Abstract
Aims
There is little evidence of beta-blocker treatment benefit in patients with heart failure and reduced left ventricular ejection fraction (HFREF) and atrial fibrillation (AF). We investigated the effects of bucindolol in HFREF patients with AF enrolled in the Beta-blocker Evaluation of Survival Trial (BEST).
Methods and results
A post-hoc analysis of patients in BEST with and without AF was performed to estimate the effect of bucindolol on mortality and hospitalization. Patients were also evaluated for treatment effects on heart rate and the influence of beta1-adrenergic receptor position 389 (β1389) arginine (Arg) vs. glycine (Gly) genotypes. In the 303/2708 patients in AF, patients receiving bucindolol were more likely to achieve a resting heart rate ≤80 b.p.m. at 3 months (P < 0.005) in the absence of treatment-limiting bradycardia. In AF patients and sinus rhythm (SR) patients who achieved a resting heart rate ≤80 b.p.m., there were beneficial treatment effects on cardiovascular mortality/cardiovascular hospitalization [hazard ratio (HR) 0.61, P = 0.025, and 0.79, P = 0.002]. Without achieving a resting heart rate ≤80 b.p.m., there were no treatment effects on events in either group. β1389-Arg/Arg AF patients had nominally significant reductions in all-cause mortality/HF hospitalization and cardiovascular mortality/hospitalization with bucindolol (HR 0.23, P = 0.037 and 0.28, P = 0.039), whereas Gly carriers did not. There was no evidence of diminished heart rate response in β1389-Arg homozygotes.
Conclusion
In HFREF patients with AF, bucindolol was associated with reductions in composite HF endpoints in those who achieved a resting heart rate ≤80 b.p.m. and nominally in those with the β1389-Arg homozygous genotype.
Keywords: Atrial fibrillation, Heart failure, Bucindolol, Beta-blocker, Beta1-adrenergic receptor polymorphism
Introduction
Atrial fibrillation (AF) occurs in 10–40% of patients with chronic HF (HF) and has been associated with poor outcomes including death and HF progression in patients with reduced1–5 (HFREF) or preserved6 (HFPEF) left ventricular ejection fractions (LVEFs). In HFREF, beta-blocker therapy is associated with improved outcomes and ventricular reverse remodelling,7–12 but there is little evidence of benefit if AF is also present. Retrospective analyses of patients with AF in the Cardiac Insufficiency Bisoprolol Study (CIBIS) II,13 the Metoprolol CR/XL Randomization Intervention Trial in Chronic Heart Failure (MERIT-HF),14 or the Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure (SENIORS)15 trials revealed no evidence of reduction in the respective primary endpoints by any of the beta-blockers tested, in contrast to effects in patients in sinus rhythm (SR). An analysis of the US Carvedilol Heart Failure Trials Program showed improvements in LVEF, but reductions in heart rate and mortality were not significant.16 Carvedilol was superior to metoprolol in reducing mortality in AF patients in the Carvedilol or Metoprolol European Trial (COMET),3 but there was no placebo control and the degree of beta1-adrenergic receptor (β1-AR) blockade was not comparable in the two arms.17 A propensity-matched study in the Beta-Blocker Evaluation of Survival Trial (BEST) comparing patients with a history of AF with matched controls without AF suggested that bucindolol may reduce time to first HF hospitalization in AF patients compared with those in SR, but all-cause mortality was not affected, and treatment effects within rhythm subgroups were not presented.18 Furthermore, in a recent study, carvedilol had little effect on heart rate in elderly HFREF patients with AF and the β1-AR position 389 (β1389)-arginine (Arg) homozygous genotype,19 and another study showed that beta-blockers have less effect on rate reduction in AF patients with this genotype compared with β1389-glycine (Gly) carriers,20 suggesting that the effects of beta-blockers in AF patients may be pharmacogenetically influenced.
We conducted a retrospective analysis of BEST to estimate the effect of bucindolol within AF and SR subgroups, including the influence of the β1389 genotype. BEST investigated the use of the non-selective beta-blocker/sympatholytic agent bucindolol in HFREF patients who had advanced, New York Heart Association (NYHA) class III or IV HF. Patients in the placebo arm had an annual mortality of 17% and overall mortality of 33% over an average of 2 years follow-up.21,22 Because of its potent sympatholytic properties23 and relative lack of bradycardia-related side effects,22,24,25 we hypothesized that bucindolol would be safe and efficacious in HFREF patients with AF, provide adequate ventricular rate (VR) control, and that, due to its selective inhibitory effects on β1389 arginine receptors, it would be at least as effective in AF patients with the β1389-Arg/Arg genotype as compared with Gly carriers.
Methods
BEST trial design and definitions
The design and primary results of BEST have been published previously.21,25 BEST enrolled 2708 patients with an LVEF ≤35% and NYHA class III or IV symptoms who were double-blind randomized to receive bucindolol or placebo. Enrolment was stratified based on LVEF (>20% vs. ≤20%), gender, ethnicity (black vs. non-black), and presence of coronary artery disease. β1389 genotype was determined in 1040 patients enrolled in a DNA substudy as described previously.26,27 Diagnosis of AF and resting VR were determined using baseline electrocardiogram (ECG). HF endpoints examined were the BEST primary endpoint of time to all-cause mortality and the secondary endpoints of time to first all-cause mortality or HF hospitalization, time to first cardiovascular mortality or cardiovascular hospitalization, and all-cause hospital days/patient. Hospitalizations were classified by an adjudication committee as described previously.28 Non-HF endpoints were change in VR on follow-up ECG at 3 months, achievement of rate control defined in this study as a resting VR of ≤80 b.p.m. on ECG at 3 months29,30 in the of absence of symptomatic bradycardia during the first 7 months of enrolment (1 month of up-titration + 6 months follow-up on high dose study medication), and change in venous norepinephrine level at 3 months. HF endpoints and hospital days/patient were analysed with respect to the study definition of rate control in both AF and SR patients. Holter monitoring and exercise testing were not performed. All-cause mortality/HF hospitalization, cardiovascular mortality/cardiovascular hospitalization, and rate control analyses were also conducted in the DNA substudy according to β1389 genotype. All-cause mortality was not analysed in the DNA substudy due to the small number of events (n = 25 in the AF group).
