Introduction
Two of the most fundamental ways in which cells and animals interact with their environment are through obtaining nutrients sufficient to sustain life and defending against attack of potentially pathogenic organisms. It is not surprising with some reflection, therefore, that immune cell metabolism and function are linked and that the immune system as a whole is connected and closely interacts with basic metabolic tissues and processes. This basic premise is now well accepted, but, until recently, was overlooked in both studies of the immune system and in studies of nutrition and metabolism. It had been apparent nearly one hundred years ago, however, that nutrition and immunological state could be associated, as malnutrition was clearly defined as an immunosuppressive condition, with increased infections in poorly fed populations (1, 2). Evolutionarily, it appears that the highly energy-dependent process of immune protection was simply selected as a system to be quickly sacrificed in times of famine, so as to maintain enough energy for immediately vital functions, such as neuronal and cardiovascular systems.
This nutrition and immunity association now provides context for the rise of immunometabolism as a field. Current thinking acknowledges that the links between immunity and metabolism are not limited to malnutrition and include basic cellular processes as well as close interactions between immune cells and metabolic tissues. In particular, immune responses are highly energy dependent, and how lymphocytes obtain their energy offers a new approach to modify immunity and define specific immune functions. Metabolic stress response pathways, such as autophagy, are also intimately tied to immunity, to activate the innate immune response, to allow adaptive immunity, as well as to directly eliminate some pathogens. In addition, the growing incidence of obesity and the metabolic syndrome in the western world are now known to have inflammation and overactive immunity as a root source of pathology. As recognition of the importance of these areas grows, the field of immunometabolism is now of broad importance in basic biologic understanding of immunity as well as in a variety of pathological settings. This volume includes reviews to discuss current literature and thought on each of these topics and to point toward future directions how metabolic links with immunity ultimately may be exploited to treat a wide array of metabolic and immunologic diseases.
Metabolic reprogramming in lymphocytes themselves
One of the first criteria for proper immunity is that the immunologic cells themselves must have appropriate and sufficient energy to support their demands. The study of regulation of cell metabolism in the immune system was of great interest in the past and has re-emerged in recent years to better understand basic biology of immunity as well as transformation. One of the earliest pioneers of this work on leukocyte metabolism, not surprisingly, was the noted Otto Warburg. Warburg showed that glycolysis rather than oxidative metabolism is the favored metabolic program for stimulated leukocytes (3). This study followed a series of seminal papers by Warburg starting in the 1920s that showed cancer metabolism was highly characterized by a transition from an aerobic oxidative metabolism to glycolysis, even in the presence of oxygen. This metabolic program is now found in a wide range of cancers and is termed aerobic glycolysis (4). Warburg’s essential finding, therefore, was that stimulated leukocytes are metabolically similar to cancer cells and favor glycolysis over mitochondrial oxidative pathways. Others soon followed to also measure how leukocyte activation increased anaerobic glucose metabolism (5, 6), including detailed NMR flux analyses of T-cell activation that showed increased glycolysis as well as glutamine oxidation (7). It has been evident, therefore, that lymphocyte activation leads to a metabolic reprogramming similar to cancer cells as far back as the 1950s and 1960s.
The driving force behind the transition of a resting lymphocyte from an oxidative to a highly glycolytic metabolism is to support the change in cellular metabolic demands to support immunity. Cell metabolism must fundamentally meet the functional requirements of the cell. Resting cells are quiescent and require primarily adenosine triphosphate (ATP) for basal cell functions and energy-demanding process of chemotaxis in immune surveillance. Upon stimulation, however, lymphocytes enter the cell cycle and can divide as often as every 4–6 h (8, 9). In this state, lymphocytes require both ATP and tremendous quantities of biosynthetic precursors to support rapid growth. Oxidative metabolism is highly efficient at generating energy, but converting carbon sources to carbon dioxide for ATP production leaves little capacity for macromolecular synthesis. Glycolysis coupled with glutamine metabolism, however, can provide both ATP and intermediates for nucleotide, lipid, and amino acid synthesis (10). At the end of an immune response, T cells no longer require rapid growth and decrease glycolytic metabolism as cells are selected for long-term immunological memory. Memory T cells have been shown to revert to oxidation of lipids as primary energy source (11, 12). The transition of T cells from oxidative to glycolytic and back to oxidative metabolism is reviewed by Van Der Windt and Pearce (13). Ultimately, the ability of cells to undergo these metabolic transitions may link tightly to cell survival and the ability of antigen-specific T-cell clones to rapidly expand to allow competitive selection of high-affinity clones (14–16) and is reviewed by Wensveen et al. (17).
