Abstract
Objectives
Osteopathic manipulative treatment (OMT) focused on the upper cervical spine is theorized to affect the function of the vagus nerve and thereby influence the parasympathetic branch of the autonomic nervous system. This study was designed to determine the acute effect of upper cervical spine manipulation on cardiac autonomic control as measured by heart rate variability.
Design
Nineteen healthy, young adult subjects underwent three different experimental interventions administered in random order: cervical OMT, sham manipulation, and time control. Six minutes of electrocardiographic data were collected before and after each intervention, and heart rate variability was assessed by both time-domain and frequency-domain measures.
Results
No differences in resting heart rate or any measure of heart rate variability were observed between the baseline periods prior to each intervention. The OMT protocol resulted in an increase in the standard deviation of the normal-to-normal intervals (0.12±0.082 seconds, p<0.01), an increase in the high frequency spectral power (p=0.03), and a decrease in the low/high frequency spectral ratio (p=0.01) relative to the sham and time control conditions. No significant differences between sham and time control were observed (p>0.11 for all variables).
Conclusions
These data support the hypothesis that upper cervical spine manipulation can acutely affect measures of heart rate variability in healthy individuals.
Introduction
The anatomical relationship of the efferent vagus nerve to the musculoskeletal structures at the occiput lends credence to the hypothesis that osteopathic manipulative treatment (OMT) at this location, such as suboccipital decompression, could affect vagal functions. Limited previous studies have shown that manual therapies can affect vagal control1–3; however, none have targeted these particular anatomical structures and these particular therapeutic techniques. In theory, OMT may have direct rather than indirect effects on the parasympathetic nervous system via manipulation of these cervical structures. Specifically, decompression of the occipito-atlantal (O-A) junction, a technique that focuses on treating an articular compression between the occiput and the atlas, may improve conditions relating to the path of the vagus as it exits the skull.
Because of the proximity of the vagus to the musculoskeletal structures in the suboccipital region, it is plausible that local inflammation, edema, muscle hypertonicity or spasm, or other somatic dysfunctions could cause either a chemical or compressive effect on the vagus, thereby affecting its optimum function. Since the vagus plays a significant role in the autonomic control of heart rate, it is therefore also plausible that if optimum performance of the vagus is impeded by dysfunction in the surrounding structures, its ability to contribute effectively to the autonomic control of heart rate might also be affected. Therefore, the purpose of this study was to determine whether OMT in the cervical region using O-A decompression affects autonomic control of the heart by assessment of measures of heart rate variability.
Materials and Methods
Three experimental interventions (OMT, sham treatment, and time control) were used to test the hypothesis that cervical OMT enhances vagal control of heart rate as measured by indices of heart rate variability. The osteopathic manipulative techniques chosen included soft tissue manipulation in the cervical region and suboccipital decompression. A crossover design was used in which each subject underwent each of the three interventions in randomized order. This study was performed on 19 healthy volunteer subjects who were normotensive. Each subject provided signed written informed consent before participation in any aspect of the study. Because this study depended on the normal function of the autonomic nervous system, the following exclusion criteria were used: consumption of caffeine within the past 4 hours, use of tobacco within the past 48 hours, history of cardiovascular disease, neuropathic disorders, or unexplained episodes of syncope. Furthermore, all subjects were screened for symptoms of somatic dysfunction, thus, the treatments did not provide relief of a pre-existing musculoskeletal injury or condition.
Subjects were instrumented for the measurement of a standard lead II electrocardiogram and respiration by plethysmographic bands placed around the chest and abdomen. Once instrumented, subjects underwent a period of quiet supine rest for 20 minutes prior to each intervention. Prior to the initial intervention, a 6-minute baseline period of data was recorded. Following the baseline period, three interventions (OMT, sham manipulation, and time control) were performed for ∼15 minutes (including the post-treatment recovery) in random order. Each intervention was separated by an additional 20-minute period of rest and a new 6-minute baseline period. Data were collected during the last 6 minutes of each experimental condition. In addition, the subject was allowed to ambulate for 5 to 10 minutes before starting the subsequent rest period.
