Abstract
This report describes the use of a novel model of multispecies biofilms to stimulate profiles of cytokines/chemokines from oral epithelial cells that contribute to local inflammation in the periodontium. Streptococcus gordonii (Sg)/S. oralis (So)/S. sanguinis (Ss) and Sg/Fusobacterium nucleatum (Fn)/Porphyromonas gingivalis (Pg) biofilms elicited significantly elevated levels of IL-1α and showed synergistic stimulatory activity compared with an additive effect of the 3 individual bacteria. Only the Sg/Actinomyces naeslundii (An)/Fn multispecies biofilms elicited IL-6 levels above those of control. IL-8 was a primary response to the Sg/An/Fn biofilms, albeit the level was not enhanced compared with a predicted composite level from the monospecies challenges. These results represent some of the first data documenting alterations in profiles of oral epithelial cell responses to multispecies biofilms.
Keywords: oral health, cytokines, chemokines, host-pathogen interactions, gingivitis, periodontitis
Introduction
Microbiological studies have expanded knowledge of the genera and species that form the human oral microbiome (Zaura et al., 2009; Jakubovics, 2010; Kolenbrander et al., 2010). A range of gingival cells responds to this microbial challenge in the oral cavity (Kebschull and Papapanou, 2011). To maintain homeostasis, these responses have evolved with pro- and anti-inflammatory molecules and cell communication molecules arising from the resident cells of the periodontium (Bodet et al., 2006; Stathopoulou et al., 2010). Additional molecules derive from infiltrating inflammatory and immune cells in the affected tissues. Current evidence supports that these responses can result in chronic inflammation that generates both soft- and hard-tissue damage, defined as periodontitis (Preshaw and Taylor, 2011).
Historically, most in vitro studies have evaluated how individual planktonic bacteria or their component products would trigger host responses (Ji et al., 2007; Stathopoulou et al., 2010). The investigations were designed to identify specific response molecules (Ji et al., 2007; Mans et al., 2009), as well as the receptors and ligand interactions that account for the responses (Hayashi et al., 2010). These studies have provided some understanding of the capacity of individual micro-organisms or bacterial consortia to elicit patterns of responses from specific host cell types, the net result of which are identified by in situ response profiles. However, there are few data elucidating specific stimulatory capabilities of oral multispecies biofilms or comparing these responses with the individual bacteria within the microbial complexes (Guggenheim et al., 2009; Belibasakis et al., 2011). This report describes seminal data on the use of a novel multispecies biofilm model, created on rigid gas-permeable contact lens (RGPL) material, used to challenge oral epithelial cell cultures and contrasting responses with those occurring after challenge with monospecies biofilms of the component bacteria (Peyyala et al., 2011a,b).
Materials & Methods
Bacteria and Biofilm Growth
Bacterial strains have been described previously: Fusobacterium nucleatum ATCC 25586, Actinomyces naeslundii ATCC 49840, Streptococcus gordonii ATCC 10558, S. sanguinis ATCC 10556, S. oralis ATCC 1055, and Porphyromonas gingivalis FDC38 (Peyyala et al., 2011b). Bacteria were grown in Brain Heart Infusion (Becton Dickinson, Sparks, MD, USA) medium supplemented with 5 µg hemin mL-1 and 1 µg menadione mL-1 (F. nucleatum, A. naeslundii, S. gordonii) or Trypticase Yeast Extract salts medium (S. sanguinis, S. oralis) under anaerobic conditions (85% N2, 10% H2, 5% CO2) at 37oC (Peyyala et al., 2011b).