The details of this metabolic transition are now becoming apparent and may provide new directions to modulate immunity (Fig. 1). With similar metabolic demands to maximally support cell growth of both cancer cells and stimulated lymphocytes, the field of cancer metabolism has provided important metabolic clues to understand immune function. Also, because the key signaling pathways that stimulate an immune response are often oncogenic when constitutively active, it is now apparent that the molecular signals that promote aerobic glycolysis are shared between these otherwise potentially quite disparate populations. In normal cells, signals to promote metabolism are initiated by cell extrinsic stimuli, such as cytokines or antigen receptor stimulation. Indeed, even resting T cells require cell extrinsic signals to maintain basal rates of metabolism (18). Of these signals in resting cells, conditional deletion of the interleukin-7 (IL-7) receptor showed this pathway to be essential to maintain basal glucose metabolism in vivo (19). T-cell receptor and CD28 costimulation can then lead to a rapid and dramatic increase in metabolism and transition to aerobic glycolysis (20). The molecular and metabolic details of this switch in acute T-cell activation are now becoming apparent and are reviewed by Wang and Green (21). Chronic T-cell activation, such as in autoimmunity or in graft versus host disease, may lead to a different metabolic phenotype, with T cells reverting from aerobic glycolysis back to favor a more oxidative metabolism (22). Wahl et al. (23) review these metabolic changes and the potential of specifically targeting the oxidative metabolism of chronically stimulated T cells in inflammatory diseases.
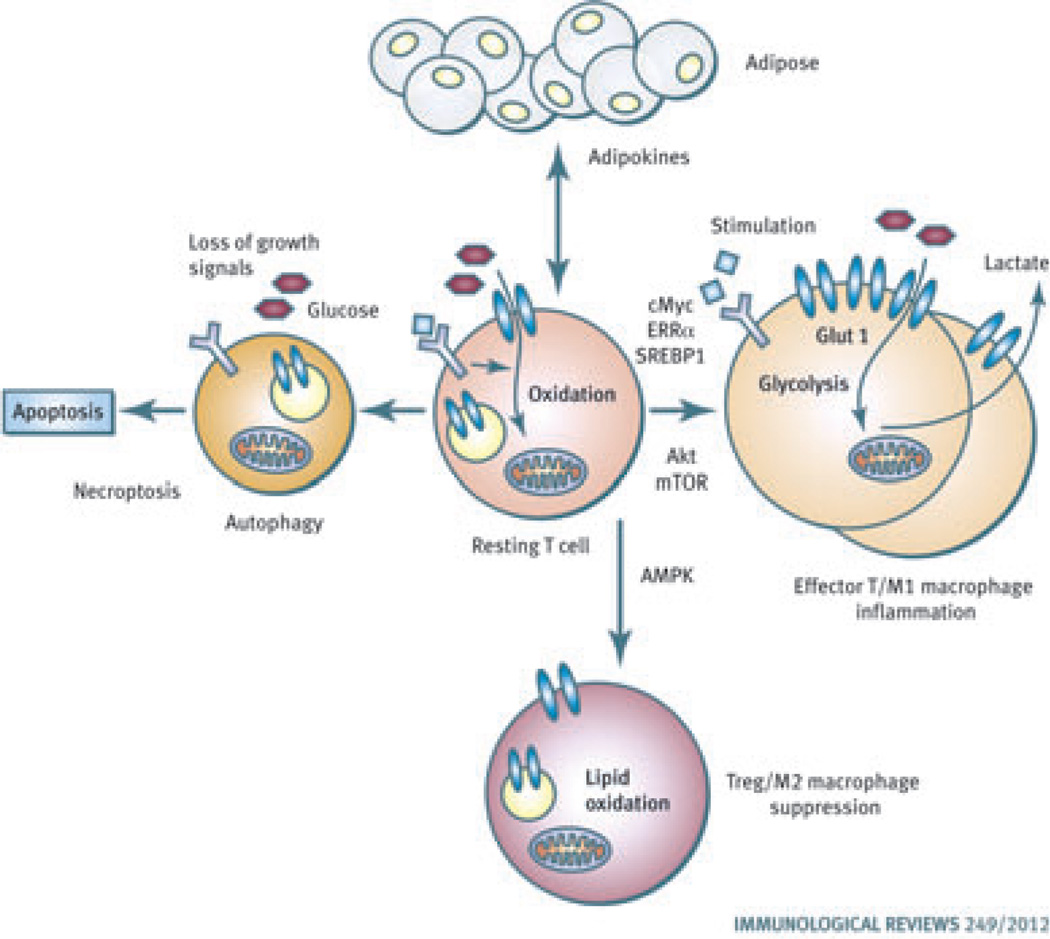
Fig. 1. Links between metabolism and immunity.
Resting lymphocytes use an oxidative metabolism for maximal energy generation. Upon stimulation, the Akt/mammalian target of rapamycin (mTOR), c-Myc, and estrogen related receptor-α pathways promote effector T cells to switch to a glycolytic metabolism that promotes cell growth for rapid proliferation. Classical M1 macrophages are also highly glycolytic and inflammatory. If AMP-activated protein kinase is stimulated, however, to suppress mTOR and promote lipid oxidation, regulatory T cells (Tregs) are favored. Suppressive M2 macrophages are also oxidative. Autophagy, apoptosis, and necroptosis pathways can also be metabolically regulated and can shape the immune response. Lymphocytes and macrophages are also closely tuned to adipose tissue through lipid signaling mechanisms and adipokines. T cells and macrophages then can play key roles to regulate insulin resistance and the metabolic syndrome.