Interventions
The three interventions that each subject completed in this study were (1) an OMT intervention, (2) a sham manipulation intervention, and (3) time control as described below.
OMT
The OMT protocol involved the treatment of the subject's posterior cervical musculature by using the two soft tissue techniques of kneading and stretching. Kneading is a technique in which a force is applied perpendicular to the long axis of the muscle, whereas stretching is a separation of the origin and insertion of a muscle. This soft tissue treatment was performed for approximately 5 minutes. Following this, the treatment provider performed a suboccipital (O-A) decompression for 2–3 minutes.4 To achieve the O-A decompression, the treatment provider used his or her index fingers to contact the occiput as near to the occipital condyles as possible. The index fingers were reinforced with the middle fingers. Tension was then applied toward the orbits to make firm contact with the occiput and constant traction was directed superiorly. Minor adjustments in all three planes of motion (flexion/extension, sidebending, and rotation) were made as needed to maintain ligamentous balance. The study treatment provider held the point of ligamentous balance until the best motion in the joint was obtained.4
Sham treatment
A sham treatment protocol involved the placement of fingers near the occipital condyles. However, no tension was applied in any direction, the subject's head was simply held in the treatment provider's hands for approximately 8 minutes.
Time control condition
The time control intervention involved no physical contact with the subject. The subject lay quietly in the supine position for a 15-minute period.
All physiologic data were recorded digitally on a desktop computer using a customized data acquisition system (WINDAQ, Akron, OH). All analyses were performed post hoc using the software and techniques described below.
Heart rate variability measures
Heart rate variability was measured as an index of autonomic control using both time-domain and frequency-domain measures. The time-domain measures included the mean heart rate and R-R interval and the standard deviation of normal-to-normal heart beats (SDNN). The time series was converted into the frequency domain by fast Fourier transformation using commercially available software (DADiSP/AdvDSP, Cambridge, MA). Specifically, the spectral analysis was performed as follows. After resampling, the data were detrended with a third order polynomial fit, Hanning filtered, and divided into 128 point segments with 50% overlap for fast Fourier transform (Welch method) using data analysis and display software (DSP Development Corporation, Cambridge, MA). The harmonic low frequency (LF; 0.04–0.12 Hz) and high-frequency (HF; 0.15–0.30 Hz) power was extracted for each subject during each measurement period and estimated in squared milliseconds per Hertz, and the HF/LF ratio was calculated as described previously.5
Statistical analyses
All data sets were initially tested for normality using a Shapiro-Wilk test. For all normally distributed data, parametric statistics were used, whereas for data sets that failed the normality test, nonparametric analyses were performed. Initially, the pretreatment baseline data for each treatment condition were compared using a one-way analysis of variance. Since these baseline data were equivalent for all variables, pairwise comparisons were used for all other analyses: paired t-test when the data were normally distributed or Wilcoxon signed rank test when the data were not normally distributed. For all analyses, significance was set an alpha level of 0.05. All data are reported as mean±SEM.
Results
A total of 19 subjects were used in the analysis of this study. Of these subjects, six were mean and 13 were women. The average age of the subjects was 25±2 years with a range of 22–31 years. The average body mass index for the study was 23±3 kg/m2.
All pretreatment physiologic baseline heart rate and heart rate variability data were not significantly different between each baseline period (p>0.12) as shown in Table 1. In addition, respiratory rate remained between 12 and 14 breaths/min throughout each baseline period and all interventions. Specifically, respiratory rate was 13.2±0.3 breaths/min for time control, 13.0±0.2 breaths/min for sham, and 12.6±0.3 for OMT, with no main effect difference observed (p=0.22).
Table 1.
Baseline Data for Each Condition
| OMT | Sham | Time control | p value | |
|---|---|---|---|---|
| R-R interval (msec) | 938±13 | 929±17 | 917±21 | 0.59 |
| SDNN (msec) | 129±26 | 143±21 | 146±32 | 0.57 |
| HF power | 46±15 | 40±9 | 55±24 | 0.24 |
| LF power | 345±69 | 301±48 | 279±65 | 0.11 |
OMT, osteopathic manipulative treatment; HF, high frequency; LF, low frequency; SDNN, standard deviation of the normal-to-normal.
