Abstract
Objectives
Emerging research is revealing that direct physical contact of the human body with the surface of the earth (grounding or earthing) has intriguing effects on human physiology and health, including beneficial effects on various cardiovascular risk factors. This study examined effects of 2 hours of grounding on the electrical charge (zeta potential) on red blood cells (RBCs) and the effects on the extent of RBC clumping.
Design/interventions
Subjects were grounded with conductive patches on the soles of their feet and palms of their hands. Wires connected the patches to a stainless-steel rod inserted in the earth outdoors. Small fingertip pinprick blood samples were placed on microscope slides and an electric field was applied to them. Electrophoretic mobility of the RBCs was determined by measuring terminal velocities of the cells in video recordings taken through a microscope. RBC aggregation was measured by counting the numbers of clustered cells in each sample.
Settings/location
Each subject sat in a comfortable reclining chair in a soundproof experiment room with the lights dimmed or off.
Subjects
Ten (10) healthy adult subjects were recruited by word-of-mouth.
Results
Earthing or grounding increased zeta potentials in all samples by an average of 2.70 and significantly reduced RBC aggregation.
Conclusions
Grounding increases the surface charge on RBCs and thereby reduces blood viscosity and clumping. Grounding appears to be one of the simplest and yet most profound interventions for helping reduce cardiovascular risk and cardiovascular events.
“Erythrocytes have a strong net negative charge called the zeta potential produced by the scialoglycoprotein coat such that approximately 18 nm is the shortest span between two cells.”
—Wintrobe's Clinical Hematology1
Introduction
Cardiovascular disease (CVD) is a leading cause of death worldwide. The latest statistics (2009) for the United States show that CVD is the leading cause of death for persons age 65 and over.2 Interventions that reduce the incidence of CVD are therefore of profound importance. Blood viscosity and aggregation are major factors in hypertension and other cardiovascular pathologies, including myocardial infarction. Cardiologists are gradually losing interest in low-density lipoprotein (LDL) cholesterol as the major cardiovascular risk factor.3 From the perspective of the health care practitioner, it is essential to have a better understanding of the relationships between other well-documented factors in CVD, including blood viscosity, blood pressure (BP), peripheral resistance, coagulation, left-ventricular hypertrophy, and inflammation.
Blood is a complex fluid containing a variety of formed elements (cells), proteins, nutrients, and metabolic waste products, along with dozens of clotting factors. In spite of this complexity, measurement of the electrophoretic mobility or zeta potential of red blood cells (RBCs) is a simple method for measuring blood viscosity.4–8 This is because blood viscosity is strongly influenced by the RBC surface charge that governs the spacing between erythrocytes. A higher repulsive surface charge increases spacing between erythrocytes, reduces clumping, lowers viscosity, and lowers peripheral resistance to flow.9 Conditions that reduce RBC surface charge correlate with occlusive arterial disease because of a higher incidence of RBC aggregation.5 It is accepted that blood viscosity and resistance to blood flow are related and are elevated in patients who have hypertension.10–12 Total resistance is the product of vascular resistance and viscosity. Small changes in viscosity produce large differences in total resistance,13 especially in peripheral vessels <30 μm in diameter, in which the relative effective viscosity can increase six- to sevenfold.14 These results confirm the existence of a blood hyperviscosity syndrome in hypertension. Positive correlations in rheologic variables with arterial pressure and with indices of left-ventricular hypertrophy suggest that these changes may be involved in the pathophysiology of hypertension and its serious complications.15,16
The electrophoretic mobility or zeta potential can be measured by determining the mobility of RBCs in an imposed electric field. The classic text on zeta potential is Control of Colloid Stability Through Zeta Potential (with a closing chapter on its relationship to CVD by Riddick).4 Riddick's perspectives on CVD are important but have not been widely recognized, probably because rheology is a highly specialized and interdisciplinary subject. Moreover, blood is a very complex material, and many variables affect its ability to carry oxygen, nutrients, and metabolic waste products.
In this report the terms earthing and grounding are used interchangeably. The branch of physics known as electrostatics teaches that, when two conductive objects with different electrical potential touch each other, there is a virtually instantaneous transfer of charge so that the two objects equilibrate to the same electrical potential. The human body is a conductor of electricity17 and so is earth (soil), except in very dry areas such as deserts. Consequently, grounding leads to rapid equalization of the electrical potential of the body with the potential of the Earth (planet) through an almost instantaneous transfer of electrons from soil to the body.18,19 This has been the natural bioelectrical environment of the human body and of other organisms throughout most of evolutionary history.
Given that earthing or grounding alters many electrical properties of the body,18–21 it was logical to evaluate an electrical property of the blood. The goal was to find if grounding affects RBC zeta potential and RBC aggregation in an ordinary office environment. The results show that grounding the body to soil increases the zeta potential and thereby decreases aggregation of RBCs.
Materials and Methods
Subjects
Ten (10) healthy subjects were screened using the Health History Inventory.22 Each subject had one grounding session. Table 1 details age and gender distribution of subjects; Table 2 documents their pain levels before and after each session, as well as medications and general health condition of each subject. Informed consent was obtained from all subjects prior to their participation. The Biomedical Research Institute of America provided institutional review board supervision of this project (www.biomedirb.com). The McGill Pain Questionnaire (MPQ) was used to evaluate the level and location of pain before and after grounding sessions.23
Table 1.
