Abstract
Mastication is one of the most important orofacial functions. The neurobiological mechanisms of masticatory control have been investigated in animal models, but less so in humans. This project used functional connectivity magnetic resonance imaging (fcMRI) to assess the positive temporal correlations among activated brain areas during a gum-chewing task. Twenty-nine healthy young-adults underwent an fcMRI scanning protocol while they chewed gum. Seed-based fcMRI analyses were performed with the motor cortex and cerebellum as regions of interest. Both left and right motor cortices were reciprocally functionally connected and functionally connected with the post-central gyrus, cerebellum, cingulate cortex, and precuneus. The cerebellar seeds showed functional connections with the contralateral cerebellar hemispheres, bilateral sensorimotor cortices, left superior temporal gyrus, and left cingulate cortex. These results are the first to identify functional central networks engaged during mastication.
Keywords: brain function, eating behavior(s), imaging, mastication, mathematical modeling, nervous system
Introduction
Chewing is a vital orofacial function. Many studies have identified brain areas associated with chewing, and some investigations have begun identifying the connections among these areas. Face sensorimotor cortical regions are connected with brainstem central pattern generator circuits (CPG) and may play key roles in adaptive and maladaptive modifications involving orofacial functions (for reviews, see: Lund et al., 1998; Lund and Kolta, 2006; Avivi-Arber et al., 2011). The CPG are responsible for generating chewing rhythmicity as well as for coordinating masticatory muscle activity (Lund et al., 1998; Lund and Kolta, 2006). Sensory afferents modulate CPG circuitry directly or ascend to synapse within the ventral posterior medial thalamic nuclei and subsequently pass information to suprabulbar areas (Lund and Kolta, 2006; Avivi-Arber et al., 2011; Manto et al., 2012).
Recent work has demonstrated orofacial somatotopic maps within the thalamic nuclei, sensorimotor cortices, and cerebella in humans (DaSilva et al., 2002; Moulton et al., 2009; Avivi-Arber et al., 2011; Manto et al., 2012). Descending sensorimotor cortical neurons synapse with trigeminal motoneurons, and many appear to be involved in specific glossomandibular movements or functionally specific chewing cycles associated with ingestion, reduction, or pre-swallowing (Sauerland et al., 1967; Olsson et al., 1986; Yao et al., 2002; Avivi-Arber et al., 2011).
The basal ganglia (Sesay et al., 2000; Masuda et al., 2001), red nucleus (Kennedy et al., 1986), and other cortical and cerebellar regions (Sesay et al., 2000; Onozuka et al., 2002; Avivi-Arber et al., 2011) are also involved in oral movements. However, the precise roles of these areas are unclear.
To our knowledge, no studies have used functional connectivity MRI (fcMRI) to study brain activity during chewing. fcMRI is a novel set of MRI methodologies. fcMRI does not provide direct information about anatomical connections between and among regions, and correlation does not mean causation. However, fcMRI is used to identify reliable and reproducible functional networks in the brain. One fcMRI method defines seed regions of interest (ROI) and then treats the mean time series in the ROI as a reference waveform to identify other brain regions manifesting activity patterns that are temporally correlated with the ROI (for review, see Van Dijk et al., 2010). This study used this method to evaluate brain connectivity during chewing.
Materials & Methods
Study Participants
Twenty-nine healthy right-handed individuals (15 men:14 women; mean age 24 yrs, SD = 3.5) with fully dentate Class I occlusions were selected. The research diagnostic criteria for temporomandibular disorders (RDC-TMD) (Dworkin and LeResche, 1992) were used to exclude those with masticatory myogenous or arthrogenous conditions.