Measurement of beta1- adrenergic receptor position 389 Arg/Gly polymorphisms
Amino acid position 389 Arg or Gly β1-AR polymorphisms were measured as previously described.31 Genotypes analysed were the major allele homozygote Arg/Arg vs. the combination of heterozygotes and Gly homozygotes (Gly carriers).
Statistical analyses
Student's t-test and the χ2 test were used for continuous and categorical variables to identify differences in baseline characteristics. The log-rank test was used to compare treatment group rates of HF endpoints within the AF and SR subgroups and according to β1389 genotype within each subgroup. Kaplan–Meier curves were generated, and Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals (CIs) between bucindolol and placebo. All Cox proportional hazards models were adjusted for the four randomization stratification variables. A test for interaction between baseline rhythm and treatment arm was also performed. Achievement of study-defined rate control was compared between treatment arms using the χ2 test. Total hospital days/patient, changes in norepinephrine levels, and changes in VR at 3 months according to rhythm status, treatment arm, and genotype were evaluated using the Wilcoxon rank sum test. A P-value <0.05 was considered significant.
Results
Baseline characteristics
At randomization, 303/2708 (11.1%) patients were in AF, 2176 (80.4%) were in SR, and 229 (8.5%) had other rhythms or missing data. Baseline characteristics of SR and AF patients are shown in Table 1 (patients with other rhythms/missing data are not shown). Compared with SR, AF patients were older, more likely to be male, less likely to be black, and less likely to have a history of hypertension, diabetes mellitus, or previous coronary artery interventions. AF patients had a longer duration of HF and more severe symptoms, were more likely to be hypervolaemic, had a higher LVEF and serum creatinine, and had a lower body mass index, heart rate, and diastolic blood pressure. Norepinephrine levels were higher in AF patients than SR patients (624 ± 446 vs. 496 ± 320 pg/mL, respectively, P < 0.0001), and there was no difference in the frequency of the β1389-Arg/Arg genotype between AF and SR patients (52/111 or 46.8% vs. 402/846 or 47.5%, P = 0.89). The mean bucindolol dose in mg/day was 120 ± 69 in AF and 125 ± 64 in SR (P = 0.39). Data regarding concomitant medications are also found in Table 1. Most patients were on angiotensin-converting enzyme inhibitors, and a large proportion (>90%) of both AF and SR patients were on digoxin. SR patients were more likely to be on vasodilators, statins, and aspirin, while AF patients were more likely to be on oral anticoagulation. Few patients in either group were on spironolactone, calcium channel blockers, or other antiarrhythmics.
Table 1.
Baseline characteristics
| Characteristic | Active atrial fibrillation |
Sinus rhythm |
||
|---|---|---|---|---|
| Placebo (n = 157) | Bucindolol (n = 146) | Placebo (n = 1086) | Bucindolol (n = 1090) | |
| Age (years ± SD)‡ | 65.5 ± 10.3 | 65.7 ± 11.7 | 59.0 ± 12.2 | 58.8 ± 12.4 |
| Female‡ | 11 (7%) | 18 (12%) | 279 (26%) | 252 (23%) |
| Black‡ | 19 (12%) | 19 (13%) | 259 (24%) | 283 (26%) |
| Ischaemic aetiology | 85 (54%) | 84 (58%) | 632 (58%) | 638 (59%) |
| Previous MI | 67 (43%) | 62 (42%) | 464 (43%) | 462 (42%) |
| Coronary angioplasty† | 13 (8%) | 17 (12%) | 187 (17%) | 170 (16%) |
| AF history‡ | 152 (97%) | 141 (97%) | 112 (10%) | 141 (13%) |
| Ventricular arrhythmia | 11 (7%) | 19 (13%) | 102 (9%) | 100 (9%) |
| Hypertension† | 81 (52%) | 82 (56%) | 642 (59%) | 659 (60%) |
| Diabetes‡ | 37 (24%) | 45 (31%) | 384 (35%) | 423 (39%) |
| Previous smoker | 83 (53%) | 90 (62%) | 603 (56%) | 576 (53%) |
| CHF duration (months ± SD)‡ | 64.7 ± 57.1 | 51.7 ± 43.0* | 48.2 ± 47.8 | 46.3 ± 45.9 |
| NYHA class† | ||||
| III | 141 (90%) | 129 (88%) | 1005 (93%) | 1005 (92%) |
| IV | 16 (10%) | 17 (12%) | 81 (7%) | 85 (8%) |
| Heart rate (b.p.m. ± SD)‡ | 80 ± 14 | 79 ± 14 | 83 ± 13 | 83 ± 14 |
| Systolic BP (mmHg ± SD) | 117.1 ± 17.8 | 117.8 ± 17.9 | 117.4 ± 17.8 | 117.3 ± 18.5 |
| Diastolic BP (mmHg ± SD)† | 69.5 ± 11.1 | 70.4 ± 11.4 | 71.4 ± 10.9 | 71.6 ± 11.5 |
| Body mass index (kg/m2 ± SD)† | 27.7 ± 5.9 | 27.4 ± 5.1 | 28.2 ± 6.1 | 28.3 ± 6.1 |
| Euvolaemica‡ | 77 (49%) | 86 (59%) | 718 (66%) | 713 (65%) |
| LVEF (% ± SD)† | 23.7 ± 7.2 | 24.1 ± 6.9 | 23.0 ± 7.2 | 22.8 ± 7.3 |
| RVEF (% ± SD) | 32.3 ± 11.3 | 35.0 ± 12.0 | 35.7 ± 14.1 | 35.0 ± 13.8 |
| Norepinephrine (pg/mL ± SD)‡ | 639 ± 383 | 608 ± 508 | 477 ± 303 | 510 ± 335 |
| Creatinine (mg/dL ± SD)‡ | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 |
| Medication | ||||
| ACE inhibitor | 155 (99%) | 142 (97%) | 1075 (99%) | 1076 (99%) |
| Vasodilator† | 48 (41%) | 59 (40%) | 482 (44%) | 467 (43%) |
| Spironolactone | 8 (5%) | 2 (1%) | 32 (3%) | 34 (3%) |
| Calcium channel blocker | 1 (1%) | 1 (1%) | 4 (<1%) | 9 (1%) |
| Antiarrhythmic agent | 2 (1%) | 5 (3%) | 23 (2%) | 35 (3%) |
| Digoxin | 149 (95%) | 139 (95%) | 995 (92%) | 1006 (92%) |
| Statin‡ | 26 (17%) | 18 (12%) | 300 (28%) | 292 (27%) |
| ASA‡ | 28 (18%) | 28 (19%) | 530 (49%) | 511 (47%) |
| Oral anticoagulant‡ | 135 (86%) | 120 (82%) | 395 (36%) | 392 (36%) |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ASA, acetylsalicylic acid; BP, blood pressure; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; RVEF, right ventricular ejection fraction; SD, standard deviation; SR, sinus rhythm.
