Abstract
This study investigated the effects and possible mechanism of ferulic acid, a naturally occurring phenolic compound, on endogenous glutamate release in the nerve terminals of the cerebral cortex in rats. Results show that ferulic acid inhibited the release of glutamate evoked by the K+ channel blocker 4-aminopyridine (4-AP). The effect of ferulic acid on the evoked glutamate release was prevented by chelating the extracellular Ca2+ ions, but was insensitive to the glutamate transporter inhibitor DL-threo-beta-benzyl-oxyaspartate. Ferulic acid suppressed the depolarization-induced increase in a cytosolic-free Ca2+ concentration, but did not alter 4-AP–mediated depolarization. Furthermore, the effect of ferulic acid on evoked glutamate release was abolished by blocking the Cav2.2 (N-type) and Cav2.1 (P/Q-type) channels, but not by blocking ryanodine receptors or mitochondrial Na+/Ca2+ exchange. These results show that ferulic acid inhibits glutamate release from cortical synaptosomes in rats through the suppression of presynaptic voltage-dependent Ca2+ entry.
Key Words: antioxidants, brain, calcium, cerebrocortical nerve terminals, ferulic acid, glutamate release, neuroprotection, phenolic compounds, voltage-dependent Ca2+ channels
Introduction
Ferulic acid is a phenolic compound commonly found in fruits, vegetables, and grains. Several biological activities of ferulic acid (4-hydroxy-3-methoxy cinnamic acid, Fig. 1) have emerged, for example, antioxidant, anti-inflammatory, anticancer, antiapoptotic, antidiabetic, and hepatoprotective properties.1 In addition to these properties, ferulic acid attenuates oxidative stress, glutamate, or amyloid-induced neurotoxicity,2–8 protects against cerebral ischemia-induced brain damage,9 and ameliorates β-amyloid–induced memory impairment.10 These findings suggest a neuroprotective role for ferulic acid; however, the mechanism whereby ferulic acid exerts this capability is unclear.
FIG. 1.

Chemical structure of ferulic acid.
In the brain, glutamate is a major excitatory neurotransmitter and plays an important role in functions, such as synaptic plasticity, learning, and memory.11,12 Excessive release of glutamate induces an increase in intracellular Ca2+ levels. This, in turn, triggers a cascade of cellular responses, including an enhanced oxygen-free radical production, disturbed mitochondrial function, and protease activation, ultimately leading to neuronal cell death.13,14 The neuronal damage induced by over-excitation is likely involved in a number of neuropathological conditions, ranging from acute insults, such as stroke, epileptic seizures, traumatic brain, and spinal cord injury, to chronic neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis.15–17 Consequently, if a compound can attenuate glutamate release from nerve terminals, it may have a neuroprotective effect against the pathological conditions related to excessive glutamate release. Some neuroprotective agents have been revealed to decrease glutamate release in human and rat brain tissues.18–20 Likewise, ferulic acid has a neuroprotective-like effect and whether ferulic acid has an effect on endogenous glutamate release should be evaluated.
To our knowledge, there are no studies addressing whether ferulic acid directly affects glutamate release at the presynaptic level. Thus, the current study used isolated nerve terminals (synaptosomes) purified from the rat cerebral cortex as a model to determine the effects of ferulic acid on glutamate release. In contrast to brain slices, synaptosomes do not suffer from any postsynaptic interactions and are, therefore, extensively used to evaluate presynaptic phenomena.21 In addition, the possible underlying mechanism was also investigated.
Materials and Methods
Materials
3′,3′,3′-Dipropylthiadicarbocyanine iodide [DiSC3(5)] and Fura-2-acetoxy-methyl ester (Fura-2-AM) were obtained from Invitrogen (Carlsbad, CA, USA). Dantrolene, DL-threo-β-benzyloxyaspartate (DL-TBOA), 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157), and ω-conotoxin MVIIC (ω-CgTX MVIIC) were obtained from Tocris Cookson (Bristol, United Kindom). Ethylene glycol bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), sodium dodecyl sulfate (SDS), ferulic acid, and all other reagents were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Animals
Adult male Sprague-Dawley rats (100–200 g) were used in these studies. All animal procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the Fu Jen Catholic University Animal Care and Utilization Committee. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Synaptosomal preparation and glutamate release assay
Synaptosomes were prepared as described previously22 from the cerebral cortices of rats.
Synaptosomes (0.5 mg) were resuspended in 2 mL of the HEPES-buffered incubation medium (HBM: 140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1 mM MgCl2·6H2O, 1.2 mM Na2HPO4, 10 mM glucose, and 10 mM HEPES, pH 7.4) that contained 16 μM bovine serum albumin, and incubated in a cuvette maintained at 37°C with stirring in a Perkin-Elmer LS-50B spectrofluorimeter (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). Glutamate release was assayed in the presence of NADP+ (2 mM), glutamate dehydrogenase (50 U/mL), and CaCl2 (1 mM) by on-line fluorimetry, as described previously.23 Release was stimulated as indicated with 4-aminopyridine (4-AP, 1 mM), or KCl (15 mM), 10 min after the start of incubation and monitored at 2-sec intervals. A standard of exogenous glutamate (5 nmol) was added at the end of each experiment, and the fluorescence response used to calculate released glutamate was expressed as nanomoles glutamate per milligram synaptosomal protein (nmol/mg). Values quoted in the text and expressed in bar graphs represent levels of glutamate cumulatively released after 5 min of depolarization.