Effect of interventions
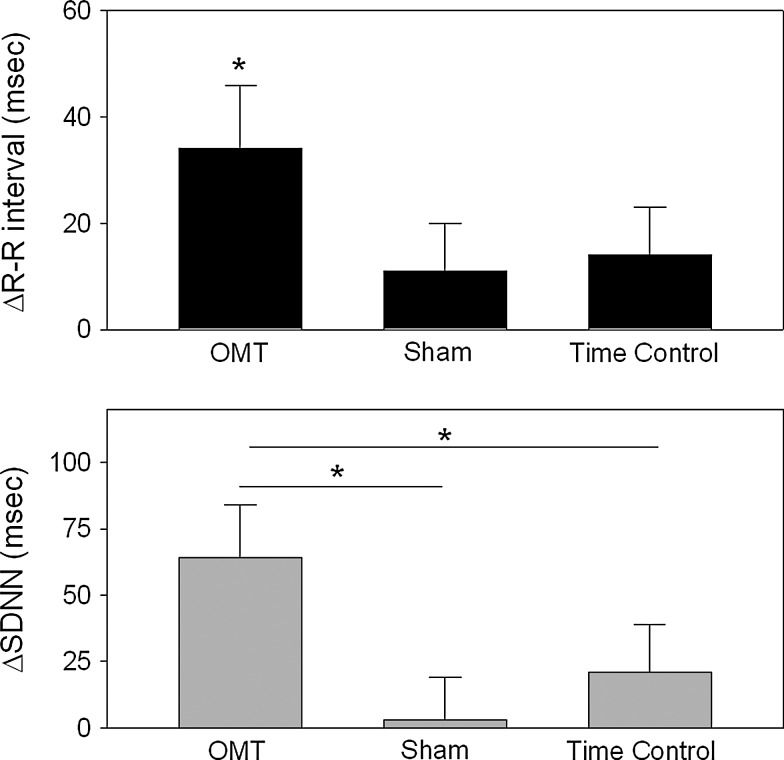
The change evoked on heart rate and indices of heart rate variability by each intervention is illustrated in Figs. 1 and 2. As shown in Figure 1, resting R-R interval was significantly increased with the OMT treatment (+34±12 milliseconds, p=0.02), but not the sham (+11±9 milliseconds) or time control (+16±9 milliseconds, p>0.22); however, a main effect difference between the groups was not observed (p=0.07). Also illustrated in Fig. 1, the time-domain index of heart rate variability, SDNN, was found to be increased significantly by the OMT intervention (Δ64±20 milliseconds) and this change was significantly greater than either sham or time control (main effect difference, p<0.01). The change in SDNN after the sham (Δ3±16 milliseconds) and time control protocols (Δ21±18 milliseconds) were not found to be significant (p>0.14).
FIG. 1.
The changes in R-R interval (top panel) and SDNN (bottom panel) are illustrated from baseline to immediate post-treatment for each of the treatment conditions in which OMT was suboccipital decompression. The asterisks represent a significant change from baseline for R-R interval during OMT (top panel) and significant difference for the post hoc comparisons between the three treatment conditions (bottom panel); significance was defined as p<0.05.