The study was approved by the University of Michigan medical institutional review board, and volunteers signed informed consents before participating. Exclusion criteria included: using medications with known neuromotor effects, e.g., neuroleptics or antidepressants; use of over-the-counter medications ≤ 3 days before the scanning session; a diagnosis of systemic, vascular, or central nervous system disease; and the presence of medical devices or conditions, e.g., pregnancy, that could be dangerous or incompatible with the MRI environment.
fcMRI Protocol
Participants were trained to chew gum on the right side only in response to “Chew on your right side”. They were also trained to follow the command, “Stop chewing, rest and place the gum in your right cheek”. They were guided until they performed the tasks as instructed by one investigator, to standardize the performance across participants. All questions and concerns about the commands were addressed before scanning occurred.
Participants were placed in the scanner (3 Tesla GE Signa scanner, LX [VH3] release, GE Healthcare, Milwaukee, WI, USA; Neuro-optimized gradients) with their heads secured with wedge-shaped pillows, which filled space between the RF coil and their heads, and Velcro straps placed firmly over their foreheads and secured to the MRI headrest. This method decreases movement artifacts to acceptable levels (Quintero et al., 2012). Participants were re-briefed on the tasks, shown the commands, and allowed to practice prior to scanning. T1 images (TR = 12.3 ms, TE = 5.2 ms, flip angle = 15 degrees, bandwidth = 15.63, field of view = 26 cm, number of slices = 144 and slice thickness = 1 mm, voxel size = 1.02 mm x 1.02 mm x 1 mm) were used for pre-processing anatomical and functional data.
Participants used mirrored glasses to watch projected instructions that guided them through the experiment, which included 25-second blocks of chewing gum on the right side, followed by 25-second blocks of holding the gum in the right cheek and remaining quietly at rest. These blocks were repeated 10 times. Each participant completed one functional scanning session. They chewed only on the right to avoid confounding signals induced by changing chewing sides.
Functional imaging was performed with a blood-oxygenation-dependent level (BOLD) contrast-sensitive pulse sequence (TR = 2500 ms, TE = 30 ms, flip angle = 90 degrees, field of view = 22 cm, slice thickness = 3.0 mm, number of scans = 200, number of slices = 53 and voxel size = 3.44 mm x 3.44 mm x 3 mm, spiral acquisition) (Glover and Thomason, 2004) recorded continuously during the experiment. For each individual’s fMRI run, the first 5 images were discarded to allow for MR signal stabilization.
fcMRI Pre-processing
The Statistical Parametric Mapping software (SPM, Ver. 8, Functional Imaging Laboratories, London, UK) and the functional connectivity toolbox, CONN (Whitfield-Gabrieli and Nieto-Castanon, 2012) were used to pre-process and analyze the data. Data pre-processing consisted of slice-time correction and motion correction to minimize time-locked chewing-related movement artifacts, normalization, and smoothing (Isotropic Gaussian kernel with a 6-mm full width at half maximum). Each participant’s functional and structural images were used in fcMRI processing.
MarsBar software (http://marsbar.sourceforget.net) and Montreal Neurological Imaging (MNI) atlas coordinates were used to create 6-mm-diameter spherical seeds in the right and left motor cortices, and right and left cerebellar hemispheres (see Table for coordinates). Seed locations were selected based on results from a previous study, wherein these areas demonstrated significant activations related to chewing (Quintero et al., 2012). Rest and chewing block onsets and durations were identified for each participant for statistical purposes.
Table.