Synaptosomal plasma membrane potential
The synaptosomal membrane potential can be monitored by positively charged, membrane potential-sensitive carbocyanine dyes, such as DiSC3(5), which is a positively charged carbocyanine that accumulates in polarized synaptosomes that are negatively charged on the inside. At high concentrations, the dye molecules accumulate and the fluorescence is quenched. Upon depolarization, the dye moves out and, hence, the fluorescence increases.24 Synaptosomes were preincubated and resuspended as described for the glutamate release experiments. After 3-min incubation, 5 μM DiSC3(5) was added and allowed to equilibrate before the addition of CaCl2 (1 mM) after 4-min incubation. Then, 4-AP (1 mM) was added to depolarize the synaptosomes at 10 min, and DiSC3(5) fluorescence was monitored at excitation and emission wave lengths of 646 and 674 nm, respectively.
Cytosolic-free Ca2+concentration
The Ca2+ concentration ([Ca2+]C) was measured using the Ca2+ indicator Fura-2-AM. Synaptosomes (0.5 mg/mL) were preincubated in HBM with 16 μM BSA in the presence of 5 μM Fura-2-AM and 0.1 mM CaCl2 for 30 min at 37°C in a stirred test tube. After Fura-2-AM loading, synaptosomes were centrifuged in a microcentrifuge for 30 sec at 3000 g (5000 rpm). The synaptosomal pellets were resuspended in HBM with BSA, and the synaptosomal suspension was stirred in a thermostated cuvette in a Perkin-Elmer LS-50B spectrofluorometer. CaCl2 (1 mM) was added after 3 min, and further additions were made after an additional 10 min. Fluorescence data were accumulated at excitation wave lengths of 340 and 380 nm (emission wavelength of 505 nm) at 7.5-sec intervals. Calibration procedures were performed as described previously,25 using 0.1% SDS to obtain the maximal fluorescence with Fura-2 saturation with Ca2+, followed by 10 mM EGTA (Tris buffered) to obtain minimum fluorescence in the absence of any Fura-2/Ca2+complex. [Ca2+]C was calculated using equations described previously.26
Data analysis
Data were analyzed using Lotus 1-2-3, Microcal Excel, and Microcal Origin. Data are expressed as mean±SEM. To test the significance of the effect of a drug versus control, an unpaired Student's t-test was used. When an additional comparison was required (such as whether a second treatment influenced the action of ferulic acid), a one-way analysis of variance was used followed by a post hoc LSD comparison. Analysis was completed via software SPSS (17.0; SPSS, Inc., Chicago, IL, USA).
Results
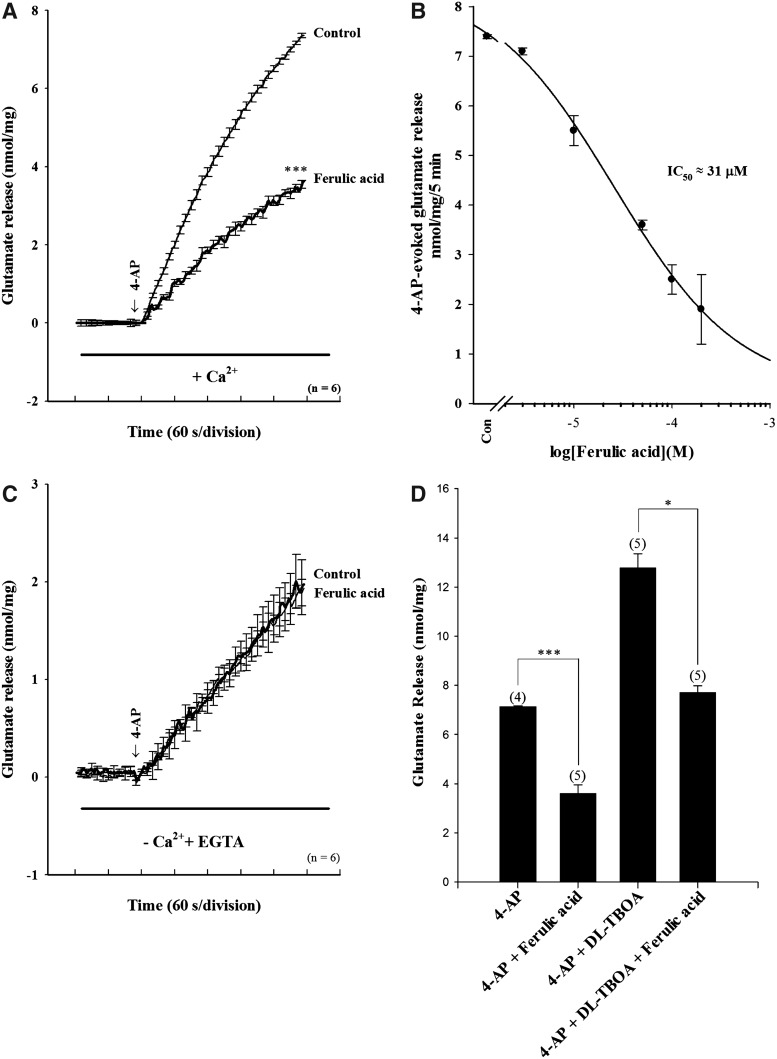