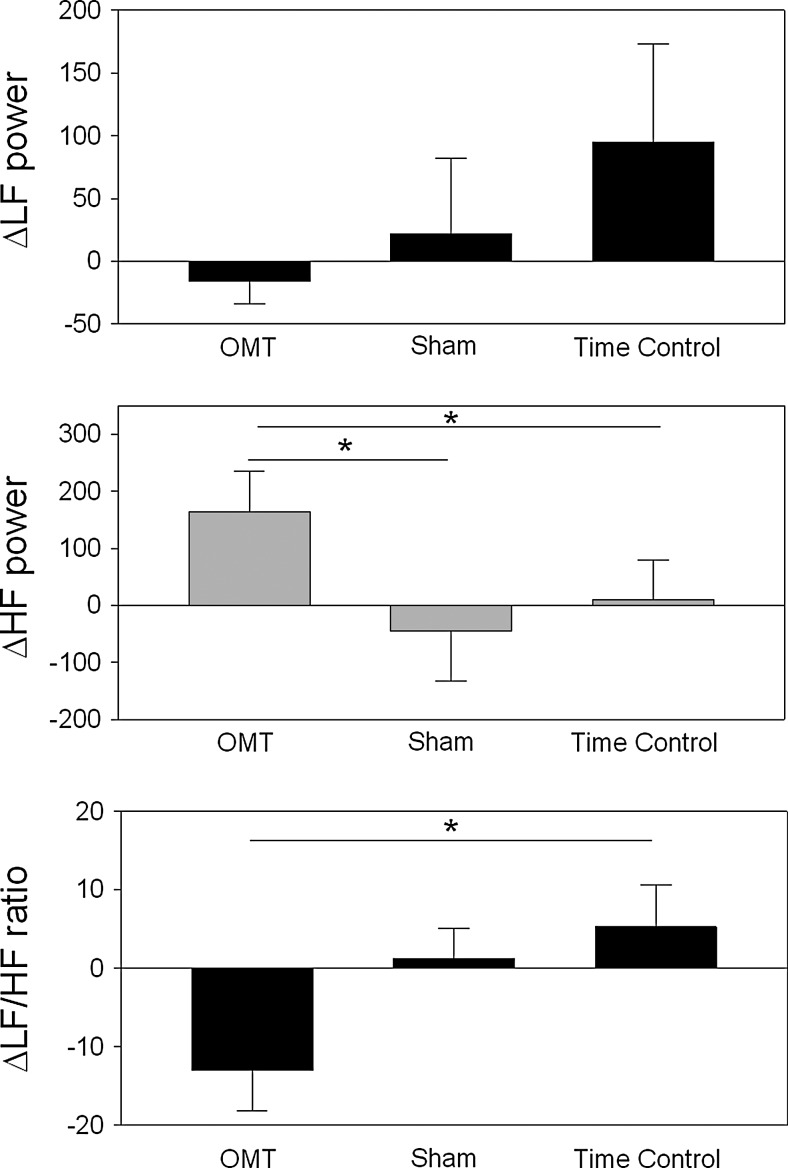
FIG. 2.
The changes in low frequency spectral power (LF power, 0.05–0.15 Hz), high frequency power (HF power, 0.15–0.30 Hz), and calculated LF/HF ratio are illustrated. The asterisks represent a significant difference for the post hoc comparisons between the three treatment conditions; significance was defined as p<0.05.
The changes in the frequency domain indices of heart rate variability were also consistent with an enhanced parasympathetic control associated with the OMT treatment (see Fig. 2). LF power was not significantly different between the three interventions (main effect difference, p=0.29), whereas HF power was significantly increased with OMT (main effect difference, p=0.02) and unchanged with both sham and time control interventions (p>0.32). The resulting LF/HF ratio was also significantly decreased during OMT both as a change from baseline (p<0.01) and in comparison with sham and time control conditions (main effect difference, p=0.033).
Discussion
These data demonstrate that suboccipital decompression, a soft tissue manipulative technique, can affect indices of heart rate variability. This effect was seen in healthy subjects free of injury or somatic dysfunction, thus the positive effect was not due to a treatment effect associated with an underlying musculoskeletal condition. Moreover, there was no effect of a sham treatment or time control; thus, the effect of the OMT appears to be related to the specific techniques of the treatment.
