Abstract
BACKGROUND
Lung contusion (LC) induces inflammation with high local concentrations of proinflammatory mediators stimulating chemotaxis and activation of neutrophils. LC is also a risk factor for development of pneumonia; however, the reason for this increased susceptibility is not clearly identified. We hypothesize that LC creates acute changes in the host pulmonary innate immune system that leads to vulnerability from a “second” hit bacterial infection.
METHODS
Female C57Bl/6 mice underwent LC injury at time −6 hours. At 0 hours, these mice were inoculated intratracheally with 1,000 colony forming unit (CFU) of Klebsiella pneumoniae (LC+Pneu) or vehicle (LC). Control animals underwent a sham LC injury followed by pneumonia (Sham+Pneu). Bronchoalveolar lavage (BAL) fluid and lung tissue specimens were collected. Lung bacteria levels were quantified by serial dilution, plating, and counting CFUs. Cytokine levels were assayed by ELISA. Cell type identification and quantification was performed using flow cytometry.
RESULTS
Survival at 72 hours was markedly different for the LC, Sham+Pneu, and LC+Pneu groups (100%, 80%, 20%, p < 0.05 Sham+Pneu vs. LC+Pneu). LC+Pneu animals had decreased pulmonary bacterial clearance at 24 hours compared with the Sham+Pneu group (4 × 107 vs. 8 × 106 CFUs, p < 0.05). BAL levels of IL-1β, IL-6, and keratinocyte chemoattractant were all significantly elevated in LC+Pneu mice compared with the Sham+Pneu group at 24 hours. Conversely, the Sham+Pneu mice had increased levels of macrophage inflammatory protein-2, total cells, macrophages, and neutrophils in BAL compared with the LC+Pneu group at 24 hours. LC+Pneu animals demonstrated changes in macrophage apoptosis and necrosis in BAL samples obtained 2 hours after induction of pneumonia when compared with the Sham+Pneu group. Both Sham+Pneu and LC+Pneu animals demonstrated an increase in the level of IL-10 in BAL fluid compared with LC animals.
CONCLUSION
Acute inflammation after LC acts to modulate the presence of inflammatory cells necessary to combat gram-negative bacteria. This results in decreased bacterial clearance and increased mortality from pneumonia.
Keywords: Chest trauma, lung contusion, inflammation, pneumonia
Severely injured patients are at high risk for pneumonia.1 Chest trauma resulting in lung contusion (LC) affects 30% of trauma admissions to the intensive care unit.2 Injury from multiple trauma including LC places patients at substantial risk for pneumonia, acute respiratory distress syndrome, and multiple organ system dysfunction syndrome.3 This risk begins almost immediately after the acute injurious episode. When caring for these patients, it is not unusual to conduct a fever work-up shortly after admission and find microbiologic evidence of bacterial pneumonia. Organisms from both gram-positive and gram-negative species of bacteria are responsible for ventilator-associated pneumonia in trauma patients.4 However, patients who develop early ventilator-associated pneumonia are more likely to have Streptococcus sp. or Staphylococcus aureus as the etiology of their infection, whereas those with a late pneumonia have an equal mix of gram-positive and gram-negative bacteria as the offending microbe that is isolated on culture. Trauma patients who develop a respiratory complication have an increase in their median hospital length of stay by 15 days and a $63,000 increase in their hospital costs.5 Current therapy for severe pulmonary failure consists of lung protective mechanical ventilator strategies, physiologic intensive care unit monitoring, and broad-spectrum antibiotics when pneumonia is present. Despite decades of research and adherence to best clinical practices, nosocomial pneumonia continues to be the principal cause of death from a hospital-acquired infection.6
The innate immune system represents the primary line of defense in the lung after invasion by bacterial organisms. Within the alveolus of the lung, the principle cell regulating the innate immune response is the macrophage.7,8 In addition to the macrophages ability to phagocytose whole bacteria cells, these cells are capable of recognizing bacteria through cell-membrane receptors for specific pathogen-associated molecular patterns such as lipopolysaccharide.9 Often, the invading microbial challenge is too great for the alveolar macrophages to handle alone. Under these conditions, the macrophages are capable of initiating an acute inflammatory response which results in increased production of proinflammatory mediators chemokine (C-X-C motif) ligand-1 (CXCL1)/keratinocyte chemoattractant (KC), chemokine (C-X-C motif) ligand-2 (CXCL2)/macrophage inflammatory protein-2 (MIP-2) that induce chemotactic migration of polymorphonuclear leukocytes (PMNs).10 These mediators act to recruit large numbers of neutrophils out of the vascular space into the lung tissue and alveolar spaces. Ensuing phagocytosis and bacterial killing by recruited neutrophils constitutes an essential component of a functional innate immune response to bacterial pathogens and is vital to controlling the spread of gram-negative bacteria within the lung.8,11
Trauma, specifically LC injury, alters the innate immune system by triggering reduced alveolar macrophage HLA-DR expression, a parameter that correlates inversely with the development of infectious complications and mortality in trauma patients.12 When studied in the context of sepsis, as the severity of injury increases, the number of apoptotic T cells ingested by macrophages is increased.13 Macrophages become deactivated, down-regulate MHC II, and increase their production of transforming growth factor beta (TGF-β) and interleukin-10 (IL-10).14,15 This change in macrophage phenotype creates immune suppression, decreased inflammation, and an increased bacterial load.
Our goal was to examine the mechanisms responsible for this increased susceptibility to develop pneumonia after LC. We hypothesize that the acute inflammatory response after LC results in changes that impede the effective clearance of gram-negative bacteria.
MATERIALS AND METHODS
Animals
Female C57BL/6 wild-type mice (Harlan, Indianapolis, IN) were used in all experiments. Mice were housed under specific pathogen-free conditions and were allowed to acclimate to their new surroundings for 1 week before being used in experiments. All experiments were performed in accordance with the National Institutes of Health guidelines for care and use of animals. Approval for the experimental protocol was obtained from the University of Michigan Committee on Use and Care of Animals.
LC Injury
At time −6 hours, a blunt LC injury was created using a cortical contusion impactor apparatus.16 Under ketamine anesthesia, fur was clipped from the planned contusion area and mice were fixed onto an adjustable height table. After correct positioning, the right chest of the mouse is struck by the cortical contusion impactor along the posterior axillary line 1 cm above the costal margin using a velocity of 5.8 m/s and a depth of 10 mm. Immediately after contusion, all animals received buprenorphine to avoid posttreatment discomfort. Mice were then allowed to recover spontaneously. Sham LC animals underwent all of the interventions outlined above except actually being stuck by the contusion apparatus.