To examine the effect of ferulic acid on glutamate release, nerve terminals were depolarized with the K+ channel blocker 4-AP, which, by increasing the excitability of the synaptosomal plasma membrane. This increases the opening of voltage-dependent Ca2+ channels (VDCCs) to elevate cytoplasmic-free [Ca2+]C and, thus, induces the release of glutamate.27 In synaptosomes incubated in the presence of 1 mM CaCl2, 4-AP (1 mM) evoked a glutamate release of 7.4±0.04 nmol/mg/5 min. Application of ferulic acid (50 μM) produced an inhibition of 4-AP–evoked glutamate release to 3.6±0.1 nmol/mg/5 min (n=6; P<.001), without altering the basal release of glutamate (P>.05; Fig. 2A). A maximal inhibition of 66% occurred with 100 μM ferulic acid. The IC50 value for ferulic acid inhibition of 4-AP–evoked glutamate release, derived from a concentration–response curve, was 31 μM (Fig. 2B). In addition, glutamate release evoked by 4-AP (1 mM) in the absence of extracellular Ca2+ was unaffected by ferulic acid (50 μM; n=6; Fig. 2C). Furthermore, ferulic acid (50 μM) still caused a significant inhibition on 4-AP–induced release of glutamate in the presence of DL-TBOA (10 μM), a nonselective inhibitor of all excitatory amino acid transporter subtypes (P<.05; n=5; Fig. 2D). DL-TBOA by itself increased control 4-AP–evoked glutamate release (because of inhibition of reuptake of released glutamate; P<.001; n=5). These results demonstrate that the inhibition of 4-AP–evoked glutamate release by ferulic acid is mediated by a reduction in the Ca2+-dependent exocytotic component of glutamate release.
FIG. 2.
Ferulic acid inhibits 4-aminopyridine (4-AP)–induced glutamate release from rat cerebrocortical nerve terminals; this effect is due to a decrease in vesicular exocytosis. Synaptosomes were resuspended in incubation medium at a final protein concentration of 0.5 mg/mL and incubated for 3 min before the addition of 1 mM CaCl2. 4-AP (1 mM) was added after a further 10 min to effect depolarization (arrow). Total glutamate release (+Ca2+; A) and Ca2+-independent glutamate release (−Ca2+; C) was measured under control conditions or in the presence of 50 μM ferulic acid added 10 min before the addition of 4-AP. (B) Concentration–response curve for ferulic acid inhibition of 4-AP (1 mM)–evoked glutamate release. (D) Quantitative comparison of the extent of glutamate release by 1 mM 4-AP in the absence and presence of 50 μM ferulic acid and absence and presence of 10 μM DL-threo-β-benzyloxyaspartate (DL-TBOA). DL-TBOA was added 20 min before depolarization, while ferulic acid was added 10 min before depolarization. Results are mean±SEM of four to five independent experiments. *P<.05, one-way analysis of variance (ANOVA). ***P<.001, unpaired Student's t-test. EGTA, ethylene glycol bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid.
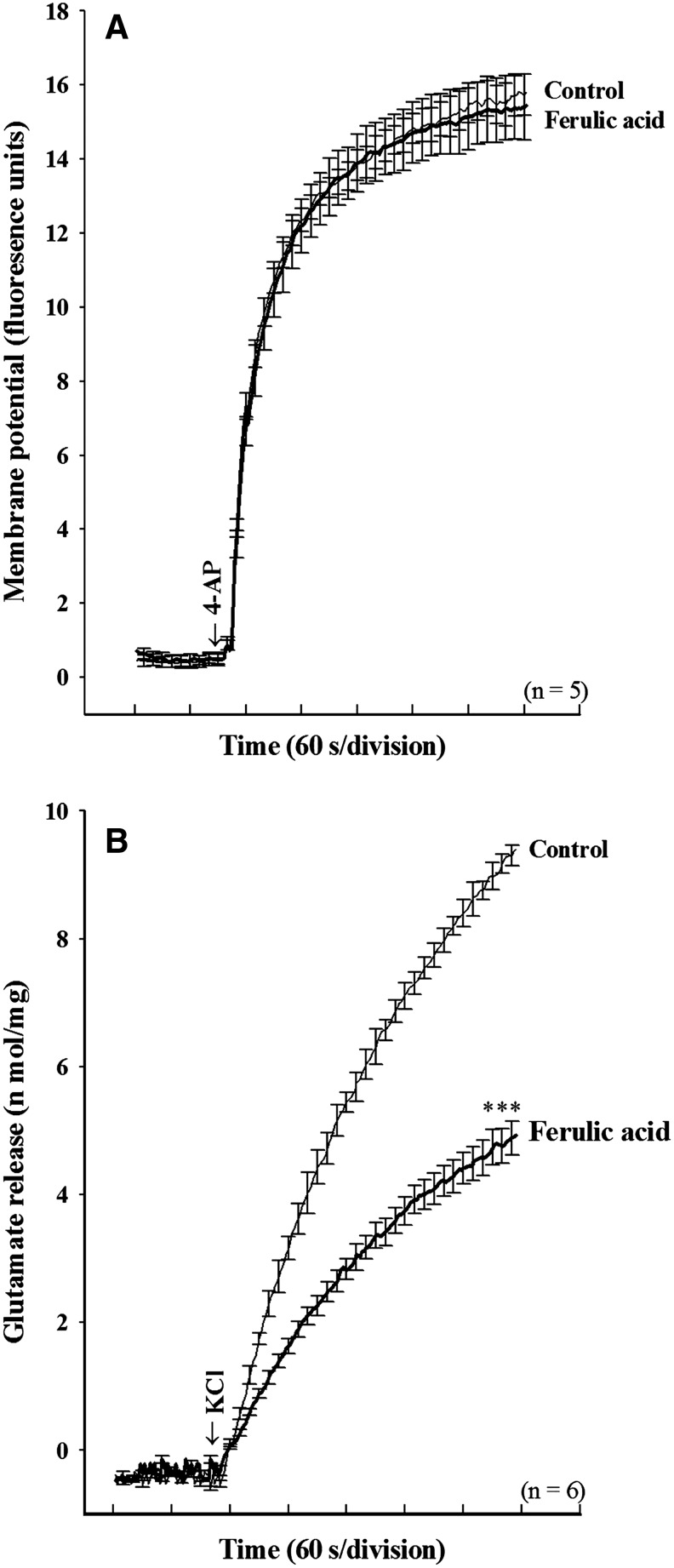
