP, Moore DJ, Gouaux B, Tatro ET, Gospodarev V, et al. Increased cortical expression of FK506 binding protein-51 in HIV-associated neurocognitive disorders. J Neurovirol. 2012;18:313–322. doi: 10.1007/s13365-011-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 33.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 34.Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, et al. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–496. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 36.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Metsaars WP, Hauw JJ, van Welsem ME, Duyckaerts C. A grading system of Alzheimer disease lesions in neocortical areas. Neurobiol Aging. 2003;24:563–572. doi: 10.1016/s0197-4580(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 38.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 39.Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, et al. APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology. 2012;78:232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69:72–77. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klunk WE. Amyloid imaging as a biomarker for cerebral β-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol Aging. 2011;32 (Suppl 1):S20–36. doi: 10.1016/j.neurobiolaging.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 43.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, et al. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi H, Nakazato Y, Hirai S, Shoji M, Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol. 1989;135:593–597. [PMC free article] [PubMed] [Google Scholar]
- 46.Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 51.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 52.Iversen LL, Iversen SD, Bloom FE, Roth RH. Introduction to neuropsychopharmacology. Oxford: Oxford University Press; 2009. Psychostimulants; pp. 447–472. [Google Scholar]
- 53.Cirrito JR, Disabato BM, Restivo JL, Verges DK, Goebel WD, Sathyan A, et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci U S A. 2011;108:14968–14973. doi: 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green AJ, Giovannoni G, Hall-Craggs MA, Thompson EJ, Miller RF. Cerebrospinal fluid tau concentrations in HIV infected patients with suspected neurological disease. Sex Transm Infect. 2000;76:443–446. doi: 10.1136/sti.76.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellis RJ, Seubert P, Motter R, Galasko D, Deutsch R, Heaton RK, et al. Cerebrospinal fluid tau protein is not elevated in HIV-associated neurologic disease in humans. HIV Neurobehavioral Research Center Group (HNRC) Neurosci Lett. 1998;254:1–4. doi: 10.1016/s0304-3940(98)00549-7. [DOI] [PubMed] [Google Scholar]