AF vs. SR comparisons: †P < 0.05, ‡P < 0.001.
Treatment group comparisons within AF and SR groups: *P < 0.05, **P < 0.001.
aEuvolaemia defined as absence of jugular venous distension, peripheral oedema, hepatomegaly, or rales.
Outcome analyses
Clinical endpoints—entire cohort
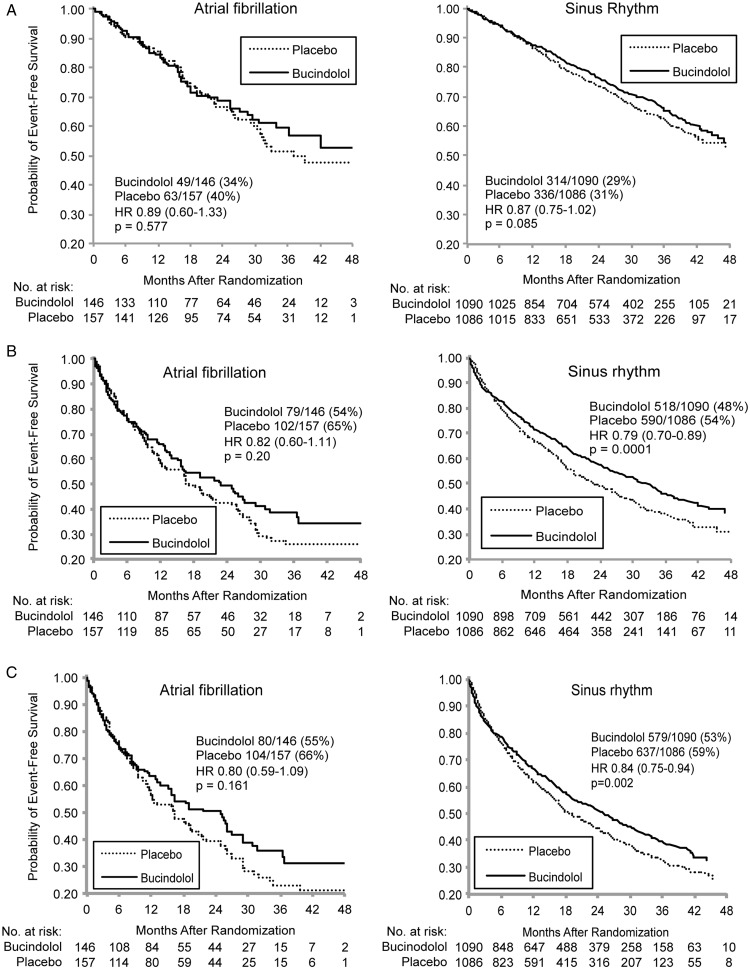
Kaplan–Meier curves for HF endpoints are shown in Figure 1. In patients with SR, bucindolol was associated with significant reductions in all-cause mortality/HF hospitalization and cardiovascular mortality/cardiovascular hospitalization, with a trend (P = 0.085) for a reduction in all-cause mortality. In the smaller sample size AF patients, hazard ratios for HF endpoints were numerically similar to those in SR patients but with higher P-values ( ≥0.16) that did not approach statistical significance. Interactions between treatment arm and rhythm status were non-significant for all three endpoints. Total hospital days/patient were not significantly different between bucindolol and placebo arms in either the AF (16.4 ± 2.5 vs. 19.3 ± 3.0 days, risk ratio = 0.85, P = 0.24) or the SR cohorts (12.3 ± 0.6 vs. 14.9 ± 0.8 days, risk ratio = 0.83, P = 0.057).
Figure 1.
(A) All-cause mortality by rhythm status and treatment group. (B) All-cause mortality/heart failure by rhythm status and treatment group. (C) Cardiovascular hospitalization or mortality by rhythm status and treatment group.
Heart rate effects—entire cohort
The VR at baseline and 3 months, and study-defined rate control status at 3 months are shown in Table 2 by baseline rhythm and treatment group. Bucindolol was associated with highly significant reductions in VR in the AF group when corrected for changes in the placebo groups (bucindolol – placebo heart rate in AF group = –9.1 b.p.m., SR group = –7.9 b.p.m., AF vs. SR P = 0.43). At month 3, 92/138 (66.7%) AF patients receiving bucindolol and 74/150 (49.3%) patients receiving placebo achieved the study definition of rate control (relative risk = 1.35, 95% CI 1.11–1.65, P = 0.003). Among AF patients receiving bucindolol, 32/146 patients (21.9%) had bradycardia events resulting in 3 (2.1%) treatment withdrawals. In comparison, 16/157 patients in the placebo arm had bradycardia events (10.2%, P = 0.005 vs. bucindolol) resulting in 2 (1.3%) withdrawals (P = 0.59 vs. bucindolol). A total of 16/131 (12.2%) AF patients receiving bucindolol and 13/144 (9.0%) AF patients receiving placebo had converted to SR by their last available ECG (P = 0.39).
Table 2.
Heart rate and achievement of study definition of rate control according to rhythm status and treatment group
| Baseline rhythm status | Treatment arm | Baseline | Month 3 |
Δ heart rate at month 3 | P-value, Δ from baseline | |
|---|---|---|---|---|---|---|
| Mean ± SD (n) | Mean ± SD (n) | Rate controlled | ||||
| Atrial fibrillation | Placebo | 80 ± 14 (157) | 81 ± 15 (149) | 74/150 (49%) | 2.2 ± 1.1 | 0.02 |
| Bucindolol | 79 ± 14 (146) | 70 ± 13 (138)** | 92/138 (67%)** | –6.9 ± 1.3** | <0.0001 | |
| Sinus rhythm | Placebo | 83 ± 13 (1086) | 80 ± 13 (1021) | 555/1021 (54%) | –2.0 ± 0.4 | <0.0001 |
| Bucindolol | 83 ± 14 (1090) | 73 ± 12 (1008)** | 756/1008 (75%)** | –9.9 ± 0.4** | <0.0001 | |
Rate-controlled ventricular rate ≤80 b.p.m at month 3, no bradycardia adverse events through month 7.