Biofilms were grown on Rigid Gas Permeable Lenses (RGPL) (Advanced Vision Technologies, Golden, CO, USA), 10.5 mm in diameter, in a single well of a 48-well plate, which allows the RGPLs to cover the entire surface of the well. Prior to biofilm formation, RGPLs were coated with 1% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) diluted in PBS to support the adherence of bacteria and incubated at room temperature for 2 to 4 hrs until dry, then stored at room temperature until further use. A 5-mL quantity of monospecies planktonic culture at 0.3 OD A600 or multispecies planktonic culture where an equal volume of each representative monospecies planktonic culture at 0.3 OD A600 was mixed to obtain a mixed culture was used to inoculate each RGPL in a single well of a 6-well polystyrene tissue culture plate (BD Falcon, Franklin Lakes, NJ, USA) and incubated in an anaerobic chamber for 3 days for static development of biofilms. At each 24-hour interval, a 3-mL quantity of culture fluid was aspirated from each well and replaced with 4 mL of media to replenish nutrients. After incubation, RGPLs with adherent biofilms were washed in PBS twice to remove loosely adherent cells. Biofilms grown on 3 additional RGPLs were used for bacterial enumeration by qPCR analysis (Peyyala et al., 2011b). The monospecies biofilms contained from 6-16 x 108 bacteria, dependent upon the species. The Sg/So/Ss multispecies biofilms were approximately 3 x 108 total bacteria (ratio: 2%/2%/96%); the Sg/An/Fn biofilms approximated 1-2 x 109 total bacteria (ratio: 29%/63%/8%); and the Sg/Fn/Pg biofilms were 2-3 x 109 total bacteria (ratio: 92%/2%/6%).
To constrain the multispecies biofilms from replicating in the keratinocyte media during the 24-hour challenge, biofilms were treated with green fluorescent nucleic acid stain SYTO 24. SYTO 24 was chosen to treat all 3 multispecies biofilms since it yielded the lowest optical density with high fluorescence intensity for the bacteria, indicating that this stain inhibited replication, while not affecting viability (Peyyala et al., 2012). Prior to challenging OKF4 cells, biofilms were immersed in 10 µg/mL SYTO 24 stain in keratinocyte media for 5 min, after which they were immersed in PBS twice to remove excess stain.
OKF4 Challenge
An immortalized epithelial cell line, OKF4 (Rheinwald et al., 2002), was cultured to form a confluent monolayer (Peyyala et al., 2011a). Biofilm and control treatments were each carried out in 6 wells in 1-mL/well fresh media and continuously incubated for 6 hrs under anaerobic conditions (85% N2, 5% CO2, and 10% H2). Three-day-old biofilms grown on contact lenses were overlaid with the biofilm surface juxtaposed to the epithelial cells. OKF4 cells with or without overlaid RGPL were used as controls and maintained high viability and function for the 24-hour experimental interval (Peyyala et al., 2011a).
Detection of Cytokines/Chemokines
Interleukin (IL)1α, IL-6, IL-8, transforming growth factor (TGF)α, Gro-1α (CXCL1), regulated upon activation, normal T-cell expressed and secreted (RANTES; CCL5), Fractalkine (CX3CL1), interferon gamma-induced protein (IP)-10 (CXCL10), monocyte chemotactic protein (MCP)-1 (CCL2), and macrophage inhibitory protein (Mip)-1α (CCL3) levels that accumulated in the supernatants were determined (R&D Systems, Minneapolis, MN, USA) with a Luminex IS100 instrument. The amount of cytokine/chemokine produced was also evaluated relative to the total bacterial challenge that was administered. The wet weight of each bacterial species contained in the monospecies and multispecies biofilms was estimated from cultivated bacteria and the cytokine/chemokine level expressed per mg wet weight of the species. The means ± standard error of the biofilm stimulation of OKF4 were compared by an analysis of variance (ANOVA) on ranks test, with Dunn’s test for multiple comparisons to evaluate the data from stimulated cells compared with unchallenged and RGPL-overlaid OKF4 cells (SigmaStat 3.5; Systat Software, Inc., Chicago, IL, USA).