The inherent variability of normal beat-to-beat fluctuations in heart rate are well-recognized to be a function of the autonomic nervous system.6,7 Many forms of manipulative medicine have been theorized to affect the autonomic nervous system, and it has been espoused that this can include both stimulatory and inhibitory effects on the respective branches of the autonomic nervous system. Heart rate variability has been used to assess changes in autonomic control of heart rate under a variety of conditions but only recently has been used in the context of manual therapies. In this study we used the premise, based on autonomic blockade studies, that HF power (0.15–0.30 Hz) is a function of parasympathetic modulation of heart rate, that LF power (0.04–0.15 Hz) is a function of both parasympathetic and sympathetic modulation of heart rate, and that the LF/HF ratio is reflective of sympathovagal balance.6–9 Moreover, it is recognized that short-term time-domain variability (such as SDNN) is a function of both sympathetic and parasympathetic modulation, but it is predominantly influenced by parasympathetic effects.7 Based on the application of these principles, the data in our study consistently point to an effect of suboccipital decompression to moderately enhance parasympathetic control of heart rate and/or shift the sympathovagal balance to a more predominant vagal control. This is evidenced by the significant increase in HF spectral power and significant decrease in the LF/HF ratio. These effects were associated with a very modest slowing of heart rate accompanying suboccipital decompression. The latter finding has less impact due to a general trend for heart rate slowing with both the sham treatment and the time control, although these were not statistically significant. Perhaps more importantly, the effect of OMT was distinct from the sham treatment, which also involved physical contact between the treatment provider and the subject. This finding suggests that the effect on heart rate variability was related to the technique of suboccipital decompression. This conclusion is made with the limitation that this technique could potentially result in a more relaxed state of the subject. However, respiration was the same throughout all interventions and the subjects did not transition into a state of sleep because their eyes remained open throughout the protocols. Finally, the practitioner–subject interaction was similar between the OMT treatment and the sham treatment, yet significant differences were observed. Thus, it is likely that the primary effect was related to the specific technique of suboccipital decompression.
A limited number of studies have assessed autonomic control of either heart rate or pupil function after manipulative treatments. The effect of high-velocity low-amplitude cervical manipulation has been shown to increase the LF power and increase the LF/HF ratio, suggesting that there is a shift in the sympathovagal balance toward a more sympathetic predominant state as evidenced by these changes in heart rate variability.3,10 In a separate study, Sillevis and colleagues11 treated patients with chronic neck pain using high-velocity low-amplitude manipulation at the T3–T4 spinal level, but they did not see an effect on the pupil diameter changes and concluded that there was no effect on the sympathetic nervous system associated with this treatment. Cervical (C1–C2) high-velocity low-amplitude manipulation has been shown to alter the edge pupil cycle time, which is mediated by sympathetic and parasympathetic influences, thereby suggesting an acute effect of cervical articulation on autonomic function.12 Cui and colleagues1 studied the effect of Tuina treatment, a Chinese form of soft-tissue massage of the cervical spine (C2–C5) that is intended to be stimulatory. The authors reported a significant increase in total spectral power of heart rate and a subtle increase in the SDNN. They also found a modest slowing of the heart rate. There was no effect on the HF spectral power, and although they concluded that this form of cervical manipulation produced an “increase in sympathetic tone,” the findings are unclear. The technique is a very dynamic form of manipulation that may directly affect the baroreceptors of the neck in addition to effects on other sensory afferent nerves. Finally, in a study by Henley and colleagues,2 cervical myofascial manipulation was shown to reduce the increase in LF spectral power and LF/HF ratio, and to increase HF power during head-up tilting. The treatments were delivered while the subject was in the upright position, so it is not clear whether there was also an effect on the baseline state. Nevertheless, these findings are consistent with our data showing changes in the heart rate variability indices relating to a reduced sympathetic and enhanced parasympathetic control specifically in the stress state of head-up tilt.