Subjects' Age and Gender Distribution
| Subject # | Age | Gender | Age Men | Age Women |
|---|---|---|---|---|
| 1 | 61 | M | 61 | |
| 2 | 62 | F | 62 | |
| 3 | 47 | F | 47 | |
| 4 | 61 | M | 61 | |
| 5 | 56 | M | 56 | |
| 6 | 42 | M | 42 | |
| 7 | 63 | F | 63 | |
| 8 | 45 | F | 45 | |
| 9 | 55 | F | 55 | |
| 10 | 57 | F | 57 | |
| Average: | 54.9 | 55.0 | 54.8 | |
| SD: | 7.6 | 9.0 | 7.5 |
SD, standard deviation.
Table 2.
Pain, Medication and Health Condition of Each Subject
| Subject # | Pain before | Pain after | Medication | Self-described health condition and exercise |
|---|---|---|---|---|
| 1 | No pain | No pain | None | No health complaint |
| (0) | (0) | Does fast walking on the beach 4×/wk, strength training 3×/wk, swims | ||
| 3×/wk & goes to an athletic club 4×/wk | ||||
| 2 | No pain | No pain | None | Experiences extreme heat sometimes in ankles & lower extremities |
| (0) | (0) | Had heart murmur as a child | ||
| Does running, cycling, & rowing; uses elliptic machine; swims 3-4×/wk 20 min to 1 hr; walks on the beach often; goes to a club; & trains outdoors | ||||
| 3 | No pain | No pain | None | No health complaint |
| (0) | (0) | Had 5 children & eats only raw food | ||
| Runs 3 days/wk and does yoga 2 days/wk outdoors & at home | ||||
| 4 | No pain | No pain | None | Contractor with no physical problems |
| (0) | (0) | Likes surfing & fishing; indulges in sugar & sodas moderately | ||
| 5 | Low back pain (5–6) | No pain (0) | Voltaren 0.5 g/day | Real estate manager & handyman has lower back pain when stressed |
| Surfs daily & likes hiking | ||||
| 6 | No pain | No pain | Ibuprofen | Did not mention why he takes ibuprofen |
| (0) | (0) | 800 mg once/wk | Does cycling 2×/wk in neighborhood | |
| 7 | Middle back pain | Middle back pain | Nature-Throid | Has knee osteoarthritis, Lyme disease & candidiasis Likes swimming but stopped 4 weeks before the study because of knee injury |
| (3) | (0.5) | 0.5 g/d | ||
| 8 | No pain | No pain | None | No physical problem to report |
| (0) | (0) | Does brisk walking ∼30 min at home & outdoors | ||
| Very conscious about food & eats only healthy fats from vegetables | ||||
| 9 | Neck/shoulder (1) | No pain | None | Experienced arrhythmia 2 years ago that was immediately corrected with potassium supplementation |
| Numness in arms+thumbs (1) | (0) | Experiences shortness of breath with activity Does not exercise but eats as much high-quality fat her body accepts | ||
| 10 | No pain | No pain | None | Is seeing a chiropractor for a stiff & sore neck |
| (0) | (0) | Does not exercise currently | ||
| Does meditation in groups & takes a singing class |
Numbers in parenthesis () represent level of pain: 0=no pain, 10=intolerable pain.
wk, week; d, day; min, minutes; hr, hour.
Exclusion criteria were: (1) pregnancy; (2) age <18 or >80; (3) taking pain, anti-inflammatory medications, sedatives, or prescription sleeping medications (<5 days prior to testing); (4) taking psychotropic drugs or diagnosis with a mental disorder; (5) recent surgery (<1 year); (6) documented life-threatening disease (such as cancer, AIDS, etc.); (7) consumption of alcohol within 48 hours of participation; and (8) use of recreational drugs. Subjects were recruited by word-of-mouth.
Grounding system
Four (4) transcutaneous electrical nerve stimulation (TENS) type conductive patches were placed on the soles of each subject's feet and on each subject's palms. Wires from a standard electrostatic discharge ground system were snap-attached to the patches and connected to a box (Fig. 1). The grounding system consisted of a 300″- long (91.44 m) ground cord attached to the box on one end and to a 12′ (30.48 cm) stainless-steel rod inserted in the soil outdoors at the other end. Another parallel cord was used to check the status of the connection with the ground. The ground cord contained an Underwriters Laboratories (UL) approved 10 milliamp fuse.*
FIG. 1.

Grounding system showing patches, wires, and box connecting to a ground rod planted outside through a switch (not shown) and a fuse (not shown). Similar patches and wires from the hands were also connected to the box to ground the hands.