Functional Connections between Seed Regions and Other Brain Areas during Chewing
| Coordinates1 |
|||||||
|---|---|---|---|---|---|---|---|
| Seed Region | Connectivity Region | BA | Cluster Size | z-Score | x | y | z |
| Right motor cortex x = 42, y = −14, z = 361 | Left pre-central gyrus | 6 | 14,159 | 7.35 | −46 | −12 | 34 |
| Left cerebellum posterior lobe | − | 3,314 | 7.28 | −18 | −64 | −18 | |
| Left cerebellum inferior semilunar lobule | − | 394 | 5.33 | −8 | −72 | −46 | |
| Left cuneus | − | 827 | 4.99 | −20 | −90 | 22 | |
| Right cerebellum posterior lobe | 245 | 4.95 | 22 | −86 | −50 | ||
| Cingulate cortex | 30 | 254 | 4.73 | −12 | −66 | 8 | |
| Right cuneus/precuneus | 269 | 4.42 | 14 | −82 | 40 | ||
| Left motor cortex x = −44, y = −12, z = 341 | Right pre-central gyrus/post-central gyrus | 4/6 | 20,719 | 6.98 | 50 | −10 | 30 |
| Left cerebellum posterior lobe | − | 8,8672 | 6.68 | −18 | −60 | −18 | |
| Right cerebellum posterior lobe | − | See key2 | 6.39 | 16 | −60 | −18 | |
| Left cerebellum inferior semilunar lobule | − | 278 | 5.58 | −10 | −70 | −48 | |
| Right cerebellum inferior semilunar lobule | − | 308 | 4.93 | 14 | −66 | −52 | |
| Right middle/superior frontal gyrus | 10/46 | 828 | 4.76 | 34 | 46 | 28 | |
| Left middle/superior frontal gyrus | 10/46 | 529 | 4.42 | −44 | 42 | 20 | |
| Right posterior cerebellum x = 10, y = −68, z = −481 | Left cerebellum posterior lobe | − | 11,385 | 6.18 | −14 | −64 | −22 |
| Right superior temporal gyrus/pre-central gyrus/post-central gyrus3 | 22 | 523 | 4.92 | 62 | −6 | 10 | |
| Left superior parietal lobe | 7 | 264 | 4.55 | −24 | −62 | 58 | |
| Right posterior cerebellum x = 14, y = −58, z = −181 | Left cerebellum posterior lobe | − | 43,445 | 7.38 | −24 | −74 | −22 |
| Left middle temporal gyrus | 20 | 495 | 4.41 | −42 | 2 | −28 | |
| Left cingulate cortex | 24 | 303 | 4.24 | −4 | −22 | 36 | |
| Left posterior cerebellum x = −16, y = −62, z = −181 | Right superior parietal gyrus | 7 | 385 | 4.94 | 26 | −58 | 62 |
| Left inferior temporal gyrus | 20 | 347 | 4.85 | −40 | 2 | −48 | |
| Left posterior cerebellum x = −6, y = −68, z = −481 | Right cerebellum inferior semilunar lobule | 14,931 | 6.95 | 10 | −64 | −54 | |
| Right medial frontal gyrus/paracentral gyrus | 6 | 2,631 | 5.94 | 4 | −24 | 80 | |
| Left superior temporal gyrus | 22 | 650 | 5.16 | −64 | −6 | 4 | |
| Right pre-central/post-central gyrus | 6 | 562 | 5.01 | 48 | −12 | 34 | |
| Right inferior temporal gyrus | 20 | 627 | 4.91 | 58 | −8 | −24 | |
| Right pre-central gyrus | 6 | 302 | 4.85 | 60 | 0 | 6 | |
| Left pre-central/post-central gyrus | 6 | 346 | 4.67 | −46 | −14 | 28 | |
Coordinates are lateral (x), anteroposterior (y), and superior-inferior (z) in mm. In the Connectivity region column: all data are uncorrected, p < 0.001. BA = Brodmann’s area. 2Cluster (8,867 voxels) included both the left and right cerebellum posterior lobes. 3This cluster included the superior temporal gyrus (95 voxels), the Rolandic operculum (150 voxels), insula (76 voxels), and the supplementary motor area (68 voxels).