The interpretation of these findings are made with the caveat that changes in parameters of heart rate variability are not a direct measure of the control of heart rate imparted by the sympathetic or parasympathetic nervous systems. Despite the landmark mechanistic studies that have shown that the two branches of the autonomic nervous system influence the LF and HF elements of a power spectral analysis of heart rate variability,5,6,13 the interpretation of a change in these values remains inconclusive.6,14 This has consistently been a concern of data derived from changes in the frequency and time-domain indices of heart rate variability when an interpretation is introduced. However, the clear role that autonomic factors play in mediating, in part, heart rate variability cannot be discounted, and thus these data point to a possible change in autonomic regulation of heart rate. This study may also be limited because in quiet resting healthy subjects the sympathovagal state is shifted to a predominance of high vagal control. Therefore, it is likely that interventions designed to increase parasympathetic tone or control may not be effective under these conditions. Moreover, suboccipital decompression is typically used for somatic dysfunction to treat some form of restriction in this region, so it is possible that this treatment would not be expected to have an effect in a healthy subject. Despite these potential reasons to expect little or no effect with suboccipital decompression, we observed significant changes consistent with an enhanced parasympathetic control of heart rate.
A potential limitation also lies in the possibility that subjects who received the OMT first, may have altered the response to a subsequent sham treatment. The data reflect the change in physiological parameters from a prior baseline; thus, the effects of sham or OMT were consistent regardless of the prior intervention. In addition, these studies were performed on subjects naïve to manipulative medicine and the specific OMT treatments, thus a prior knowledge of the treatment should not have created a bias. Finally, it is possible that OMT affected respiration and thereby could have altered the autonomic response. Importantly, we did not observe a change in respiratory rate, and although we did not quantify tidal volume, it is unlikely that total ventilation changed significantly when the rate remained unchanged.
Conclusions
In summary, these data demonstrate that suboccipital decompression, often used in a manipulative medicine regimen, can affect heart rate variability acutely. This effect is consistent with potential changes in the control of heart by the parasympathetic nervous system. Further investigation in patient populations to determine the acute and long-term benefits of this and other manipulative medicine treatments is needed.
Acknowledgments
This research was supported by a grant from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (grant # 5U19AT002023-03 and grant #K23AT003304).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cui K. Li W. Liu X, et al. Effect of cervical manipulation on autonomic nervous function in healthy volunteers. Journal of Acupuncture and Tuina Science. 2006;4:267–270. [Google Scholar]
- 2.Henley CE. Ivins D. Mills M, et al. Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: a repeated measures study. Osteopath Med Prim Care. 2008;2:7. doi: 10.1186/1750-4732-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budgell B. Polus B. The effects of thoracic manipulation on heart rate variability: a controlled crossover trial. J Manipulative Physiol Ther. 2006;29:603–610. doi: 10.1016/j.jmpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Kimberly P. Funk S. Outline of Osteopathic Manipulative Procedures: The Kimberly Manual, Millennium Edition. Marceline, MO: Walsworth Publishing Co; 2000. [Google Scholar]
- 5.Wray DW. Formes KJ. Weiss MS, et al. Vagal cardiac function and arterial blood pressure stability. Am J Physiol Heart Circ Physiol. 2001;281:H1870–1880. doi: 10.1152/ajpheart.2001.281.5.H1870. [DOI] [PubMed] [Google Scholar]
- 6.Akselrod S. Gordon D. Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 7.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 8.Malliani A. Pagani M. Lombardi F. Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 9.Pagani M. Lombardi F. Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 10.Budgell B. Hirano F. Innocuous mechanical stimulation of the neck and alterations in heart-rate variability in healthy young adults. Auton Neurosci. 2001;91:96–99. doi: 10.1016/S1566-0702(01)00306-X. [DOI] [PubMed] [Google Scholar]
- 11.Sillevis R. Cleland J. Hellman M. Beekhuizen K. Immediate effects of a thoracic spine thrust manipulation on the autonomic nervous system: a randomized clinical trial. J Man Manip Ther. 2010;18:181–190. doi: 10.1179/106698110X12804993427126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbons PF. Gosling CM. Holmes M. Short-term effects of cervical manipulation on edge light pupil cycle time: a pilot study. J Manipulative Physiol Ther. 2000;23:465–469. doi: 10.1067/mmt.2000.108820. [DOI] [PubMed] [Google Scholar]
- 13.Malliani A. Lombardi F. Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994;71:1–2. doi: 10.1136/hrt.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossman P. Karemaker J. Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]