Three main signaling mechanisms have been identified to drive aerobic glycolysis in T-cell activation. In each case, pathway activation frequently occurs in cancer due to oncogenic mutations or as a result of cell receptor signals in T cells. The phosphatidylinositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is activated in a wide range of cancers and in T-cell activation and is reviewed by Waickman and Powell (24). This pathway plays a major role to promote anabolic metabolism. Activation of the PI3K/Akt/mTOR pathway promotes Glut1 trafficking to the cell surface (25) to drive glucose uptake and glycolysis and also leads to increased protein translation and induction of the transcription factor SREBP1 that stimulates lipid synthesis (26). If mTOR complex 1 (mTORC1) is inhibited, T cells fail to switch to aerobic glycolysis or grow and instead become tolerized or anergic. Interestingly, anergic T cells remain metabolically oxidative and blocking aerobic glycolysis itself appears sufficient to drive, in turn, T-cell anergy (27). Upstream of mTORC1, PI3K produces phosphatidylinositol-3 phosphate that activates phosphoinositide-dependent kinase 1 (PDK1), which then phosphorylates Akt to promote activation of this kinase. This signaling pathway is not entirely linear, however, as PDK1 may also have roles to regulate glucose metabolism in mechanisms both dependent and independent of Akt (28).
Opposing the PI3K/Akt/mTOR pathway is 5′AMP-activated protein kinase (AMPK), which inhibits mTORC1 and stimulates catabolic rather than anabolic metabolic pathways to promote glucose and lipid oxidation (29). AMPK is activated by elevated ratio of AMP or ADP to ATP and by tumor suppressor LKB1 or CamKKβ. The roles of LKB1 and AMPK in immunity are complex, but appear to both play roles in survival and maintaining immune homeostasis (30–32). Given the prevalence of AMPK activating drugs, such as metformin, as treatments in obesity and Type II diabetes, it is important to better understand the role of this kinase in T-cell metabolism and function. It is possible that some or even many of the symptoms of the metabolic syndrome that are relieved by these drugs are mediated through direct modulation of immune cell metabolism rather than indirectly through generally improved metabolic health and this topic is reviewed in Blagih et al. (33).
A second key pathway that promotes aerobic glycolysis in lymphocytes is through induction of the oncogenic transcription factor, c-Myc. c-Myc is highly associated with a wide variety of cancers and has been shown to induce both glycolytic genes as well as genes essential for glutamine metabolism (34). c-Myc is also rapidly upregulated upon T-cell activation. Recently, conditional c-Myc deletion in T cells showed this factor essential for increased expression of nearly all genes involved in glycolysis, and glutaminolysis, and other pathways, including the polyamine pathway (35). As a consequence of this prominent regulatory role, c-Myc-deficient T cells failed to grow and were unable to proliferate. As reviewed by Wang and Green (21), it is critical that dividing cells have appropriate metabolic support, and there is a growing appreciation that c-Myc plays a central role to promote metabolic pathways to meet the needs of proliferative cells. Stimulation of the glutamine oxidative pathway may be a particularly important for c-Myc, as glutamine is essential to maintain anapleurotic flux through the tri-carboxylic cycle (TCA) in rapidly growing cells. By stimulating this pathway, however, c-Myc can render cells glutamine dependent (34), a finding that may be exploited in cancer therapies and could also have impact in immunosuppression.
A third class of proteins shown to regulate T-cell metabolism are nuclear hormone receptors. As reviewed by Kidani and Bensinger (36), the liver X receptor (LXR), peroxisome proliferator-activated receptors (PPARs), and estrogen-related receptor-α (ERRα) can play key roles in lymphocyte metabolic regulation. Most strikingly, LXR-deficient T cells have increased activation, leading to elevated proliferation and production of inflammatory cytokines (37). LXR normal opposes SREBP action and promotes the efflux of cholesterol, whereas SREBP promotes lipid and cholesterol synthesis. LXR-deficient T cells, therefore, have excess cholesterol and sterol generation and accumulation that can drive T-cell inflammatory function. PPARs (PPARα, β/δ, γ) promote lipid metabolic pathways and lipid oxidation by inducing genes such as carnitine palmitoyl transferase 1a (CPT1a), a rate-limiting component of lipid oxidation. CPT1a is also upregulated as CD8+ T cells switch from proliferating effectors using glycolysis to memory cells dependent on lipid oxidation (38). Consistent with promoting oxidation of lipids rather than lipid synthesis for cell growth, synthetic PPAR ligands have been used to treat metabolic disease and have immunosuppressive properties. Lastly, ERRα is well known to promote a variety of metabolic pathways, including the TCA cycle and mitochondrial electron transport (39), and is upregulated in T-cell activation (40) and macrophage function (41). ERRα expression is associated with poor prognosis in a variety of cancers (42) and has also been shown to be important for carbohydrate metabolism in developing Drosophila (43). ERRα is essential for macrophage metabolism and protection against Listeria monocytogenes (41), and our laboratory recently showed a key role for ERRα to coordinate mitochondrial and glucose metabolism in proliferating lymphocytes. Inhibition of this pathway can suppress immunity and an experimental autoimmune encephalomyelitis (EAE) response (40).