Statistical Analysis
White matter, cerebral spinal fluid, and motion artifacts were modeled as confounds during data pre-processing. After realignment, movement parameters (translation and rotation, each in 3 dimensions) were plotted and evaluated for each participant (Fig. 1). Motion parameter thresholds were set at ± 2 mm of translation and ± 1 degree of rotation. Participants exceeding these thresholds were excluded from all analyses. The CONN toolbox was used to model motion parameters and rest blocks as covariates and to remove high-frequency noise (0 to 0.03 Hz band pass filter). A first-level model compiled all 10 of the 25-second chew blocks per participant. Averaged and de-trended seed signals were used as a reference waveform. The time-courses of clusters, defined as ≥ 5 voxels, throughout the whole brain were correlated with the reference waveform, creating connectivity maps for each seed. A second-level analysis combined and averaged data for all 29 participants. The second-level analysis results were corrected for multiple comparisons by means of a cluster-level family-wise error (FWE) correction (p < 0.001), based on an initial voxel-level threshold set at p < 0.001. Connectivity maps (Figs. 2, 3) were constructed based on a height threshold set at the voxel-level p < 0.001, uncorrected, since the images of the maps at the cluster-level p < 0.001, corrected, resulted in several large clusters where individual brain regions were difficult to distinguish.
Figure 1.
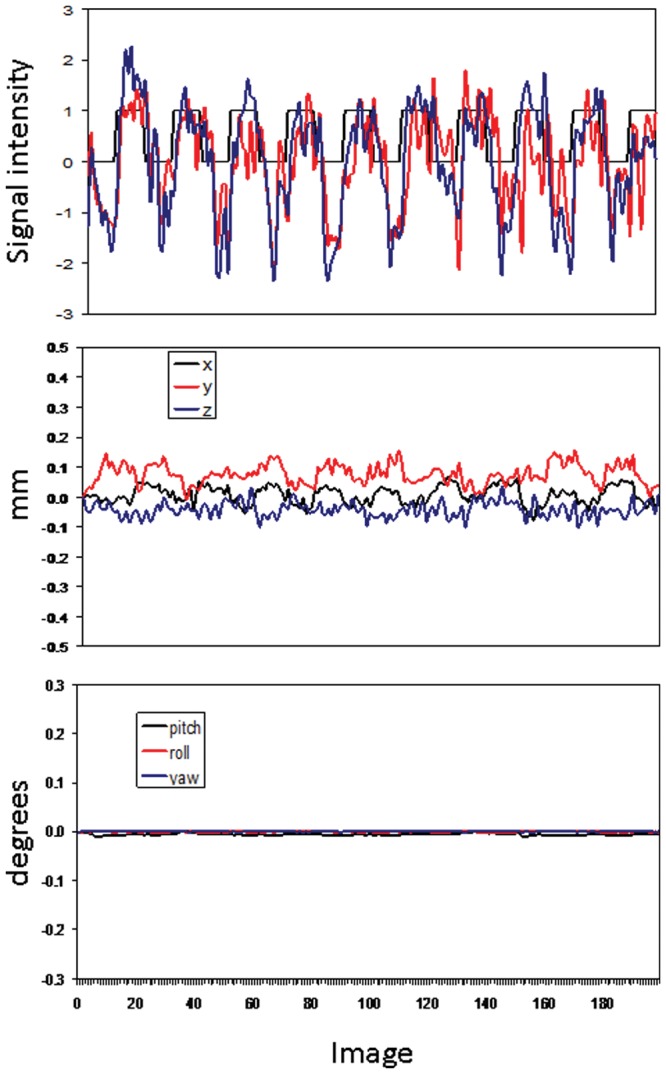
Example data from one participant. The horizontal axis for all 3 plots is the number of images in sequential order. Top: BOLD signal sampled from the left motor cortical seed (black) and from the region in the right motor cortex that showed functional connectivity with the left seed (gray). The vertical axis is signal intensity in arbitrary units. The gray square-wave in the plot shows the block design; rest blocks occurred when the square wave is at zero, and chew blocks occurred during the time periods when the square wave is at unity. The reported functional connectivity results are based on temporal correlations unique to the chewing blocks, e.g., images 10-20, 30-40, 50-60…170-180, 190-200. Middle and bottom: Plots of movement parameters for one participant showing translation (middle) and rotational (bottom) movement artifacts.