Pneumonia Model
Klebsiella pneumoniae, strain 43816, serotype 2 was purchased from American Type Culture Collection, Manassas, VA. This microbial strain was cultured in trypticase soy broth (Becton Dickinson, Franklin Lakes, NJ) with reinoculation the following morning into fresh medium to bring the bacteria into log-growth phase. The bacteria were centrifuged at 600g at 4°C for 10 minutes, washed with sterile 0.9% normal saline, centrifuged again, and then resuspended in sterile saline. Optical density was read on a spectrophotometer (Milton Roy, Rochester, NY) at a wavelength of 620 nm. Appropriate serial dilutions were subsequently made to achieve a concentration of 1,000 colony forming units (CFUs) of bacteria per 30 µL of inoculum, the LD50 for C57BL/6 mice using this strain of Klebsiella. At time 0 (6 hours after LC injury or sham LC injury), mice were inoculated with 30 µL of the bacterial suspension or saline control via sterile cutdown and intratracheal injection under isoflurane anesthesia.17
Bronchoalveolar Lavage
At 2 hours or 24 hours after inoculation, mice were killed and a tracheal cutdown was performed. The lungs were lavaged with 1.5 mL of sterile saline using a syringe, angiocatheter, and three-way stopcock apparatus. Bronchoalveolar lavage (BAL) samples were centrifuged at 400g for 8 minutes. After centrifugation, the supernatant was separated from the cells and these samples were stored frozen at −80°C until use. Spun down cells were resuspended in appropriate buffer and used in the flow cytometry assay. BAL samples were also collected from mice that underwent the same handling but were not subjected to LC or intratracheal injection of bacteria. These samples were used to obtain baseline values.
Lung Homogenate and Quantification of Lung Bacteria
The thoracic cavity was opened, and lungs flushed of blood with 2 mL of 0.9% sterile saline. Both lungs were removed and homogenized in 1 mL of 0.9% sterile saline. Serial 10-fold dilutions were made in 0.9% sterile saline and 100 µL volumes of homogenate were plated on 5% blood agar plates (Thermo Fisher Scientific, Remel Products, Lenexa, KS). Plates were incubated overnight at 37°C and CFUs counted after 16 hours. The remaining volumes of lung homogenate were centrifuged at 3,000g for 15 minutes at 4°C. Supernatants were collected and diluted 1:1 in lysis buffer (1 × phosphate buffer saline, 1% Triton X-100, 1 tablet Complete X protease inhibitor [Roche, Indianapolis, IN], pH 7.4). Samples were stored at −80°C until use.
Cytokine and Chemokine Analysis (ELISA)
Cytokines, chemokines, and albumin level were measured in BAL supernatant using prefabricated ELISA kits, according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN). Plates were read using a microplate reader (Biotek Instruments, Winooski, VT) at 450 nm and 540 nm and cytokine concentrations were calculated using an eight-point standard curve and are expressed as pg/mL or µg/mL.
Histopathological Examination
Lungs of individual mice were fixed in 4% formaldehyde solution, and 4-µm thick sections were cut, stained with hematoxylin and eosin, and examined by light microscopy.
Flow Cytometry
BAL samples were collected, red blood cells lysed, and cells counted using a hemacytometer. Lung tissue was digested for 1 hour at 37°C with 1 mg/mL of collagenase A (Roche). The resulting single cell suspension was filtered and washed, red blood cells lysed, and cells counted. To measure membrane changes associated with apoptosis, cells were labeled with Annexin V (BioLegend, San Diego, CA) and a Molecular Probes LIVE/DEAD Fixable Dead Cell Stain kit (Invitrogen, Carlsbad, CA). A flow cytometric approach was used for analysis. After washing with Annexin V binding buffer, samples were incubated with Annexin V for 20 minutes at room temperature and removed from exposure to light. The LIVE/DEAD stain was added, and the cells were incubated for 10 minutes more under light-free conditions. After appropriate washing in flow buffer (phosphate buffer saline + 1% fetal calf syndrome) and blocking with Fc block (CD16/32), cells were divided into two sets and 1 × 106 cells were surface-stained with the following fluorochrome-conjugated mouse antibodies: Ly6C-FITC, Gr-1-PE, CD11c-APC-Cy7, F4/80-AF488, CD11b-PE-Cy7 (BioLegend and BD Biosciences, San Jose, CA). Stained cells were then washed, pelleted, and fixed with 1% formalin for 20 minutes. After two final washes, flow cytometric analysis was performed using a BD LSR II flow cytometer machine (BD Biosciences). Obtained data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Apoptotic/Live/Dead cells were gated for according to the manufacturer’s instructions. Live cells react with the LIVE/DEAD fluorescent dye only on their surface to yield weak total fluorescence (LIVE/DEAD+). Dead cells with damaged membranes react with the dye both on their surface and inside the cell, yielding a bright total fluorescence signal (LIVE/DEAD+++; Table 1).
TABLE 1.
Apoptosis and Live/Dead Gating Patterns
| Early Stage of Apoptosis |
Late Stage of Apoptosis |
Dead Cells | Live Cells |
|---|---|---|---|
| Annexin V+ | Annexin V+ | Annexin V− | Annexin V− |
| LIVE/DEAD+ | LIVE/DEAD+++ | LIVE/DEAD+++ | LIVE/DEAD+ |
The cells within each of the above gated regions were further analyzed to determine the proportions of each cell type based on the following cell marker patterns: neutrophils are those cells which are Gr-1+, CD11b+, CD11c−, and F4/80+/−; macrophages are Gr-1−, CD11b+/−, CD11c++, and F4/80+.
Statistical Methods
All statistical analysis and graphs were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). Results are presented as mean values ± the standard error of mean unless otherwise noted. Continuous variables were analyzed using an unpaired two-tailed Student’s t test. Multiple groups were compared by one-way analysis of variance with Tukey’s multiple comparison test used for post hoc analysis. Survival curves were generated using the Kaplan-Meier method. Chi-square and the log-rank (Mantel-Cox) tests were used to compare the survival data between experimental groups. Statistical significance was defined as a p value < 0.05.