Experimental setup
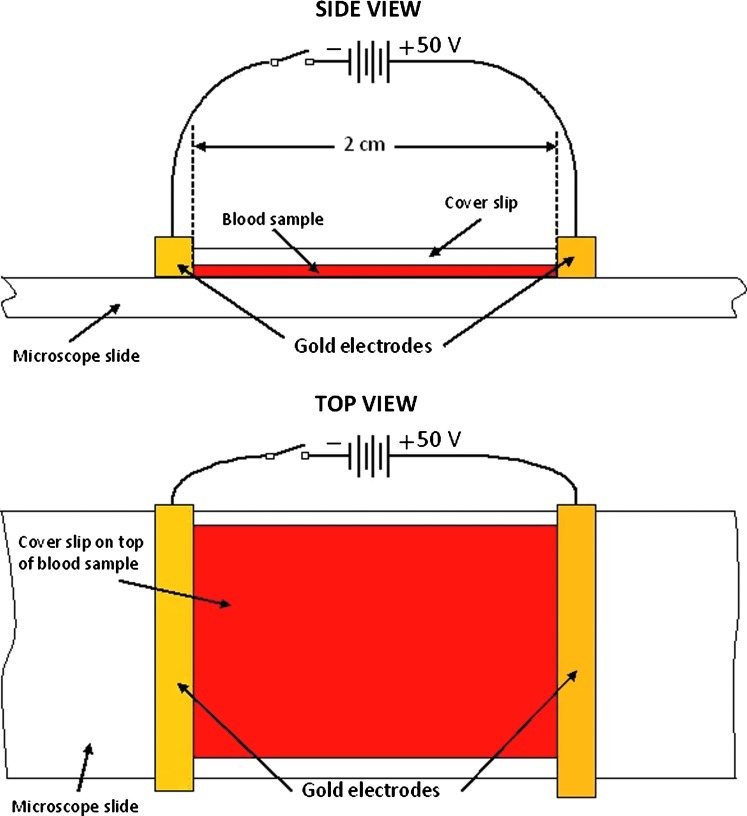
Standard microscope slides (75 mm×25 mm, 1-mm thick) and cover slips (20 mm×20 mm, or 22 mm×22 mm, ∼0.2-mm thick) were used. The electrode system consisted of 2 gold bars (2.0 mm×2.0 mm square cross-section and 5.0 cm in length) placed directly on the microscope slide at the sides of the cover slip (Fig. 2). The gold bars were connected to two 9-volt batteries in series. A switch controlled the application of the electric field. The field between the electrodes ranged from 14.3 volts/cm to 28.0 volts/cm (mean±standard deviation [SD]=23.1±3.7 volts/cm).24
FIG. 2.
Side and top views of the experimental setup for zeta potential measurement.
For each sample, a drop of solution containing minerals and trace elements in the same proportions as they occur in blood serum (Quinton Isotonic Water) was added to the drop of blood to decrease RBC concentration and to prevent electroendosmosis from affecting the RBCs' mobility. The proportion was 20% blood to isotonic solution. A cover slip was then placed over the sample and the gold bars moved into position. A drop of isotonic solution was added on each side of the cover slip to insure conductive contact between the gold electrodes and the diluted blood sample. A video camera mounted on a darkfield microscope (Richardson RTM-3.0; combined magnification factor of 1000) recorded the movement of the RBCs. Observations were made for a few minutes, which was enough time to record the RBCs' terminal velocities for a period of at least 10 seconds at 3 different locations. A micrometer stage allowed for moving the sample to find areas with appropriate RBC density for zeta potential and aggregation measurement. When a suitable area was located, the power to the gold bars was switched on. Suitable areas had a low enough RBC density that most of the RBCs could move about freely without collision for at least 10 seconds. Three separate measurements were made at each of 3 different such areas, yielding a total of 9 measurements on each sample. The video images were recorded on digital video discs for subsequent determination of velocity of RBC migration.
Zeta potential (ζ) and RBC aggregation measurements
The zeta potential (ζ) of RBCs maintains the fluidity of blood by preventing RBC aggregation.12,25,26 The combination of zeta potential and aggregation are important determinants of blood viscosity.
For zeta potential calculations the Smoluchowski equation was used as follows27:
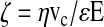 |
where η is the solution's viscosity, vc is the terminal velocity of the RBCs, ɛ is the electrical permittivity of the solution, and E is the electric field to which the RBCs were submitted.
The electric field was calculated from the electric potential and the distance between electrodes. The terminal velocities of the RBCs were measured directly from the recordings by clocking the time it took for an RBC to go through a predetermined distance (the stopwatch used had a precision of 0.01 second). In the Smoluchowski equation, the remaining parameters were taken to be:
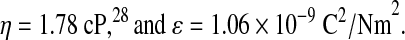 |
With these values and the electrode system previously described, zeta potentials were obtained for healthy persons in good agreement with the normal range, according to Fontes (between −9.30 mV and −15.0 mV with an average of −12.5 mV).27
To measure RBC aggregation, the stage of the darkfield microscope was moved step by step to observe the whole sample. Each move was followed by a brief pause of 1 second. The goal was to determine which locations had an appropriate RBC-cluster density for counting the clusters. For each blood sample, six locations with relatively similar RBC-cluster density (∼25%–50% of the area seen through the microscope's objective covered with RBCs) were randomly selected. For each location, a standardized area was used to count clusters. The area was a circle with a diameter of 100 μm (corresponding to a surface of 7854 μm2). Cell clusters were counted as follows: each individual cell was counted as a cluster of 1 cell; each pair of cells was counted as a cluster of 2 cells; each group of 3 cells was counted as a cluster of 3 cells; and so on up to 8 cells per cluster. Clusters of nine cells or more were counted together and put in one cluster group (the 9+ cells cluster group; in no case was more than 12 cells found in one cluster).
Experimental procedure and study design
After each subject's arrival, the study coordinator verified that the consent form was signed and that all the subject's questions were answered. The subject's responses to the Health History Inventory (HHI) were reviewed to check for compliance with respect to the exclusion criteria as well as to gather basic information regarding the subject's general health. Next, the questions in the McGill Pain Questionnaire (MPQ) were asked. Then two blood samples were taken from the subject. Because the amount of blood required was minimal (0.01 mL or 0.01 cm3), each sample was obtained by the finger-prick method. The subject was then asked to sit in a comfortable reclining chair in the soundproof experiment room with the lights dimmed or off, depending on the subject's level of comfort with darkness. After 2 hours, two more blood samples were drawn while the subject was still grounded.
