Abstract
The humoral innate immune response consists of multiple components, including the naturally occurring antibodies (NAb), pentraxins and the complement and contact cascades. As soluble, plasma components, these innate proteins provide key elements in the prevention and control of disease. However, pathogens and cells with altered self proteins utilize multiple humoral components to evade destruction and promote pathogy. Many studies have examined the relationship between humoral immunity and autoimmune disorders. This review focuses on the interactions between the humoral components and their role in promoting the pathogenesis of bacterial and viral infections and chronic diseases such as atherosclerosis and cancer. Understanding the beneficial and detrimental aspects of the individual components and the interactions between proteins which regulate the innate and adaptive response will provide therapeutic targets for subsequent studies.
Keywords: Complement, Contact cascade, Pentraxins, Natural antibodies, Cancer, Infection
Highlights
► Humoral innate proteins remove pathogens, apoptotic cells, altered cells and debris. ► Bacteria utilize complement and contact proteins to evade the immune response. ► Viruses compromise multiple humoral proteins to circumvent host cell lysis. ► Humoral innate immune proteins drive the progression of atherosclerosis. ► Tumor cells use natural antibodies and contact proteins to escape immunosurveillance.
1. Introduction
As the first line of defense, the innate immune response consists of both cellular and humoral components. The cellular component encompasses multiple cell types that use pattern recognition molecules to recognize and remove pathogens and cellular debris. The interactions of pattern recognition molecules within the cellular component have received significant attention in the last two decades. In contrast, less is known about the interactions between the humoral components. The humoral innate immune response consists of the serine protease cascades of the complement and contact systems as well as naturally occurring antibodies (NAb) and pentraxins. Recent data indicate that each component may be beneficial or detrimental during infection or chronic disease depending on concentration and interactions with other components. This review will focus on the interactions and roles of humoral components in bacterial and viral infections as well as the chronic diseases, atherosclerosis and cancer.
2. Humoral innate immune components
2.1. Complement
Complement activation occurs by one of three initiation pathways, the classical, alternative, or mannose binding lectin (MBL) pathway. Each pathway contains a C3 convertase that cleaves C3 producing C3b and subsequently a C5 convertase. Cleavage of C5 by the C5 convertase results in C5b deposition and initiates the common terminal pathway. The terminal pathway forms the membrane attack complex (MAC), a pore in the cellular membrane, and lysis of the host or pathogenic cell. The action of the C3 and C5 convertases also produces potent anaphylatoxins, C3a and C5. Although not specifically part of the humoral immune response, complement receptor 3 (CR3) found on neutrophils and macrophages enhances the innate immune response by recognizing C3b opsonized pathogens. Recent evidence indicates that complement plays a significant role in directing the adaptive immune response as well as in tissue regeneration [1], [2]. Specifically, as part of the B cell receptor complex, CR2 recognition of cleavage products iC3b, C3dg, and C3d significantly increases Ab production [3]. Thus, maintaining homeostasis requires tight regulation of the cascade. Regulation of this potentially damaging cascade occurs at multiple levels with soluble and membrane bound inhibitors including C1 inhibitor (C1INH), CD55, CD59, CD46, Factor H and related proteins.
2.2. Contact cascade
The plasma also contains components of a second proteolytic cascade, the contact system. Factor XII (FXII; Hageman factor) of the contact system is proteolytically cleaved to FXIIa by negatively charged surfaces of damaged cells. FXIIa initiates the coagulation cascade leading to clot formation and cleaves prekallikrein to kallikrein for subsequent release of bradykinin. Through an endothelial G-coupled receptor (bradykinin receptor 1; BKR1), bradykinin induces vasodilation, neutrophil chemotaxis and vascular permeability [4]. Furthermore, the bradykinin degradation product, des-arg9-bradykinin regulates the adaptive response and alters the blood–brain barrier through a second receptor, bradykinin receptor 2 (BKR2) [5]. Importantly, both FXIIa and kallikrein activate the complement cascade independent of known complement initiators [6]. Several components of the activated contact system including, FXa, FXIa and plasmin, cleave C5 and C3 producing C5a and C3a [7]. The complement inhibitor, C1INH, also inhibits FXIIa indicating multiple interactions between the two pathways [6]. These data suggest crosstalk between two cascades of humoral innate immune response.
2.3. Naturally occurring antibodies
Produced primarily by B1 B lymphocytes, NAb are germline-encoded Ab with restricted epitope specificities and are produced in the absence of external antigen stimulations. NAb are usually of the IgM isotype but may include IgG and IgA isotypes as well [8]. Natural IgM Abs mediate clearance of cellular debris, aging or apoptotic cells by oponization and recruitment of complement components [9]. As part of the innate immune response, NAb recognize a wide range of pathogens, albeit with low affinity and modulate the adaptive immune response by interacting with B, T and dendritic cells [10]. Finally, NAb are potent initiators of the complement cascade suggesting additional interactions between components of the innate humoral response.
2.4. Pentraxins
As a family of evolutionarily conserved multimeric pattern recognition proteins, pentraxins are acute phase proteins which are rapidly synthesized and serve as markers of infection, inflammation, and tissue damage [11]. Each pentraxin contains a common domain in the C terminus. The presence or absence of additional domains divides the family into long or short pentraxins, respectively. The short pentraxins include C-reactive protein (CRP) and serum amyloid P protein (SAP), both of which are produced by the liver [11]. Produced by a multitude of cell types, pentraxin 3 (PTX3) is the primary long pentraxin active in humoral innate immunity. Similar to NAb, pentraxins recognize and bind multiple pathogens as well as intrinsic ligands, including apoptotic cells and extracellular matrix components [12], [13], [14]. Macrophages and other innate immune cells recognize pentraxins, CRP, SAP and PTX3, via Fcγ receptors [15], [16], [17]. Binding of pentraxins to a target facilitates clearance of pathogens and cell debris by complement activation indicating additional interactions between components of the innate immune response [12]. Overall, pentraxins are multifunctional and nonredundant components of the humoral innate immune response. Therefore, pentraxins play a critical role in human disease by interacting with multiple components of the humoral response.
Together the interactions of the components of the humoral arm of the innate immune response are critical in protecting the host from invading pathogens, interacting with the cellular component and instructing the adaptive immune response. However, inappropriate activation of any one of the humoral components may be detrimental to the host. This review focuses on how infectious organisms evade these devastating circulating proteins and how chronic disease may be enhanced by the interactions of NAb, pentraxins and the complement and contact cascades.
3. Humoral innate immunity and infection
3.1. Humoral innate immune component interactions with bacteria
A coordinated attack by multiple components of the humoral innate immune system is crucial in protecting the host from bacterial infections. A compromised humoral innate immune system increases susceptibility to bacteria [3], [18]. Successful development of therapeutics that decrease bacterial infections requires understanding of the interactions between the complement and contact cascades as well as NAb and pentraxins.
Complement contributes to the humoral innate immune response directly by the MAC complex forming pores and lysing the bacteria as well as indirectly by activating other components of the immune system that combat the bacteria. Invading bacteria induce complement activation by all three initiation pathways [19], [20], [21]. NAb recognize invading bacteria and activate the classical and MBL complement pathways. The MBL pathway also recognizes sugar moieties on the bacterial surfaces. In addition, the presence of bacterial carbohydrates, lipids and proteins trigger the alternative complement pathway [19]. Activation of all three pathways results in C3 cleavage to C3b. C3b coats the bacterial surface, and enhances recognition by neutrophils and macrophages by opsonization. C3b coated bacteria also bind CR2 to enhance Ab production. Initiation by each of the above pathways may also result in bacterial lysis by MAC.
Although the activation of the complement cascade is essential for immune protection, bacteria have evolved several strategies to evade the immune response. Evasion strategies of bacteria can be broadly classified into four types: a) recruitment or mimicking of complement regulators; b) inhibition or modulation of complement proteins; c) enzymatic degradation of complement proteins; and d) blockage of MAC penetration [22]. For example, Enterococcus faecalis and Streptococcus pyogenes, as Gram positive bacteria, are endowed with a thick polysaccharide capsule that prevents complete penetration of the capsule and cell wall by the MAC and subsequent bacterial lysis [23].
Many bacteria evade complement by recruiting complement regulatory proteins to the bacterial surface to prevent lysis. Specific examples are provided in Table 1 . Several important pathogens including S. pyogenes, Streptococcus pneumonia, Neisseria gonorroheae and Borrelia burgdorferi produce proteins which recruit Factor H of the alternative pathway and/or C4bBP of the classical and lectin pathways [24], [25], [26], [27]. Other bacteria produce proteins that directly interact and inhibit central complement components. For example, the S. pneumoniae protein, PspA, inhibits C3b deposition on the pneumococcal surface [28]. Similarly, other bacterial proteins directly inhibit C3 convertase [29], [30] or MAC formation [31]. Additional bacterial enzymes degrade critical complement components including the anaphylatoxins, C3a and C5a. This prevents chemotaxis of phagocytic cells towards the site of infection. E. faecalis, a leading nosocomial pathogen, produces gelatinase, an enzyme that directly cleaves C5a, preventing neutrophil recruitment to the site of infection [32]. Due to incomplete complement activation, each of the above mechanisms frequently allows bacterial proliferation and deleterious effects on the host. A better understanding of the bacterial proteins that inhibit complement activation may lead to therapeutics that also curb excessive complement activation.
Table 1.
Complement evasion proteins recruited by different bacterial species to prevent complement‐mediated lysis.