RESULTS
LC Induces Parenchymal Injury and Infiltration of Inflammatory Cells
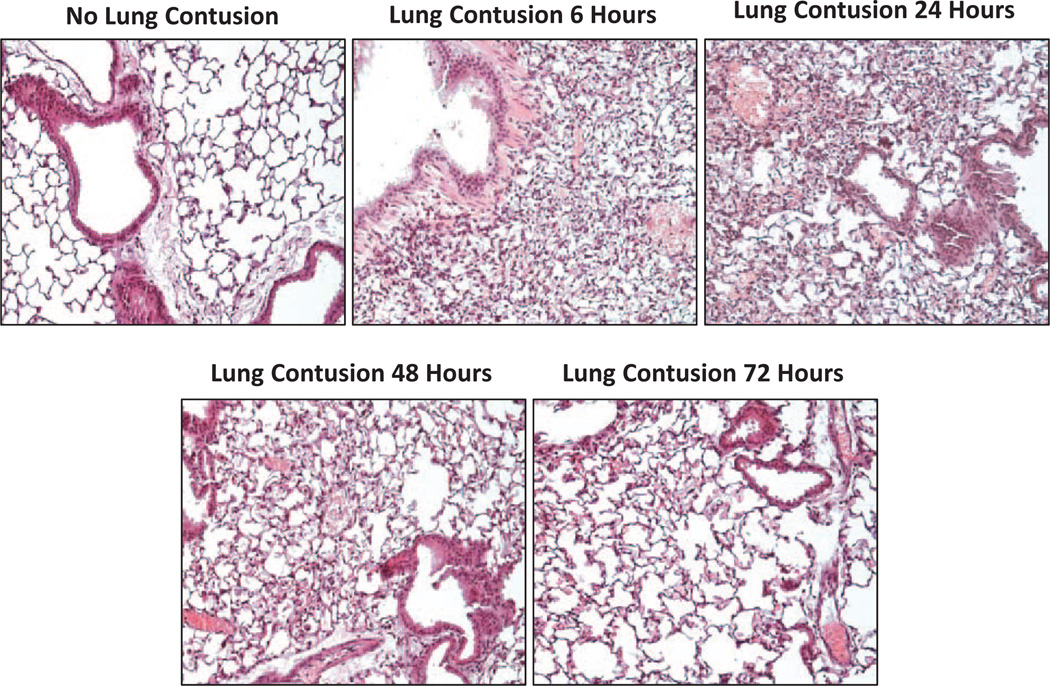
Assessment of right-side lung tissue at the time of euthanasia revealed a uniform pattern of lung injury between animals. Visually, areas of contusion were hemorrhagic at 6 hours and had a deep purple color at 24 hours consistent with hemorrhage and pulmonary consolidation. By 48 hours the contused areas appeared light brown in color. Histologic evaluation of contused lungs showed extensive hemorrhage within the contused area and infiltration of inflammatory cells into the lung parenchyma (Fig. 1). At 24 hours after contusion, alveoli remain collapsed and the dense infiltration of inflammatory cells has peaked. By 48 hours, there was evidence of resolution of hemorrhage, reinflation of alveoli, and a decrease in cellular infiltration with continued improvement to 72 hours.
Figure 1.
Hematoxylin and eosin-stained lung tissue (original magnification, ×40). Lung tissue sections obtained from contused portion of the right mouse lung at 0, 6, 24, 48, and 72 hours after lung contusion. There is dense infiltration of inflammatory cells into the lung parenchyma and collapse of the alveoli within 6 hours after lung contusion. By 24 hours this cellular infiltration has peaked and resolution occurs from 24 to 72 hours after traumatic injury.
Exposure to K. pneumoniae After LC Results in Increased Mortality
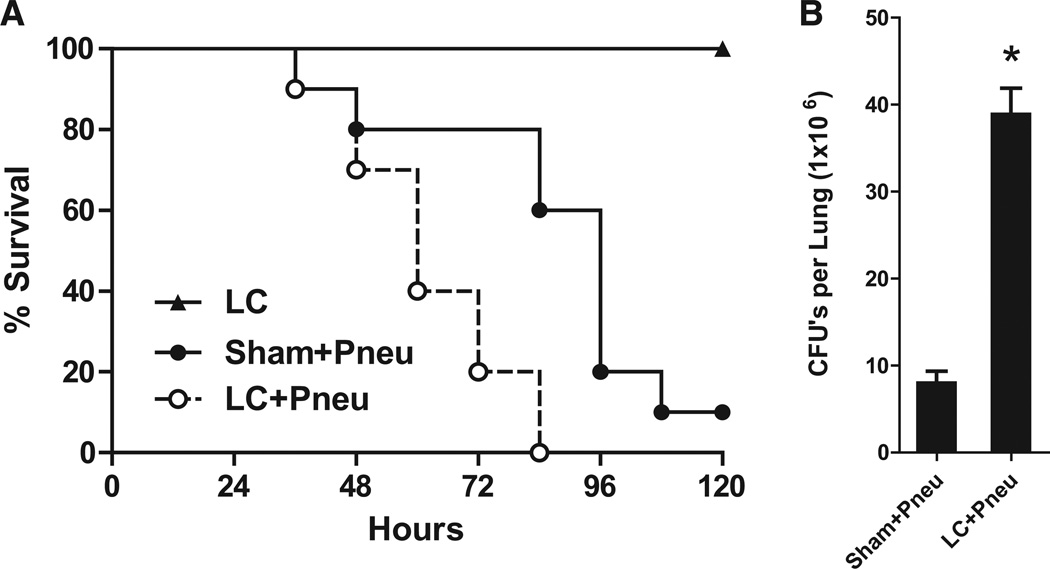
Mice underwent LC or sham injury at time −6 hours. All of the sham and half of the LC animals were then inoculated with K. pneumoniae at time 0 hours and followed for survival during the next 5 days. None of the animals died from LC alone (Fig. 2, A). Exposure to gram-negative bacteria 6 hours after LC resulted in 100% mortality by 84 hours after inoculation. Survival at 72 hours was 80% for Sham+Pneu animals, but reduced to 20% for the LC+Pneu group (p = 0.03, χ2). The two survival curves for the Sham+Pneu and LC+Pneu groups were significantly different (p = 0.004, log-rank test). Traumatically injured mice with LC were less effective at clearing bacteria and had significantly higher CFU counts in the lung 24 hours after inoculation (Fig. 2, B). These data suggest a defective ability to clear Klebsiella, 6 hours after LC.
Figure 2.
(A) Survival after lung contusion and/or Klebsiella pneumonia created 6 hours after chest injury. LC: lung contusion, Sham+Pneu: sham lung contusion and pneumonia, LC+Pneu: lung contusion and pneumonia. N = 10 mice per group. p < 0.05 (Sham+Pneu vs. LC+Pneu) log-rank (Mantel-Cox) test. (B) Quantitative culture of lung homogenate obtained 24 hours after induction of Klebsiella pneumonia and 30 hours after lung contusion. Results expressed as mean CFUs per lung. *p < 0.05 Student’s t test.