Data analyses
Prior to applying statistical tests, each data set was checked for normality using Lillifors test for normality.29 Most of the data samples tested were found to satisfy the Lillifors test.† Statistical analyses were performed using the Student's t-test, using the statistical package of Microsoft Office Excel (2007 Microsoft Office System, version 12.0.6524.0). t-Tests were performed even when a data set showed moderate evidence against normality when compared to a data set conforming to normality. One reason for doing this is that the t-test is a robust method with respect to moderate departures from the hypothesis of homogeneity of variance.30 Another reason is that, as an exploratory pilot research project, it was felt that these results could be indicative of real differences if there were more datapoints. The t-test method could also provide useful information for investigators planning future research projects with a larger number of subjects. The common statistical level of significance □=0.05 was used throughout this article. When the Lillifors test showed strong evidence against normality, no t-tests were performed.
Results
Zeta potential
Table 3 shows RBC velocity and zeta potential (ζ) before and after grounding (earthing) for each of the 10 subjects. As explained previously, for each blood draw, RBC velocity was measured 9 times. Given that there were 2 blood draws before and 2 blood draws after a session (for a total of 4 blood draws per subject per session), each RBC velocity presented in Table 3 represents the average of 18 measurements. The average, SD and standard error of the mean (SEM) were computed between subjects. Thus, these statistical parameters reflected the distribution of velocities among subjects (which were consistent with a normal distribution according to the Lillifors test for normality). The zeta potentials in this table were computed using the Smoluchowski equation from the corresponding velocities (as previously explained). All subjects had an increase in the absolute value of zeta potential after 2 hours of grounding. The smallest absolute increase was by a factor of 1.27 and the largest was by a factor of 5.63. On average, the absolute value of zeta potential increased by a factor of 2.70 (a highly statistically significant result, as can be seen from the one-tailed t-test; this statistical test was used because an increase in the absolute value of zeta potential of ∼ 20%–30% was expected after grounding). This increase effectively brought the average zeta potential from a very small average value of −5.28 mV into a normal value (–14.3 mV). It seems that the healthier a subject was, the less significant the increase was (see Table 2 for subjects' health conditions).
Table 3.
Velocities and Zeta Potentials for the 10 Subjects
|
Subject |
Velocity (μm/s) |
Zeta Potential (mV) |
||||
|---|---|---|---|---|---|---|
| # | Before | During | Dur/Bef | Before | During | Dur/Bef |
| 1 | 11.9 | 29.2 | 2.46 | −7.96 | −19.6 | 2.46 |
| 2 | 3.65 | 13.6 | 3.73 | −2.45 | −9.14 | 3.73 |
| 3 | 9.36 | 11.6 | 1.24 | −5.62 | −7.12 | 1.27 |
| 4 | 12.1 | 21.6 | 1.79 | −7.29 | −13.6 | 1.86 |
| 5 | 9.46 | 20.8 | 2.20 | −5.87 | −13.0 | 2.22 |
| 6 | 5.78 | 32.0 | 5.53 | −3.61 | −20.3 | 5.63 |
| 7 | 11.8 | 42.7 | 3.61 | −7.40 | −26.8 | 3.63 |
| 8 | 7.42 | 24.4 | 3.29 | −4.66 | −15.4 | 3.30 |
| 9 | 5.26 | 11.4 | 2.16 | −4.14 | −8.96 | 2.16 |
| 10 | 4.80 | 10.7 | 2.23 | −3.80 | −8.50 | 2.24 |
| Total | 81.5 | 218 | −52.8 | −143 | ||
| Average | 8.15 | 21.8 | 2.68 | −5.28 | −14.3 | 2.70 |
| SD | 3.19 | 10.6 | 1.24 | 1.85 | 6.37 | 1.26 |
| SEM | 1.01 | 3.34 | 0.39 | 0.585 | 2.02 | 0.40 |
| t-test: | 5.63E-04 | 3.57E-04 | ||||
S, second; Dur/Bef, During Earthing divided by Before Earthing; SD; standard deviation; SEM; standard error of the mean.
RBC aggregation
With respect to RBC aggregation results for the 10 subjects, there were significantly more aggregates or (clusters) during grounding (after 2 hours of grounding while still grounded) than before grounding (p=0.0000153). This is because there were significantly more clusters with 1 or 2 cells after 2 hours of grounding (p=0.0000269 and p=0.000354, respectively), simultaneously significantly fewer clusters of 3 cells (p=0.0451), and far fewer clusters with 4+ cells (although no statistical evaluation was done for clusters with 4+ cells, the last column of Table 4 shows that the average number of cells during earthing, which was 15.0, was less than half the average number of cells before earthing which was 34.7, a ratio of 34.7/15.0=2.3>2.0). There was clearly less clumping after 2 hours of grounding than before grounding.
Table 4.
Cell Aggregate Results for the 10 Subjects
| |
|
|
|
Total number of cells in cluster |
|||
|---|---|---|---|---|---|---|---|
| Corrected to 100 cells (number of cells shown) | Total Clusters | Total Cells | 1 | 2 | 3 | 4+ | |
| Before | Average: | 49.5 | 100 | 26.8 | 21.4 | 17.1 | 34.7 |
| Earthing | SD | 5.53 | 0 | 5.90 | 2.96 | 1.68 | 3.14 |
| SEM | 1.60 | 0 | 1.70 | 0.855 | 0.485 | 0.370 | |
| During | Average | 64.5 | 100 | 43.1 | 26.4 | 15.5 | 15.0 |
| Earthing | SD | 2.93 | 0 | 3.99 | 1.35 | 0.731 | 2.55 |
| SEM | 0.845 | 0 | 1.15 | 0.391 | 0.211 | 0.301 | |
| During–Before | 15.0 | 0 | 16.3 | 4.92 | −1.59 | −19.6 | |
| Before vs. During | Clusters | 1 vs. 1 | 2 vs. 2 | 3 vs. 3 | 4 vs. 4 | ||
| t-tests | 1.53E-05 | 2.69E-05 | 3.54E-04 | 0.0451 | |||
SD, standard deviation; SEM, standard error of the mean.