Results
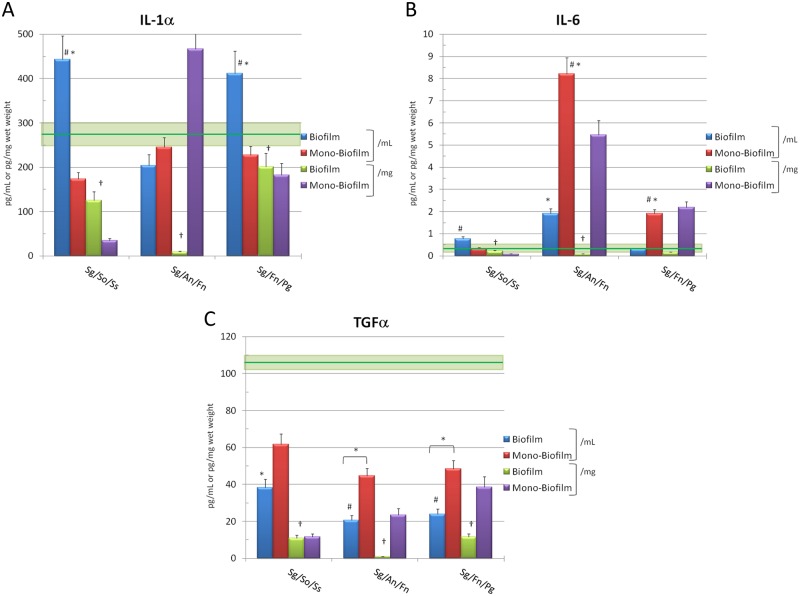
Selected cytokine/cell communication factor responses of the epithelial cells to challenge with the 3 multispecies biofilms, compared with a composite summation of responses to the individual bacterial biofilms, are demonstrated in Figs. 1A-1C. The Sg/So/Ss and Sg/Fn/Pg biofilms elicited significantly elevated levels of IL-1α and showed synergistic stimulatory activity compared with that expected from an additive effect of the individual bacteria. As importantly, both of the multispecies biofilms stimulated significantly greater IL-1α production than the mass of the bacteria that was present (i.e., biofilm pg/mL vs. biofilm pg/mg wet weight). The Sg/An/Fn multispecies biofilms were generally inactive in enhancing production of this cytokine. However, we observed that a summation of IL-1α levels with the monospecies biofilms related to the mass of the challenge was significantly elevated and directly related to the capacity of F. nucleatum to stimulate IL-1α (Peyyala et al., 2012).
Figure 1.
Levels of cytokines elicited by challenge of oral epithelial cells with multispecies biofilms (Biofilm). Mono-Biofilm denotes a composite of responses for the 3 monospecies biofilms represented in the multispecies biofilms. Each bar denotes mean level of triplicate determinations for each condition, and the vertical brackets signify 1 SD. The asterisk (*) denotes a significant difference from basal controls (RGPL-overlaid epithelial cells) at least at p < 0.05. The green horizontal line denotes mean of cells incubated in media and overlaid with RGPL absent bacteria for comparison of responses, and the light green box denotes 1 SD. The pound sign (#) denotes significant difference between multispecies biofilms and composite sum of monospecies biofilms for the 3 bacteria at least at p < 0.05. Basal epithelial cell levels of IL-1α = 245 ± 27; IL-6 = 0.64 ± 0.12; TGFα = 118 ± 16.
The Sg/So/Ss and Sg/An/Fn biofilms induced elevated IL-6 levels compared with the monospecies biofilms. The Sg/An/Fn biofilms were very active in inducing IL-6, as both monospecies and multispecies biofilms, with F. nucleatum demonstrating the greatest stimulatory capacity. Finally, the Sg/Fn/Pg multispecies biofilms were inactive compared with levels expected from a summation of the individual species. The multispecies and monospecies biofilms showed an overall inhibition of production of TGFα below basal levels of the cells.