Differentiation of effector and regulatory T cells
Because metabolism must match cell function, it is not surprising that T cells with distinct immunological roles utilize specific metabolic programs (9). A key clue to define these pathways was the finding that conditional deletion of mTOR in mature CD4+ T cells allowed only the generation of regulatory T cells (Tregs) (44). Further studies to specifically modify the mTORC1 and mTORC2 pathways also led to selective generation of T-cell subsets, with mTORC1 essential for Th1 and Th17 cells while mTORC2 was essential for Th2 (45, 46). This topic is reviewed in detail by Waickman and Powell (24). Given the clear role for mTOR to promote glucose uptake and glycolysis (26), we reasoned that Tregs and effector [T-helper 1 (Th1), Th2, Th17] CD4+ T cells may differ metabolically. Indeed, while all stimulated CD4+ T cells are more metabolically active than resting cells, Tregs are reliant on lipid oxidation for a primary metabolic fuel, whereas effectors utilize glucose and glutamine oxidation (47). Consistent with a role for AMPK to promote oxidative metabolism and antagonize mTORC1, phospho-AMPK levels were elevated in both induced and natural Treg and in vivo stimulation of AMPK could both decrease glucose metabolism and increase Tregs in a model of murine asthma (47). Furthermore, targeting metabolic pathways could shift T-cell differentiation, as inhibition of glucose metabolism with 2-deoxyglucose blocked generation of Th1 and Th17 cells, but favored production of Treg both in vitro and in vivo (47, 48) and is reviewed by Wang and Green (21).
In addition to mTOR and AMPK, the hypoxia inducible factor 1α (HIF1α) has been found to play a key role in generation of CD4+ T-cell subsets, but in this case, specifically Th17 cells. HIF1α is a transcription factor that is tightly regulated by oxygen availability and has a role to upregulate glycolytic genes and promote anaerobic metabolism (49, 50). Interestingly, HIF1α is highly expressed in Th17 cells but not in other CD4 lineages (48, 51). Although HIF1α deficiency does not disrupt normal T-cell activation and metabolic reprogramming in the first day or two of activation (35), HIF1αnull T cells ultimately fail to become Th17 cells even under optimal conditions (48, 51). The mechanisms by which HIF1α to promote Th17 fate is not certain and may involve increased glycolysis (48) or potentially direct biochemical regulation of the Th17 transcription factor RORγT (51). This topic is reviewed in Wang and Green (21) and may have significant implications for modulating the balance of inflammatory Th17 and suppressive Treg cells in immunity.
Amino acid metabolism is also critical for lymphocyte proliferation and differentiation of T cells into effector or Treg lineages and is reviewed by McGaha et al. (52). Amino acid pathways are linked to mTOR, as mTOR is sensitive to amino acid levels and decreased amino acids suppress mTOR activation. Beyond mTOR signaling, however, availability of essential amino acids is critical for immune function through uncharged tRNA stress and other metabolic intermediates. Tryptophan catabolism, in particular, is induced through upregulation of indoleamine 2,3 dioxygenase (IDO1, IDO2) in response to inflammatory cytokines. IDO1 can promote tryptophan metabolism that can have both cell intrinsic and extrinsic effects on T cells and can both deplete tryptophan and generate metabolites with signaling properties that suppress inflammation and immunity and instead promote Treg generation. Conversely, IDO1 inhibition can increase tryptophan availability and promote immunity (53, 54).
Autophagy in metabolism and immunity
Nutrient limitation in the microenvironment is a concern of cells throughout evolution, and the process of autophagy is one of the primary responses to this stress. Autophagy involves a series of ubiquitination-like protein modifications that ultimately lead to the engulfment of cytosolic contents into double membrane lipid vesicles for transport into lysosomes. Upon fusion with lysosomes, then termed autophagolysosomes, the contents are degraded, and small molecules constituents are released into the cytosol. This has the benefit of potentially clearing unneeded cytosolic contents as well as to generate an intracellular source of nutrients (55, 56). This process is reviewed in detail in McLeod et al. (57). The metabolic role for autophagy was clearly shown in response to growth factor deprivation of apoptosis-deficient cells (58), and we have since shown that hematopoietic cells rely on autophagy to provide a source of lipids for oxidative metabolism when glycolysis decreases (59). Autophagy has numerous other functions as well, however, and acts as a means to dispose and recycle a variety of intracellular components, including bulk cytosolic material, protein aggregates, and full organelles.