Treatment group comparisons within rhythm subgroups: *P < 0.05, *P < 0.005.
Outcomes as a function of successful rate control—entire cohort
Hazard ratios for HF endpoints by treatment arm and the study-defined rate control at 3 months are given in Table 3 for AF patients compared with SR patients. Among AF patients who achieved study-defined rate control, 30/92 (32.7%) and 34/74 (45.9%) died in the bucindolol and placebo arms, respectively (hazard ratio = 0.62, 95% CI 0.36–1.06, P = 0.077), compared with 178/756 (23.5%) and 155/555 (27.9%) SR patients who achieved study-defined rate control (P = 0.14). In patients who achieved study-defined rate control, there were significant decreases in cardiovascular mortality/cardiovascular hospitalization and in all-cause mortality/HF hospitalization associated with bucindolol in both AF and SR patients. For total hospital days/patient, only SR patients who achieved study-defined rate control had a significant reduction in the bucindolol group. There were no reductions in any HF outcomes for either AF or SR patients who did not achieve study-defined rate control.
Table 3.
Outcomes according to study definition of rate control at 3 months
| Rate control subgroups | Placebo, n (%) | Bucindolol, n (%) | Hazard ratio (95% CI) | Log-rank P-value |
|---|---|---|---|---|
| All-cause mortality | ||||
| Atrial fibrillation | ||||
| Ventricular rate control | 34/74 (46%) | 30/92 (33%) | 0.62 (0.36–1.06) | 0.077 |
| Not rate controlled | 23/76 (30%) | 11/46 (24%) | 1.13 (0.51–2.49) | 0.761 |
| Sinus rhythm | ||||
| Heart rate control | 155/555 (28%) | 178/756 (24%) | 0.85 (0.68–1.05) | 0.135 |
| Not rate controlled | 138/467 (30%) | 83/256 (32%) | 0.97 (0.74–1.28) | 0.839 |
| All-cause mortality/heart failure hospitalization | ||||
| Atrial fibrillation | ||||
| Ventricular rate control | 47/74 (64%) | 47/92 (51%) | 0.72 (0.46–1.10) | 0.129 |
| Not rate controlled | 49/76 (64%) | 24/46 (52%) | 0.92 (0.53–1.58) | 0.750 |
| Sinus rhythm | ||||
| Heart rate control | 285/555 (51%) | 322/756 (43%) | 0.78 (0.66–0.91) | 0.002 |
| Not rate controlled | 252/467 (54%) | 135/256 (53%) | 0.90 (0.72–1.11) | 0.323 |
| Cardiovascular mortality/cardiovascular hospitalization | ||||
| Atrial fibrillation | ||||
| Ventricular rate control | 49/74 (66%) | 47/92 (51%) | 0.61 (0.40–0.95) | 0.025 |
| Not rate controlled | 50/76 (66%) | 26/46 (57%) | 1.06 (0.62–1.80) | 0.833 |
| Sinus rhythm | ||||
| Heart rate control | 318/555 (57%) | 358/756 (47%) | 0.79 (0.67–0.92) | 0.002 |
| Not rate controlled | 271/467 (58%) | 163/256 (64%) | 1.09 (0.89–1.33) | 0.408 |
| Total hospital days (mean ± SE) | ||||
| Atrial fibrillation | ||||
| Rate control | 22 ± 5.9, n = 74 | 17 ± 3.5, n = 92 | Δ = –5.2 | 0.127 |
| Not rate controlled | 17 ± 2.2, n = 76 | 16 ± 3.7, n = 46 | Δ = –0.9 | 0.132 |
| Sinus rhythm | ||||
| Heart rate control | 14 ± 1.2, n = 555 | 11 ± 0.7, n = 756 | Δ = –3.7 | 0.021 |
| Not rate controlled | 16 ± 1.2, n = 467 | 18 ± 1.7, n = 256 | Δ = 1.2 | 0.508 |
| Norepinephrine change, 3 months (mean ± SE) | ||||
| Atrial fibrillation | ||||
| Rate control | 87 ± 7.7, n = 54 | –22 ± 4.3, n = 65 | Δ = –108.5 | 0.029 |
| Not rate controlled | –37 ± 6.2, n = 54 | –50 ± 27, n = 31 | Δ = –12.8 | 0.800 |
| Sinus rhythm | ||||
| Heart rate control | 23 ± 14.1, n = 373 | –67 ± 0.5, n = 519 | Δ = –89.3 | <0.0001 |
| Not rate controlled | 28 ± 17.4, n = 304 | –128 ± 2.6, n = 155 | Δ = –155.5 | <0.0001 |
Outcomes by β1389 Arg/Gly genotype
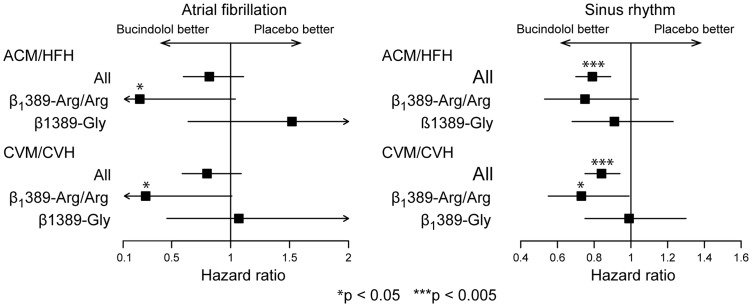
Hazard ratios for HF endpoints for the entire cohort and by β1389 genotype are shown in Figure 2. Bucindolol was associated with substantial reductions in all-cause mortality/HF hospitalization (hazard ratio = 0.23) and cardiovascular mortality/cardiovascular hospitalization (hazard ratio = 0.28) in AF patients with the β1389-Arg/Arg genotype that were significant according to P-values in log-rank analysis (P = 0.037 and P = 0.039, respectively) but not according to Cox proportional hazards analysis (95% CI 0.05–1.04 and 0.08–1.01, respectively). In contrast, there was no evidence of benefit in Gly carriers for either all-cause mortality/HF hospitalization or cardiovascular mortality/cardiovascular hospitalization (hazard ratio = 1.07, P = 0.89; and hazard ratio = 1.52, P = 0.43, respectively). Among SR patients, there was a significant reduction in cardiovascular mortality/cardiovascular hospitalization events in the β1389-Arg/Arg group but not in the Gly carriers (hazard ratio = 0.73, 95% CI 0.55–0.99, P = 0.039 vs. hazard ratio = 0.99, 95% CI 0.75–1.30, P = 0.93).