| Bacteria | Complement evasion protein (CEP) | Target | Action of CEP | Reference |
|---|---|---|---|---|
| Borrelia spp. | 1) CRASP: Complement regulator-acquiring surface proteins. | Factor H, FHL-1, CFHR-1, C4BP | Regulator acquisition | (24),(199), (200) |
| 2) Erp: OspE/F-related proteins. | Factor H | Regulator acquisition | (201),(202) | |
| 3) CD59 like protein. | C8 and C9 | Prevention of MAC formation | (203) | |
| Enterococcus faecalis | 1) Capsular polysaccharide | C3b | Renders bound C3b Inaccessible to antibodies | (204) |
| 2) GelE: gelatinase | C3a, C3b, C5a | Inhibits C3 activity, Cleaves C5a proteolytically | (205),(29) | |
| Escherichia spp. | 1) OmpA: outer membrane protein A | C4BP | Regulator acquisition | (206) |
| 2) TraT: TraT outer membrane protein | C1-INH | C1-INH acquisition | (207) | |
| 3) StcE: secreted protease of C1 esterase inhibitor | C5B6 | Prevention of MAC formation | (208) | |
| Haemophilus influenzae | 1) Unknown factor | C4BP, Factor H, FHL-1 | Regulator acquisition | (209), (210) |
| 2) HSF: Haemophilus surface fibril | Vitronectin | Prevention of MAC formation | (211) | |
| Neisseria spp. | 1) GNA1870: genome-derived neisserial antigen 1870. | Factor H, FHL-1 | Regulator acquisition | (23) |
| 2) LOS: lipooligosaccharide | Factor H, FHL-1 | Regulator acquisition | (212) | |
| 3) Por: outer membrane porins | C4BP, Factor H, FHL-1 | Regulator acquisition | (213) | |
| 4) Type IV pili | Membrane co-factor protein | Attachment to epithelial cells | (214) | |
| 5) OpaA− heparin binding outer membrane protein | Vitronectin | Regulator acquisition | (215) | |
| Pseudomonas spp. | 1) PaE: Pseudomonas elastase | C1q, C3 | Degradation of C1q and C3 | (216) |
| 2) PaAP: Pseudomonas alkaline protease | C1q, C3 | Degradation of C1q and C3 | (216) | |
| 3) Tuf: elongation factor | Factor H, FHL-1 | Regulator acquisition | (217) | |
| Staphylococcus spp. | 1) CHIPS: chemotaxis inhibitory protein of S. aureus. | C5Ar | Antagonizes C5a | (218) |
| 2) Efb: extracellular fibrinogen-binding protein | C3, C3b and C3d | Inhibition of C3 and C3b containing convertases | (26), (27), (219) | |
| 3) SAK: Staphylokinase | C3b, IgG | Degradation of IgG, removes C3b from surface | (220) | |
| 4) Sbi: S. aureus IgG-binding protein | IgG | Inhibits IgG interaction with C1q | (221) | |
| 5) SCIN: staphylococcal complement inhibitor | C3 convertase | Prevents cleavage of C3 into C3a and C3b | (219), (220) | |
| 6) SpA: S. aureus protein A | IgG, C1q | Inhibits IgG interaction withC1q | (222) | |
| 7) SSL-7: staphylococcal superantigen-like protein 7 | C5 | Inhibits C5 cleavage | (223) | |
| Streptococcus spp. | 1) Bac protein | IgA, Factor H | Regulator acquisition | (224) |
| 2) Fba: fibronectin-binding protein | Factor H, FHL-1 | Regulator acquisition | (225) | |
| 3) Hic b: factor H-binding inhibitor of complement | Factor H | Regulator acquisition | (21) | |
| 4) M protein: surface proteins M family (Arp, Sir, etc.) | Factor H, FHL-1, C4BP | Regulator acquisition | (22) | |
| 5) PLY: pneumolysin | IgG, C1q | Complement activation and depletion | (226), (227) | |
| 6) PspA: pneumococcal surface protein A | Unknown | Impairing complement receptors | (25) | |
| 7) PspC: pneumococcal surface protein C | Factor H, C3 | Regulator acquisition, degradation of C3 | (228), (229) | |
| 8) scpA/B: streptococcal C5a peptidase | C5a | Degradation of C5a | (230) | |
| 9) SIC: streptococcal inhibitor of complement | C5b-7, C5b-8 | MAC formation prevention | (28), (231) | |
| 10) SPE B: streptococcal pyrogenic exotoxin B | Properdin, Ig's | Degrades Properdin | (232) | |
| 11) SpG: Streptococcus protein G | IgG | Inhibits IgG interaction with C1q | (233) | |
| Yersinia spp. | YadA − yersinia adhesin | Factor H | Regulator acquisition | (234) |
Although the contact cascade may activate complement for bacterial lysis, contact activation directly on the surface of bacteria also eliminates invading bacteria in a complement independent manner. Bacterial activation of the contact system releases kinin which in turn leads to the production of potent anti-microbial peptides and recruits other immune components to the site of infection [33], [34]. Despite activation of the immune response, kinin release and subsequent vasodilation may favor the bacteria due to an influx of plasma nutrients to the site of infection and increasing microcirculation of bacteria [34]. A massive activation of the contact system also causes excessive consumption of thrombin which results in hypovolemic shock and sepsis-induced coagulation [35]. Bacteria initiate the contact cascade and induce kinin release by a) producing proteases to degrade kininogens; b) activating FXII; or c) using structural elements and surface proteins that activate the contact cascade [36], [37], [38]. Several pathogens, including Porphyromonas gingivalis [39], Staphylococcus aureus [5] and S. pyogenes [40], produce proteins that degrade kininogens to kinins, with or without the release of bradykinin. As expected, the excess kinin release results in an array of pathological complications for the host.
Other bacteria produce proteases which activate FXII to produce kinin. For example, alkaline proteinase and elastase produced by Pseudomonas spp., Vibrio proteinase produced by Vibrio vulnificus, and V8 proteinase produced by S. aureus all activate FXII [41]. Many structural elements on bacterial surfaces including lipopolysaccharide of Gram negative bacteria and lipoteichoic of Gram positive bacteria activate the contact system [38]. Surface proteins of pathogens such as M protein of S. pyogenes and curli fibers of Escherichia coli and Salmonella typhii also bind to contact system components and initiate the release of bradykinin. Recent evidence suggests that patients with severe sepsis have abnormally high levels of kinins [42] which increase expression of bradykinin receptors BKR1 and BKR2 [43]. Binding of bradykinin to these receptors causes a massive pro-inflammatory response which is often detrimental to the host.
As key participants in the humoral innate immune response, NAb specific for pathogenic bacteria exist in the sera of uninfected individuals and play a critical role in the early clearance of invading bacteria by activating complement. Saliva contains secretory-IgA which recognizes and clears S. pyogenes [44]. Others demonstrated that NAb titers increase with bacterial load during Pseudomonas spp. infections, correlating with bacterial elimination [45]. N. gonorrhoeae induces NAb (IgM, IgG and IgA isotypes) which recognize the heat-stable but not the heat-labile bacterial antigens [46]. In contrast, after immunization, Ab recognize both heat-stable and heat-labile antigens.
Many NAb recognize commensals including the enterics, E. coli and Salmonella typhosa. The presence of NAb prevents the overgrowth of enterics and the gut microbiota [47]. Similarly, NAb mediate protection against the respiratory pathogen, S. pneumonia. As early respiratory tract colonizers of infants, pneumococcal antigens may stimulate the production of these Ab [48]. NAb not only keep commensals in check but also play a crucial role in preventing dissemination of intracellular bacteria. For example, NAb enhance antigen-trapping of Listeria monocytogenes in secondary lymphoid organs [10].
Although NAb are extremely effective in initiating an early response against invading bacteria, reports as early as 1972 indicated that NAb concentrations decrease with severity of bacterial sepsis [49]. Late in sepsis, when the bacterial burden surpasses a specific threshold, the plasma NAb concentrations plummet due to the large number of antigen-Ab complexes [49]. Together, the complexes and bacterial endotoxin increase the permeability of the capillaries, allowing the reticuloendothelial system to rapidly clear the Ab complexes.
As clinical biomarkers of infection, the concentration of CRP, PTX3 and other pentraxins increase within 6–12 h post infection [50]. The increased levels appear to be produced by human monocytes, macrophages and dendritic cells in response to whole bacteria or bacterial cell wall components. For example, Pseudomonas aeruginosa and Mycobacterium bovis Bacille Calmette–Guerin stimulate production of PTX3 [51], [52]. In addition, mycobacterial cell wall component lipoarabinomannan and lipopolysaccharide stimulate PTX3 expression in human peripheral mononuclear cells [52], [53]. In contrast, CRP, produced in response to Neisseria meningitides, opsonizes the bacteria for enhanced phagocytosis by macrophages [54]. Recent studies demonstrate that C1q recognizes pentraxins bound to pathogen surfaces and target the bacteria for destruction [55], [56]. Other pentraxin–bacterial interactions may also exist. Thus, additional studies examining the complex interactions between bacteria and pentraxins will enhance our understanding of host-bacteria interactions.
Together the components of the humoral innate immune system play an indispensible role in the removal of bacteria from a host. NAb, the contact system and pentraxins aid in eliminating bacteria and activate the complement system which lyses additional bacteria. However, bacteria have evolved several escape mechanisms which may be used against the host. In addition, bacterial complement inhibitors may be used for treatment of diseases involving excessive complement activation. Understanding this complex interaction between the bacteria and humoral innate immune response is crucial in designing effective therapeutic interventions against bacterial infections.
3.2. Humoral innate immune component interactions with viruses
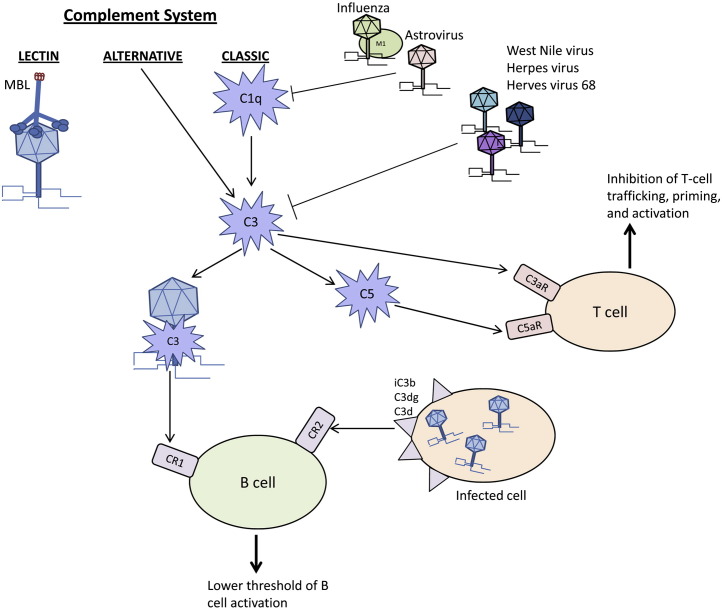
As a component of humoral immunity and a mediator between the innate and adaptive immune response, complement can directly neutralize viruses and modulate pathogen elimination (Fig. 1 ). MBL directly binds multiple viral glycoproteins including those of HIV, SARS coronavirus, and Marburg virus [57], [58], [59]. Additionally, C3-coated glycoproteins bind CR1 and enhance the humoral immune response [60], [61]. In a similar fashion, CR2 recognizes C3 cleavage products iC3b, C3dg and C3d to lower the threshold of B cell activation [62]. Importantly, C1q, C3, C4 and CR1 and CR2 contribute to the normal antiviral IgM or IgG responses and modulate humoral immunity indicating a crucial role for complement activation in the immune response to viruses [10], [63].
Figure 1.
Schematic representation of the interaction between the three pathways of the complement cascade and viruses. Complement removes virions and initiates the adaptive response to remove virally-infected cells. Viruses also inhibit complement at C1q and C3.
Viruses use different mechanisms to counter and disrupt the carefully regulated complement cascade of enzymes, protein complexes and receptors. Viruses evade the host immune response by a) modifying C1q or C3; b) entering host cells; or c) using host or virally produced complement inhibitors to prevent cell lysis. Viral proteins directly interact with the components of the complement cascade to disrupt complement-mediated destruction. C1q and C3 are frequent targets of the viral proteins. For example, the coat protein of human astrovirus type 1 binds C1q, displaces the C1r/C1s tetramer and inhibits the activation of the classical complement pathway [64]. Similarly, the matrix (M1) protein of influenza A virus binds C1q but this viral protein blocks the interaction between C1q and IgG [65]. Many other viruses modulate C3 as a central component of the complement cascade. West Nile virus synthesizes two isoforms of NS1 protein to regulate C3. Soluble NS1 increases Factor I-mediated cleavage of C3b to iC3b while the cell surface-bound NS1 decreases deposition of C3b and MAC [66]. Herpes virus synthesizes trans-membrane glycoprotein gC1 and gC2, which bind C3b and specifically accelerate the decay of the alternative C3 convertase and inhibit the interaction of C3b with C5 and properdin [67], [68]. Together these studies indicate that viruses interact with multiple complement components to prevent complement-mediated host cell death.
Viruses also modulate the regulators of complement activation by encoding viral proteins with structural and functional homology to host proteins or by recruiting host complement regulatory proteins to the virion. For example, gamma-herpes virus 68 induces expression of a complement inhibitory protein on the cell surface which may be detected in supernatants of infected cells. In vitro studies demonstrated that the viral inhibitory proteins block C3 deposition by both the classical and alternative activation pathways [69]. Kaposi's sarcoma-associated herpes virus, herpes virus saimiri, variola virus, vaccinia virus, monkeypox virus and ectromelia virus also encode regulators of the complement cascade which bind C3b or C4b and block activity [70], [71], [72], [73], [74], [75], [76], [77]. Other viruses recruit host complement regulatory proteins to virions to evade complement-mediated destruction. Human immunodeficiency virus-1 (HIV-1), human T-lymphotropic virus-1 (HTLV-1) and human cytomegalovirus (HCMV) incorporate the complement control proteins CD55 and CD59 into their virions to circumvent the complement response [78], [79]. Thus, complement inhibitors, either from the host or virally synthesized, protect the infected cell from lysis and allow virus proliferation.
Viruses induce complement activity for their own benefit. HIV enters human CD4+ T cells through complement receptors. HIV gp41 and gp120 proteins activate complement through the classical and lectin pathways, respectively. At the same time, the above two proteins inhibit MAC formation by recruiting Factor H and CD59 to the surface of the virally-infected host cell to abolish complement-mediated lysis [80]. Therefore, complement aids in viral entry but the virus prevents cell lysis by inhibiting the remainder of the complement cascade.