Autophagy is regulated via AMPK and mTORC1 phosphorylation of the serine/threonine kinases Ulk1/2. In nutrient-rich conditions, Ulk1 and Ulk2 are phosphorylated and inhibited by mTORC1. When nutrients become limiting, however, such as with reduced amino acid levels or decreased ratios of ATP to ADP or AMP that lead to AMPK activation, mTORC1 activity decreases to reduce Ulk1/2 inhibitory phosphorylation, and AMPK can directly phosphorylate and activate Ulk1/2 (60). Activated Ulk1/2 then initiates autophagy, including processes such as mitochondrial degradation, or mitophagy (61). This may play a key role to provide an alternate cell-intrinsic source of nutrients to help promote the survival of tumor cells under metabolic stress. There is a strong interest, therefore, in inhibiting autophagy to eliminate cancer cells. This approach, however, may have some unintended consequences on immune cells and also inhibit any potential anti-tumor immune response or other immune responses, as reviewed by Townsend et al. (62).
It is now clear from studies that have directly targeted autophagy in immune cells that this process plays multiple roles in immunity. These functions include and go beyond providing a source of nutrients to metabolically stressed cells. Autophagy appears not only to be an important source of nutrients to support metabolism of nutrient-deprived leukocytes (59) but also to play a prominent role in initiating and supporting immune responses. Because the autophagic response is essentially a means to direct cytosolic components to the lysosome, this process is used to rid the cell of intracellular pathogens. Mycobacteria, for example, are targeted in macrophages to autophagolysosomes for destruction and inhibition of autophagy can exacerbate infection (63). Furthermore, it is now clear that this metabolic stress pathway is exploited broadly in innate immunity as a means to recognize danger signals and initiate the inflammatory innate immune response. Both damage-associated molecular patterns and pathogen-associated molecular patterns are recognized in autophagolysosomes and are essential for normal immune protection in tissue damage or infection, as reviewed by Tang et al. (64).
Autophagy plays a key role in lymphocyte development and activation. The precise mechanism of this reliance is not clear, but autophagy-incompetent B and T cells fail to develop properly (65, 66). Further lymphocyte homeostasis and activation are defective. This may be in part due to a partial reliance of lymphocytes on autophagy as a metabolic source in the initial phase of activation, when the rapid increase in metabolic demand may outpace the upregulation of aerobic glycolysis and glutaminolysis. In addition, autophagy may play key roles in regulation of signaling and clearance of excess mitochondria as reviewed by Mcleod et al. (57). In particular, developing thymocytes have more mitochondria than mature peripheral lymphocytes and autophagy appears to play a key role to eliminate excess mitochondria that otherwise appear to sensitize cells to death (66).
In addition to autophagy, metabolic stress can ultimately lead to apoptosis or necrosis (67). The process of necroptosis, however, has features of both necrosis and apoptosis and is reviewed by Lu and Walsh (68). Necroptosis is induced through activation of RIP1 and RIP3 kinases downstream of death receptor activation. Caspase activation can override this process to induce apoptosis, and necroptosis is most clearly evident in cells with caspase inhibition. As many pathogens can suppress caspase activity, this process may have important implication in host defense. In addition, reactive oxygen species (ROS) that can vary based on cell metabolism have become apparent as a key initiator of necroptosis. The precise link between necroptosis and autophagy is not understood, but caspase inhibition that leads to necroptosis also can promote autophagy. Inhibition of necroptosis, in turn, decreases autophagy. The mechanisms and role of necroptosis remains uncertain, but regulation of autophagy and the influence of ROS on necroptosis show clear connections to metabolism.
Nutrition in immunity and immunity in metabolic diseases
Beyond cell-intrinsic metabolism in the immune system, associations between systemic nutritional status and immunity are becoming increasingly apparent. In particular, this topic has become of great interest as the rates of obesity and metabolic syndrome increase and a low level systemic inflammation has been shown to be a driving force in much of the associated pathology (69). Why nutrition and immunity are so closely linked is unclear, but just as undernutrition leads to immune suppression, overnutrition leads to dysfunctional immune responses and chronic inflammation. It may be that the evolutionary need to conserve energy in times of food scarcity has linked energy-expensive immune responses to nutritional status to balance the energy cost of immunity with needs to support necessary physiological processes (70). Obesity links systemic metabolism to the immune system through hormones and adipocyte-derived cytokines, or adipokines, that can modulate immunity to promote inflammation and metabolic syndrome (71). Importantly, these links affect nearly all cells in the immune system and are reviewed by Nikolajczyk et al. (72). The role of lymphocytes, in particular, as initiators of many of the inflammatory phenotypes of obesity is being supported by findings that Tregs accumulate in lean visceral fat but are decreased in obesity, as Th1 and inflammatory T-cell subsets become favored (73, 74). Furthermore, CD8+ T cells may play a key role to recruit inflammatory macrophages and T-cell immunotherapy can suppress adipose inflammation. T-cell activation and inflammation, therefore, may be among the first drivers of adipose inflammation and the metabolic syndrome (73, 75, 76).