Figure 2.
Treatment effect of bucindolol on composite heart failure outcomes according to baseline rhythm status and β1389 genotype. ACM, all-cause mortality; CVH, cardiovascular hospitalization; CVM, cardiovascular mortality; HFH, heart failure hospitalization.
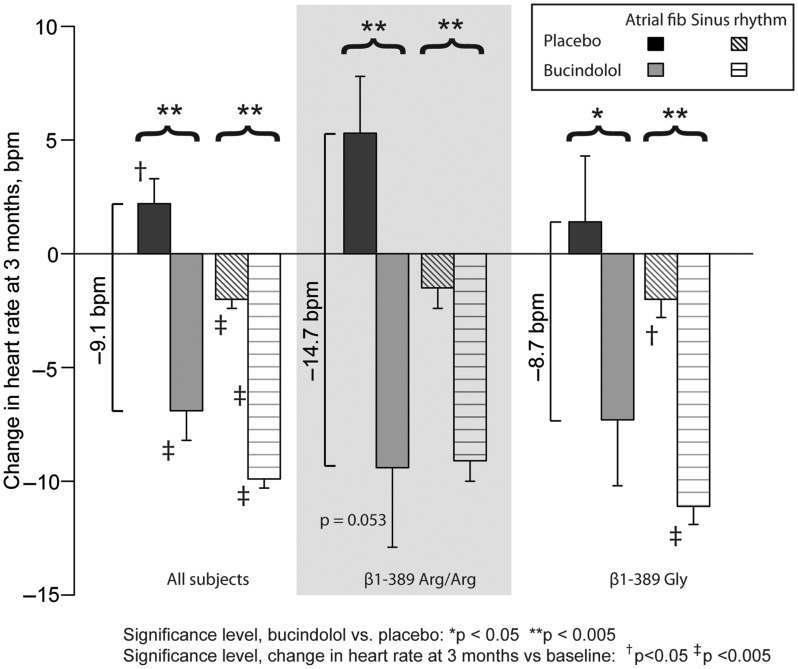
Changes in heart rate from baseline to 3 months are shown in Figure 3 according to rhythm status, treatment group, and β1389 genotype. The difference between bucindolol and placebo VR change at 3 months was greater for β1389-Arg/Arg compared with Gly carrier AF patients (bucindolol – placebo difference of –14.7 and –8.7 b.p.m., respectively) but the difference was not significant (P = 0.32). Small numbers of patients and events in the AF genotype groups precluded a comparison of treatment effects in subjects with or without study-defined rate control. A total of 5/51 (9.8%) β1389-Arg/Arg patients with AF converted to SR compared with 9/57 (15.8%, P = 0.40) Gly carriers. There were no treatment effects on conversion from AF to SR in either genotype (Arg/Arg bucindolol vs. placebo = 9.1% vs. 10.3%, P = 0.88; and Gly carrier bucindolol vs. placebo = 16.0% vs. 15.6%, P = 0.97).
Figure 3.
Change in ventricular rate at 3 months according to treatment group, rhythm status, and β1389 genotype.
Treatment effect on systemic norepinephrine levels
Bucindolol was associated with a significant reduction in venous norepinephrine levels at 3 months compared with placebo in SR patients (–82 ± 12 vs. 25 ± 11 pg/mL, P < 0.0001), but not in AF patients (–31 ± 54 vs. 27 ± 37 pg/mL, P = 0.38). Norepinephrine levels at 3 months decreased significantly in AF patients who achieved study-defined rate control receiving bucindolol vs. placebo (difference = –108 pg/mL, P = 0.029), but not in patients who failed to achieve study-defined rate control (–13 pg/mL, P = 0.80). However, the difference in norepinephrine change between rate control groups was not significant (P = 0.65). SR patients had significant decreases in norepinephrine regardless of achievement of study-defined rate control (rate control bucindolol – placebo difference = –90 pg/mL vs. non-rate control difference = –156 pg/mL, P <0.0001 for both), and there was no difference in 3-month change in norepinephrine levels between SR patients receiving bucindolol who achieved study-defined rate control and those who did not (P = 0.14).
Discussion
BEST trial—entire cohort
In this retrospective analysis of BEST, we found that compared with placebo bucindolol was associated with treatment effects in AF patients that were not obviously quantitatively different from those in patients in SR, who had significant respective reductions in the combined endpoint of all-cause mortality or first hospitalization and cardiovascular mortality or first cardiovascular hospitalization of 21% and 16%. In AF patients, bucindolol was associated with a significantly greater likelihood of achievement of rate control, defined in this study as a resting heart rate ≤80 b.p.m. in the absence of bradycardia-related side effects, compared with placebo. In the study-defined rate control population, bucindolol was also associated with significant reductions in the combined endpoint of time to cardiovascular mortality/cardiovascular hospitalization. Furthermore, the salutary effect of bucindolol on study-defined rate control was observed in the setting of high rates of digoxin use (>90%). These findings suggest that bucindolol may be safe and efficacious in treating AF in the setting of HFREF, with possible enhancement of benefit in patients with the β1389-Arg/Arg genotype, and these results warrant further study.