Cell-cluster sizes were counted separately for clusters containing up to 8 cells (9 cells and above being grouped together); cell clusters with 4 or more cells were grouped together in Table 4 (column labeled “4+”). This was done because cell clusters of 4 to 9+ cells did not pass the Lillifors test, probably because there were too few RBC aggregates of this size. For each blood draw, the number of cell clusters for each cell cluster size was counted at 6 different locations under the microscope. Given that there were 2 blood draws before earthing and 2 after earthing, 12 counts were done for each cluster size per subject before earthing and 12 counts were done after 2 hours of earthing. The during earthing samples were taken while the subjects were still earthed. Because there were 10 subjects, each value presented in Table 4 for cluster sizes 1, 2, and 3, is the average of 120 cell-cluster counts for each size before and 120 after 2 hours of earthing. Because the column to the right shows the grouping of cluster sizes 4 to 9+, and each of these clusters sizes had 120 cell-cluster values, the average values presented in this column are the average of 720 cell cluster values. To ensure there was no statistical bias caused by a difference in number of RBCs forming all cluster sizes counted at one microscope objective location for one blood draw, when compared to another location for the same or another blood draw, the results presented in Table 4 were adjusted to the number of clusters per 100 cells counted. For example, the number of RBCs counted before earthing for clusters of 1, 2, 3, and 4+ RBCs was 100 (i.e., 26.8+21.4+17.1+34.7=100). The number of clusters of each size was determined by dividing the number of cells counted by the cluster size.
Pain
Most subjects came to the research premises with no pain (Table 2). Of the 3 subjects who reported that they had pain at the beginning of the session, 2 reported that they were pain-free after 2 hours of grounding. The other subject reported that her pain had nearly vanished by the end of the session.
Subjects # 5, 7, and 9 presented with pain. The zeta potentials (in mV) before and after 2 hours of grounding for subject #5 were 9.46 and 20.8, respectively (an increase by a factor of 20.8/9.46=2.20), for subject #7 they were 11.8 and 42.7, respectively (an increase by a factor of 3.61), and for subject #9 they were 5.26 and 11.4, respectively (an increase by a factor of 2.16), respectively. When combining the three subjects with pain, one obtains an average of 8.84 before grounding and 24.97 after 2 hours of grounding (an average increase by a factor of 2.82). The average zeta potentials before and after 2 hours of grounding for the 7 subjects with no pain were 7.85 before grounding and 20.45 after grounding, respectively (an average increase by a factor of 2.61), which shows less of an improvement. So it appears that the zeta potential of subjects with pain improved slightly more than the zeta potential of subjects with no pain. Interestingly, the subject with the largest increase in zeta potential after 2 hours of grounding, with a factor of 5.63 (subject #6), did not have pain upon arriving at the clinic to be tested. However, he indicated that he takes 800 mg of ibuprofen once a week. On the other hand, the subject with the lowest increase in zeta potential, with a factor of only 1.27 (subject #3), was perhaps the healthiest, eating only raw food, running 3 times per week, and doing yoga 2 times per week outdoors and at home.
Discussion
A number of clinical studies on the physiologic effects of grounding the human body have indicated improvements in various cardiovascular and heart-related parameters. One of the first investigations reported normalization of the day–night cortisol rhythms in subjects who were grounded by sleeping on a conductive mattress pad connected via a wire to a rod inserted into soil.31 It is known that chronic elevation of cortisol can result in disruption of circadian rhythms and chronic activation of the sympathetic nervous system, both of which can contribute to insomnia and its many well-documented health effects, including hypertension, CVD, stroke and other disorders.32,33
Subsequent research has repeatedly confirmed the positive effects of grounding on the autonomic nervous system (ANS), including increases in parasympathetic activity18,34 and, most recently, increases in heart rate variability (HRV).35 The significance of the latter study is that HRV is an important indicator of the status of autonomic balance and stress on the cardiovascular system. A decrease in HRV indicates autonomic dysfunction and is a predictor of the severity of progression of coronary artery disease.36,37 The positive effects of grounding on HRV suggest that simple grounding techniques can be utilized as a basic strategy for supporting the cardiovascular system, especially during situations of heightened autonomic tone and/or hypertension.35 The present study demonstrated a profound increase in zeta potential and a corresponding decrease in blood viscosity.