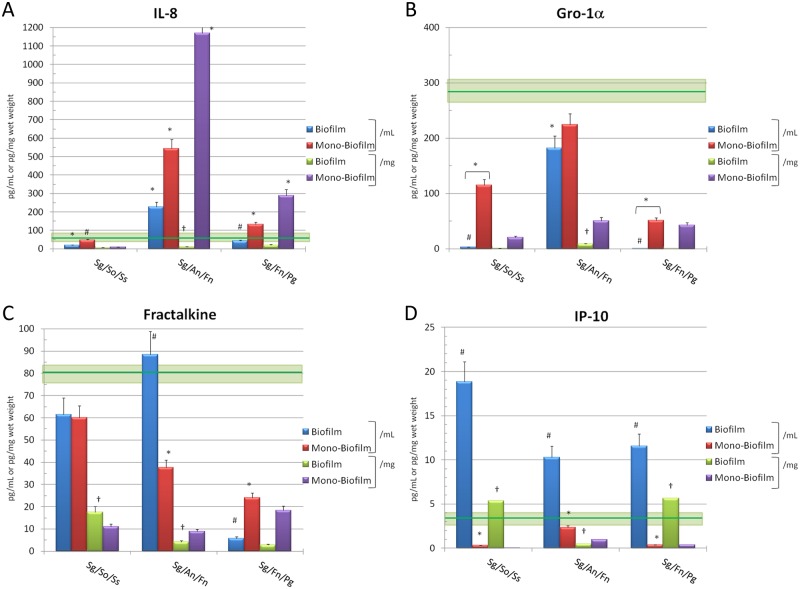
IL-8 was a primary response to the Sg/An/Fn biofilms, albeit the level to the multispecies biofilm was not enhanced compared with a predicted composite level from the individual monospecies challenges (Fig. 2A). The F. nucleatum monospecies biofilms induced exceptional levels of IL-8 (Peyyala et al., 2011a), which is reflected in the composite values for the mono- biofilms. The Sg/So/Ss biofilms appeared to inhibit the production of IL-8. Finally, minimal responses were observed with the Sg/Fn/Pg biofilms, and the combination of these micro-organisms lowered the levels that were expected from challenge with the individual bacterial biofilms, primarily elicited by Fn.
Figure 2.
Levels of chemokines elicited by challenge of oral epithelial cells with multispecies biofilms (Biofilm). Mono-Biofilm denotes a composite of responses for the 3 monospecies biofilms represented in the multispecies biofilms. Each bar denotes mean level of triplicate determinations for each condition, and the vertical brackets signify 1 SD. The asterisk (*) denotes a significant difference from basal controls (RGPL-overlaid epithelial cells) at least at p < 0.05. The green horizontal line denotes mean of cells incubated in media and overlaid with RGPL absent bacteria for comparison of responses, and the light green box denotes 1 SD. The pound sign (#) denotes significant difference between multispecies biofilms and composite sum of monospecies biofilms for the 3 bacteria at least at p < 0.05. Basal epithelial cell levels of IL-8 = 81 ± 11; Gro-1α (CXCL1) = 321 ± 43; Fractalkine (CX3CL1) = 56 ± 17; IP-10 (CXCL10) = 1.8 ± 0.5.
No Gro-1α was observed with either the Sg/So/Ss or Sg/Fn/Pg multispecies biofilms (Fig. 2B). There was a more dramatic decrease from basal production than that which occurred with the monospecies biofilms of the individual bacteria. This chemokine was detected in supernatants of the Sg/An/Fn biofilms, with a level significantly lower than basal epithelial cell production. While the levels were consistently below the basal levels produced by the cells, it appeared that higher levels were detected with the multispecies biofilms considered as a unit (i.e., pg/mL) vs. expression of the levels related to the mass of the bacteria (pg/mg wet weight).