A major adipokine with critical roles in both nutrition and immune regulation that contributes to T-cell function and inflammation is leptin (77). Adipocytes produce leptin, which can promote satiety and act to reduce food intake. In obesity, adipocytes continue to produce leptin, and leptin levels are raised. The effects of elevated levels of this adipokine have been most apparent in patients with congenital leptin deficiency and in mutant mice that lack either leptin (Ob/Ob) or the leptin receptor (Db/Db). In each of these cases, leptin deficiency leads to metabolic dysfunction (78), with increased appetite and excessive eating leading to obesity, and also immune deficiencies. Conversely, lipodystrophy results in very low leptin levels and metabolic dysfunction that can be restored with leptin replacement (79). Although all branches of the immune system are affected by leptin, macrophages and T cells in particular stand out. In particular, leptin is known not only in metabolic regulation but also as an inflammatory cytokine, promoting Th1 and suppressing Th2 CD4+ T-cell subsets (77). Tregs are also highly impacted by leptin, which can suppress their proliferation. Matarese et al. (80) review the interaction of leptin with T cells in the immune system. Thus, increased adipocyte mass and adipokine secretion has direct impact on T-cell subsets and function as a mechanism to link nutrition to immunity. Conversely, malnutrition decreases leptin, which leads to lower T-cell numbers and the potential of increased Treg function (77, 81) that may contribute to immune suppression.
In addition to lymphocytes, macrophages are key effectors of obesity-related inflammation. Like T-cell subsets, macrophages can be associated with multiple phenotypes. While these phenotypes are somewhat plastic and macrophages can reprogram from one mode to another, the classic inflammatory macrophage is termed M1, whereas M2 macrophages are immune regulatory and immune suppressive. M2 macrophages dominant in adipose tissues of lean individuals and produce anti-inflammatory cytokines, such as IL-10, to maintain metabolic homeostasis through active insulin signaling. Lipid accumulation in obesity, however, leads to a polarization of M2 macrophages to the M1 phenotype, with production of inflammatory cytokines, such as TNF-α and IL-1β. The signals that cause this switch from M2 to M1 are complex, but fatty acids themselves can promote Toll-like receptor 4 signaling (82) and NLRP3 inflammasome-mediated processing of IL-1β (83). Parallel to the glycolytic phenotype of effector T cells and oxidative phenotype of Tregs, M1 macrophages are glycolytic, whereas M2 rely on AMPK and are oxidative (84). Johnson et al. (85) review signals and metabolic processes that regulate M1 and M2 macrophage metabolism and their roles in insulin resistance. The M1 macrophages and production of inflammatory cytokines can then promote insulin resistance in adipocytes, to perpetuate the pro-inflammatory response and metabolic dysregulation through multiple cytokines, such as IL-1β, which may have a particularly important role in this process (86), as reviewed by Tack et al. (87).
In addition to the direct activation of inflammation by lipids signaling mechanisms, changes in nutrient availability for lymphocytes and macrophages may also influence inflammation. Indeed, changes in fuel availability can influence in vitro differentiation of effector and Treg cells, with increased palmitate/oleate levels suppressing effector T cells while stimulating Treg (47). In vivo, nutrient changes are more complex, and obesity and diabetes-related hyperlipidemia and hyperglycemia may instead promote effector inflammatory T cells. Changes in lipid composition and oxidation may also be important. This apparent reversal in the role of lipids to stimulate Treg in the normal state to suppression of Treg in obesity and diabetes shows that metabolic disease has multiple and complex regulation of immune cells. Indeed, the accumulation of Tregs in lean visceral adipose tissue is reversed with obesity and diabetes (73), demonstrating an in vivo switch that may be a key event in the pathology of obesity-related inflammation and the metabolic syndrome.
Targeting metabolism in immunological diseases and immunity in metabolic disease
Recognition of the role of metabolism in immunity and that immune cells play critical roles to drive the pathology of metabolic diseases has raised a number of important questions and opportunities. The tight association of immunity and metabolism works at numerous levels. On the most basic level, lymphocytes and innate immune cells need energy to function. How these pathways are regulated and impact T-cell function are still not clear, but blocking glucose or glutamine metabolism can potently inhibit lymphocyte activation and proliferation (35, 47, 88, 89). Pharmacologically targeting these metabolic pathways, therefore, may provide a new direction in immunosuppression. Furthermore, with the distinct metabolic phenotypes of effector T cells and Tregs (47, 48, 51) and parallel patterns for M1 and M2 macrophages, it may be possible to tailor immune responses by modifying select metabolic programs. Initial data show that each T-cell subset requires its specific metabolic program and increasing or decreasing this program can enhance or suppress, respectively, these cell functions. For example, addition of lipids increased Treg generation by fueling lipid oxidation, whereas overexpression of Glut1 to increase glucose uptake can increase effector T-cell proliferation (47, 89). Likewise, CD8+ memory T cells utilize lipid oxidation (12), and enhancing or repressing this pathway by modulation of the mitochondrial lipid transporter CPT1a can directly impact memory cell formation and survival (12, 38).