LC Alters the Presence and Balance of Inflammatory Cells Present Within the Alveoli in the Setting of Pneumonia
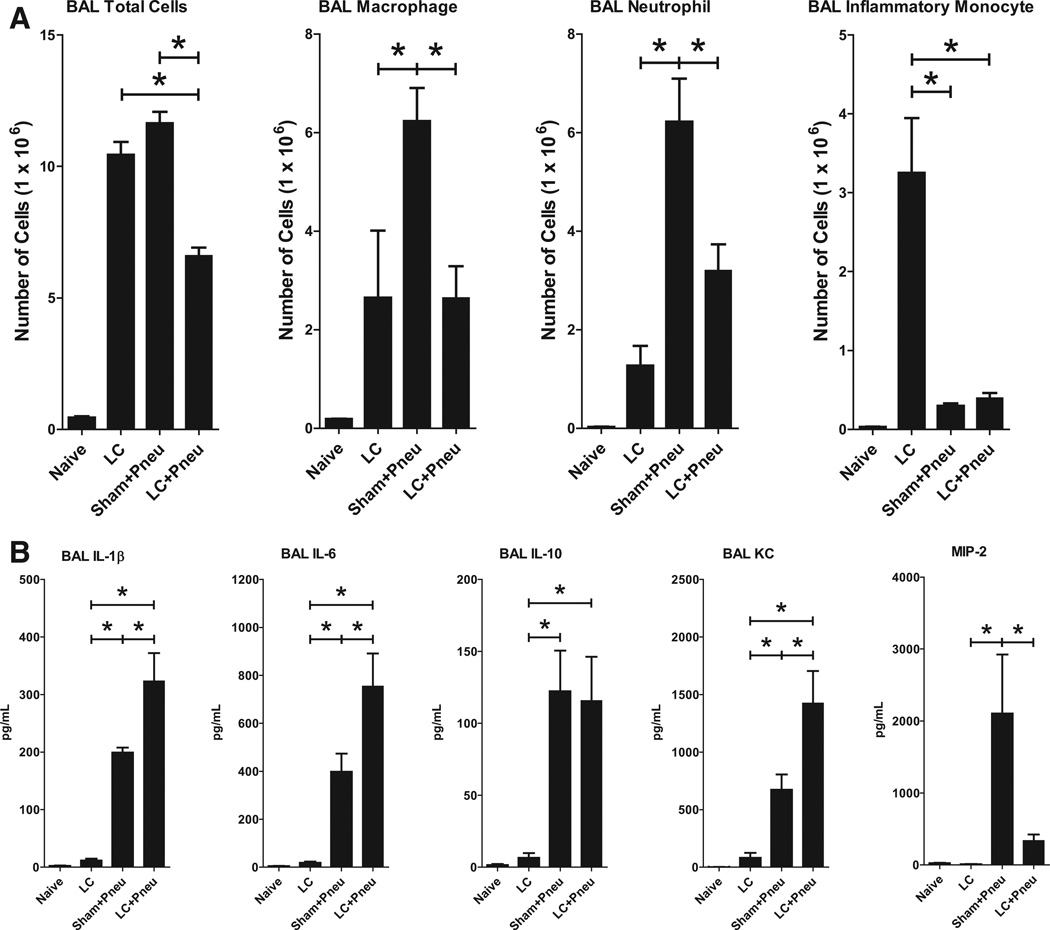
LC results in a robust time-dependent proinflammatory state. Within the alveoli, there is an increase in total cells, macrophages, neutrophils, and inflammatory monocytes which peaks by 24 hours and declines back toward the uninjured state by 48 hours (data not shown). In this experiment, naive mice received no interventions. The other groups consisted of LC alone at time −6 hours given saline vehicle intratracheally at time 0 (LC), sham LC at −6 hours and administered Klebsiella at time 0 (Sham+Pneu), and LC at −6 hours administered Klebsiella at time 0 (LC+Pneu). BAL sampling was performed 24 hours after inoculation with bacteria or saline vehicle. Cells in the BAL fluid were counted and then analyzed for cell type using flow cytometry. Sham+Pneu generated the largest influx of total cells, macrophages, and neutrophils into the alveoli (Fig. 3, A). LC alone did not generate as robust an inflammatory response as pneumonia. LC animals exposed to Klebsiella (LC+Pneu) could not generate an inflammatory response equivalent to noninjured animals inoculated with bacteria (Sham+Pneu). This is evidence for consumption and incomplete repriming of the innate immune system in the early time period after LC injury.
Figure 3.
Cell counts and types of cells present in equal volumes of BAL fluid as identified using flow cytometry (A). Samples were collected 24 hours after induction of Klebsiella pneumonia and 30 hours after lung contusion. Analysis of results for the LC, Sham+Pneu, and LC+Pneu groups was performed using one-way analysis of variance and Tukey’s multiple comparison test. BAL total cells p < 0.0001, BAL macrophages p = 0.004, BAL neutrophil p = 0.003, and BAL inflammatory monocyte p < 0.0001. *p < 0.05 post hoc test. Analysis of cytokine and chemokine levels by ELISA in BAL samples (B). Analysis of results for the LC, Sham+Pneu, and LC+Pneu groups was performed using one-way analysis of variance and Tukey’s multiple comparison test. For BAL IL-1β p = 0.0001, IL-6 p = 0.01, IL-10 p = 0.2, KC p = 0.007, and MIP-2 p = 0.01. *p < 0.05 post hoc test.
Cytokine and Chemokine Levels in BAL Fluid Are Elevated in the Setting of Pneumonia
LC alone produces a modest proinflammatory cytokine response characterized by elevations of interleukin-1β (IL-1β), and interleukin-6 (IL-6), in BAL fluid (Fig. 3, B). Exposure to bacteria as pneumonia alone (Sham+Pneu) or after LC (LC+Pneu) causes a markedly higher cytokine/chemokine response. LC+Pneu results in significantly more production of IL-1β, IL-6, and KC by 24 hours than pneumonia alone in the absence of LC injury (Sham+Pneu). Exposure to bacteria also increases the level of the anti-inflammatory cytokine IL-10.