The Sg/An/Fn biofilms demonstrated an elevated level of Fractalkine compared with what would have been predicted from the individual monospecies biofilms (Fig. 2C). A substantial inhibition was observed with the Sg/Fn/Pg biofilms that showed less than 5% of the basal levels of this chemokine. Of interest was that with both the pioneer and bridging micro-organism biofilms, the levels related to the biofilms as a unit (i.e., pg/mL) were consistently increased compared with the levels of this chemokine expressed per mass of the bacteria (pg/mg wet weight). Generally, IP-10 levels were triggered by all the multispecies biofilms to a significantly greater level than noted with individual bacterial biofilms (Fig. 2D). As noted with the other chemokines, levels observed related to the multispecies biofilms as a unit were routinely increased when compared with levels related to simply the mass of the bacteria in the biofilms. MIP-1α, RANTES, and MCP-1 were present in minimal levels in the basal cell cultures, as well as following stimulation of the cells with various combinations of bacteria (data not shown).
Discussion
This report extends our findings using novel RGPL material to build oral bacterial biofilms that were used to challenge epithelial cells. The oral bacterial ecology is acquired early in life and evolves over time with the host to form a complex of numerous genera and species occupying the various ecological niches in the oral cavity (Paster et al., 2006; Kishi et al., 2009). We provide some unique data documenting the patterns of cytokines/chemokines that are induced in oral epithelial cells following challenge with targeted multispecies bacterial biofilms, representing bacterial consortia associated with periodontal health, gingivitis, and periodontitis (Socransky and Haffajee, 2005). Cytokines/chemokines that have been reported to be induced by various stimuli interacting with a range of epithelial cell types were targeted, including IL-1α, IL-6, TGFα, IL-8, Gro-1α, Fractalkine, IP-10, MIP-1α, RANTES, and MCP-1 (Gemmell et al., 2001; Ji et al., 2007; Pathirana et al., 2010).
Extensive results have documented the responses of epithelial cells after challenge with planktonic oral bacteria (Huang et al., 2004; Hasegawa et al., 2007; Ji et al., 2007; Stathopoulou et al., 2010). However, current models of the host-microbe interactions in the oral cavity emphasize the critical nature of complex microbial biofilms that form unique microbial ecologies (Colombo et al., 2009; Darveau, 2009) and that change during transition from health to disease (Marsh, 2010). Importantly, we currently know very little concerning how this multicellular organized microbial structure, i.e., biofilm, alters the characteristics of the host responses to individual bacterial species. The results demonstrated unique characteristics of the multispecies biofilms dependent upon the composition of the ecology, and whether the biofilms reflected bacteria associated with periodontal health, gingivitis, or periodontitis. Moreover, we identified specific mediators that were significantly elevated with the multispecies biofilms compared with what would have been expected based upon a simple additive effect of individual monospecies biofilms challenge. Finally, a number of the biofilms appeared to inhibit either the normal basal level of message, basal level of translation and secretion, and/or degradation of various mediators in this biofilm model system. This was often in contrast to mediator levels expected from the monospecies biofilms, as well as the levels in response to planktonic bacterial challenge (Peyyala et al., 2012).
A few recent reports of hydroxyapatite discs used to create multispecies biofilms determined levels of IL-1ß, IL-6, IL-8 (Guggenheim et al., 2009), and RANKL/OPG (Belibasakis et al., 2011) produced by epithelial cells, periodontal ligament cells, or dental pulp cells in response to biofilms with multiple species. Analysis of the data indicated an apparent increase in apoptosis and degradation of Il-1ß, IL-6, and IL-8 in this system. These complex multispecies biofilms were vastly dominated by a very limited number of species, and the model did facilitate identification of the types of responses that might occur to various members of the ecology that are observed with disease. Consequently, the stimulatory capacity of individual bacteria in biofilms, how they compare with planktonic stimuli, and how these responses would be altered in the presence of complex multispecies biofilms remain to be elucidated.