Targeting the metabolism of immune cells to suppress inflammation is a challenge. Blocking basic pathways, such as glycolysis, may or may not be feasible. Certainly, treatment with the glycolytic inhibitor 2-deoxyglucose has been shown to suppress EAE (48). Given the potentially narrow therapeutic window for a potent direct glycolytic inhibitor, however, the impact of this approach is not clear in the long term. The selective reliance of chronically stimulated cells on oxidative metabolism may allow drugs that inhibit aspects of this pathway to reduce the function of autoreactive lymphocytes. The drug Bz-423, for example, binds the F1F0ATPase of mitochondria and increases ROS generation and seems to be selectively toxic to chronically stimulated lymphocytes (22). Normal and resting cells appear to survive this drug, potentially due to their ability to handle ROS stress or ability to adapt metabolically and use alternate pathways. This approach is reviewed by Wahl et al. (23) and supports the notion that selective inhibition of even basic metabolic components or pathways, that would appear to be fundamental to all cells, can still provide some selectivity. This may depend on the degree of reliance of cells on those pathways but nevertheless may provide a therapeutic opportunity. Alternatively, targeting regulators of metabolism that promote specific metabolic pathways can provide an indirect means to target metabolism. We have shown, for example, that metformin can lead to AMPK activation and reduce T-cell responses in vivo while maintaining Tregs (40). Also, nuclear hormone receptors, such as PPARs and ERRα, are excellent drug targets that may act in part through modulation of lymphocyte metabolism. Together, these topics are reviewed by Townsend et al. (62) and Kidani and Bensinger (36).
Much of the pathology of the metabolic syndrome is now clearly mediated by inflammation. This topic is reviewed by Nikolajczyk et al. (72), Johnson et al. (85), and Tack et al. (87). Directly suppressing inflammatory pathways, therefore, are promising new approaches to treat obesity-related diseases. In addition, approaches to reduce the metabolic stress of adipocytes and other metabolic tissues can reduce inflammation. Given that metabolic therapies in obesity and diabetes generally strive to increase lipid oxidation, it is tempting to speculate that at least a part of the effects of increased lipid oxidation may be by directly promoting oxidative metabolism in immune cells because both M2 and Treg cells prefer lipid oxidation when compared to inflammatory M1 macrophages and effector T cells. Indeed, metformin is a very commonly prescribed drug that promotes AMPK activation and can relieve some symptoms of metabolic syndrome. We have shown that AMPK activation drive the balance of T cells away from effectors and toward Tregs (47), and this may be a component of the success of metformin treatment that complements general improvements in metabolic health.
The field of immunometabolism has been growing leaps and bounds in the past years. It is not, however, an entirely new field. The links between nutrition and immune status have been appreciated for many years. The newfound molecular links between metabolic tissues and immune signaling pathways as well as increased understanding and appreciation of direct metabolic pathways of the immune cells themselves offers a new and exciting direction to understand both normal immunity as well as immunity in metabolic disease. Recent studies that address direct changes in lymphocyte metabolism in immunity have much to owe to the cancer metabolism field, as activated lymphocytes and cancer cells can be very similar metabolically. In this light, it is appropriate that the father of cancer metabolism, Otto Warburg (4), was also one of the first to investigate the activation of glycolysis in leukocyte activation (3). There are many questions that remain, chief of which will be to improve our understanding of metabolic pathways in immune cells and how immunological cells interact with cells in traditional metabolic tissues. It now is clear, however, that addressing these questions has the potential to impact a wide range of diseases.
Acknowledgements
I thank Nancie MacIver and Liza Makowski for helpful suggestions and comments. This work was supported by R01HL108006, the Lupus Research Institute, and the Leukemia and Lymphoma Society.
Footnotes
The author has no conflicts of interest to declare.