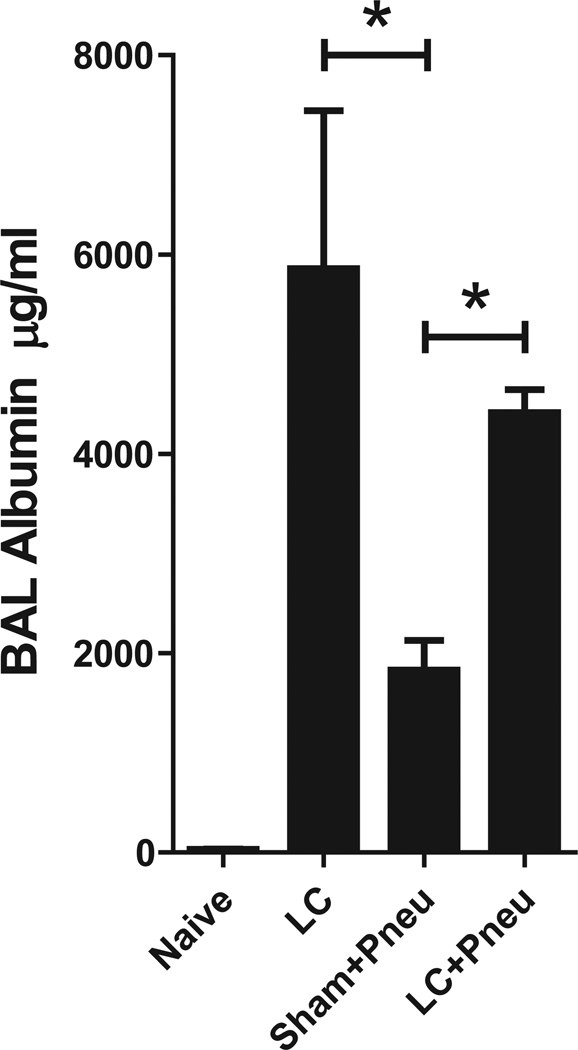
Alveolar-Capillary Integrity
Alveolar-capillary integrity was measured by assay of BAL levels of albumin which reflects the degree of permeability caused by injury. At 24 hours, BAL levels of albumin were elevated in all groups compared with the naive controls (Fig. 4). LC injury with and without ensuing pneumonia produced intensified levels of damage to the alveolar-capillary integrity than exposure to just pneumonia alone (Sham+Pneu). This increase in permeability may be attributed to the nature of mechanical tissue trauma after LC.
Figure 4.
Levels of albumin present in BAL fluid as a measure of lung injury 24 hours after induction of Klebsiella pneumonia and 30 hours after lung contusion. Analysis of results for the LC, Sham+Pneu, and LC+Pneu groups was performed using one-way analysis of variance (p < 0.0001) and Tukey’s multiple comparison test. *p < 0.05 post hoc test.
LC Injury Leads to Changes in Alveolar Macrophage Apoptosis
To determine why pneumonia after LC results in differences in mortality and the innate inflammatory response, we examined BAL fluid samples obtained early after exposure to pneumonia for differences in cellular apoptosis. We detected inflammatory cells undergoing apoptosis of inflammatory cells by determining the presence of externalization of phosphatidylserine using Annexin V staining. Phosphatidylserine, when redistributed to the outer cell membrane, serves as a marker for apoptotic cells to be recognized and ingested.18 Cellular membrane integrity was determined by exclusion of fluorescent reactive dye by living cells. Animals underwent LC or sham injury at time −6 hours, inoculation with bacteria at time 0, and BAL sampling at time 2 hours.
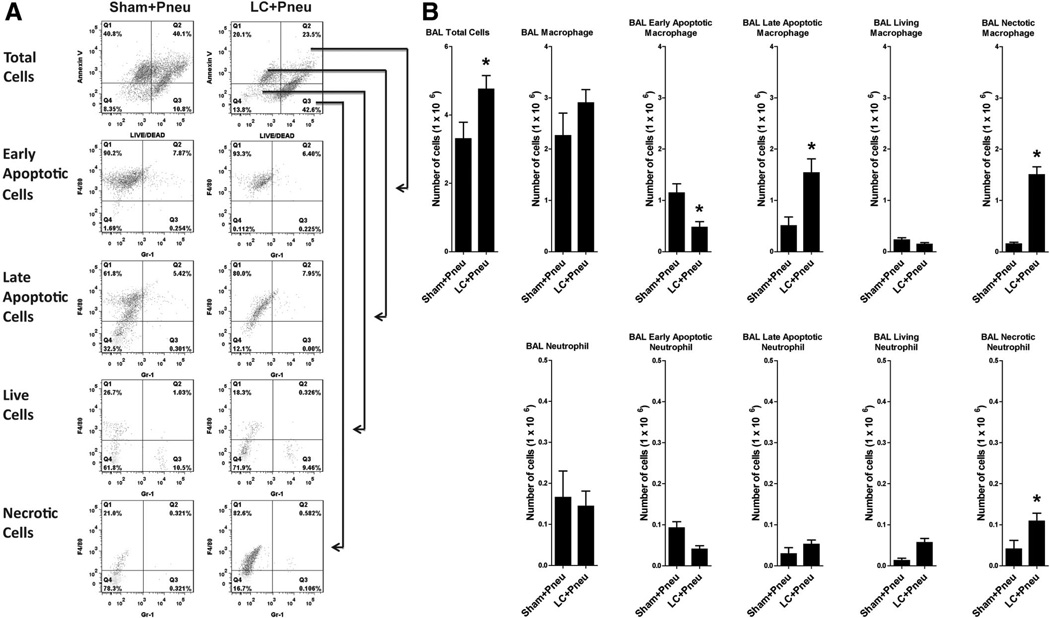
Representative histograms are shown in Figure 5, A that depict the distribution of apoptotic cells in BAL fluid. Many cells were in early stage of apoptosis (Annexin V+, LIVE/DEAD+++) or late stage of apoptosis (Annexin V+, LIVE/DEAD−). The double-negative population (Annexin V−, LIVE/DEAD−) depicts living cells. Cells negative for Annexin V and positive for LIVE/DEAD stain are considered dead cells. Two hours after inoculation with bacteria, the LC+Pneu animals had higher levels of total cells and macrophages present in BAL fluid compared with the Sham+Pneu group (Fig. 5, A and B). However, these macrophages rapidly disappeared as the LC+Pneu animals have fewer macrophages present in their BAL fluid by 24 hours when compared with the pneumonia-alone animals (Fig. 3, A). In fact, the majority of BAL macrophages in both groups of mice were in the early and late stages of apoptosis or even necrotic, thus representing abundant material for efferocytosis (Fig. 5, B). We also found many necrotic neutrophils cells in the early BAL fluid of LC+Pneu mice. The number of BAL neutrophils, either live or apoptotic, and living were similar in Sham+Pneu and LC+Pneu groups (Fig. 5, B).
Figure 5.
Flow cytometry analysis of BAL fluid obtained 8 hours after lung contusion and 2 hours after induction of pneumonia. (A) Representative flow cytometry pattern for sham lung contusion+pneumonia (Sham+Pneu) and lung contusion+pneumonia (LC+Pneu) groups. (B) Cell counts and types of cells present in equal volumes of BAL fluid as identified using flow cytometry. BAL total cells, BAL macrophage, BAL early apoptotic macrophage, BAL late apoptotic macrophage, BAL living macrophage, BAL necrotic macrophage, BAL neutrophil, BAL early apoptotic neutrophil, BAL late apoptotic neutrophil, BAL living neutrophils, and BAL necrotic neutrophils. *p < 0.05 Student’s t test.