Complement activation during viral infection frequently causes endothelial cell damage and activation of the contact cascade. Recent studies show that herpes simplex virus (HSV), HCMV, and Hanta virus enhance thrombin formation and fibrinolysis [81], [82]. The HSV glycoprotein binds Factor X to induce the generation of thrombin [83]. HSV infection also decreases endothelial heparin sulfate proteoglycan (HSPG) that recruits and binds anti-thrombin III [84]. Other viruses, including Dengue virus and HIV, are also associated with decreased thrombin generation [85]. Dengue virus produces the nonstructural protein, NS1, that binds and inhibits prothrombin activation [86].
Viruses are able to modulate cytokine expression to induce a pro-coagulant state. Mediated by IL-1, TNFα, and IL-6, Marburg virus, Ebola virus and Hanta virus induce tissue factor expression on the endothelial surface [87], [88]. Viruses also take advantage of coagulation factors to enhance viral binding and replication. Human species A adenovirus-18 (HAdV-18) and 31(HAdV-31) bind coagulation Factor IX to facilitate virus entry and infection. HAdV-5 and other human adenoviruses utilize coagulation Factor X for the infection of host cells [89]. Sindbis viruses up-regulate the expression of BKR2 receptors on endothelial cells, and subsequently enhance viral replication by reducing Sindbis virus-induced apoptosis in a BKR2 dependent manner [90]. Thus, viruses also modulate the contact cascade and thrombin activity to promote viral infection.
The broad reactivity of individual NAb allows rapid recognition and protection from pathogens never encountered before. By bridging the innate and adaptive immune response, NAb facilitate antigen uptake, processing and presentation by B cells. For example, sera from naïve mice contain IgM NAb specific for lymphocytic choriomeningitis virus, vaccinia virus and two Vesicular stomatitis virus serotypes,VSV-New Jersey and VSV-Indiana [91]. In addition, NAb bind viruses at an early stage of infection to prevent viral dissemination to vital target organs. Moreover, NAb present in IVIg bind CCR5 to inhibit CCR5-tropic HIV infection in macrophages and lymphocytes.
The relatively low levels and limited specificity of NAb is not sufficient to provide complete immune protection. They also show limited neutralization, opsonization and complement binding ability compared to specific Ab of the adaptive response. Early in influenza virus infection, NAb bind to the hemagglutinin molecules of influenza A and B, neutralize the viruses and provide initial protection before the emergence of antigen-induced Ab produced by the adaptive response [92], [93], [94]. High titers of influenza virus occur in the lungs of mice which do not secrete IgM, and in the absence of soluble IgM NAb, the survival rate decreases significantly compare to wildtype mice [95]. The broad spectrum of NAb reactivity allows pre-infection sera to bind at least 12 influenza A and B strains. However, the level of influenza specific NAb does not increase as the disease progresses. These data demonstrate that NAb are distinct from antigen-induced Ab. NAb also inhibit CCR5-tropic HIV infection. IVIg contains anti-CCR5 Ab which inhibits HIV-1 infection of human macrophages and CD4+ T cells by CCR5-tropic but not CXCR4-tropic HIV-1. IVIg also contains NAb directed against multiple other cell surface molecules, including CD4, CD5, adhesion motif and CD95 [96]. These data indicate that NAb are an important part of the innate humoral immune response to viruses.
Pentraxins are a superfamily of multimeric proteins that responds to a variety of inflammatory stimuli to activate complement and prevent infection. As described earlier, the classic short pentraxins, CRP and SAP, are generally accepted as indicators of infection. But similar to bacteria, the prototypic long pentraxin PTX3 rapidly increases in response to viral infections. As a better protein marker of dengue virus infection than CRP [97], PTX3 also demonstrates antiviral functions in human or murine cytomegalovirus (HCMV or MCMV) and influenza virus. In these studies, PTX3 binds viruses and inhibits viral-cell fusion and internalization [98]. In influenza infection, sialylated ligands on PTX3 mimic the structure of the cellular receptors and bind the viral hemagglutinin glycoprotein and block the receptor-binding site of hemagglutinin [99]. Thus, pentraxins recognize and bind viral antigens to initiate the humoral innate immune response. However, the specific role of pentraxins in viral infection has not been well studied.
As part of humoral innate immunity, complement, contact system, NAb and pentraxins work together to develop elaborate networks of cascades in response to viral infections. These networks crosstalk with each other to recognize and eliminate invading viruses. However, viruses use numerous strategies to evade the immune response by compromising multiple proteins of the innate response simultaneously. Further efforts should examine the signaling pathways that modulate each component. Due to the complex interactions between components of the humoral response, future studies may need to examine the virus/host interactions together and not as individual components. Comprehensive research on antiviral mechanisms of the host and viral evasion mechanisms will provide insight into pathogenesis and novel treatment options.
4. Humoral innate immunity in chronic disease
4.1. Humoral innate immunity in atherosclerosis
Although the humoral innate immune response is critical in preventing bacterial and viral infections, the same components are frequently pathogenic in chronic disease. For example, complement plays a key role in the pathogenesis of atherosclerosis. Compared to non-atherosclerotic arteries, fibrous plaques up‐regulate transcripts of the classical complement cascade proteins [100]. Additionally, deposition of classical complement proteins, including C1q, C3, C4 and MAC occurs in atherosclerosis [101]. The alternative complement pathway is also implicated in plaque development or atherogenesis. For example, plasma from mice with high-fat diet-induced atherosclerotic lesions contained elevated C3, properdin and factor D levels [102]. Endotoxin- and diet-induced atherosclerosis in LDL receptor-deficient mice also requires the alternative pathway, factor B [103]. Importantly, C6 deficiency protects against diet-induced atherosclerosis, indicating that the terminal complement pathway is required in progression of atherosclerotic lesions [104]. Indeed, MAC deposition correlates with the severity of arterial damage in human aortic fibrous plaque [105] and in mice, MAC deposition promotes endothelial damage [106]. Endothelial MAC deposition also preceded monocyte infiltration and foam cell formation in a rabbit model of atherosclerosis [104]. Together, the data indicate that multiple complement pathways of activation increase the risk of atherosclerosis.
Complement regulatory proteins such as CD59 maintain the balance of complement activation and inhibition. Lewis et al. [107] found that MAC contributed to atherogenesis in apolipoprotein E−/− mice and CD59 deficiency exacerbated the disease. Similarly, Wu et al. demonstrated that the loss of CD59 accelerated atherosclerosis while endothelial CD59 overexpression attenuated endothelial damage. Finally, CD59 deficiency accelerated lesion development and increased plaque vascular smooth muscle cell composition in an atherosclerotic mouse model [108]. Thus, CD59 and possibly other complement inhibitors maintain homeostasis.
Although NAb activate complement, these Ab also aid in the clearance of pro-atherogenic debris (Fig. 2 ). NAb recognize pro-inflammatory, oxidation-specific epitopes associated with oxidative stress, apoptosis and atherosclerosis [109]. Recently, Chang et al. [110] reported that the oxidized phospholipid specific, NAb clone, T15/E06 attenuated endothelial activation and subsequent atherogenesis. Other in vitro studies indicated that IgM NAb recognized malondialdehyde, malondialdehyde low density lipoprotein (LDL) and oxidized LDL. The recognition of oxidized LDL prevented macrophage uptake, inhibited foam cell formation and atherogenesis [109], [111], [112]. However, under other conditions such as myocardial infarction, NAb are pathological. Compared to wildtype mice, Ab deficient mice experience significantly less tissue damage in response to myocardial infarction. Importantly, administering a single NAb clone restores tissue damage and inflammation to the Ab deficient mice [113], [114]. In addition, peptide inhibition of the NAb binding attenuates myocardial infarction induced injury in wildtype mice [113]. NAb play a key role in the modulation of atherosclerosis by clearing apoptotic cells and oxidized structures; however, excessive complement activation in response to NAb binding may be detrimental and cause pathology.
Figure 2.
Role of humoral innate immunity in atherosclerosis. Pentraxins interact with oxidized LDL (oxLDL) or enzymatically modified LDL (eLDL) facilitating foam cell formation. Complement facilitates macrophage extravasation and foam cell formation that release proinflammatory factors and enhance atherosclerosis. NAb may recognize and prevent foam cell formation. Taken together, the humoral innate immune response modulates atherosclerosis.
Complement has an intimate relationship with the contact system, suggesting that many coagulation factors also play key roles in the development of atherosclerosis [6]. Increased cardiovascular disease risk and atherosclerotic vascular damage are associated with elevated levels of Factor XII, prekallikrein, high molecular weight kininogen and Factor XI [115], [116]. In addition, Factor XII−/− mice are protected from arterial thrombosis and stroke indicating an activation of the contact system [117]. However, the specific crosstalk between the contact and complement systems in atherosclerosis is still poorly understood and should be addressed in future investigations.
It is well established that pentraxins activate the complement cascade in atherosclerosis [118]. Recent research demonstrates pentraxins may have a direct role in inducing atherosclerosis (Fig. 2). Pentraxins deposit in the atherosclerotic plaques where they interact with enzymatically modified or oxidized lipoproteins, promote foam cell formation, induce endothelial cell dysfunction and exacerbate atherosclerosis [12].
Although controversial, CRP appears to play a role in atherosclerosis. Detectable in the arterial wall in the early stages of atherogenesis, CRP continues to accumulate with disease progression [100]. By inhibiting endothelial production of nitric oxide, CRP impairs vasodilation and angiogenesis [119] and facilitates endothelial cell apoptosis, leading to atherosclerotic lesion formation. In addition, by increasing the expression and activity of endothelial cell plasminogen activator inhibitor-1 (PAI-1), a fibrinolysis inhibitor, CRP potentially contributes to plaque instability and atherothrombosis [120]. CRP also enhances endothelial cell adhesion molecules [121], superoxide production [122] and promotes monocyte recruitment into the plaque [12], all of which may contribute to atherogenesis. CRP also binds to enzymatically modified or oxidized LDL; however, the beneficial nature of this binding remains controversial in the clinical setting [123], [124]. A meta-analysis of a large multi-nation statin trial found that increased CRP levels identified only a limited population at risk with no clinical outcomes [125]. Thus, additional clinical studies are required prior to increased CRP screening of the population.
Similar to NAb, CRP has also been implicated in myocardial infarction in a complement-dependent manner. Early studies indicated that CRP activates C1q of the classical complement pathway and that monomeric CRP binds the alternative complement pathway inhibitor, factor H (reviewed in [126]). Additional studies found CRP colocalized with C1q and C3 within the ischemic tissue following myocardial infarction [127]. Recent studies indicate that monomeric CRP on necrotic cell surfaces binds both C1q and the classical pathway inhibitor C4bp to regulate the removal of injured cells in the absence of significant inflammation [128]. Thus, CRP appears to enhance opsonization without the subsequent inflammatory response.
Additional pentraxins also play a role in atherosclerosis. SAP was also detected in human atherosclerotic lesions [129]. By binding amyloid-β (Aβ) and serum amyloid component A (SAA), SAP mediates the inflammatory response to amyloid fibrils in atherosclerosis [130]. In contrast, SAP binds and prevents the uptake of oxLDL by macrophages, possibly reducing foam cell formation [131]. Similar to CRP and SAP, elevated expression of PTX3 precedes atherosclerotic lesion development [132] and correlates with acute myocardial infarction [133]. PTX3 increases tissue factor expression in endothelial cells, potentially inducing atherothrombosis [134]. Together, these results indicate a causal role of pentraxins in atherogenesis, suggesting that they may be effective therapeutic targets for atherosclerosis.
In conclusion, the pathology of atherosclerosis is a complex, multi-factorial process involving lipid deposition, vessel wall dysfunction, inflammation and autoimmunity. The humoral innate immune components play a pivotal role in the progression of this chronic disease. Activation of each component may drive atherogenesis; whereas NAb may also protect against atherosclerosis by binding to oxidation-specific epitopes. It should be noted that these components do not act independently but interact with each other, collectively modulating the disease progression. Further research is warranted to examine the integrated role of these humoral innate immune components in atherosclerosis. Such insights may yield a new understanding of the mechanisms underlying this disease and lead to novel therapeutic approaches to attenuate the progression of atherosclerosis.