References
- 1.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutri. 1997;66:460S–463S. doi: 10.1093/ajcn/66.2.460S. [DOI] [PubMed] [Google Scholar]
- 2.Effects of nutrition on growth and resistance to infection. Am J Public Health. 1926;16:1221–1222. doi: 10.2105/ajph.16.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O, Gawehn K, Geissler AW. Metabolism of leukocytes. Z Naturforsch B. 1958;13B:515–516. [PubMed] [Google Scholar]
- 4.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 5.MacHaffie RA, Wang CH. The effect of phytohemagglutinin upon glucose catabolism in lymphocytes. Blood. 1967;29(Suppl):640–646. [PubMed] [Google Scholar]
- 6.Pachman LM. The carbohydrate metabolism and respiration of isolated small lymphocytes. in vitro studies of normal and phytohemagglutinin stimulated cells. Blood. 1967;30:691–706. [PubMed] [Google Scholar]
- 7.Bental M, Deutsch C. Metabolic changes in activated T cells: an NMR study of human peripheral blood lymphocytes. Magn Reson Med. 1993;29:317–326. doi: 10.1002/mrm.1910290307. [DOI] [PubMed] [Google Scholar]
- 8.Maciver NJ, et al. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33:168–173. doi: 10.1016/j.it.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Berardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Araki K, et al. mTOR regulates memory CD8 Tcell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Windt GJW, Pearce EJ. Metabolic switching and fuel choice during T cell differentiation and memory development. Immunol Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves NL, et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Coloff JL, et al. AKT requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. J Biol Chem. 2011;286:5921–5933. doi: 10.1074/jbc.M110.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wensveen FM, et al. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity. 2010;32:754–765. doi: 10.1016/j.immuni.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Wensveen FM, van Gisbergen KPJM, Eldering E. The fourth dimension in immunological space: how the struggle for nutrients selects high-affinity lymphocytes. Immunol Rev. 2012;249:84–103. doi: 10.1111/j.1600-065X.2012.01156.x. [DOI] [PubMed] [Google Scholar]
- 18.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 2012;249:14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatza E, et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001975. 67ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahl DR, Byersdorfer CA, Ferrara JLM, Opipari AW, Jr, Glick GD. Distinct metabolic programs in activated T cells: opportunities for selective immunomodulation. Immunol Rev. 2012;249:104–115. doi: 10.1111/j.1600-065X.2012.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macintyre AN, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, et al. The serine/threonine kinase LKB1 controls thymocyte survival through regulation of AMPK activation and Bcl-XL expression. Cell Res. 2010;20:99–108. doi: 10.1038/cr.2009.141. [DOI] [PubMed] [Google Scholar]
- 31.MacIver NJ, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamas P, et al. LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur J Immunol. 2010;40:242–253. doi: 10.1002/eji.200939677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blagih J, Krawczyk CM, Jones RG. LKB1 and AMPK: central regulators of lymphocyte metabolism and function. Immunol Rev. 2012;249:59–71. doi: 10.1111/j.1600-065X.2012.01157.x. [DOI] [PubMed] [Google Scholar]
- 34.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang RN, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kidani Y, Bensinger SJ. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol Rev. 2012;249:72–83. doi: 10.1111/j.1600-065X.2012.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villena JA, Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalek RD, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci USA. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonoda J, et al. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CY, et al. The metabolic regulator ERRalpha, a downstream target of HER2/IGF-1R, as a therapeutic target in breast cancer. Cancer Cell. 2011;20:500–510. doi: 10.1016/j.ccr.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS. The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab. 2011;13:139–148. doi: 10.1016/j.cmet.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 51.Dang EV, et al. Control of T(H)17/T(reg) Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGaha TL, et al. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. 2012;249:135–157. doi: 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 54.Sharma MD, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lum JJ, De Berardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 56.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLeod IX, Jia W, He Y-W. The contribution of autophagy to lymphocyte survival and homeostasis. Immunol Rev. 2012;249:195–204. doi: 10.1111/j.1600-065X.2012.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 59.Altman BJ, et al. Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis. Oncogene. 2011;30:1855–1867. doi: 10.1038/onc.2010.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Townsend KN, Hughson LRK, Schlie K, Poon VI, Westerback A, Lum JJ. Autophagy inhibition in cancer therapy: metabolic considerations for anti-tumor immunity. Immunol Rev. 2012;249:176–194. doi: 10.1111/j.1600-065X.2012.01141.x. [DOI] [PubMed] [Google Scholar]
- 63.Gutierrez MG, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 64.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller BC, et al. The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy. 2008;4:309–314. doi: 10.4161/auto.5474. [DOI] [PubMed] [Google Scholar]
- 66.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 67.Mason EF, Rathmell JC. Cell metabolism: an essential link between cell growth and apoptosis. Biochim Biophys Acta. 2011;1813:645–654. doi: 10.1016/j.bbamcr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu JV, Walsh CM. Programmed necrosis and autophagy in immune function. Immunol Rev. 2012;249:205–217. doi: 10.1111/j.1600-065X.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 70.Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- 71.Conde J, et al. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. BioFactors. 2011;37:413–420. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 72.Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strissel KJ, et al. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity. 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 76.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lord GM, et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 78.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 79.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 80.Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol Rev. 2012;249:116–134. doi: 10.1111/j.1600-065X.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- 81.Procaccini C, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tack CJ, Stienstra R, Joosten LAB, Netea MG. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunol Rev. 2012;249:239–252. doi: 10.1111/j.1600-065X.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 88.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]