LC Results in a Greater Presence of Neutrophils Within the Lung Early After Exposure to K. pneumoniae
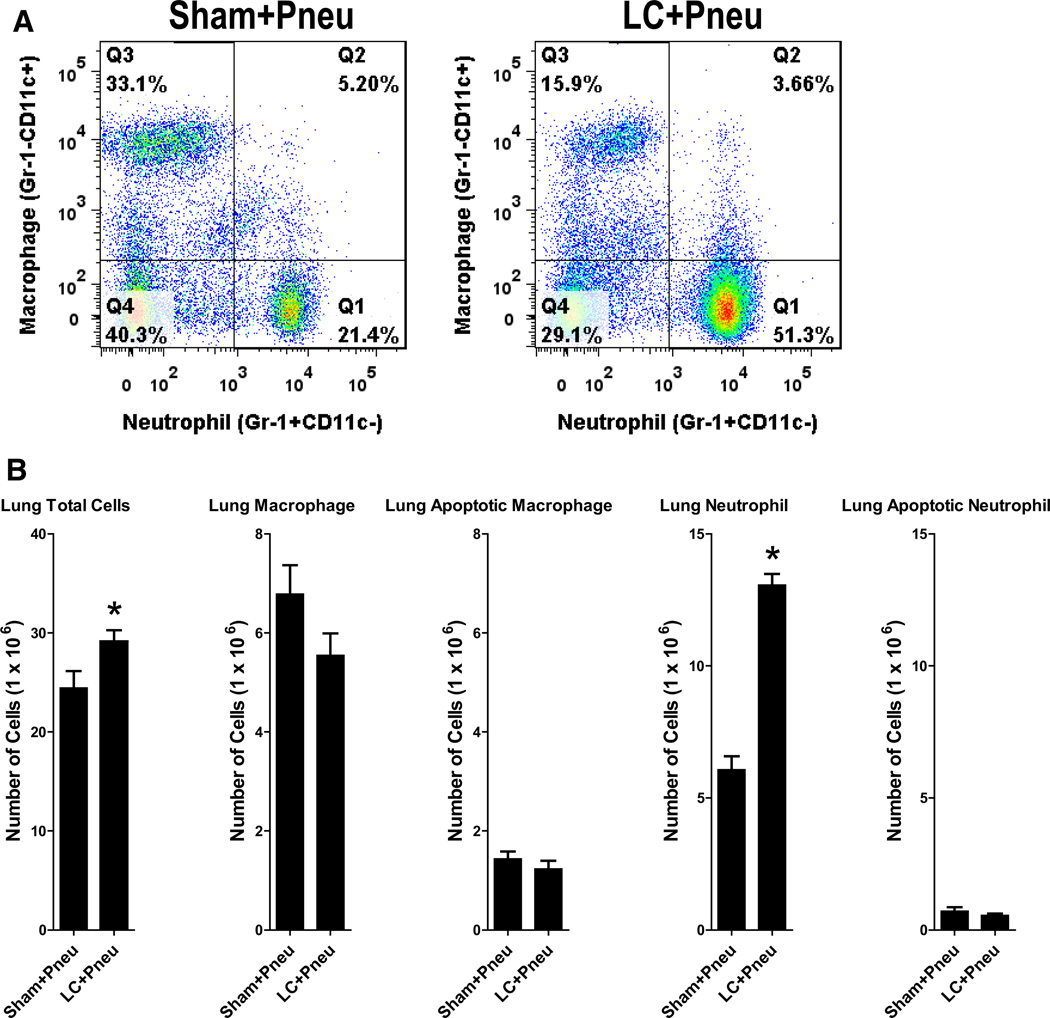
Flow cytometry analysis of whole lung tissue obtained 2 hours after bacterial inoculation in sham or LC animals revealed a slight increase in total cells and a large increase in neutrophils present in the LC+Pneu animals compared with the Sham+Pneu mice (Fig. 6). No differences in levels of lung macrophage or neutrophil apoptosis were found.
Figure 6.
Flow cytometry analysis of lung homogenate obtained 8 hours after lung contusion and 2 hours after induction of pneumonia. (A) Representative flow cytometry pattern for sham lung contusion+pneumonia (Sham+Pneu) and lung contusion+pneumonia (LC+Pneu) groups. (B) Cell counts and types of cells present in equal volumes of lung homogenate fluid as identified using flow cytometry. Lung total cells, lung macrophage, lung apoptotic macrophage, lung neutrophil, and lung apoptotic neutrophil. *p < 0.05 Student’s t test.
DISCUSSION
The pathophysiology of pneumonia involves a complex interaction between the bacterial inoculum and the host pulmonary defense system which leads to both local and systemic inflammation. The physiologic means by which bacteria are identified and cleared from the alveolar space is critical. Neutrophil recruitment, cellular function, and their working relationship with alveolar macrophages to eliminate microorganisms can all be impacted by LC injury as a result of trauma.19 Systemic activation of neutrophils after trauma is the leading cause of acute respiratory distress syndrome and multiple organ failure in these patients.3
LC primes the innate immune system and is known to result in an exaggerated response to exposure to pathogen-associated molecular patterns such as lipopolysaccharide.20 In our experiments with live gram-negative bacteria, a mixed local innate immune response was observed. In the early period after inoculation with bacteria, animals that underwent prior LC injury exhibited increased recruitment of total cells into the alveoli and lung when compared with sham-injured animals. There were high levels of macrophages present. However, most of these macrophages were in the process of undergoing apoptosis within in the alveoli or were already necrotic. This was coupled with increased recruitment of neutrophils into the lung parenchyma. We have previously reported that acute inflammatory lung injury after LC is neutrophil dependent.21 In the current experiments, there was increased permeability injury as measured by higher levels of albumin in the BAL at 24 hours in the LC+Pneu group when compared with the Sham+Pneu animals.