To further understand the mechanism responsible for the ferulic acid-mediated inhibition of glutamate release, we used a membrane-potential-sensitive dye, DiSC3(5), to determine the effect of ferulic acid on the synaptosomal plasma membrane potential. Fig. 3A shows that 4-AP (1 mM) caused an increase in DiSC3(5) fluorescence by 15.8±0.6 fluorescence units/5 min. Application of ferulic acid (50 μM) for 10 min before 4-AP addition did not alter the resting membrane potential, and produced no significant change in the 4-AP–mediated increase in DiSC3(5) fluorescence (15.5±0.9 fluorescence units/5 min; n=5). In addition, we confirmed the ferulic acid-mediated inhibition of glutamate release using an alternative secretagogue, high external [K+] (Fig. 3B). Elevated extracellular KCl depolarizes the plasma membrane by shifting the K+ equilibrium potential above the threshold potential for activation of voltage-dependent ion channels. Whereas Na+ channels are inactivated under these conditions, VDCCs are activated nonetheless to mediate Ca2+ entry, which supports neurotransmitter release.28 Addition of 15 mM KCl evoked controlled glutamate release of 9.4±0.2 nmol/mg over 5 min, which was decreased to 4.9±0.3 nmol/mg over 5 min in the presence of ferulic acid (50 μM; n=6; P<.01; Fig. 3B). This indicates that the observed inhibition of evoked glutamate release by ferulic acid is unlikely to have been caused by a hyperpolarizing effect of the drug on the synaptosomal plasma membrane potential, or attenuation of the depolarization produced by 4-AP.
FIG. 3.
Ferulic acid does not alter the synaptosomal membrane potential. (A) Synaptosomal membrane potential monitored with 3′,3′,3′-dipropylthiadicarbocyanine iodide [DiSC3(5)] in the absence (control) and in the presence of 50 μM ferulic acid, added 10 min before depolarization with 1 mM 4-AP. (B) Glutamate release was induced by 15 mM KCl in the absence (control) or presence of 50 μM ferulic acid, added 10 min before depolarization. Result are means±SEM of five to six independent experiments. ***P<.001, unpaired Student's t-test.
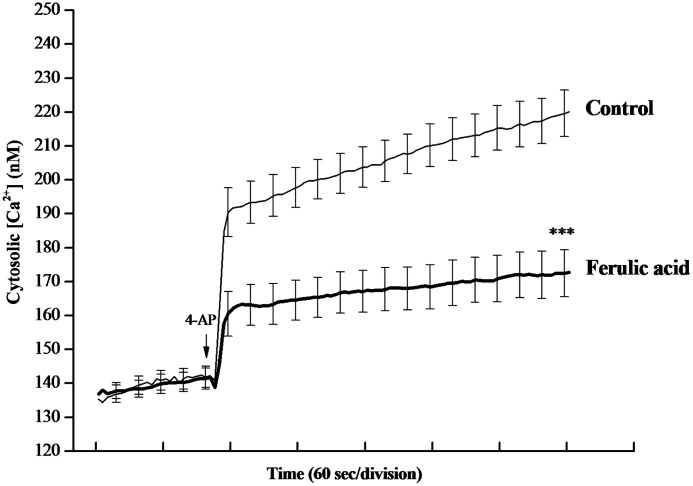
Downstream of membrane depolarization, presynaptic inhibition of neurotransmitter release can be mediated by a lowering of intraterminal Ca2+ levels. To investigate whether a decrease in [Ca2+]C was responsible for the ferulic acid-mediated inhibition of release, we used a Ca2+ indicator Fura-2 to monitor intraterminal Ca2+ levels directly. In Figure 4, after addition of 4-AP (1 mM), [Ca2+]C in synaptosomes was elevated to a plateau level of 219.5±6.8 nM. This 4-AP–evoked rise in [Ca2+]c was decreased by application of ferulic acid (50 μM) to 172.4±6.9 nM (n=6; P<.001).