We have evaluated the interactions of the host cells with monospecies biofilms created from all 6 of the oral species used in these multispecies biofilms and noted that these bacteria form multiple formats/architecture that would be detected by the cells (Peyyala et al., 2012). The results of these studies demonstrated clear differences in the profiles of epithelial cell responses to bacteria in monospecies biofilms compared with planktonic forms of the bacteria. Moreover, selected biofilms significantly up-regulated a range of mediators, while certain of the bacterial biofilms, e.g., P. gingivalis, appeared to adversely affect even basal production of the cytokines/chemokines, as has been suggested from previous studies (Huang et al., 2004; Guggenheim et al., 2009). Thus, it was of particular interest to determine how these bacteria would interact with the host cells in complex biofilms, and whether there would be synergistic, additive, or subtractive outcomes for specific cytokines/chemokines. As noted in the results, examples of each of these outcomes could be identified compared with the monospecies biofilms created with each of the species. Specifically, we included biofilms composed of a mixture of oral streptococci that have been identified as prominent members of the subgingival ecology in biofilms from healthy sulci (Zijnge et al., 2010). The Sg/An/Fn biofilm was created to reflect changes that have been suggested to occur early in the ecological shift related to gingival inflammation (Teles et al., 2007). Finally, the Sg/Fn/Pg biofilm incorporates representative bacteria considered pioneer species that directly colonize the tooth surface (i.e., S. gordonii), a species (i.e., F. nucleatum) that increases with inflammation and demonstrates a significant capacity to co-aggregate with both pioneer streptococcal species, as well as providing bridging cognate interaction with pathogens (i.e., P. gingivalis) in the climax community of diseased periodontal pockets (Jakubovics and Kolenbrander, 2010).
These studies are also novel in that the host-biofilm interactions were conducted under anaerobic conditions that reflect the ecological microenvironment at the site of a periodontal disease lesion. While the vast majority of in vitro studies have been conducted under aerobic conditions (Hasegawa et al., 2007; Ji et al., 2007; Guggenheim et al., 2009), a recent study reported that under reduced oxygen tension (i.e., 2% oxygen), selected oral bacteria, including P. gingivalis, elicited elevated levels of cytokines/chemokines in a low oxygen environment (Grant et al., 2010). This model system will enable us to explore variations in the oxygen level, volatile sulfur compounds, and elevated pH on the host-bacterial biofilm interactions that would be expected to occur in periodontal disease.
This novel biofilm model should also facilitate evaluation of variations in response profiles with differences in epithelial cell features (Seifi et al., 2011). This investigation also provided some fundamental biologic knowledge of host response outcomes to multispecies biofilms, facilitating future examination of the attributes of intracellular signaling pathways that respond to these complexes.
Acknowledgments
We thank Michelle J. Steffen and Jason Stevens for their technical assistance.
Footnotes
This work was supported by grant R21 DE018177 from the National Institute of Dental and Craniofacial Research.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Belibasakis GN, Meier A, Guggenheim B, Bostanci N. (2011). Oral biofilm challenge regulates the RANKL-OPG system in periodontal ligament and dental pulp cells. Microb Pathog 50:6-11. [DOI] [PubMed] [Google Scholar]
- Bodet C, Chandad F, Grenier D. (2006). Inflammatory responses of a macrophage/epithelial cell co-culture model to mono and mixed infections with Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. Microbes Infect 8:27-35. [DOI] [PubMed] [Google Scholar]
- Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. (2009). Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the Human Oral Microbe Identification Microarray. J Periodontol 80:1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. (2009). The oral microbial consortium’s interaction with the periodontal innate defense system. DNA Cell Biol 28:389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell E, Carter CL, Seymour GJ. (2001). Chemokines in human periodontal disease tissues. Clin Exp Immunol 125:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Kolamunne RT, Lock FE, Matthews JB, Chapple IL, Griffiths HR. (2010). Oxygen tension modulates the cytokine response of oral epithelium to periodontal bacteria. J Clin Periodontol 37:1039-1048. [DOI] [PubMed] [Google Scholar]