Magnets repel each other when the same poles come sufficiently close to one another. Similarly, electric charges of the same sign repel each other when they are in proximity to one another. The surface of RBCs has negative electrical charges that maintain spacing of the cells in the bloodstream by electrostatic repulsion. The electrophoretic mobility of RBCs is a function of net negative charge (zeta potential), provided that the viscosity of the suspending medium does not change during the measurement. In a study of 50 patients with occlusive arterial disease and 50 control counterparts (N=100), the migration time of red cells (seconds) was longer and the electrophoretic mobility (μsec/V/cm) was less in the patients with occlusive disease than in the healthy controls.5 This study on electrophoretic mobility suggested differences in RBC surface charge (zeta potential). The researchers concluded that patients with occlusive arterial disease have one or more factors in their plasma and RBCs that reduce the net negative charge (zeta potential) of the cells, thereby facilitating RBC aggregation.5 This finding supports the notion that there are definitely many factors that can reduce zeta potential, and thereby increase blood viscosity and increase RBC aggregation, both of which play a major role in the pathogenesis of arteriosclerosis.5 A meta-analysis evaluating the connection between blood viscosity and CVD demonstrates clearly that the risk of major cardiovascular events increase with higher blood-viscosity levels.38 In the Edinburgh Artery Study, a population of 4860 men 45–59 years of age was observed for 5 years. The 20% of the men with the highest blood viscosity had a 3.2 times greater risk for cardiac events, compared with the 20% of men with the lowest blood viscosity. Fifty-five percent (55%) of major cardiovascular events occurred in the highest blood-viscosity group versus only 4% in the lowest blood-viscosity group.39
The role of increased blood viscosity in the pathogenesis of occlusive arterial disease was clearly and succinctly described by Kensey.15 Endothelial dysfunction, mechanical shear forces, and alterations in blood flow mechanics at arterial bifurcations and areas of low blood flow eddies are correlated with plaque progression in the coronary vasculature. Similarly, blood viscosity is known to increase in a number of clinical situations, such as hypertension, smoking, lipid disorders, advancing age, and diabetes mellitus.
A 2008 study was the first to report on the zeta potential of red blood cells in patients with diabetes.6 Researchers from the University of Calcutta described a “remarkable alteration” in the electrodynamics of RBCs—a progressive deterioration of the zeta potential and hypercoagulability among patients with diabetes, which was even worse among those who also had CVD. The researchers also indicated that high blood sugar levels are associated with significant alterations in the electrodynamics of an RBC's outer membrane and may increase the potential for RBC clumping. It was concluded that zeta potential could and should be used as an indicator of cardiovascular disease in patients who have diabetes. 6
On the basis of a randomized placebo-controlled primary prevention trial (the West of Scotland Coronary Prevention Study), researchers suggested that pravastatin therapy may lower the risk for coronary heart disease and mortality partially by lowering both plasma viscosity and blood viscosity.40 Many subsequent investigations have demonstrated the pleiotropic effects of statins on blood rheology, including reductions in plasma viscosity,41 whole-blood viscosity, RBC deformities, and RBC aggregation.42
Grounding is the most desirable and suitable intervention for both reducing blood viscosity and reducing inflammation simultaneously. Medical imaging tomography has been used to document cases of rapid improvement in acute inflammation after grounding.19 A pilot study on delayed-onset muscle soreness demonstrated a remarkable reduction of inflammatory mediators, including a reduction in white blood cell count (lymphocytes, neutrophils, and eosinophils).43
Attenuating inflammation and reducing blood viscosity will help physicians address primary and secondary prevention issues. Blood viscosity can be modified through a number of recognized primary prevention strategies. Moderate exercise, dietary adjustments (low sodium and sugar intake, and no trans fats), smoking cessation, and blood donation all have a positive impact on viscosity as do specific blood viscosity–modifying supplements, such as omega 3 essential fatty acids and pharmaceutical drugs (statins).
Grounding to the soil represents yet another intervention that lowers blood viscosity by raising zeta potential, which results in a decrease in RBC aggregation. The Earth's surface is electrically conductive and is maintained at a negative potential by a global electrical circuit. This circuit has three main generators; the solar wind entering the magnetosphere; the ionospheric wind; and thunderstorms.44 An estimated 1000–2000 thunderstorms are continually active around the globe, emitting thousands of lightening strikes per minute. This creates a constant current of thousands of amperes transferring positive charge to the upper atmosphere and negative charge to the surface of the Earth.44 The Earth's surface is therefore an abundant source of free electrons. As soil's electrons are conducted to the human body, the grounded body assumes favorable physiologic and electrophysiologic changes. Attenuation of the inflammatory response and a favorable impact on blood viscosity and RBC aggregation have been the most recent findings. Previous studies have also demonstrated that grounding promotes favorable regulation of circadian rhythms, improved sleep with better night-time cortisol dynamics,30 and favorable ANS function.18,31,34 Skin conductance is altered within 2 seconds of grounding.18,34,35 When one is in simple direct contact with the ground (walking barefoot, sitting or laying down on the soil's surface), or if one is utilizing a grounding system for sleep, zeta potential increases, and RBC aggregation and blood viscosity decrease. Grounding may represent one of the simplest and yet most profound interventions to help reduce cardiovascular risk and cardiovascular events.
Conclusions
Increased blood viscosity in the general population may be a predictor of cardiovascular events because of its influences on hypertension, thrombogenesis, ischemia, and arthrogenesis. Unfortunately, blood viscosity has become a forgotten risk factor and is rarely measured in clinical practice.45 Interventions that reduce blood viscosity and RBC aggregation are important. Statins appear to be effective for modulating blood viscosity, but can have serious side-effects including death.3 Moreover, some patients have statin intolerance. The use of a safe effective anti-inflammatory strategy that is not dependent on isoprenoid inhibition is therefore desirable.
Grounding or earthing the body is virtually harmless. To date, there has been no systematic study of the effects of grounding on BP. However, there are anecdotal reports that patients using blood-thinning drugs, such as warfarin (Coumadin®), need to have their clotting time monitored when they begin to make more frequent conductive contact with the earth. When physicians recommend evidence-based, harmless, and simple natural interventions, alleviation of human suffering and improved quality of life can be realized. The findings in this pilot study indicate that grounding has a safe and significant effect on zeta potential and that further study is warranted.