FIG. 4.
Ferulic acid reduces the 4-AP–induced increase in cytosolic Ca2+ concentration ([Ca2+]C). Cytosolic-free [Ca2+]C (nM) was monitored using Fura-2. Synaptosomes were stimulated with 1 mM 4-AP in the absence (control) and in the presence of 50 μM ferulic acid, added 10 min before depolarization. Results are mean±SEM of six independent experiments. ***P<.001, unpaired Student's t-test.
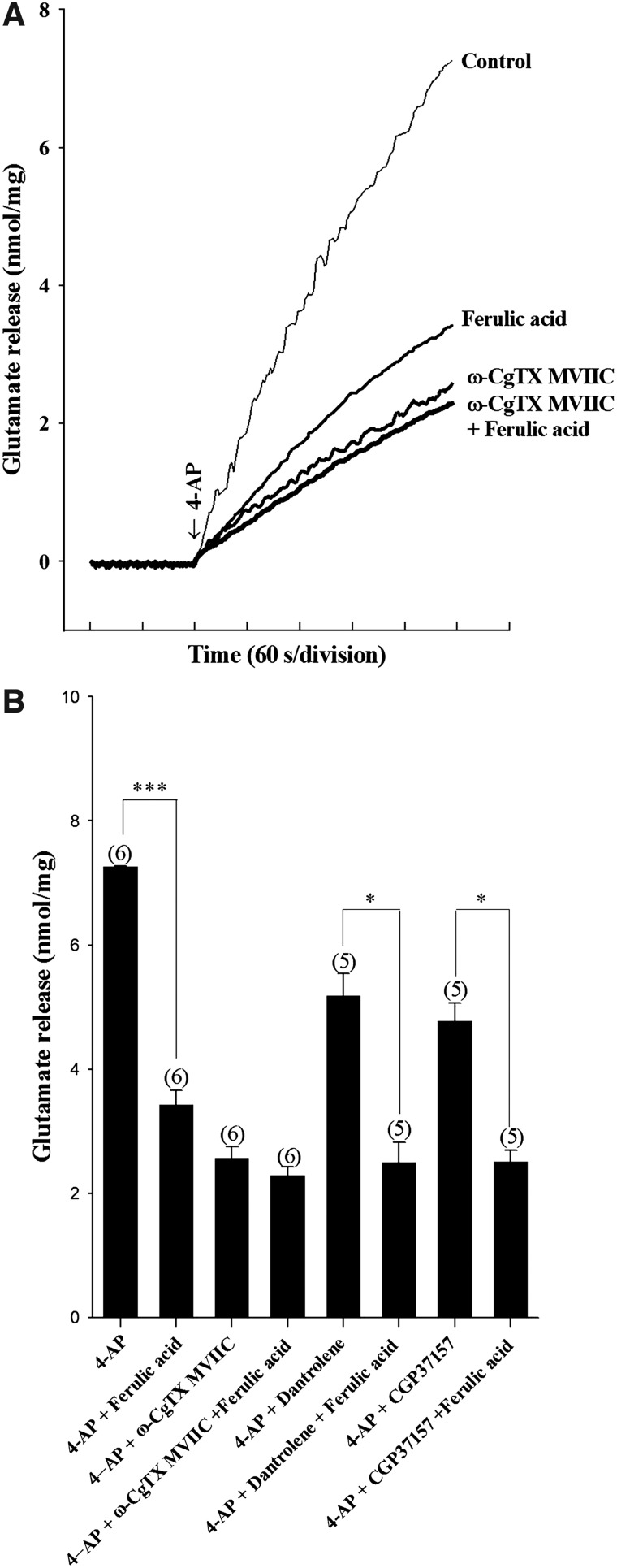
In the adult rat cerebrocortical nerve terminal preparation, the release of glutamate evoked by depolarization is reported to be caused by Ca2+ influx through Cav2.2 (N-type) and Cav2.1 (P/Q-type) channels and Ca2+ release from internal stores, such as endoplasmic reticulum (ER) and mitochondria.29–33 To assess the role of Cav2.2 and Cav2.1 channels, synaptosomes were preincubated with 2 μM ω-conotoxin MVIIC (ω-CgTX MVIIC), a wide-spectrum blocker of Cav2.2 and Cav2.1 channels. As shown in Figure 5A, glutamate release evoked by 1 mM 4-AP under control conditions was significantly decreased in the presence of 2 μM ω-CgTX MVIIC alone or 50 μM ferulic acid alone (P<.001). When ω-CgTX MVIIC (2 μM) and ferulic acid (50 μM) were applied simultaneously, the inhibition of glutamate release following 4-AP depolarization was not significantly different from the effect of ω-CgTX MVIIC alone (n=6; Fig. 5A, B). On the other hand, a potential role of intracellular Ca2+ release in ferulic acid-mediated inhibition of glutamate release was tested in the presence of dantrolene, an inhibitor of intracellular Ca2+ release from ER, and CGP37157, a membrane-permeant blocker of mitochondrial Na+/Ca2+ exchange. Dantrolene (100 μM) reduced control 4-AP–evoked release (P<.01; n=5). In the presence of dantrolene, however, ferulic acid (50 μM) still effectively inhibited 4-AP–evoked glutamate release (P<.05; Fig. 5B). Similarly to dantrolene, CGP37157 (100 μM) decreased the release of glutamate evoked by 4-AP (1 mM; P<.01), but it had no effect on the ferulic acid-mediated inhibition of 4-AP–evoked glutamate release (n=5; Fig. 5B).