- Guggenheim B, Gmür R, Galicia J, Stathopoulou P, Benakanakere M, Meier A, et al. (2009). In vitro modeling of host-parasite interactions: the ‘subgingival’ biofilm challenge of primary human epithelial cells. BMC Microbiol 9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Mans JJ, Mao S, Lopez MC, Baker HV, Handfield M, et al. (2007). Gingival epithelial cell transcriptional responses to commensal and opportunistic oral microbial species. Infect Immun 75:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi C, Gudino CV, Gibson FC, Genco CA. (2010). Review: Pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol 25:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Zhang HB, Dang HN, Haake SK. (2004). Differential regulation of cytokine genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microb Pathog 37:303-312. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS. (2010). Talk of the town: interspecies communication in oral biofilms. Mol Oral Microbiol 25:4-14. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Kolenbrander PE. (2010). The road to ruin: the formation of disease-associated oral biofilms. Oral Dis 16:729-739. [DOI] [PubMed] [Google Scholar]
- Ji S, Kim Y, Min BM, Han SH, Choi Y. (2007). Innate immune responses of gingival epithelial cells to nonperiodontopathic and periodontopathic bacteria. J Periodontal Res 42:503-510. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Papapanou PN. (2011). Periodontal microbial complexes associated with specific cell and tissue responses. J Clin Periodontol 38(Suppl 11):17-27. [DOI] [PubMed] [Google Scholar]
- Kishi M, Abe A, Kishi K, Ohara-Nemoto Y, Kimura S, Yonemitsu M. (2009). Relationship of quantitative salivary levels of Streptococcus mutans and S. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Community Dent Oral Epidemiol 37:241-249. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. (2010). Oral multispecies biofilm development and the key role of cell–cell distance. Nat Rev Microbiol 8:471-480. [DOI] [PubMed] [Google Scholar]
- Mans J, von Lackum K, Dorsey C, Willis S, Wallet S, Baker H, et al. (2009). The degree of microbiome complexity influences the epithelial response to infection. BMC Genomics 10:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. (2010). Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am 54:441-454. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Olsen I, Aas JA, Dewhirst FE. (2006). The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42: 80-87. [DOI] [PubMed] [Google Scholar]
- Pathirana RD, O’Brien-Simpson NM, Reynolds EC. (2010). Host immune responses to Porphyromonas gingivalis antigens. Periodontol 2000 52: 218-237. [DOI] [PubMed] [Google Scholar]
- Peyyala R, Kirakodu S, Novak KF, Ebersole JL. (2011a). Epithelial interleukin-8 responses to oral bacterial biofilms. Clin Vaccine Immunol 18:1770-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyyala R, Kirakodu SS, Ebersole JL, Novak KF. (2011b). Novel model for multispecies biofilms that uses rigid gas-permeable lenses. Applied Environ Microbiol 77:3413-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyyala R, Kirakodu SS, Novak KF, Ebersole JL. (2012). Oral microbial biofilm stimulation of epithelial cell responses. Cytokine 58:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshaw PM, Taylor JJ. (2011). How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol 38(Suppl 11):60-84. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, et al. (2002). A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi A, Weaver EM, Whipple ME, Ikoma M, Farrenberg J, Huang ML, et al. (2011). The lytic activation of KSHV during keratinocyte differentiation is dependent upon a suprabasal position, the loss of integrin engagement, and calcium, but not the interaction of cadherins. Virology 410:17-29. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. (2005). Periodontal microbial ecology. Periodontol 2000 38: 135-187. [DOI] [PubMed] [Google Scholar]
- Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. (2010). Epithelial cell pro-inflammatory cytokine response differs across dental plaque bacterial species. J Clin Periodontol 37:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles RP, Bogren A, Patel M, Wennstrom JL, Socransky SS, Haffajee AD. (2007). A three-year prospective study of adult subjects with gingivitis II: microbiological parameters. J Clin Periodontol 34:7-17. [DOI] [PubMed] [Google Scholar]
- Zaura E, Keijser B, Huse S, Crielaard W. (2009). Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol 9:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmür R, et al. (2010). Oral biofilm architecture on natural teeth. PLoS One 5:e9321. [DOI] [PMC free article] [PubMed] [Google Scholar]