Footnotes
The fuse was used to protect subjects in the highly unlikely possibility that they might make accidental contact with a live/hot wire in the test environment. Subsequently the fuse was replaced with a 100,000-ohm resistor in the wires for all grounding systems. This resistor makes it impossible to get an electrical shock, even if a person touches a live wire.
Strong evidence against normality was found only in aggregates of 4 or more RBCs.
Acknowledgments
This research has been supported by Earth FX Inc., in Palm Springs, CA. The authors would like to thank the Healthwalk Integrative Wellness Center in Carlsbad, CA, for providing the study premises and microscope and Dr. Anna Walden for her contribution in taking blood specimens from subjects, recording RBC motions on compact discs and for acting as a research coordinator.
Disclosure Statement
G. Chevalier, S.T. Sinatra, and J.L. Oschman are independent contractors for Earth FX, Inc., the company sponsoring earthing research, and own a small percentage of shares in the company.
References
- 1.Greer JP. Foerster J. Lukens JN. Wintrobe's Clinical Hematology. 11th. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 1167. [Google Scholar]
- 2.Miniño AM. Death in the United States. NCHS Data Brief. 2011. 2009. Jul, 2011. www.cdc.gov/nchs/data/databriefs/db64.htm. [Feb 3;2012 ]. www.cdc.gov/nchs/data/databriefs/db64.htm Number 64.
- 3.Sinatra ST. Is cholesterol lowering with statins the gold standard for treating patients with cardiovascular risk and disease? South Med J. 2003;96:220–222. doi: 10.1097/01.smj.0000051743.83926.12. [DOI] [PubMed] [Google Scholar]
- 4.Riddick TM. Control of Colloid Stability Through Zeta Potential. Wynnewood, PA: Livingston; 1968. [Google Scholar]
- 5.Begg TB. Wade IM. Bronte-Stewart B. The red cell electrophoretic mobility in atherosclerotic and other individuals. J Atheroscler Res. 1966;6:303–312. doi: 10.1016/s0368-1319(66)80042-x. [DOI] [PubMed] [Google Scholar]
- 6.Adak S. Chowdhury S. Bhattacharyya M. Dynamic and electrokinetic behavior of erythrocyte membrane in diabetes mellitus and diabetic cardiovascular disease. Biochim Biophys Acta. 2008;1780:108–115. doi: 10.1016/j.bbagen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Baskurt OK. Tugral E. Neu B. Meiselman HJ. Particle electrophoresis as a tool to understand the aggregation behavior of red blood cells. Electrophoresis. 2002;23:2103–2109. doi: 10.1002/1522-2683(200207)23:13<2103::AID-ELPS2103>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Bor-Kucukatay M. Yalcin O. Meiselman HJ. Baskurt OK. Erythropoietin-induced rheological changes of rat erythrocytes. Br J Haematol. 2000;110:82–88. doi: 10.1046/j.1365-2141.2000.02150.x. [DOI] [PubMed] [Google Scholar]
- 9.Vink H. Wieringa PA. Spaan JAE. Evidence that cell surface charge reduction modifies capillary red cell velocity–flux relationships in hamster cremaster muscle. J Physiol. 1995;489:193–201. doi: 10.1113/jphysiol.1995.sp021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letcher RL. Chien S. Pickering TG, et al. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects: Role of fibrinogen and concentration. Am J Med. 1981;70:1195–1202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- 11.Fowkes FGR. Lowe GDO. Rumley A, et al. The relationship between blood viscosity and blood pressure in a random sample of the population aged 55 to 74 years. Eur Heart J. 1993;14:597–601. doi: 10.1093/eurheartj/14.5.597. [DOI] [PubMed] [Google Scholar]
- 12.Letcher RL. Chien S. Pickering TG. Laragh JH. Elevated blood viscosity in patients with borderline essential hypertension. Hypertension. 1983;5:757–762. doi: 10.1161/01.hyp.5.5.757. [DOI] [PubMed] [Google Scholar]
- 13.Rosenson RS. Viscosity and ischemic heart disease. J Vasc Med Biol. 1993;4:206–212. [Google Scholar]
- 14.Pries AR. Secomb TW. Gessner T, et al. Resistance to blood flow in microvessels in vivo. Circ Res. 1994;75:904–915. doi: 10.1161/01.res.75.5.904. [DOI] [PubMed] [Google Scholar]
- 15.Kensey KR. Rheology: An overlooked component of vascular disease. Clin Appl Thromb/Hemostasis. 2003;9:93–99. doi: 10.1177/107602960300900201. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F. Voisin P. Brunotte F, et al. Haemorheological abnormalities in arterial hypertension and their relation to cardiac hypertrophy. J Hypertens. 1988;6:293–297. [PubMed] [Google Scholar]
- 17.Halliday D. Resnick R. Walker J. Fundamentals of Physics. 4th. New York: John Wiley & Sons; 1993. p. 638. [Google Scholar]
- 18.Chevalier G. Mori K. Oschman JL. The effect of earthing (grounding) on human physiology. Eur Biol Bioelectromagnetics. 2006 Jan;:600–621. [Google Scholar]
- 19.Oschman JL. Can electrons act as antioxidants? A review and commentary. J Altern Complement Med. 2007;13:955–967. doi: 10.1089/acm.2007.7048. [DOI] [PubMed] [Google Scholar]