Elevated local concentrations of IL-1β, IL-6, and KC were found at 24 hours in the BAL fluid of LC+Pneu animals. These proinflammatory mediators stimulate chemotaxis and activation of neutrophils.9 The initial up-regulation of the pulmonary-host defenses after LC gives way to a progressive diminution of proinflammatory mediators and a shift toward the compensatory anti-inflammatory response syndrome characterized by Th-2 anti-inflammatory cytokines such as IL-10 once pneumonia is present. We observed evidence for increased levels of IL-10 in both the Sham+Pneu and the LC+Pneu animals when compared with the LC alone group 24 hours after exposure to bacteria or vehicle. IL-10 is an important anti-inflammatory cytokine and is capable of down regulating proinflammatory mediator production (tumor necrosis factor-α, IL-1, interleukin-12, KC, macrophage inflammatory protein-1, and MIP-2) in the setting of lung inflammation, thereby resulting in diminished neutrophil influx.23 In the presence of IL-10, recruited and extravasated neutrophils are deactivated by initiating their apoptosis program and are removed from the lung by alveolar macrophages.18,24
Transient immune system down-regulation and repriming after LC create a vulnerable window during which the host is susceptible to infection and mortality from gram-negative pneumonia. In the experiments performed, LC consumed many of the cells that would normally be present or recruited to recognize and clear a bacterial insult. The most pronounced finding was the degree of alveolar macrophage apoptosis and necrosis present in animals that underwent LC before induction of pneumonia. These mice, which underwent LC+Pneu, died faster and had decreased survival and a significant increase in their pulmonary bacterial burden when compared with Sham+Pneu animals. Why is the host unable to replenish its innate immune system and repel a microbial invasion after LC injury? Apoptosis and necrosis of macrophages may play a critical role. Under normal circumstances, the alveolar macrophages are intact and recognize bacteria, setting off a proinflammatory cytokine/chemokine response. Early after LC, these macrophages are undergoing programmed cell death and may not by capable of effectively coordinating the host response to a secondary bacterial exposure. Thus, the initial inflammatory response to lung injury may in fact consume the very cells that would ordinarily be available to recognize and clear an ensuing bacterial insult.
In the setting of virulent Mycobacterium tuberculosis, infected macrophages are known to die utilizing two different forms of cell death: (1) necrosis, a form of death characterized by cell lysis and (2) apoptosis, a cellular form of death in which the plasma membrane of the cell remains intact.25,26 Apoptosis is energy dependent, leads to microbe killing, limits inflammation, and minimizes tissue injury. Necrosis, however, is energy-independent mechanism by which the bacteria exit the macrophage, escape host defenses, and proliferate. This allows the pathogen to escape intracellular digestion exposing the pathogen to the humoral immune system and activated phagocytes. Necrotic cell death also exacerbates the innate inflammatory response by spilling over of lethal intracellular components. Apoptotic cell death has been observed in lung tissue after pulmonary contusion.27 LC created by a blast wave in rats resulted in an increase in apoptotic cells inside the lung and these changes peak at 48 hours. There was also a significant increase in caspase eight levels and myeloperoxidase activity in the lung during 48 hours to 72 hours.
Apoptosis serves as an injury-limiting mechanism by holding the cell membrane intact, especially in the case of PMNs which keeps their toxic granule contents from being released. Macrophages are important in this process as they engulf apoptotic neutrophils and release anti-inflammatory mediators.28 Therefore, the role of alveolar macrophages is critical to host defense against infection because not only do they initiate the inflammatory response but also they are integral to orchestrating modulation and regulation of the inflammatory response by contributing to cytokine/chemokine changes and eliminating aged PMNs.29
K. pneumoniae has been shown to induce necrosis of monocytes and macrophages.30 In addition, depletion of alveolar macrophages in a K. pneumoniae pneumonia model resulted in 100% lethality, whereas control mice (nondepleted) all survived.31 Alveolar macrophage depleted mice had increased outgrowth of bacteria from the lungs and blood. They also had an increased influx of PMNs into the lung. Likewise, our results demonstrated increased apoptosis/necrosis of macrophages in animals with pneumonia after LC (LC+Pneu) when compared with animals with pneumonia alone (Sham+Pneu). We found large numbers of macrophages and neutrophils present in the alveolar space 6 hours after LC. After bacterial inoculation many of these inflammatory cells went on to cell death by necrosis or apoptosis. Our finding that there were large numbers of necrotic macrophages strongly suggests that the Klebsiella evaded intracellular killing and went on to multiply and spread within the lung. This is evident and explains both the quantitative culture results and survival experiment outcomes in which the LC++Pneu mice had defective bacterial clearance and decreased survival compared with the Sham+Pneu animals.
CONCLUSION
Acute inflammation after LC acts as a cellular-sump leading to consumption of inflammatory cells and in particular induces apoptotic/necrotic changes in the alveolar macrophages. This deficit and failure to complete the process of repriming results in a vulnerable window in which there is decreased bacterial clearance and increased mortality from gram-negative pneumonia.
Acknowledgments
Supported by National Institutes of Health grant R01-HL102013 (to K.R.), K08-GM078610 (to M.R.H.) with joint support from the American College of Surgeons and American Association for the Surgery of Trauma.
DISCUSSION
Dr. Frederick A. Moore (Gainesville, Florida): In the early ‘90s at the Denver General Hospital under the auspices of a SCOR grant we were studying the role of neutrophils in ARDS. And we noted that some of our patients seemed to be vulnerable to secondary inflammatory insults like aspiration or early rodding of a femur. We were studying neutrophil priming and activation in the lab and we proposed a two-hit model.
Using gut ischemia reperfusion as our surrogate for shock we showed that we could prime neutrophils, neutrophils went to the lung, we got reversible lung injury. And then if we took an otherwise innocuous second insult, such as low dose endotoxin at six hours, we could create fulminant ARDS and a lot of mortality. We went on to demonstrate in patients that this priming did occur and appeared to be a pivotal mechanism in MOF.
Today Dr. Dolgachev and colleagues present a different two-hit model. They create a pulmonary contusion using a standard cortical contusion impactor device. Six hours later they induce a pneumonia by intratracheal inoculation of Klebsiella.
They show that survival at 72 hours for pulmonary contusion alone is 100%, for pneumonia alone is 80%, but if you combine the two insults the mortality is substantially worse at 20%.
However, when they look at the innate immune response in this model, they find something very different from ours. The first hit, pulmonary contusion, inactivates the alveolar macrophage response by inducing macrophage apoptosis and necrosis. As a result, the pulmonary contusion patients appear to mount a less intense neutrophil response when inoculated with Klebsiella and presumably die of overwhelming sepsis. I have several questions which I will try to make short.
How did these animals die? Do you have evidence of overwhelming sepsis or did they die of respiratory failure?
How are you going to confirm these laboratory observations are clinically relevant? In my experience with pulmonary contusion, pneumonia is really not a series early problem; and the early pneumonias we see are gram-positive not gram-negative.
In your introduction you talk about IL-10 and I could go through a bunch of data but I’ll go right to the punch line. Tell me what you think, how IL-10 is playing a role in this increased mortality.