Pharmacogenetic effects by β1389 Arg/Gly polymorphism
Reductions in all-cause mortality/HF hospitalization and cardiovascular mortality/cardiovascular hospitalization of borderline significance were associated with bucindolol for AF patients with the β1389-Arg/Arg genotype, and reduction in VR was comparable between β1389-Arg homozygotes and Gly carriers. The β1-AR Arg389Gly polymorphism results in attenuation of receptor function, with less adenylyl cyclase activation in response to sympathetic stimulation,26,31,32 less constitutive activity,31,33 and a lower β1-AR affinity for norepinephrine.32,34 It has been shown that β1389-Arg homozygotes with HFREF demonstrate a significant reduction in HF endpoints with bucindolol, while β1389-Gly carriers do not.31 For other beta-blockers β1389-Arg homozygotes may have greater improvements in LVEF compared with β1389-Gly carriers,33,35–37 but no reduction in clinical endpoints has been demonstrated.38–40 The apparently unique ability of bucindolol to lower HF events in β1389-Arg homozygotes may be due to the drug's sympatholytic properties23,41 impacting the higher norepinephrine affinity of the β1389 Arg receptor,32,34 and/or its inverse agonist properties reducing constitutive receptor activity.31 The much higher norepinephrine affinity and adrenergic signal transduction capacity of the Arg389 β1-AR may also protect patients from excessive sympatholysis34 that has been associated with increased mortality in β1389-Gly carriers receiving bucindolol.41 Although treatment effects on HFREF endpoints in β1389-Arg homozygotes with AF in this analysis were only nominally significant, the substantial effect size in the setting of a small sample size suggests that further study of bucindolol in an adequately powered prospective trial may be warranted in this β1-AR genotype.
Differences in receptor function between β1389-Arg/Arg and Gly AR may also have implications for beta-blocker rate control in AF. It was recently reported that AF patients with the β1389-Arg/Arg genotype are more resistant to pharmacological rate control than AF patients with β1389-Gly genotypes, but only 11% of the patients in that study had HF, and separate results for beta-blockers vs. other drugs were not reported.20 A retrospective analysis of the CIBIS in the Elderly Study (CIBIS-ELD) reported that carvedilol was ineffective for rate control in β1389-Arg homozygotes, whereas bisoprolol was equally effective in all β1389 genotypes.19 Healthy patients without AF have shown a greater reduction in heart rate in β1389-Arg homozygotes compared with Gly carriers in response to beta-blocker treatment,42 whereas HFREF patients in SR have shown no difference in heart rate response to beta-blockers between β1389-Arg/Arg and Gly carriers.35–37 In the current study, there is no evidence that HFREF patients with the β1389-Arg/Arg genotype receiving bucindolol had a lower likelihood of achieving study-defined rate control vs. Gly carriers in either AF or SR groups. The discrepancy between the current and previous studies in the observed effect of the β1389 genotype on beta-blocker AF rate control may be related to the unique effects of bucindolol that inhibit β1389-Arg AR function and lower norepinephrine, or to the under-representation of HFREF in previous studies. Although these findings must be validated, the contrast between the previous negative findings in AF patients treated with beta-blockers and the current study highlights that there may be important differences between the roles of various beta-blockers in the management of HFREF patients with AF.
Ventricular rate and heart failure with reduced ejection fraction
The majority of published evidence suggests that rate control of AF is equivalent to rhythm control in HF, and that either strategy is preferable to no treatment.30,43–45 However, it has been argued that type III antiarrhythmics and other ion channel drugs used to maintain SR in these studies exert harm,46 especially in HFREF patients,44 precluding observation of the benefit of maintaining SR. Furthermore, the optimum target for rate control in HF has not been established. The results of the Rate Control Efficacy II Trial (RACE II), which compared a target heart rate ≤80 with ≤110 and a study that compared heart rate targets of ≤80 and ≤100 using the AFFIRM and the Rate Control vs. Electrical cardioversion (RACe) Trials, respectively, suggest that stringency of rate control is not associated with improved outcomes in the general AF population.47,48 A subsequent analysis of RACE II also showed no difference in atrial or ventricular remodelling indices between lenient (80–110 b.p.m.) and strict ( ≤80 b.p.m.) rate control.49 However, 74.0% of patients enrolled in AFFIRM had a normal LVEF and only 4.8% had a diagnosis of ‘cardiomyopathy’.29 Furthermore, only 4.9% of patients enrolled in RACe had a diagnosis of dilated cardiomyopathy, and only 15.1% of patients enrolled in RACE II had an LVEF ≤40%.48,50 AF-CHF, the largest trial to examine rate vs. rhythm control in HFREF which showed equivalence between rate and rhythm control, defined rate control as a resting heart rate of ≤80 b.p.m. similar to the present study, with the additional criteria of a 6 min walk heart rate of ≤110 b.p.m.30 Deleterious effects of prolonged tachycardia on ventricular function and remodelling have been well described,51 but their relevance in establishing a target resting heart rate for HFREF with AF remains unclear. Results from the Systolic HF treatment with the If inhibitor ivabradine Trial (SHIFT) showed an association between lower heart rate and improved outcomes in SR patients,52 and the magnitude of heart rate reduction, irrespective of dose of background beta-blocker therapy, was the primary determinant of subsequent outcomes.53 The findings from SHIFT suggest that the magnitude of heart rate reduction may be important in HFREF patients, and the subgroup analyses in the present study showing that benefit appeared limited to patients who achieved a resting heart rate ≤80 b.p.m. suggest that this target may be useful in future studies of rate-controlling agents in HFREF patients with AF.
Limitations
This was a retrospective analysis, and any findings must be validated prospectively. BEST was underpowered to detect differences between outcomes in AF patients receiving bucindolol vs. placebo, and firm conclusions cannot be drawn from these data. In addition, the only measures of VR were resting 12-lead ECG at baseline and at 3 months of follow-up, unlike AFFIRM and AF-CHF, which also measured VR during either a 6 min walk test or 24 h Holter monitoring.29,30 Furthermore, bradycardia was identified based on adverse event reporting and did not include ambulatory VR monitoring. This could have led to underestimation of bradycardia events, though it is unlikely that treatment-limiting events went unreported. Finally, the classification of AF was based on the baseline ECG, and thus intermittent or paroxysmal AF could not be excluded. These limitations could be addressed with a prospective clinical trial using pre-specified definitions of permanent AF, rate control, and HF endpoints powered sufficiently to detect differences in HF endpoints between treatment arms and according to β1389 genotype.
Conclusions
In patients with AF in BEST, bucindolol was associated with a reduction in cardiovascular mortality/cardiovascular hospitalization in AF patients who achieved a resting heart rate ≤80 b.p.m. without symptomatic bradycardia. In the BEST DNA substudy, bucindolol was possibly associated (nominal P-values) with a decrease in all-cause mortality/HF hospitalization or cardiovascular mortality/cardiovascular hospitalization in patients who had the β1389-Arg/Arg genotype. The likelihood of achieving defined rate control was increased in AF patients receiving bucindolol, and patients who were β1389-Arg/Arg genotype exhibited no evidence of resistance to heart rate reduction. These data suggest that bucindolol may be an effective, safe choice for HF event rate reduction and VR control of AF in HFREF, and further studies are warranted.