FIG. 5.
Ferulic acid-mediated inhibition of 4-AP–evoked glutamate release is abolished by Cav2.2 and Cav2.1 channels blockade. (A) Glutamate release was evoked by 1 mM 4-AP in the absence (control) or presence of 50 μM ferulic acid, 2 μM ω-conotoxin MVIIC (ω-CgTX MVIIC), 2 μM ω-CgTX MVIIC, and 50 μM ferulic acid. (B) Quantitative comparison of the extent of glutamate release by 1 mM 4-AP in the absence or presence of 50 μM ferulic acid, and absence and presence of 2 μM ω-CgTX MVIIC, 100 μM dantrolene, or 100 μM 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157). Ferulic acid was added 10 min before depolarization, whereas the other drugs were added 30 min before depolarization. Results are mean±SEM of five to six independent experiments. *P<.05, one-way ANOVA. ***P<.001, unpaired Student's t-test.
Discussion
In this work, isolated cerebrocortical nerve terminals (synaptosomes) retaining the morphological and functional characteristics of in vivo nerve terminals21 were used to obtain clear evidence that ferulic acid inhibits the depolarization-evoked release of glutamate. To the best of our knowledge, this study presents the first examination of the effect of ferulic acid on endogenous glutamate release at the presynaptic level. Several possible mechanisms for this effect are discussed as follows.
Neurotransmitter release is a complex phenomenon and can be modulated at several putative sites in the nerve terminal, including Na+ channels, K+ channels, Ca2+ channels, and the release process itself.12,34,35 Therefore, when addressing the mechanism responsible for the ferulic acid-mediated inhibition of Ca2+-dependent glutamate release, this study considered two scenarios that might be involved: (1) alteration of the synaptosomal plasma membrane potential and downstream modulation of Ca2+ influx into the terminal, and (2) direct regulation of VDCCs affecting Ca2+ entry. The first possibility is unlikely for three reasons. First, 4-AP– versus KCl-evoked glutamate release is significantly inhibited by ferulic acid. 4-AP–mediated depolarization involves upstream K+ and Na+ channel activity, 15 mM external KCl affects experimental clamping of the K+ electrochemical potential, and hence, the membrane potential.36 The latter type of depolarization bypasses regulation at the level of K+ channels and also effectively removes voltage-dependent Na+ involvement, in that, these channels are desensitized in response to strong and continued depolarization.28 Based on the mechanistic differences between the 4-AP– and KCl-mediated depolarization discussed above, it is unlikely that Na+ channels are involved in the ferulic acid modulation of glutamate release. Second, no significant effect of ferulic acid on synaptosomal plasma membrane potential appears either in the resting condition or upon depolarization with 4-AP (indicating a lack of effect on K+ conductance). Third, ferulic acid did not affect the 4-AP–evoked Ca2+-independent glutamate release, which depends only on the membrane potential.37 This indicates that ferulic acid does not affect glutamate release by reversing the direction of the plasma membrane glutamate transporter. The glutamate transporter inhibitor DL-TBOA failed to affect the ferulic acid inhibition of 4-AP–evoked glutamate release, supporting this suggestion. Accordingly, these results clearly suggest that the ferulic acid-mediated inhibition of 4-AP–evoked glutamate release is mediated by a decrease in the Ca2+-dependent exocytotic component of glutamate release. Moreover, this phenomenon is not because of a decrease in synaptosomal excitability caused by the modulation of Na+ or K+ ion channels.
If it is not the modulation of synaptosomal excitability, then the locus of action of ferulic acid must lie further downstream in the stimulus–exocytosis coupling cascade. Using Fura-2, this study demonstrated that ferulic acid significantly decreases the 4-AP–evoked increase in [Ca2+]C. Furthermore, the inhibition of glutamate release by ferulic acid is highly sensitive to blockade of the Cav2.2 and Cav2.1 channels, which are known to directly participate in triggering glutamate release from synaptosomes.30–33 In addition to VDCCs, however, intracellular stores of Ca2+ released through the ER ryanodine receptors and mitochondria may be responsible for [Ca2+]C increase following depolarization.29 This possibility was eliminated by the observation that the inhibitory effect of ferulic acid on 4-AP–evoked glutamate release was not affected by the ER ryanodine receptor inhibitor dantrolene, and by the mitochondrial Na+/Ca2+ exchange inhibitor CGP37157. Although there is no direct evidence that ferulic acid acts on presynaptic Ca2+ channels, these results imply that the inhibition of glutamate release by ferulic acid occurs primarily through the suppression of Ca2+ influx through Cav2.2 and Cav2.1 channels. However, the blockade of Cav2.2 and Cav2.1 channel activity did not completely eliminate the inhibitory effect of ferulic acid on the 4-AP–evoked glutamate release (∼6% of the activity remained), raising the possibility that the Cav2.2- and Cav2.1-resistant Ca2+ channel types are also involved in the action of ferulic acid.