4.2. Cancer and humoral innate immune response
Complement products such as C1q, C3, C3a, C4, C5 and the MAC are detectable in the tumor microenvironment [135]. These activated complement proteins have three mechanisms for complement-mediated destruction of tumor cells: a) complement-dependent cytotoxicity (CDC) [136], b) indirect Ab-dependent, cell-mediated cytotoxicity [137], which can be complement receptor-dependent and c) CR3-dependent cellular cytotoxicity (CR3-DCC) [138], which is relatively rare with tumors. Complement components are deposited in various tumor types, indicating that activation of complement may contribute to immunosurveillance of malignant cells. Complement proteins C5b-9 are deposited on the cellular surface of breast cancer cells and papillary thyroid carcinoma cells [139], [140]. Complement activation products, such as C1q and C3 cleavage products are detectable in ovarian carcinoma patients [141]. Complement is activated and deposited in neoplastic tissue, but the functional implications are unclear.
Tumor cells are resistant to complement‐mediated attack [142]. Tumor cells have natural mechanisms for self-protection against the complement system, specifically MAC and the cytotoxic activation of CR3. Extracellular protectors such as membrane and soluble complement inhibitors are released by tumor cells into the microenvironment and interfere with complement cascade activation and limit the quantity of complement deposition [143], [144]. Membrane complement inhibitors, including CD35 (CR1), CD46 (MCP) and CD55 (DAF) control the activation of complement at the level of C3. These serve as an important mechanism of self-protection, making the cells insensitive to the action of complement.
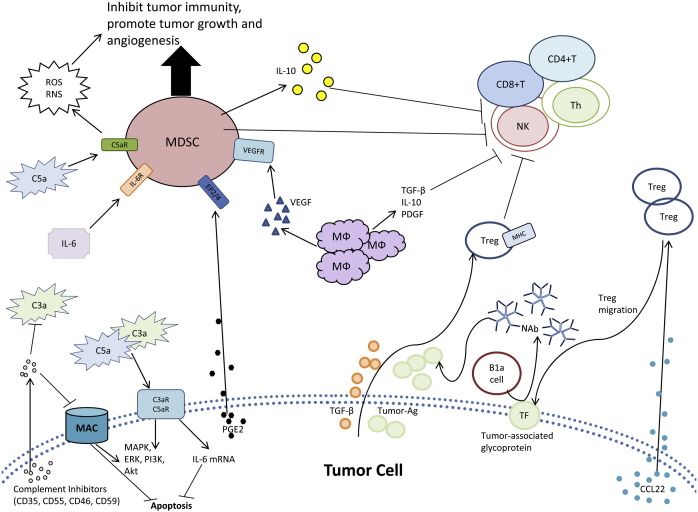
Although the complement system regulates inflammation and the innate immune response, complement proteins also aid in tumor growth and immunosuppression. For example, C3−/− or C4−/− mice display a significant decrease in tumor proliferation compared to wildtype mice [145]. These results may be linked to the anaphylatoxins, C3a and C5a, which participate in several signal transduction pathways linked to tumorigenesis (Fig. 3 ). The binding of C3a and C5a to their receptors increases IL-6, which induces transcriptional changes in cell cycle progression and inhibits apoptosis [146], [147]. Specifically, C3a receptor (C3aR) and C5a receptor (C5aR) activate MAPK, ERK, phosphatidylinosityol 3-kinase and Akt [148], [149]. Each of these signal transduction pathways may mediate oncogenic transformation and progression. Similar pathways may also be activated by the MAC to induce cellular proliferation [139], [150], [151]. Thus, complement proteins promote tumor growth through the upregulation of signaling molecules for cellular proliferation and by preventing apoptosis of neoplastic cells.
Figure 3.
Neoplastic cells exploit the humoral immune system by several mechanisms. The schematic diagram shows a variety of mechanisms tumor cells utilize to evade humoral immunity, regulate antibody effector mechanisms, and modulating leukocyte function in the microenvironment. TF, CD176; MDSC, myeloid-derived suppressor cell; MФ, macrophage.
Complement anaphylatoxins may alter cellular differentiation resulting in immune suppression. In healthy individuals, myeloid-derived suppressor (MDS) cells differentiate to macrophages, dendritic cells and neutrophils [152], [153]. However, when trapped in the intermediate stage of differentiation, MDS cells may mediate tumor-induced immune suppression [152], [153]. Tumor secreted factors induce pro-inflammatory mediators such as C5a, which recruit C5aR+ MDS to the tumor cells and disrupt normal differentiation of MDS cells (Fig. 3) [145]. Binding of C5a to C5aR on MDS increases reactive oxygen and nitrogen species that contribute to cell-mediated immunosuppression [145]. These complement stimulated MDS cells also prevent the activation of CD4+ and CD8+ T cells, inhibit Natural Killer cell cytotoxicity, stimulate cytokine production for tumorigenesis and increase angiogenesis [154], [155]. The complement system proteins such as C5a provide inflammatory protection in the tumor microenvironment.
Tumor-associated antigens are known to modulate trans‐membrane signaling that is required for proliferation, invasion and metastasis of tumor cells. The presence of NAb against tumor-associated antigens, such as gangliosides of melanoma cells, correlate with increased patient survival [156]. Tumor-reactive Ab exist in healthy wildtype animal blood samples (IgM) and peripheral blood concentrations of NAb increase shortly after initial tumor development and prior to detection of circulating antigens [157], [158], [159]. NAb may have a direct cytotoxic effect on tumor cells, while also inducing a bystander effect during a humoral anti-tumor response. Thus, NAb recognize tumor-modified cell surfaces that develop during tumorigenesis and activate complement to destroy nascently transformed cells.
Tumor cells are able to utilize NAb to escape immunosurveillance. Neoplastic transformation alters the expression of a variety of cell surface glycoproteins. An example of this is the Thomsen-Friedenreich antigen (CD176), a disaccharide (Gal beta 1–3 GalNAc alpha) with truncated glycosylations that is expressed at elevated levels on the surface of cancer cells [160]. An abnormal anti-CD176 titer correlates with primary carcinomas [161]. B1a cells produce NAb against CD176, as a form of immunosurveillance against CD176+ cancer cells [162]. Anti-CD176 NAb induce apoptosis in surface CD176+ cells [162]. Tumor cells escape Ab-dependent cytotoxicity by releasing soluble CD176 to bind NAb and protect the tumor cell from apoptosis [161], [163]. Although expression of CD176 is important for recognition of cancer cells, soluble CD176, secreted by tumor cells, prevents Ab dependent elimination. Tumor cells use the innate immune system and NAb to avoid immunosurveillance and elimination.
NAb are important for the recognition and elimination of precancerous and cancerous cells [164], [165], [166], [167], [168]. Such tumor-reactive NAb are expressed in multiple tumor types, including melanoma [169], lung [170], breast [171], head and neck [172] and ovarian cancers [173], [174]. As an example, the human monoclonal IgM Ab SAM-6, which was isolated from a gastric cancer patient, reacted with malignant tissue and induced the accumulation of intracellular lipids, cholesteryl ester and triglycerides [175], [176]. The humoral innate immune response and NAb specifically have an important role in the recognition and elimination of neoplastic cells.
NAb can be generated from tumor surface antigens. The auto-Ab produced by a patient's body in response to cancer cell formation can be utilized to detect the presence of cancer and to differentiate benign and malignant tumors [177]. Cell surface glycans that are secreted into the serum by malignant cells provide a mechanism to track tumor burden. Many malignant cells, but not normal cells, overexpress CD20, ECFR and HER2 allowing these proteins to be commonly used as diagnostic markers. These antigens also provide therapeutic targets for production of tumor antigen-specific monoclonal Ab.
Human cancers are known to produce extracellular proteolytic enzymes required for invasion and dissemination. Tumors that release these proteolytic enzymes breakdown the basement membrane and extracellular matrix (ECM) to facilitate cancer cell invasion into the surrounding normal tissue [178], [179]. By regulating production of ECM proteases, plasminogen activator (PA) is suggested to play a role in this process [178], [180]. The primary PA studied in relation to cancer metastasis is the urokinase-type (u-PA) which generates plasmin for the degradation of ECM [181]. Compared to the normal or benign tissue, malignant tumors produce elevated levels of u-PA [182]. U-PA and/or the receptor, U-PAR are prognostic markers in human malignancies, including breast cancer [183], lung cancer [184], [185], bladder cancer [186], bone marrow [187] stomach cancer [188], colorectal [189] and cervical cancer [190]. The binding of u-PA to its receptor u-PAR appears to be necessary for cancer metastasis [191], [192]. As a component of the contact system, u-PA and u-PAR levels correlate with poor prognosis and both are required for successful invasion and metastasis.
The u-PA/plasmin system is also involved in other cellular processes in tumorigenesis, including the release of growth factors. As an example, the binding of u-PA to u-PAR generates plasmin for the activation of the latent form of TGF-β, which regulates other cellular functions, including PAI-1 [193], [194]. The migration and invasion of cancer cells requires changes in cadherin and integrin expression and binding which leads to alterations in the expression of u-PA, u-PAR and PAI-1 [195]. For example, cancer cell detachment requires a down-regulation of E-cadherin expression [196], which increases expression of u-PA and invasiveness in vitro [197].
Plasminogen activators ultimately activate prekallikrein and kallikrein to generate bradykinin. Bradykinin and hydroxypropyl-(Hyp) bradykinin are elevated in the blood plasma, peritoneal and pleural fluids of cancer patients [198], [199]. Bradykinin is the most potent permeability factor and pain inducer produced in the tumor microvasculature. The elevation in bradykinin mediates the enhanced vascular permeability which allows cell invasion in cancer patients [200], [201]. BKR2 is also upregulated in human and rodent cancer tissue [90]. The kallikrein cascade is also implicated in tumor formation. Matsumura et al. [202] showed that the continual generation of kinins stimulated proliferation of tumor cells through mitogenic actions and promoted diapedesis to enhance tumor cell metastasis [202]. Together, these data indicate that the contact system supports cell migration and invasion through multiple interactions with u-PA, u-PAR, PAI-1, kallikrein, kinins, ECM proteins, integrins, receptors, and growth factors that allow reorganization of the tumor microenvironment.
As a short pentraxin family member, CRP is synthesized by the liver in response to stimulation by IL-6 and IL-1 [203], [204]. Increased levels of CRP are observed in cancer patients [205], [206], [207]. As an example, CRP was reported to be elevated in 87% of metastatic breast cancer patients [208]. Many cancer patients also have elevated IL-1 and IL-6 levels [209], [210], [211], which stimulates CRP production. PTX3 is a serum biomarker of multiple human carcinomas due to its overexpression in cancerous tissue, including soft tissue liposarcomas and lung carcinoma [212], [213]. Breast cancer cell lines that overexpress PTX3 have activated anti-fibroblast growth factor 2 and a reduction in angiogenesis in vitro and in vivo [214].
Experimental and clinical data suggest that the humoral innate immune responses initiate significant pro-tumor effects on the developing neoplasia. Humoral innate immune responses exacerbate recruitment and activate immune cells in the tumor microenvironment. They regulate tissue remodeling, pro-angiogenic and pro-survival pathways that potentiate cancer formation. Thus, the innate immune system is an important regulator of cancer development.
5. Conclusions
The humoral immune system plays a role in the initiation and regulation of the inflammatory response and elimination of pathogens. Derived from many small plasma proteins, components of the innate humoral immune response disrupt the target cell's plasma membrane and induce cytolysis. Together, the humoral and cellular immune responses control bacterial and viral replication and eliminate infected and altered cells. However, pathogens have evolved strategies to counter and evade the immune response. In addition, the humoral innate response may be exploited by uncontrolled proliferative cancer cells and atherosclerotic lesions resulting in continued expansion despite the humoral immune response. Recognizing the strategies used by pathogens and altered cells allows a better understanding of the pathological interactions between cell (infected or altered) and the host.