Funding
D.P.K. is supported by National Institutes of Health Grant 2 T32 HL007822-12 (PI: Peter Buttrick, MD). The BEST Trial was supported by the NHLBI and the VA Cooperative Studies Program. ARCA biopharma provided statistical support for the study.
Conflicts of interest: G.D. and M.R.B. are employees of ARCA biopharma. All other authors have no relevant conflicts of interest.
Acknowledgements
The authors wish to thank Rachel Rosenberg for assistance in manuscript preparation.
References
- 1.Crijns HJ, Tjeerdsma G, de Kam PJ, Boomsma F, van Gelder IC, van den Berg MP, van Veldhuisen DJ. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J. 2000;21:1238–1245. doi: 10.1053/euhj.1999.2107. [DOI] [PubMed] [Google Scholar]
- 2.Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. 1991;84:40–48. doi: 10.1161/01.cir.84.1.40. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, Metra M, Torp-Pedersen C, Poole-Wilson P. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J. 2005;26:1303–1308. doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 5.Savelieva I, John Camm A. Atrial fibrillation and heart failure: natural history and pharmacological treatment. Europace. 2004;5(Suppl 1):S5–S19. doi: 10.1016/j.eupc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Linssen GC, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011;10:1111–1120. doi: 10.1093/eurjhf/hfr066. [DOI] [PubMed] [Google Scholar]
- 7.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 8.Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA investigators. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH For the U.S. Carvedilol Heart Failure Study Group. Effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 12.CIBIS II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS II): a randomised trial. Lancet. 1999;353:146. [PubMed] [Google Scholar]
- 13.Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen-Grandjean M, Pochmalicki G, Dargie H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II trial. Circulation. 2001;103:1428–1433. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- 14.van Veldhuisen DJ, Aass H, El Allaf D, Dunselman PH, Gullestad L, Halinen M, Kjekshus J, Ohlsson L, Wedel H, Wikstrand J MERIT-HF Study Group. Presence and development of atrial fibrillation in chronic heart failure. Experiences from the MERIT-HF study. Eur J Heart Fail. 2006;8:539–546. doi: 10.1016/j.ejheart.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Mulder BA, van Veldhuisen DJ, Crijns HJ, Böhm M, Cohen-Solal A, Babalis D, Roughton M, Flather MD, Coats AJ, Van Gelder IC. Effect of nebivolol on outcome in elderly patients with heart failure and atrial fibrillation: insights from SENIORS. Eur J Heart Fail. 2012;14:1171–1178. doi: 10.1093/eurjhf/hfs100. [DOI] [PubMed] [Google Scholar]
- 16.Joglar JA, Acusta AP, Shusterman NH, Ramaswamy K, Kowal RC, Barbera SJ, Hamdan MH, Page RL. Effect of carvedilol on survival and hemodynamics in patients with atrial fibrillation and left ventricular dysfunction: retrospective analysis of the US Carvedilol Heart Failure Trials Program. Am Heart J. 200;142:498–501. doi: 10.1067/mhj.2001.117318. [DOI] [PubMed] [Google Scholar]
- 17.Bristow MR. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176–1194. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed MI, White M, Ekundayo OJ, Love TE, Aban I, Liu B. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rau T, Düngen HD, Edelmann F, Waagstein F, Lainščak M, Dimković S, Apostolović S, Nešković AN, Haverkamp W, Gelbrich G, Eschenhagen T. Impact of the β1-adrenoceptor arg389gly polymorphism on heart-rate responses to bisoprolol and carvedilol in heart-failure patients. Clin Pharmacol Ther. 2012;92:21–28. doi: 10.1038/clpt.2012.18. [DOI] [PubMed] [Google Scholar]
- 20.Parvez B, Chopra N, Rowan S, Vaglio JC, Muhammad R, Roden DM, Darbar D. A common β1-adrenergic receptor polymorphism predicts favorable response to rate-control therapy in atrial fibrillation. J Am Coll Cardiol. 2012;59:49–56. doi: 10.1016/j.jacc.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 22.Domanski MJ, Krause-Steinrauf H, Massie BM, Deedwania P, Follmann D, Kovar D, Murray D, Oren R, Rosenberg Y, Young J, Zile M, Eichhorn E BEST Investigators. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail. 2003;9:354–363. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 23.Bristow MR, Abraham WT, Yoshikawa T, White M, Hattler BG, Crisman TS, Lowes BD, Robertson AD, Larrabee P, Gilbert EM. Second- and third-generation beta-blocking drugs in chronic heart failure. Cardiovasc Drugs Ther. 1997;11:291–296. doi: 10.1023/a:1007748131847. [DOI] [PubMed] [Google Scholar]
- 24.Bristow MR, O'Connell JB, Gilbert EM, French WJ, Leatherman G, Kantrowitz NE, Lowes BD, Robertson AD, Larrabee P, Gilbert EM. Dose-response of chronic beta-blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Bucindolol investigators. Circulation. 1994;89:1632–1642. doi: 10.1161/01.cir.89.4.1632. [DOI] [PubMed] [Google Scholar]
- 25.Design of the beta-blocker evaluation survival trial (BEST) The BEST steering committee. Am J Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- 26.Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 27.Bristow MR, Murphy GA, Krause-Steinrauf H, Anderson JL, Carlquist JF, Thaneemit-Chen S, Krishnan V, Abraham WT, Lowes BD, Port JD, Davis GW, Lazzeroni LC, Robertson AD, Lavori PW, Liggett SB. An alpha2c-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail. 2010;3:21–28. doi: 10.1161/CIRCHEARTFAILURE.109.885962. [DOI] [PubMed] [Google Scholar]