Now, lastly, how long does this defect in alveolar macrophage response persist? Could it last for days? If so, would a therapeutic immunomodulator such as GM CSF make sense?
Have you tried to bolster macrophage function-viability in your pulmonary contusion model to see if you can improve the PMN response and increase resistance to the intratracheal inoculation? And what about prophylactic antibiotics?
In sum, I would like to congratulate the investigators on performing some very enticing trauma basic research. I’d like to acknowledge the wisdom of the AAST Board for creating a funding mechanism to augment the KO8 grant that supported this research. Finally, I would like to thank the Association for the privilege of the floor.
Dr. Vladislav A. Doglachev (Ann Arbor, Michigan): Thank you very much, Dr. Moore. Regarding to the first question, we do not see any sepsis. Animals die because of pulmonary failure.
How do we compare them? Basically we suggest that our models are more close to a real clinical situation because most of the patients have a complications due to gram-negative organisms. And basically, gram-positive organisms are very easy to fight by mice with no harm to the animal done.
Regarding IL-10, this is a much tougher question and one that we are actually studying right now. We have IL-10 over-producing animals in our facilities and the study is underway, so I’m not ready to comment on this question.
How does a deficit of alveolar macrophages response persist? Basically this is why lung contusion and pneumonia-changed animals die very fast, because we do not have any macrophages at this early time point after contusion to fight second bacterial insult. Basically macrophages are overwhelmed and all dead.
Have I tried to bolster macrophage function? We have a very nice study presented a few months ago at the Shock meeting where we used stem cells to overcome this problem. We got 60% survival over 20% survival after stem cell administration.
Footnotes
Presented at the American Association for the Surgery of Trauma Meeting September 14–17, 2011, Chicago, Illinois.
AUTHORSHIP
All authors participated in designing this study. V.A.D., B.Y., and J.M.R. collected the data, which were analyzed and interpreted by V.A.D., K.R., and M.R.H. V.A.D., K.R., and M.R.H. wrote the manuscript, which was critically revised by K.R. and M.R.H. V.A.D. and M.R.H. prepared the figures.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Michelet P, Couret D, Bregeon F, et al. Early onset pneumonia in severe chest trauma: a risk factor analysis. J Trauma. 2010;68:395–400. doi: 10.1097/TA.0b013e3181a601cb. [DOI] [PubMed] [Google Scholar]
- 2.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68:845–850. [PubMed] [Google Scholar]
- 3.Moore EE, Moore FA, Harken AH, Johnson JL, Ciesla D, Banerjee A. The two-event construct of postinjury multiple organ failure. Shock. 2005;24(Supp 1):71–74. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 4.Evans HL, Warner K, Bulger EM, Sharar SR, Maier RV, Cuschieri J. Pre-hospital intubation factors and pneumonia in trauma patients. Surg Infect (Larchmt) 2011;12:339–344. doi: 10.1089/sur.2010.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmila MR, Jakubus JL, Maggio PM, et al. Real money: Complications and hospital costs in trauma patients. Surgery. 2008;144:307–316. doi: 10.1016/j.surg.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nader ND, McQuiller PS, Raghavendran K, Knight PR. The role of alveolar macrophages in the pathogenesis of aspiration pneumonitis. Immunol Invest. 2007;36:457–471. doi: 10.1080/08820130701361053. [DOI] [PubMed] [Google Scholar]
- 8.Nelson S, Summer WR. Innate immunity, cytokines, and pulmonary host defense. Infect Dis Clin North Am. 1998;12:555–567. doi: 10.1016/s0891-5520(05)70198-7. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 10.Lukacs NW, Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol. 1999;72:102–120. doi: 10.1159/000058729. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Doerschuk CM, Mizgerd JP. Neutrophils in innate immunity. Semin Respir Crit Care Med. 2004;25:33–41. doi: 10.1055/s-2004-822303. [DOI] [PubMed] [Google Scholar]
- 12.Hoth JJ, Scott MJ, Owens RK, et al. Trauma alters alveolar effector cell apoptosis. Surgery. 2003;134:631–637. doi: 10.1016/s0039-6060(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 13.Kasten KR, Tschop J, Adediran SG, Hildeman DA, Caldwell CC. T cells are potent early mediators of the host response to sepsis. Shock. 2010;34:327–336. doi: 10.1097/SHK.0b013e3181e14c2e. [DOI] [PubMed] [Google Scholar]
- 14.Reddy RC, Chen GH, Newstead MW, et al. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect Immun. 2001;69:1394–1401. doi: 10.1128/IAI.69.3.1394-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 16.Hoth JJ, Hudson WP, Brownlee NA, et al. Toll-like receptor 2 participates in the response to lung contusion injury in a murine model of pulmonary contusion. Shock. 2007;28:447–452. doi: 10.1097/shk.0b013e318048801a. [DOI] [PubMed] [Google Scholar]
- 17.Hemmila MR, Fan MH, Kim J, et al. Improved survival in mice given systemic gene therapy in a gram negative pneumonia model. J Trauma. 2005;58:1110–1118. doi: 10.1097/01.ta.0000170855.37686.91. [DOI] [PubMed] [Google Scholar]
- 18.Huynh ML, Fadok VA, Henson PM, et al. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32:122–130. doi: 10.1097/SHK.0b013e31819c385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoth JJ, Wells JD, Brownlee NA, et al. Toll-like receptor 4 dependent responses to lung injury in a murine model of pulmonary contusion. Shock. 2009;31:376–381. doi: 10.1097/SHK.0b013e3181862279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raghavendran K, Davidson BA, Woytash JA, et al. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock. 2005;24:132–138. doi: 10.1097/01.shk.0000169725.80068.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanley TP, Vasi N, Denenberg A. Regulation of chemokine expression by IL-10 in lung inflammation. Cytokine. 2000;12:1054–1064. doi: 10.1006/cyto.1999.0655. [DOI] [PubMed] [Google Scholar]
- 23.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 24.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behar SM, Martin CJ, Booty MG, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4:279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liener UC, Knoferl MW, Sträter J, et al. Induction of apoptosis following blunt chest trauma. Shock. 2003;20:511–516. doi: 10.1097/01.shk.0000095057.62263.fb. [DOI] [PubMed] [Google Scholar]
- 28.Fadok VA, Bratton DL, Knowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knapp S, Schultz M, van Der Poll T. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock. 2005;24:12–18. doi: 10.1097/01.shk.0000191385.41689.f3. [DOI] [PubMed] [Google Scholar]
- 30.Willingham SB, Allen IC, Bergstralh DT, et al. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and –independent pathways. J Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broug-Holub E, Toews GB, van Iwaarden JF, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immunol. 1997;65:2278–2282. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]