- 20.Oschman JL. Perspective: Assume a spherical cow. The role of free or mobile electrons in bodywork, energetic and movement therapies. J Bodywork Move Ther. 2008;12:40–57. doi: 10.1016/j.jbmt.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Applewhite R. Effectiveness of a conductive patch and a conductive bed pad in reducing induced human body voltage via the application of earth ground. Eur Biol Bioelectromagn. 2005;1:23–40. [Google Scholar]
- 22.American Council on Exercise. Health History Inventory Form. www.acefitness.org/acestore/p-369-health-history-inventory-form.aspx. [Mar 29;2008 ]. www.acefitness.org/acestore/p-369-health-history-inventory-form.aspx
- 23.Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 24.Jan K-M. Chien S. Role of surface electric charge in red blood cell interactions. J Gen Physiol. 1973;61:638–654. doi: 10.1085/jgp.61.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Çinar Y. Senyol MA. Duman K. Blood viscosity and blood pressure: Role of temperature and hyperglycemia. Am J Hypertens. 2001;14(5[pt1]):433–438. doi: 10.1016/s0895-7061(00)01260-7. [DOI] [PubMed] [Google Scholar]
- 26.Johnston-Lavis HJ. Hypertension, blood viscosity, and capillary spasm. BMJ. 1911;2:111. [Google Scholar]
- 27.Fontes A. Fernandes HP. de Thomaz AA, et al. Measuring electrical and mechanical properties of red blood cells with double optical tweezers. J Biomed Optics. 2008;13:014001-1–014001-6. doi: 10.1117/1.2870108. [DOI] [PubMed] [Google Scholar]
- 28.Alonzo C. Pries AR. Gaehtgens P. Time-dependent rheological behavior of blood at low shear in narrow vertical tubes. Am J Physiol. 1993;265(2[pt2]):H553–H561. doi: 10.1152/ajpheart.1993.265.2.H553. [DOI] [PubMed] [Google Scholar]
- 29.Lilliefors H.On the Kolmogorov–Smirnov test for normality with mean and variance unknown J Am Stat Assoc 1967621 399–402. [Google Scholar]
- 30.Winer BJ. Brown DR. Michels KM. Statistical Principles in Experimental Design. 3rd. Boston: McGraw-Hill; 1991. p. 864. [Google Scholar]
- 31.Ghaly M. Teplitz D. The biological effects of grounding the human body during sleep, as measured by cortisol levels and subjective reporting of sleep, pain, and stress. J Altern Complement Med. 2004;10:767–776. doi: 10.1089/acm.2004.10.767. [DOI] [PubMed] [Google Scholar]
- 32.Alschuler LN. Stress: Thief in the night. Int J Integ Med. 2001;3:27–34. [Google Scholar]
- 33.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obesity Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 34.Chevalier G. Mori K. The effect of earthing on human physiology: Part 2. Electrodermal measurements. Subtle Energies Energy Med. 2008;18:11–34. [Google Scholar]
- 35.Chevalier G. Sinatra ST. Emotional stress, heart rate variability, grounding and improved autonomic tone: Clinical applications. Integr Med. 2011;10:16–21. [Google Scholar]
- 36.Kupari M. Virolainen J. Koskinen P. Tikkanen MJ. Short term heart rate variability and factors modifying the risk of coronary artery disease in a population sample. Am J Cardiol. 1993;72:897–903. doi: 10.1016/0002-9149(93)91103-o. [DOI] [PubMed] [Google Scholar]
- 37.Huikuri HV. Jokinen V. Syvänne M, et al. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 38.Danesh J. Collins R. Peto R. Lowe GDO. Haematocrit, viscosity, erythrocyte sedimentation rate: Meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–520. doi: 10.1053/euhj.1999.1699. comment in: Eur Heart J 2000;21:513–514. [DOI] [PubMed] [Google Scholar]
- 39.Lowe GD. Lee AJ. Rumley A, et al. Blood viscosity and risk of cardiovascular events: The Edinburgh Artery Study. Br J Haematol. 1997;96:168–173. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- 40.Lowe G. Rumley A. Norrie J, et al. Blood rheology, cardiovascular risk factors, and cardiovascular disease: The West of Scotland Coronary Prevention Study. Thromb Haemost. 2000;84:553–558. erratum in: Thromb Haemost 2001;85:946. [PubMed] [Google Scholar]
- 41.Doncheva NI. Nikolov KV. Vassileva DP. Lipid-modifying and pleiotropic effects of gemfibrozil, simvastatin and pravastatin in patients with dyslipidemia. Folia Med (Plodiv) 2006;48(3–4):56–61. [PubMed] [Google Scholar]
- 42.Muravyov AV. Yakusevich VV. Surovaya L. Petrochenko A. The effect of simvastatin therapy on hemorheological profile in coronary heart desease (CHD) patients. Clin Hemorheol Microcirc. 2004;31:251–256. [PubMed] [Google Scholar]
- 43.Brown D. Chevalier G. Hill M. Pilot study on the effect of grounding on delayed-onset muscle soreness. J Altern Complem Med. 2010;16:265–273. doi: 10.1089/acm.2009.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volland H. Atmospheric electrodynamics. In: Lanzerotti LJ, editor. Physics and Chemistry in Space. Vol. 11. Berlin & New York: Springer-Verlag; 1984. [Google Scholar]
- 45.Késmárky G. Kenyeres P. Rábai M. Tóth K. Plasma viscosity: A forgotten variable. Clin Hemorheol Micro. 2008;39(1–4):243–246. [PubMed] [Google Scholar]