- 28.Carson P, Fiuzat M, O'Connor C, Anand I, Plehn J, Lindenfeld JA, Silver M, White M, Miller A, Davis G, Robertson AD, Bristow M, Gottlieb S. Determination of hospitalization type by investigator case report form or adjudication committee in a large heart failure clinical trial (β-blocker evaluation of survival trial [BEST]) Am Heart J. 2010;160:649–654. doi: 10.1016/j.ahj.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 30.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 31.Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, Hodne D, Nelson B, Morrison J, Domanski MJ, Wagoner LE, Abraham WT, Anderson JL, Carlquist JF, Krause-Steinrauf HJ, Lazzeroni LC, Port JD, Lavori PW, Bristow MR. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci USA. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandilands J, O'Shaughnessy M, Brown J. Greater inotropic and cyclic AMP responses evoked by noradrenaline through arg389 β1-adrenoceptors versus gly389 β1-adrenoceptors in isolated human atrial myocardium. Br J Pharmacol. 2003;138:386–392. doi: 10.1038/sj.bjp.0705030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, Liggett SB. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor CM, Fiuzat M, Carson PE, Anand IS, Plehn JF, Gottlieb SS, Silver MA, Lindenfeld J, Miller AB, White M, Walsh R, Nelson PB, Medway AM, Davis G, Robertson AD, Port JD, Carr J, Murphy GA, Lazzeroni LC, Abraham WT, Liggett SB, Bristow MR. Combinatorial pharmacogenetic interactions of bucindolol and β1, α2C adrenergic receptor polymorphisms. PLoS One. 2012;7:pe44324. doi: 10.1371/journal.pone.0044324. Published online 10 October 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molenaar P, Chen L, Semmler AB, Parsonage WA, Kaumann AJ. Human heart beta-adrenoceptors: beta1-adrenoceptor diversification through ‘affinity states’ and polymorphism. Clin Exp Pharmacol Physiol. 2007;34:1020–1028. doi: 10.1111/j.1440-1681.2007.04730.x. [DOI] [PubMed] [Google Scholar]
- 36.de Groote P, Helbecque N, Lamblin N, Hermant X, Mc Fadden E, Foucher-Hossein C, Amouyel P, Dallongeville J, Bauters C. Association between beta-1 and beta-2 adrenergic receptor gene polymorphisms and the response to beta-blockade in patients with stable congestive heart failure. Pharmacogenet Genomics. 2005;15:137–142. doi: 10.1097/01213011-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Terra SG, Hamilton KK, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS, Belgado BS, Hill JA, Aranda JM, Yarandi HN, Johnson JA. Beta1-adrenergic receptor polymorphisms and left ventricular remodeling changes in response to beta-blocker therapy. Pharmacogenet Genomics. 2005;15:227–234. doi: 10.1097/01213011-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Liu WN, Fu KL, Gao HY, Shang YY, Wang ZH, Jiang GH, Zhang Y, Zhang W, Zhong M. β1 adrenergic receptor polymorphisms and heart failure: a meta-analysis on susceptibility, response to β-blocker therapy and prognosis. PLoS One. 2012;7:e37659. doi: 10.1371/journal.pone.0037659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sehnert AJ, Daniels SE, Elashoff M, Wingrove JA, Burrow CR, Horne B, Muhlestein JB, Donahue M, Liggett SB, Anderson JL, Kraus WE. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol. 2008;52:644–651. doi: 10.1016/j.jacc.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 40.White HL, de Boer RA, Maqbool A, Greenwood D, van Veldhuisen DJ, Cuthbert R, Ball SG, Hall AS, Balmforth AJ MERIT-HF Study Group. An evaluation of the beta-1 adrenergic receptor arg389gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail. 2003;5:463–468. doi: 10.1016/s1388-9842(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 41.Bristow MR, Krause-Steinrauf H, Nuzzo R, Liang CS, Lindenfeld J, Lowes BD, Hattler B, Abraham WT, Olson L, Krueger S, Thaneemit-Chen S, Hare JM, Loeb HS, Domanski MJ, Eichhorn EJ, Zelis R, Lavori P. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation. 2004;110:1437–1442. doi: 10.1161/01.CIR.0000141297.50027.A4. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Liu ZQ, Tan ZR, Chen XP, Wang LS, Zhou G, Zhou HH. Gly389Arg polymorphism of beta1-adrenergic receptor is associated with the cardiovascular response to metoprolol. Clin Pharmacol Ther. 2003;74:372–379. doi: 10.1016/S0009-9236(03)00224-8. [DOI] [PubMed] [Google Scholar]
- 43.Caldeira D, David C, Sampaio C. Rate vs rhythm control in patients with atrial fibrillation and heart failure: a systematic review and meta-analysis of randomised controlled trials. Eur J Intern Med. 2011;22:448–455. doi: 10.1016/j.ejim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc M, Guerra PG, Hohnloser SH, Lee KL, Macle L, Nattel S, Pedersen OD, Stevenson LW, Thibault B, Waldo AL, Wyse DG, Roy D AF-CHF Investigators. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;55:1796–1802. doi: 10.1016/j.jacc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 45.Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, Bosker HA, Kamp O, Tijssen JG, Veeger NJ, Van Gelder IC RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the rate control versus electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–1111. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Lafuente-Lafuente C, Longas-Tejero MA, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2012;5:pCD005049. doi: 10.1002/14651858.CD005049.pub3. [DOI] [PubMed] [Google Scholar]
- 47.Van Gelder IC, Wyse DG, Chandler ML, Cooper HA, Olshansky B, Hagens VE, Crijns HJ RACE and AFFIRM Investigators. Does intensity of rate-control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies. Europace. 2006;8:935–942. doi: 10.1093/europace/eul106. [DOI] [PubMed] [Google Scholar]
- 48.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 49.Smit MD, Crijns HJ, Tijssen JG, Hillege HL, Alings M, Tuininga YS, Groenveld HF, Van den Berg MP, Van Veldhuisen DJ, Van Gelder IC RACE II Investigators. Effect of lenient versus strict rate control on cardiac remodeling in patients with atrial fibrillation data of the RACE II (rate control efficacy in permanent atrial fibrillation II) study. J Am Coll Cardiol. 2011;58:942–949. doi: 10.1016/j.jacc.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 50.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 51.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 52.Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 53.Swedberg K, Komajda M, Böhm M, Borer J, Robertson M, Tavazzi L, Ford I SHIFT Investigators. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose? Findings from the SHIFT (Systolic Heart Failure Treatment with the I(f) Inhibitor Ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. doi: 10.1016/j.jacc.2012.01.020. [DOI] [PubMed] [Google Scholar]