A wide spectrum of humoral components actively play a role in disease and we present conclusive evidence that the humoral immune system modifies the course of disease to enhance pathogenesis. Understanding the relationship between pathogenesis and humoral immune components will enhance the probability of targeted therapeutic treatments and patient survival. Further research is needed to determine the mechanisms of action and interactions of multiple components of humoral immunity, as well as their role in the pathogenesis of infection, neoplasia, and atherosclerosis.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by grants from Kansas Bioscience Authority, KBA-CBRI 611310 (ZL), Johnson Center for Basic Cancer Research (SDF) and NIH grants: AI061691 (SDF), 1R15CA152922 (SNS). AI077782 (SV) as well as P20 RR017686 (SDF), and P20 RR016475 (SDF) from the Institutional Development Award Program of the National Center for Research Resources.
References
- 1.Carroll M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 2.Kimura Y., Madhavan M., Call M.K., Santiago W., Tsonis P.A., Lambris J.D., Del Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- 3.Molina H., Holers V.M., Li B., Fung Y., Mariathasan S., Goellner J., Strauss-Schoenberger J., Karr R.W., Chaplin D.D. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renne T. The procoagulant and proinflammatory plasma contact system. Semin. Immunopathol. 2012;34:31–41. doi: 10.1007/s00281-011-0288-2. [DOI] [PubMed] [Google Scholar]
- 5.Imamura T., Tanase S., Szmyd G., Kozik A., Travis J., Potempa J. Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J. Exp. Med. 2005;201:1669–1676. doi: 10.1084/jem.20042041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamad O.A., Back J., Nilsson P.H., Nilsson B., Ekdahl K.N. Platelets, complement, and contact activation: partners in inflammation and thrombosis. Adv. Exp. Med. Biol. 2012;946:185–205. doi: 10.1007/978-1-4614-0106-3_11. [DOI] [PubMed] [Google Scholar]
- 7.Amara U., Rittirsch D., Flierl M., Bruckner U., Klos A., Gebhard F., Lambris J.D., Huber-Lang M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avrameas S. Natural autoantibodies — from horror autotoxicus to gnothi seauton. Immunol. Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 9.Binder C.J., Shaw P.X., Chang M.K., Boullier A., Hartvigsen K., Horkko S., Miller Y.I., Woelkers D.A., Corr M., Witztum J.L. The role of natural antibodies in atherogenesis. J. Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R.M. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 11.Du Clos T.W., Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol. Res. 2004;30:261–277. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 12.Bassi N., Zampieri S., Ghirardello A., Tonon M., Zen M., Cozzi F., Doria A. Pentraxins, anti-pentraxin antibodies, and atherosclerosis. Clin. Rev. Allergy Immunol. 2009;37:36–43. doi: 10.1007/s12016-008-8098-6. [DOI] [PubMed] [Google Scholar]
- 13.Pepys M.B., Baltz M.L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv. Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 14.Hirschfield G.M., Herbert J., Kahan M.C., Pepys M.B. Human C-reactive protein does not protect against acute lipopolysaccharide challenge in mice. J. Immunol. 2003;171:6046–6051. doi: 10.4049/jimmunol.171.11.6046. [DOI] [PubMed] [Google Scholar]
- 15.Moalli F., Doni A., Deban L., Zelante T., Zagarella S., Bottazzi B., Romani L., Mantovani A., Garlanda C. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 16.Mold C., Baca R., Du Clos T.W. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J. Autoimmun. 2002;19:147–154. doi: 10.1006/jaut.2002.0615. [DOI] [PubMed] [Google Scholar]
- 17.Mold C., Gresham H.D., Du Clos T.W. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J. Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 18.Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 19.Qu H., Ricklin D., Lambris J.D. Recent developments in low molecular weight complement inhibitors. Mol. Immunol. 2009;47:185–195. doi: 10.1016/j.molimm.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallis R. Interactions between mannose-binding lectin and MASPs during complement activation by the lectin pathway. Immunobiology. 2007;212:289–299. doi: 10.1016/j.imbio.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen R., Thiel S., Jensenius J.C. Mannan-binding-lectin-associated serine proteases, characteristics and disease associations. Springer Semin. Immunopathol. 2005;27:299–319. doi: 10.1007/s00281-005-0006-z. [DOI] [PubMed] [Google Scholar]
- 22.Lambris J.D., Ricklin D., Geisbrecht B.V. Complement evasion by human pathogens. Nat. Rev. Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laarman A., Milder F., van Strijp J., Rooijakkers S. Complement inhibition by gram-positive pathogens: molecular mechanisms and therapeutic implications. J. Mol. Med. (Berl.) 2010;88:115–120. doi: 10.1007/s00109-009-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janulczyk R., Iannelli F., Sjoholm A.G., Pozzi G., Bjorck L. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 2000;275:37257–37263. doi: 10.1074/jbc.M004572200. [DOI] [PubMed] [Google Scholar]
- 25.Jarva H., Jokiranta T.S., Wurzner R., Meri S. Complement resistance mechanisms of streptococci. Mol. Immunol. 2003;40:95–107. doi: 10.1016/s0161-5890(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 26.Madico G., Welsch J.A., Lewis L.A., McNaughton A., Perlman D.H., Costello C.E., Ngampasutadol J., Vogel U., Granoff D.M., Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraiczy P., Skerka C., Kirschfink M., Brade V., Zipfel P.F. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur. J. Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Ren B., McCrory M.A., Pass C., Bullard D.C., Ballantyne C.M., Xu Y.Y., Briles D.E., Szalai A.J. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 2004;173:7506–7512. doi: 10.4049/jimmunol.173.12.7506. [DOI] [PubMed] [Google Scholar]
- 29.Hammel M., Sfyroera G., Ricklin D., Magotti P., Lambris J.D., Geisbrecht B.V. A structural basis for complement inhibition by Staphylococcus aureus. Nat. Immunol. 2007;8:430–437. doi: 10.1038/ni1450. [DOI] [PubMed] [Google Scholar]
- 30.Lee L.Y.L., Hook M., Haviland D., Wetsel R.A., Yonter E.O., Syribeys P., Vernachio J., Brown E.L. Inhibition of complement activation by a secreted Staphylococcus aureus protein. J. Infect. Dis. 2004;190:571–579. doi: 10.1086/422259. [DOI] [PubMed] [Google Scholar]
- 31.Akesson P., Sjoholm A.G., Bjorck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- 32.Thurlow L.R., Thomas V.C., Narayanan S., Olson S., Fleming S.D., Hancock L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect. Immun. 2010;78:4936–4943. doi: 10.1128/IAI.01118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalter E.S., Daha M.R., Tencate J.W., Verhoef J., Bouma B.N. Activation and inhibition of hageman factor-dependent pathways and the complement-system in uncomplicated bacteremia or bacterial shock. J. Infect. Dis. 1985;151:1019–1027. doi: 10.1093/infdis/151.6.1019. [DOI] [PubMed] [Google Scholar]
- 34.Frick I.M., Bjorck L., Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb. Haemost. 2007;98:497–502. [PubMed] [Google Scholar]
- 35.Lottenberg R. Contact activation proteins and the bacterial surface. Trends Microbiol. 1996;4:413–414. doi: 10.1016/0966-842x(96)30031-0. (discussion 414–415) [DOI] [PubMed] [Google Scholar]
- 36.Ben Nasr A., Olsen A., Sjobring U., Muller-Esterl W., Bjorck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol. Microbiol. 1996;20:927–935. doi: 10.1111/j.1365-2958.1996.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 37.Herwald H., Morgelin M., Bjorck L. Contact activation by pathogenic bacteria: a virulence mechanism contributing to the pathophysiology of sepsis. Scand. J. Infect. Dis. 2003;35:604–607. doi: 10.1080/00365540310016268. [DOI] [PubMed] [Google Scholar]
- 38.Kalter E.S., van Dijk W.C., Timmerman A., Verhoef J., Bouma B.N. Activation of purified human plasma prekallikrein triggered by cell wall fractions of Escherichia coli and Staphylococcus aureus. J. Infect. Dis. 1983;148:682–691. doi: 10.1093/infdis/148.4.682. [DOI] [PubMed] [Google Scholar]
- 39.Scott C.F., Whitaker E.J., Hammond B.F., Colman R.W. Purification and characterization of a potent 70-Kda thiol lysylproteinase (Lys-Gingivain) from porphyromonas-gingivalis that cleaves kininogens and fibrinogen. J. Biol. Chem. 1993;268:7935–7942. [PubMed] [Google Scholar]
- 40.Herwald H., Collin M., Muller-Esterl W., Bjorck L. Streptococcal cysteine proteinase releases kinins: a virulence mechanism. J. Exp. Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molla A., Yamamoto T., Akaike T., Miyoshi S., Maeda H. Activation of hageman-factor and prekallikrein and generation of kinin by various microbial proteinases. J. Biol. Chem. 1989;264:10589–10594. [PubMed] [Google Scholar]
- 42.Mattsson E., Herwald H., Cramer H., Persson K., Sjobring U., Bjorck L. Staphylococcus aureus induces release of bradykinin in human plasma. Infect. Immun. 2001;69:3877–3882. doi: 10.1128/IAI.69.6.3877-3882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leeb-Lundberg L.M., Marceau F., Muller-Esterl W., Pettibone D.J., Zuraw B.L. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 44.Quan C., Berneman A., Pires R., Avrameas S., Bouvet J.P. Salivary natural antibodies as a basic immune barrier against group A streptococci. Streptococci Host. 1997;418:881–885. doi: 10.1007/978-1-4899-1825-3_207. [DOI] [PubMed] [Google Scholar]
- 45.Petras G., Nemeth C. Natural antibodies in Pseudomonas aeruginosa infections. Acta Microbiol. Acad. Sci. Hung. 1979;26:265–272. [PubMed] [Google Scholar]
- 46.Cohen I.R. Natural and immune human antibodies reactive with antigens of virulent Neisseria gonorrhoeae — immunoglobulins G M and A. J. Bacteriol. 1967;94:141–148. doi: 10.1128/jb.94.1.141-148.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michael J.G., Whitby J.L., Landy M. Studies on natural antibodies to gram-negative bacteria. J. Exp. Med. 1962;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briles D.E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Anti-phosphocholine antibodies found in normal mouse serum are protective against intravenous infection with Type-3 Streptococcus-pneumoniae. J. Exp. Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Backhausz R., Meretey K., Petras G. Changes in the natural antibody titer in course of the infectious complications developing in patients given artificial respiration. Ann. Immunol. Hung. 1972;16:51–63. [PubMed] [Google Scholar]