Ferulic acid is widely found in fruits, vegetables, cereals, and coffee. Consumption of these foods may result in ∼150–250 mg/day of FA intake.38 Ferulic acid can cross the blood–brain barrier and enter the brain by rapid kinetics.39 Studies have shown that ferulic acid improves memory deficits and brain damage induced by a variety of toxins, including glutamate and Aβ1–42.7,10,40 Although the exact mechanisms responsible for the neuroprotective effect of ferulic acid remain to be elucidated, the possible involvement of scavenging free radicals or antioxidant properties has been reported.3,41,42 In this work, the ability of ferulic acid to depress glutamate release is of particular significance and presents an additional explanation for the neuroprotective effect of ferulic acid in several neuronal injuries, especially in glutamate-induced neurotoxicity; this is because excessive glutamate release has been proposed to be involved in the pathophysiology of several neurological states, including ischemic brain damage and neurodegenerative diseases.43,44 On the other hand, the concentration of ferulic acid used to inhibit glutamate release in our study in vitro (50 μM) is high, but the action of ferulic acid is specific. The observation supporting this statement revealed the following: (1) the effect of ferulic acid on the evoked glutamate release was prevented by chelating the extracellular Ca2+ ions, but was insensitive to the glutamate transporter inhibitor; (2) ferulic acid decreased the depolarization-induced increase in [Ca2+]C, whereas it did not alter 4-AP–mediated depolarization; and (3) ferulic acid-mediated inhibition of glutamate release was abolished by the N-, P-, and Q-type Ca2+ channel blocker, but not by the ryanodine receptor blocker, or the mitochondrial Na+/Ca2+ exchanger blocker.
In conclusion, the results described in this report demonstrate that ferulic acid inhibits glutamate release from cerebrocortical synaptosomes via a suppression of voltage-dependent Ca2+ entry, supporting the hypothesis that ferulic acid may have efficacy as a brain therapeutic agent against excitotoxicity, but the relevance of our finding to in vivo clinical situations remains to be determined. However, this finding may provide further understanding of the mode of ferulic acid action in the brain, thereby emphasizing the therapeutic potential of this compound in treating neurodegenerative disease.
Acknowledgment
This work was supported by grants from the National Science Council (NSC 99-2628-B-030-001-MY3 and NSC 100-2320-B-030-006-MY3), Taiwan.
Author Disclosure Statement
The authors affirm that no competing financial interests exist.
References
- 1.Gohil KJ. Kshirsagar SB. Sahane RS. Ferulic acid-A comprehesive pharmacology of an important bioflavonoid. IJPSR. 2012;3:700–710. [Google Scholar]
- 2.Mohmmad Abdul H. Butterfield DA. Protection against amyloid beta-peptide (1–42)-induced loss of phospholipid asymmetry in synaptosomal membranes by tricyclodecan-9-xanthogenate (D609) and ferulic acid ethyl ester: implications for Alzheimer's disease. Biochim Biophys Acta. 2005;1741:140–148. doi: 10.1016/j.bbadis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Sultana R. Ravagna A. Mohmmad-Abdul H. Calabrese V. Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1–42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 4.Picone P. Bondi ML. Montana G. Bruno A. Pitarresi G. Giammona G. Di Carlo M. Ferulic acid inhibits oxidative stress and cell death induced by Aβ oligomers: improved delivery by solid lipid nanoparticles. Free Radic Res. 2009;43:1133–1145. doi: 10.1080/10715760903214454. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z. Wei T. Hou J. Li G. Yu S. Xin W. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur J Pharmacol. 2003;467:41–47. doi: 10.1016/s0014-2999(03)01597-8. [DOI] [PubMed] [Google Scholar]
- 6.Joshi G. Perluigi M. Sultana R. Agrippino R. Calabrese V. Butterfield DA. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane) dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem Int. 2006;48:318–327. doi: 10.1016/j.neuint.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Yu L. Zhang Y. Ma R. Bao L. Fang J. Yu T. Potent protection of ferulic acid against excitotoxic effects of maternal intragastric administration of monosodium glutamate at a late stage of pregnancy on developing mouse fetal brain. Eur Neuropsychopharmacol. 2006;16:170–177. doi: 10.1016/j.euroneuro.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CY. Su SY. Tang NY. Ho TY. Chiang SY. Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y. Guan Y. Xu Y. Li Y. Wu W. Sodium ferulate combined with bone marrow stromal cell treatment ameliorating rat brain ischemic injury after stroke. Brain Res. 2012;1450:157–165. doi: 10.1016/j.brainres.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 10.Yan JJ. Cho JY. Kim HS. Kim KL. Jung JS. Huh SO. Suh HW. Kim YH. Song DK. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol. 2001;133:89–96. doi: 10.1038/sj.bjp.0704047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 12.Greenamyre JT. Porter RH. Anatomy and physiology of glutamate in the CNS. Neurology. 1994;44:S7–S13. [PubMed] [Google Scholar]
- 13.Coyle JT. Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 14.Schinder AF. Olson EC. Spitzer NC. Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massieu L. Garcia O. The role of excitotoxicity and metabolic failure in the pathogenesis of neurological disorders. Neurobiology. 1998;6:99–108. [PubMed] [Google Scholar]