- 50.Simon L., Gauvin F., Amre D.K., Saint-Louis P., Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin. Infect. Dis. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 51.Doni A., Peri G., Chieppa M., Allavena P., Pasqualini F., Vago L., Romani L., Garlanda C., Mantovani A. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur. J. Immunol. 2003;33:2886–2893. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 52.Vouret-Craviari V., Matteucci C., Peri G., Poli G., Introna M., Mantovani A. Expression of a long pentraxin, PTX3, by monocytes exposed to the mycobacterial cell wall component lipoarabinomannan. Infect. Immun. 1997;65:1345–1350. doi: 10.1128/iai.65.4.1345-1350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polentarutti N., Bottazzi B., Di Santo E., Blasi E., Agnello D., Ghezzi P., Introna M., Bartfai T., Richards G., Mantovani A. Inducible expression of the long pentraxin PTX3 in the central nervous system. J. Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- 54.Casey R., Newcombe J., McFadden J., Bodman-Smith K.B. The acute-phase reactant C-reactive protein binds to phosphorylcholine-expressing Neisseria meningitidis and increases uptake by human phagocytes. Infect. Immun. 2008;76:1298–1304. doi: 10.1128/IAI.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sjoberg A.P., Trouw L.A., Blom A.M. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Bottazzi B., Doni A., Garlanda C., Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 57.Alexandre K.B., Gray E.S., Lambson B.E., Moore P.L., Choge I.A., Mlisana K., Karim S.S., McMahon J., O'Keefe B., Chikwamba R., Morris L. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins. Griffithsin, Cyanovirin-N and Scytovirin, Virology. 2010;402:187–196. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H., To Y.F., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Jensenius J.C., Turner M.W., Peiris J.S., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji X., Olinger G.G., Aris S., Chen Y., Gewurz H., Spear G.T. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 2005;86:2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez S.F., Lukacs-Kornek V., Kuligowski M.P., Pitcher L.A., Degn S.E., Turley S.J., Carroll M.C. Complement-dependent transport of antigen into B cell follicles. J. Immunol. 2010;185:2659–2664. doi: 10.4049/jimmunol.1000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang Y., Xu C., Fu Y.X., Holers V.M., Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J. Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 62.Bradbury L.E., Kansas G.S., Levy S., Evans R.L., Tedder T.F. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J. Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- 63.Mehlhop E., Whitby K., Oliphant T., Marri A., Engle M., Diamond M.S. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 2005;79:7466–7477. doi: 10.1128/JVI.79.12.7466-7477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonaparte R.S., Hair P.S., Banthia D., Marshall D.M., Cunnion K.M., Krishna N.K. Human astrovirus coat protein inhibits serum complement activation via C1, the first component of the classical pathway. J. Virol. 2008;82:817–827. doi: 10.1128/JVI.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J., Li G., Liu X., Wang Z., Liu W., Ye X. Influenza A virus M1 blocks the classical complement pathway through interacting with C1qA. J. Gen. Virol. 2009;90:2751–2758. doi: 10.1099/vir.0.014316-0. [DOI] [PubMed] [Google Scholar]
- 66.Avirutnan P., Fuchs A., Hauhart R.E., Somnuke P., Youn S., Diamond M.S., Atkinson J.P. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J. Exp. Med. 2010;207:793–806. doi: 10.1084/jem.20092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lubinski J., Nagashunmugam T., Friedman H.M. Viral interference with antibody and complement. Semin. Cell Dev. Biol. 1998;9:329–337. doi: 10.1006/scdb.1998.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kostavasili I., Sahu A., Friedman H.M., Eisenberg R.J., Cohen G.H., Lambris J.D. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 1997;158:1763–1771. [PubMed] [Google Scholar]
- 69.Kapadia S.B., Molina H., van Berkel V., Speck S.H., Virgin H.W.t. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 1999;73:7658–7670. doi: 10.1128/jvi.73.9.7658-7670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albrecht J.C., Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J. Virol. 1992;66:3937–3940. doi: 10.1128/jvi.66.6.3937-3940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullick J., Bernet J., Singh A.K., Lambris J.D., Sahu A. Kaposi's sarcoma associated herpesvirus (human herpesvirus 8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins. J. Virol. 2003;77:3878–3881. doi: 10.1128/JVI.77.6.3878-3881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spiller O.B., Robinson M., O'Donnell E., Milligan S., Morgan B.P., Davison A.J., Blackbourn D.J. Complement regulation by Kaposi's sarcoma-associated herpesvirus ORF4 protein. J. Virol. 2003;77:592–599. doi: 10.1128/JVI.77.1.592-599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Virgin H.W.t., Latreille P., Wamsley P., Hallsworth K., Weck K.E., Dal Canto A.J., Speck S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liszewski M.K., Leung M.K., Hauhart R., Buller R.M., Bertram P., Wang X., Rosengard A.M., Kotwal G.J., Atkinson J.P. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J. Immunol. 2006;176:3725–3734. doi: 10.4049/jimmunol.176.6.3725. [DOI] [PubMed] [Google Scholar]
- 75.Moulton E.A., Bertram P., Chen N., Buller R.M., Atkinson J.P. Ectromelia virus inhibitor of complement enzymes protects intracellular mature virus and infected cells from mouse complement. J. Virol. 2010;84:9128–9139. doi: 10.1128/JVI.02677-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosengard A.M., Liu Y., Nie Z., Jimenez R. Variola virus immune evasion design: expression of a highly efficient inhibitor of human complement. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8808–8813. doi: 10.1073/pnas.112220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahu A., Isaacs S.N., Soulika A.M., Lambris J.D. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 1998;160:5596–5604. [PubMed] [Google Scholar]
- 78.Saifuddin M., Parker C.J., Peeples M.E., Gorny M.K., Zolla-Pazner S., Ghassemi M., Rooney I.A., Atkinson J.P., Spear G.T. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J. Exp. Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spear G.T., Lurain N.S., Parker C.J., Ghassemi M., Payne G.H., Saifuddin M. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV) J. Immunol. 1995;155:4376–4381. [PubMed] [Google Scholar]
- 80.Datta P.K., Rappaport J. HIV and complement: hijacking an immune defense. Biomed. Pharmacother. 2006;60:561–568. doi: 10.1016/j.biopha.2006.07.087. [DOI] [PubMed] [Google Scholar]
- 81.Popovic M., Paskas S., Zivkovic M., Burysek L., Laumonnier Y. Human cytomegalovirus increases HUVEC sensitivity to thrombin and modulates expression of thrombin receptors. J. Thromb. Thrombolysis. 2010;30:164–171. doi: 10.1007/s11239-010-0447-7. [DOI] [PubMed] [Google Scholar]
- 82.Laine O., Makela S., Mustonen J., Huhtala H., Szanto T., Vaheri A., Lassila R., Joutsi-Korhonen L. Enhanced thrombin formation and fibrinolysis during acute Puumala hantavirus infection. Thromb. Res. 2010;126:154–158. doi: 10.1016/j.thromres.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 83.Etingin O.R., Silverstein R.L., Friedman H.M., Hajjar D.P. Viral activation of the coagulation cascade: molecular interactions at the surface of infected endothelial cells. Cell. 1990;61:657–662. doi: 10.1016/0092-8674(90)90477-v. [DOI] [PubMed] [Google Scholar]
- 84.Kaner R.J., Iozzo R.V., Ziaie Z., Kefalides N.A. Inhibition of proteoglycan synthesis in human endothelial cells after infection with herpes simplex virus type 1 in vitro. Am. J. Respir. Cell Mol. Biol. 1990;2:423–431. doi: 10.1165/ajrcmb/2.5.423. [DOI] [PubMed] [Google Scholar]
- 85.Hsue P.Y., Scherzer R., Grunfeld C., Nordstrom S.M., Schnell A., Kohl L.P., Nitta E., Martin J.N., Deeks S.G., Weiss E.J. HIV Infection Is Associated With Decreased Thrombin Generation. Clin. Infect. Dis. 2012;54:1196–1203. doi: 10.1093/cid/cis014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin S.W., Chuang Y.C., Lin Y.S., Lei H.Y., Liu H.S., Yeh T.M. Dengue virus nonstructural protein NS1 binds to prothrombin/thrombin and inhibits prothrombin activation prothrombin/thrombin and inhibits prothrombin activation. J. Infect. 2012:325–334. doi: 10.1016/j.jinf.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 87.Butthep P., Bunyaratvej A., Bhamarapravati N. Dengue virus and endothelial cell: a related phenomenon to thrombocytopenia and granulocytopenia in dengue hemorrhagic fever. Southeast Asian J. Trop. Med. Public Health. 1993;24(Suppl. 1):246–249. [PubMed] [Google Scholar]
- 88.Van Dam-Mieras M.C., Muller A.D., van Hinsbergh V.W., Mullers W.J., Bomans P.H., Bruggeman C.A. The procoagulant response of cytomegalovirus infected endothelial cells. Thromb. Haemost. 1992;68:364–370. [PubMed] [Google Scholar]
- 89.Lenman A., Muller S., Nygren M.I., Frangsmyr L., Stehle T., Arnberg N. Coagulation factor IX mediates serotype-specific binding of species A adenoviruses to host cells. J. Virol. 2011;85:13420–13431. doi: 10.1128/JVI.06088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhoola K.D., Figueroa C.D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 91.Battegay M., Cooper S., Althage A., Banziger J., Hengartner H., Zinkernagel R.M. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 92.Baumgarth N., Chen J., Herman O.C., Jager G.C., Herzenberg L.A. The role of B-1 and B-2 cells in immune protection from influenza virus infection. Curr. Top. Microbiol. Immunol. 2000;252:163–169. doi: 10.1007/978-3-642-57284-5_17. [DOI] [PubMed] [Google Scholar]
- 93.Clarke S.H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J. Exp. Med. 1985;161:687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baccala R., Quang T.V., Gilbert M., Ternynck T., Avrameas S. Two murine natural polyreactive autoantibodies are encoded by nonmutated germ-line genes. Proc. Natl. Acad. Sci. U. S. A. 1989;86:4624–4628. doi: 10.1073/pnas.86.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baumgarth N., Herman O.C., Jager G.C., Brown L.E., Herzenberg L.A., Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thornton B.P., Vetvicka V., Ross G.D. Natural antibody and complement-mediated antigen-processing and presentation by B-lymphocytes. J. Immunol. 1994;152:1727–1737. [PubMed] [Google Scholar]
- 97.Mairuhu A.T., Peri G., Setiati T.E., Hack C.E., Koraka P., Soemantri A., Osterhaus A.D., Brandjes D.P., van der Meer J.W., Mantovani A., van Gorp E.C. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J. Med. Virol. 2005;76:547–552. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- 98.Bozza S., Bistoni F., Gaziano R., Pitzurra L., Zelante T., Bonifazi P., Perruccio K., Bellocchio S., Neri M., Iorio A.M., Salvatori G., De Santis R., Calvitti M., Doni A., Garlanda C., Mantovani A., Romani L. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108:3387–3396. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 99.Reading P.C., Bozza S., Gilbertson B., Tate M., Moretti S., Job E.R., Crouch E.C., Brooks A.G., Brown L.E., Bottazzi B., Romani L., Mantovani A. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J. Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 100.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001;21:1214–1219. doi: 10.1161/hq0701.092160. [DOI] [PubMed] [Google Scholar]
- 101.Niculescu F., Rus H. Complement activation and atherosclerosis. Mol. Immunol. 1999;36:949–955. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 102.Recinos A., III, Carr B.K., Bartos D.B., Boldogh I., Carmical J.R., Belalcazar L.M., Brasier A.R. Liver gene expression associated with diet and lesion development in atherosclerosis-prone mice: induction of components of alternative complement pathway. Physiol. Genomics. 2004;19:131–142. doi: 10.1152/physiolgenomics.00146.2003. [DOI] [PubMed] [Google Scholar]
- 103.Malik T.H., Cortini A., Carassiti D., Boyle J.J., Haskard D.O., Botto M. The alternative pathway is critical for pathogenic complement activation in endotoxin- and diet-induced atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2010;122:1948–1956. doi: 10.1161/CIRCULATIONAHA.110.981365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmiedt W., Kinscherf R., Deigner H.P., Kamencic H., Nauen O., Kilo J., Oelert H., Metz J., Bhakdi S. Complement C6 deficiency protects against diet-induced atherosclerosis in rabbits. Arterioscler. Thromb. Vasc. Biol. 1998;18:1790–1795. doi: 10.1161/01.atv.18.11.1790. [DOI] [PubMed] [Google Scholar]
- 105.Vlaicu R., Niculescu F., Rus H.G., Cristea A. Immunohistochemical localization of the terminal C5b-9 complement complex in human aortic fibrous plaque. Atherosclerosis. 1985;57:163–177. doi: 10.1016/0021-9150(85)90030-9. [DOI] [PubMed] [Google Scholar]
- 106.Wu G., Hu W., Shahsafaei A., Song W., Dobarro M., Sukhova G.K., Bronson R.R., Shi G.P., Rother R.P., Halperin J.A., Qin X. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ. Res. 2009;104:550–558. doi: 10.1161/CIRCRESAHA.108.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis R.D., Jackson C.L., Morgan B.P., Hughes T.R. The membrane attack complex of complement drives the progression of atherosclerosis in apolipoprotein E knockout mice. Mol. Immunol. 2010;47:1098–1105. doi: 10.1016/j.molimm.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yun S., Leung V.W., Botto M., Boyle J.J., Haskard D.O. Brief report: accelerated atherosclerosis in low-density lipoprotein receptor-deficient mice lacking the membrane-bound complement regulator CD59. Arterioscler. Thromb. Va c. Biol. 2008;28:1714–1716. doi: 10.1161/ATVBAHA.108.169912. [DOI] [PubMed] [Google Scholar]
- 109.Hartvigsen K., Chou M.Y., Hansen L.F., Shaw P.X., Tsimikas S., Binder C.J., Witztum J.L. The role of innate immunity in atherogenesis. J. Lipid Res. 2009;50:S388–S393. doi: 10.1194/jlr.R800100-JLR200. (Suppl.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang M.K., Binder C.J., Miller Y.I., Subbanagounder G., Silverman G.J., Berliner J.A., Witztum J.L. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horkko S., Bird D.A., Miller E., Itabe H., Leitinger N., Subbanagounder G., Berliner J.A., Friedman P., Dennis E.A., Curtiss L.K., Palinski W., Witztum J.L. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Binder C.J., Horkko S., Dewan A., Chang M.K., Kieu E.P., Goodyear C.S., Shaw P.X., Palinski W., Witztum J.L., Silverman G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 113.Haas M.S., Alicot E.M., Schuerpf F., Chiu I., Li J., Moore F.D., Carroll M.C. Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc. Res. 2010;87:618–627. doi: 10.1093/cvr/cvq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M., Michael L.H., Grosjean S.A., Kelly R.A., Carroll M.C., Entman M.L. The role of natural IgM in myocardial ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2006:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Miller G.J., Esnouf M.P., Burgess A.I., Cooper J.A., Mitchell J.P. Risk of coronary heart disease and activation of factor XII in middle-aged men. Arterioscler. Thromb. Vasc. Biol. 1997;17:2103–2106. doi: 10.1161/01.atv.17.10.2103. [DOI] [PubMed] [Google Scholar]
- 116.Merlo C., Wuillemin W.A., Redondo M., Furlan M., Sulzer I., Kremer-Hovinga J., Binder B.R., Lammle B. Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease. Atherosclerosis. 2002;161:261–267. doi: 10.1016/s0021-9150(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 117.Gailani D., Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 118.Manolov D.E., Koenig W., Hombach V., Torzewski J. C-reactive protein and atherosclerosis. Is there a causal link? Histol. Histopathol. 2003;18:1189–1193. doi: 10.14670/HH-18.1189. [DOI] [PubMed] [Google Scholar]
- 119.Verma S., Wang C.H., Li S.H., Dumont A.S., Fedak P.W., Badiwala M.V., Dhillon B., Weisel R.D., Li R.K., Mickle D.A., Stewart D.J. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 120.Devaraj S., Xu D.Y., Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 121.Verma S., Yeh E.T. C-reactive protein and atherothrombosis–beyond a biomarker: an actual partaker of lesion formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1253–R1256. doi: 10.1152/ajpregu.00170.2003. (discussion R1257-1258) [DOI] [PubMed] [Google Scholar]
- 122.Ryu J., Lee C.W., Shin J.A., Park C.S., Kim J.J., Park S.J., Han K.H. FcgammaRIIa mediates C-reactive protein-induced inflammatory responses of human vascular smooth muscle cells by activating NADPH oxidase 4. Cardiovasc. Res. 2007;75:555–565. doi: 10.1016/j.cardiores.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 123.Bhakdi S., Torzewski M., Klouche M., Hemmes M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler. Thromb. Vasc. Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- 124.Chang M.K., Binder C.J., Torzewski M., Witztum J.L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abd T.T., Eapen D.J., Bajpai A., Goyal A., Dollar A., Sperling L. The role of C-reactive protein as a risk predictor of coronary atherosclerosis: implications from the JUPITER trial. Curr. Atheroscler. Rep. 2011;13:154–161. doi: 10.1007/s11883-011-0164-5. [DOI] [PubMed] [Google Scholar]
- 126.Mold C., Gewurz H., Du Clos T.W. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 127.Distelmaier K., Adlbrecht C., Jakowitsch J., Winkler S., Dunkler D., Gerner C., Wagner O., Lang I.M., Kubicek M. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb. Haemost. 2009;102:564–572. doi: 10.1160/TH09-02-0103. [DOI] [PubMed] [Google Scholar]
- 128.Mihlan M., Blom A.M., Kupreishvili K., Lauer N., Stelzner K., Bergstrom F., Niessen H.W., Zipfel P.F. Monomeric C-reactive protein modulates classic complement activation on necrotic cells. FASEB J. 2011;25:4198–4210. doi: 10.1096/fj.11-186460. [DOI] [PubMed] [Google Scholar]
- 129.Rocken C., Tautenhahn J., Buhling F., Sachwitz D., Vockler S., Goette A., Burger T. Prevalence and pathology of amyloid in atherosclerotic arteries. Arterioscler. Thromb. Vasc. Biol. 2006;26:676–677. doi: 10.1161/01.ATV.0000201930.10103.be. [DOI] [PubMed] [Google Scholar]
- 130.Myers S.L., Jones S., Jahn T.R., Morten I.J., Tennent G.A., Hewitt E.W., Radford S.E. A systematic study of the effect of physiological factors on beta2-microglobulin amyloid formation at neutral pH. Biochemistry. 2006;45:2311–2321. doi: 10.1021/bi052434i. [DOI] [PubMed] [Google Scholar]
- 131.Stewart C.R., Tseng A.A., Mok Y.F., Staples M.K., Schiesser C.H., Lawrence L.J., Varghese J.N., Moore K.J., Howlett G.J. Oxidation of low-density lipoproteins induces amyloid-like structures that are recognized by macrophages. Biochemistry. 2005;44:9108–9116. doi: 10.1021/bi050497v. [DOI] [PubMed] [Google Scholar]
- 132.Savchenko A., Imamura M., Ohashi R., Jiang S., Kawasaki T., Hasegawa G., Emura I., Iwanari H., Sagara M., Tanaka T., Hamakubo T., Kodama T., Naito M. Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J. Pathol. 2008;215:48–55. doi: 10.1002/path.2314. [DOI] [PubMed] [Google Scholar]
- 133.Peri G., Introna M., Corradi D., Iacuitti G., Signorini S., Avanzini F., Pizzetti F., Maggioni A.P., Moccetti T., Metra M., Cas L.D., Ghezzi P., Sipe J.D., Re G., Olivetti G., Mantovani A., Latini R. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 134.Napoleone E., Di Santo A., Bastone A., Peri G., Mantovani A., de Gaetano G., Donati M.B., Lorenzet R. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler. Thromb. Vasc. Biol. 2002;22:782–787. doi: 10.1161/01.atv.0000012282.39306.64. [DOI] [PubMed] [Google Scholar]
- 135.de Visser K.E., Korets L.V., Coussens L.M. Early neoplastic progression is complement independent. Neoplasia. 2004;6:768–776. doi: 10.1593/neo.04250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ostrand-Rosenberg S. Cancer and complement. Nat. Biotechnol. 2008;26:1348–1349. doi: 10.1038/nbt1208-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gelderman K.A., Tomlinson S., Ross G.D., Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 138.Xia Y., Vetvicka V., Yan J., Hanikyrova M., Mayadas T., Ross G.D. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 139.Niculescu F., Badea T., Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 140.Yamakawa M., Yamada K., Tsuge T., Ohrui H., Ogata T., Dobashi M., Imai Y. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994;73:2808–2817. doi: 10.1002/1097-0142(19940601)73:11<2808::aid-cncr2820731125>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 141.Bjorge L., Hakulinen J., Vintermyr O.K., Jarva H., Jensen T.S., Iversen O.E., Meri S. Ascitic complement system in ovarian cancer. Br. J. Cancer. 2005;92:895–905. doi: 10.1038/sj.bjc.6602334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fishelson Z., Donin N., Zell S., Schultz S., Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 143.Jurianz K., Ziegler S., Garcia-Schuler H., Kraus S., Bohana-Kashtan O., Fishelson Z., Kirschfink M. Complement resistance of tumor cells: basal and induced mechanisms. Mol. Immunol. 1999;36:929–939. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 144.Donin N., Jurianz K., Ziporen L., Schultz S., Kirschfink M., Fishelson Z. Complement resistance of human carcinoma cells depends on membrane regulatory proteins, protein kinases and sialic acid. Clin. Exp. Immunol. 2003;131:254–263. doi: 10.1046/j.1365-2249.2003.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Markiewski M.M., DeAngelis R.A., Benencia F., Ricklin-Lichtsteiner S.K., Koutoulaki A., Gerard C., Coukos G., Lambris J.D. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin W.W., Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haura E.B., Turkson J., Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 148.Schraufstatter I.U., Trieu K., Sikora L., Sriramarao P., DiScipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J. Immunol. 2002;169:2102–2110. doi: 10.4049/jimmunol.169.4.2102. [DOI] [PubMed] [Google Scholar]
- 149.Venkatesha R.T., Berla Thangam E., Zaidi A.K., Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol. Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 150.Fosbrink M., Niculescu F., Rus H. The role of c5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol. Res. 2005;31:37–46. doi: 10.1385/IR:31:1:37. [DOI] [PubMed] [Google Scholar]
- 151.Peng H., Takano T., Papillon J., Bijian K., Khadir A., Cybulsky A.V. Complement activates the c-Jun N-terminal kinase/stress-activated protein kinase in glomerular epithelial cells. J. Immunol. 2002;169:2594–2601. doi: 10.4049/jimmunol.169.5.2594. [DOI] [PubMed] [Google Scholar]
- 152.Bronte V., Apolloni E., Cabrelle A., Ronca R., Serafini P., Zamboni P., Restifo N.P., Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 153.Li Q., Pan P.Y., Gu P., Xu D., Chen S.H. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–1139. doi: 10.1158/0008-5472.can-03-1715. [DOI] [PubMed] [Google Scholar]
- 154.Bronte V., Serafini P., Apolloni E., Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J. Immunother. 2001;24:431–446. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 155.Young M.R., Wright M.A., Matthews J.P., Malik I., Prechel M. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J. Immunol. 1996;156:1916–1922. [PubMed] [Google Scholar]
- 156.Livingston P.O., Wong G.Y., Adluri S., Tao Y., Padavan M., Parente R., Hanlon C., Calves M.J., Helling F., Ritter G. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J. Clin. Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 157.Martin S.E., Martin W.J. Anti-tumor antibodies in normal mouse sera. Int. J. Cancer. 1975;15:658–664. doi: 10.1002/ijc.2910150415. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz-Albiez R., Laban S., Eichmuller S., Kirschfink M. Cytotoxic natural antibodies against human tumours: an option for anti-cancer immunotherapy? Autoimmun. Rev. 2008;7:491–495. doi: 10.1016/j.autrev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 159.Anderson K.S., LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J. Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Springer G.F. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 161.Springer G.F., Desai P.R., Scanlon E.F. Blood group MN precursors as human breast carcinoma-associated antigens and “naturally” occurring human cytotoxins against them. Cancer. 1976;37:169–176. doi: 10.1002/1097-0142(197601)37:1<169::aid-cncr2820370124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 162.Cao Y., Merling A., Karsten U., Goletz S., Punzel M., Kraft R., Butschak G., Schwartz-Albiez R. Expression of CD175 (Tn), CD175s (sialosyl-Tn) and CD176 (Thomsen–Friedenreich antigen) on malignant human hematopoietic cells. Int. J. Cancer. 2008;123:89–99. doi: 10.1002/ijc.23493. [DOI] [PubMed] [Google Scholar]