- 16.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 17.Raiteri L. Stigliani S. Zappettini S. Mercuri NB. Raiteri M. Bonanno G. Excessive and precocious glutamate release in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology. 2004;46:782–792. doi: 10.1016/j.neuropharm.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Klein J. Mdzinarishvili A. Sambria RK. Lang D. Ginkgo extract EGb761 confers neuroprotection by reduction of glutamate release in ischemic brain. J Pharm Pharm Sci. 2012;15:94–102. doi: 10.18433/j3ps37. [DOI] [PubMed] [Google Scholar]
- 19.Niebroj-Dobosz I. Janik P. Kwieciński H. Effect of Riluzole on serum amino acids in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2002;106:39–43. doi: 10.1034/j.1600-0404.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang SJ. Wang KY. Wang WC. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) Neuroscience. 2004;125:191–201. doi: 10.1016/j.neuroscience.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Dunkley PR. Jarvie PE. Heath JW. Kidd GJ. Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- 22.Lin TY. Lu CW. Wang SJ. Astaxanthin inhibits glutamate release in rat cerebral cortex nerve terminals via suppression of voltage-dependent Ca2+ entry and mitogen-activated protein kinase signaling pathway. J Agric Food Chem. 2010;58:8271–8278. doi: 10.1021/jf101689t. [DOI] [PubMed] [Google Scholar]
- 23.Yang TT. Wang SJ. Pyridoxine inhibits depolarization-evoked glutamate release in nerve terminals from rat cerebral cortex: a possible neuroprotective mechanism? J Pharmacol Exp Ther. 2009;331:244–253. doi: 10.1124/jpet.109.155176. [DOI] [PubMed] [Google Scholar]
- 24.Akerman KE. Scott IG. Heikkila JE. Heinonen E. Ionic dependence of membrane potential and glutamate receptor-linked responses in synaptoneurosomes as measured with a cyanine dye, DiSC3(5) J Neurochem. 1987;48:552–559. doi: 10.1111/j.1471-4159.1987.tb04128.x. [DOI] [PubMed] [Google Scholar]
- 25.Sihra TS. Bogonez E. Nicholls DG. Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release. J Biol Chem. 1992;267:1983–1989. [PubMed] [Google Scholar]
- 26.Grynkiewicz G. Poenie M. Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 27.Tibbs GR. Barrie AP. Van Mieghem FJ. McMahon HT. Nicholls DG. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: effects on cytosolic free Ca2+ and glutamate release. J Neurochem. 1989;53:1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- 28.Barrie AP. Nicholls DG. Sanchez-Prieto J. Sihra TS. An ion channel locus for the protein kinase C potentiation of transmitter glutamate release from guinea pig cerebrocortical synaptosomes. J Neurochem. 1991;57:1398–1404. doi: 10.1111/j.1471-4159.1991.tb08306.x. [DOI] [PubMed] [Google Scholar]
- 29.Berridge M J. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 30.Millan C. Sanchez-Prieto J. Differential coupling of N and P/Q type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci Lett. 2002;330:29–32. doi: 10.1016/s0304-3940(02)00719-x. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez E. Sanchez-Prieto J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur J Neurosc. 1997;9:2009–2018. doi: 10.1111/j.1460-9568.1997.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 32.Turner TJ. Adams ME. Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leenders AG. van den Maagdenberg AM. Lopes da Silva FH. Sheng ZH. Molenaar PC. Ghijsen WE. Neurotransmitter release from tottering mice nerve terminals with reduced expression of mutated P- and Q-type Ca2+ channels. Eur J Neurosci. 2002;15:13–18. doi: 10.1046/j.0953-816x.2001.01839.x. [DOI] [PubMed] [Google Scholar]
- 34.Sihra TS. Nichols RA. Mechanisms in the regulation of neurotransmitter release from brain nerve terminals: current hypotheses. Neurochem Res. 1993;18:47–58. doi: 10.1007/BF00966922. [DOI] [PubMed] [Google Scholar]
- 35.Thompson SM. Capogna M. Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls DG. Presynaptic modulation of glutamate release. Prog Brain Res. 1998;116:15–22. doi: 10.1016/s0079-6123(08)60427-6. [DOI] [PubMed] [Google Scholar]
- 37.Attwell D. Barbour B. Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;375:645–653. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z. Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109:691–702. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 39.Chang MX. Xu LY. Tao JS. Researchs on the matabolism and pharmacokinetics of Ferulic acid in rats. Chin Pharm J. 1993;18:300–300. [PubMed] [Google Scholar]
- 40.Hsieh MT. Tsai FH. Lin YC. Wang WH. Wu CR. Effects of ferulic acid on the impairment of inhibitory avoidance performance in rats. Planta Med. 2002;68:754–756. doi: 10.1055/s-2002-33800. [DOI] [PubMed] [Google Scholar]
- 41.Cheng CY. Ho TY. Lee EJ. Su SY. Tang NY. Hsieh CL. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008;36:1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 42.Kanski J. Aksenova M. Stoyanova A. Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J Nutr Biochem. 2002;13:273–281. doi: 10.1016/s0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 43.Choi DW. Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 44.Meldrum B. Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]