- 163.Cao W., Bao C., Padalko E., Lowenstein C.J. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J. Exp. Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brandlein S., Beyer I., Eck M., Bernhardt W., Hensel F., Muller-Hermelink H.K., Vollmers H.P. Cysteine-rich fibroblast growth factor receptor 1, a new marker for precancerous epithelial lesions defined by the human monoclonal antibody PAM-1. Cancer Res. 2003;63:2052–2061. [PubMed] [Google Scholar]
- 165.Brandlein S., Eck M., Strobel P., Wozniak E., Muller-Hermelink H.K., Hensel F., Vollmers H.P. PAM-1, a natural human IgM antibody as new tool for detection of breast and prostate precursors. Hum. Antibodies. 2004;13:97–104. [PubMed] [Google Scholar]
- 166.Brandlein S., Pohle T., Ruoff N., Wozniak E., Muller-Hermelink H.K., Vollmers H.P. Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res. 2003;63:7995–8005. [PubMed] [Google Scholar]
- 167.Vollmers H.P., Brandlein S. The “early birds”: natural IgM antibodies and immune surveillance. Histol. Histopathol. 2005;20:927–937. doi: 10.14670/HH-20.927. [DOI] [PubMed] [Google Scholar]
- 168.Vollmers H.P., Brandlein S. Death by stress: natural IgM-induced apoptosis. Methods Find. Exp. Clin. Pharmacol. 2005;27:185–191. doi: 10.1358/mf.2005.27.3.890876. [DOI] [PubMed] [Google Scholar]
- 169.Dummer R., Mittelman A., Fanizzi F.P., Lucchese G., Willers J., Kanduc D. Non-self-discrimination as a driving concept in the identification of an immunodominant HMW-MAA epitopic peptide sequence by autoantibodies from melanoma cancer patients. Int. J. Cancer. 2004;111:720–726. doi: 10.1002/ijc.20310. [DOI] [PubMed] [Google Scholar]
- 170.Brichory F., Beer D., Le Naour F., Giordano T., Hanash S. Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res. 2001;61:7908–7912. [PubMed] [Google Scholar]
- 171.Conroy S.E., Gibson S.L., Brunstrom G., Isenberg D., Luqmani Y., Latchman D.S. Autoantibodies to 90 kD heat-shock protein in sera of breast cancer patients. Lancet. 1995;345:126. doi: 10.1016/s0140-6736(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 172.Vlock D.R., Scalise D., Schwartz D.R., Richter D.E., Krause C.J., Baker S.R., Carey T.E. Incidence of serum antibody reactivity to autologous head and neck cancer cell lines and augmentation of antibody reactivity following acid dissociation and ultrafiltration. Cancer Res. 1989;49:1361–1365. [PubMed] [Google Scholar]
- 173.Taylor D.D., Homesley H.D., Doellgast G.J. Identification of antigenic components recognized by “membrane-bound” antibodies from ovarian cancer patients. Am. J. Reprod. Immunol. 1984;6:179–184. doi: 10.1111/j.1600-0897.1984.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 174.Vogl F.D., Frey M., Kreienberg R., Runnebaum I.B. Autoimmunity against p53 predicts invasive cancer with poor survival in patients with an ovarian mass. Br. J. Cancer. 2000;83:1338–1343. doi: 10.1054/bjoc.2000.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pohle T., Brandlein S., Ruoff N., Muller-Hermelink H.K., Vollmers H.P. Lipoptosis: tumor-specific cell death by antibody-induced intracellular lipid accumulation. Cancer Res. 2004;64:3900–3906. doi: 10.1158/0008-5472.CAN-03-3149. [DOI] [PubMed] [Google Scholar]
- 176.Brandlein S., Rauschert N., Rasche L., Dreykluft A., Hensel F., Conzelmann E., Muller-Hermelink H.K., Vollmers H.P. The human IgM antibody SAM-6 induces tumor-specific apoptosis with oxidized low-density lipoprotein. Mol. Cancer Ther. 2007;6:326–333. doi: 10.1158/1535-7163.MCT-06-0399. [DOI] [PubMed] [Google Scholar]
- 177.Taylor D.D., Gercel-Taylor C., Parker L.P. Patient-derived tumor-reactive antibodies as diagnostic markers for ovarian cancer. Gynecol. Oncol. 2009;115:112–120. doi: 10.1016/j.ygyno.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 178.Dano K., Andreasen P.A., Grondahl-Hansen J., Kristensen P., Nielsen L.S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv. Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- 179.Liotta L.A., Steeg P.S., Stetler-Stevenson W.G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 180.Blasi F. Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the u-PA-u-PAR-PAI-1 system. Thromb. Haemost. 1999;82:298–304. [PubMed] [Google Scholar]
- 181.Andreasen P.A., Kjoller L., Christensen L., Duffy M.J. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int. J. Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 182.Duffy M.J. Urokinase-type plasminogen activator: a potent marker of metastatic potential in human cancers. Biochem. Soc. Trans. 2002;30:207–210. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 183.Duffy M.J., O'Grady P., Devaney D., O'Siorain L., Fennelly J.J., Lijnen H.J. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report. Cancer. 1988;62:531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 184.Oka T., Ishida T., Nishino T., Sugimachi K. Immunohistochemical evidence of urokinase-type plasminogen activator in primary and metastatic tumors of pulmonary adenocarcinoma. Cancer Res. 1991;51:3522–3525. [PubMed] [Google Scholar]
- 185.Pedersen H., Grondahl-Hansen J., Francis D., Osterlind K., Hansen H.H., Dano K., Brunner N. Urokinase and plasminogen activator inhibitor type 1 in pulmonary adenocarcinoma. Cancer Res. 1994;54:120–123. [PubMed] [Google Scholar]
- 186.Hasui Y., Marutsuka K., Suzumiya J., Kitada S., Osada Y., Sumiyoshi A. The content of urokinase-type plasminogen activator antigen as a prognostic factor in urinary bladder cancer. Int. J. Cancer. 1992;50:871–873. doi: 10.1002/ijc.2910500607. [DOI] [PubMed] [Google Scholar]
- 187.Heiss M.M., Allgayer H., Gruetzner K.U., Funke I., Babic R., Jauch K.W., Schildberg F.W. Individual development and uPA-receptor expression of disseminated tumour cells in bone marrow: a reference to early systemic disease in solid cancer. Nat. Med. 1995;1:1035–1039. doi: 10.1038/nm1095-1035. [DOI] [PubMed] [Google Scholar]
- 188.Nekarda H., Schmitt M., Ulm K., Wenninger A., Vogelsang H., Becker K., Roder J.D., Fink U., Siewert J.R. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res. 1994;54:2900–2907. [PubMed] [Google Scholar]
- 189.Mulcahy H.E., Duffy M.J., Gibbons D., McCarthy P., Parfrey N.A., O'Donoghue D.P., Sheahan K. Urokinase-type plasminogen activator and outcome in Dukes' B colorectal cancer. Lancet. 1994;344:583–584. doi: 10.1016/s0140-6736(94)91968-2. [DOI] [PubMed] [Google Scholar]
- 190.Duffy M.J. Plasminogen activators and cancer. Blood Coagul. Fibrinolysis. 1990;1:681–687. [PubMed] [Google Scholar]
- 191.Crowley C.W., Cohen R.L., Lucas B.K., Liu G., Shuman M.A., Levinson A.D. Prevention of metastasis by inhibition of the urokinase receptor. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kook Y.H., Adamski J., Zelent A., Ossowski L. The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J. 1994;13:3983–3991. doi: 10.1002/j.1460-2075.1994.tb06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saito A., Pietromonaco S., Loo A.K., Farquhar M.G. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Odekon L.E., Blasi F., Rifkin D.B. Requirement for receptor-bound urokinase in plasmin-dependent cellular conversion of latent TGF-beta to TGF-beta. J. Cell. Physiol. 1994;158:398–407. doi: 10.1002/jcp.1041580303. [DOI] [PubMed] [Google Scholar]
- 195.Werb Z., Tremble P., Damsky C.H. Regulation of extracellular matrix degradation by cell-extracellular matrix interactions. Cell Differ. Dev. 1990;32:299–306. doi: 10.1016/0922-3371(90)90043-v. [DOI] [PubMed] [Google Scholar]
- 196.Van Roy F., Mareel M. Tumour invasion: effects of cell adhesion and motility. Trends Cell Biol. 1992;2:163–169. doi: 10.1016/0962-8924(92)90035-l. [DOI] [PubMed] [Google Scholar]
- 197.Frixen U.H., Nagamine Y. Stimulation of urokinase-type plasminogen activator expression by blockage of E-cadherin-dependent cell-cell adhesion. Cancer Res. 1993;53:3618–3623. [PubMed] [Google Scholar]
- 198.Matsumura Y., Maruo K., Kimura M., Yamamoto T., Konno T., Maeda H. Kinin-generating cascade in advanced cancer patients and in vitro study. Jpn. J. Cancer Res. 1991;82:732–741. doi: 10.1111/j.1349-7006.1991.tb01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maeda H., Matsumura Y., Kato H. Purification and identification of [hydroxyprolyl3]bradykinin in ascitic fluid from a patient with gastric cancer. J. Biol. Chem. 1988;263:16051–16054. [PubMed] [Google Scholar]
- 200.Maeda H., Wu J., Okamoto T., Maruo K., Akaike T. Kallikrein-kinin in infection and cancer. Immunopharmacology. 1999;43:115–128. doi: 10.1016/s0162-3109(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 201.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 202.Matsumura Y., Kimura M., Yamamoto T., Maeda H. Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn. J. Cancer Res. 1988;79:1327–1334. doi: 10.1111/j.1349-7006.1988.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hurlimann J., Thorbecke G.J., Hochwald G.M. The liver as the site of C-reactive protein formation. J. Exp. Med. 1966;123:365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ganapathi M.K., Rzewnicki D., Samols D., Jiang S.L., Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J. Immunol. 1991;147:1261–1265. [PubMed] [Google Scholar]
- 205.Lee J.G., Cho B.C., Bae M.K., Lee C.Y., Park I.K., Kim D.J., Ahn S.V., Chung K.Y. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer. 2009;63:106–110. doi: 10.1016/j.lungcan.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 206.Chiu H.M., Lin J.T., Chen T.H., Lee Y.C., Chiu Y.H., Liang J.T., Shun C.T., Wu M.S. Elevation of C-reactive protein level is associated with synchronous and advanced colorectal neoplasm in men. Am. J. Gastroenterol. 2008;103:2317–2325. doi: 10.1111/j.1572-0241.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 207.Groblewska M., Mroczko B., Wereszczynska-Siemiatkowska U., Kedra B., Lukaszewicz M., Baniukiewicz A., Szmitkowski M. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patients. Clin. Chem. Lab. Med. 2008;46:1423–1428. doi: 10.1515/CCLM.2008.278. [DOI] [PubMed] [Google Scholar]
- 208.Coombes R.C., Powles T.J., Gazet J.C., Ford H.T., Sloane J.P., Laurence D.J., Neville A.M. Biochemical markers in human breast cancer. Lancet. 1977;1:132–134. doi: 10.1016/s0140-6736(77)91719-6. [DOI] [PubMed] [Google Scholar]
- 209.Duffy S.A., Taylor J.M., Terrell J.E., Islam M., Li Y., Fowler K.E., Wolf G.T., Teknos T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 210.Liao W.C., Lin J.T., Wu C.Y., Huang S.P., Lin M.T., Wu A.S., Huang Y.J., Wu M.S. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin. Cancer Res. 2008;14:428–434. doi: 10.1158/1078-0432.CCR-07-1032. [DOI] [PubMed] [Google Scholar]
- 211.Macri A., Versaci A., Loddo S., Scuderi G., Travagliante M., Trimarchi G., Teti D., Famulari C. Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006;11:184–193. doi: 10.1080/13547500600565677. [DOI] [PubMed] [Google Scholar]
- 212.Willeke F., Assad A., Findeisen P., Schromm E., Grobholz R., von Gerstenbergk B., Mantovani A., Peri S., Friess H.H., Post S., von Knebel Doeberitz M., Schwarzbach M.H. Overexpression of a member of the pentraxin family (PTX3) in human soft tissue liposarcoma. Eur. J. Cancer. 2006;42:2639–2646. doi: 10.1016/j.ejca.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 213.Diamandis E.P., Goodglick L., Planque C., Thornquist M.D. Pentraxin-3 is a novel biomarker of lung carcinoma. Clin. Cancer Res. 2011;17:2395–2399. doi: 10.1158/1078-0432.CCR-10-3024. [DOI] [PubMed] [Google Scholar]
- 214.Margheri F., Serrati S., Lapucci A., Anastasia C., Giusti B., Pucci M., Torre E., Bianchini F., Calorini L., Albini A., Ventura A., Fibbi G., Del Rosso M. Systemic sclerosis-endothelial cell antiangiogenic pentraxin 3 and matrix metalloprotease 12 control human breast cancer tumor vascularization and development in mice. Neoplasia. 2009;11:1106–1115. doi: 10.1593/neo.09934. [DOI] [PMC free article] [PubMed] [Google Scholar]