Figure 2.
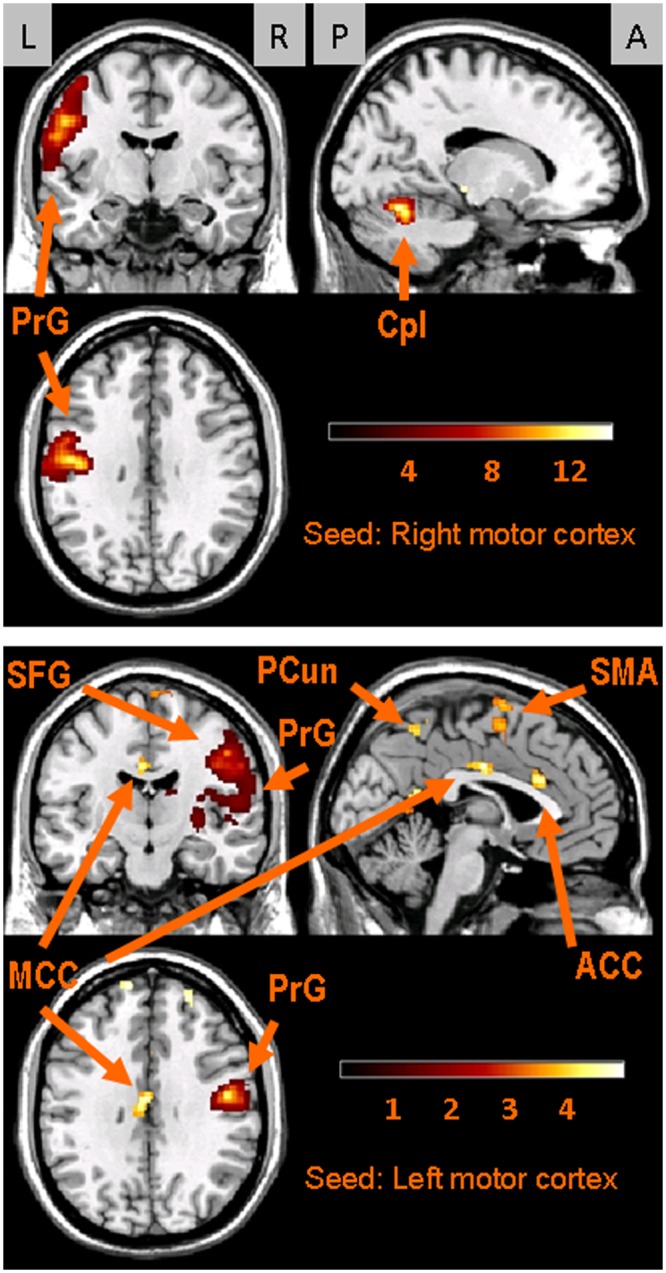
Images of the functional connectivity maps for the seeds in the motor cortices. Upper panel: Results for the right motor cortex seed. Three sections are shown in the upper panel: top left, coronal (y = -8); top right, sagittal (x = -16); and bottom left, axial (z = 32). Lower panel: Results for the left motor cortex seed. The 3 sections in the lower panel are: top left, coronal (y = -14); top right, sagittal (x = 4); and bottom left, axial (z = 32). The gray rectangles at the top of the Fig. indicate participant orientations, viz., L, left; R, right; P, posterior; and A, anterior (for axial sections, anterior is toward the top of the page). Color-coded bars display z-scores; results are based on voxel-level, p < 0.001, uncorrected. Key: Anterior cingulate cortex (ACC); cerebellum posterior lobe (Cpl); middle cingulate cortex (MCC); pre-central gyrus (PrG); precuneus (PCun); superior frontal gyrus (SFG); supplementary motor area (SMA). The labels indicate where the peak values occurred; however, several clusters expand into other areas that are not named in the Fig. (see text and Table).
Figure 3.
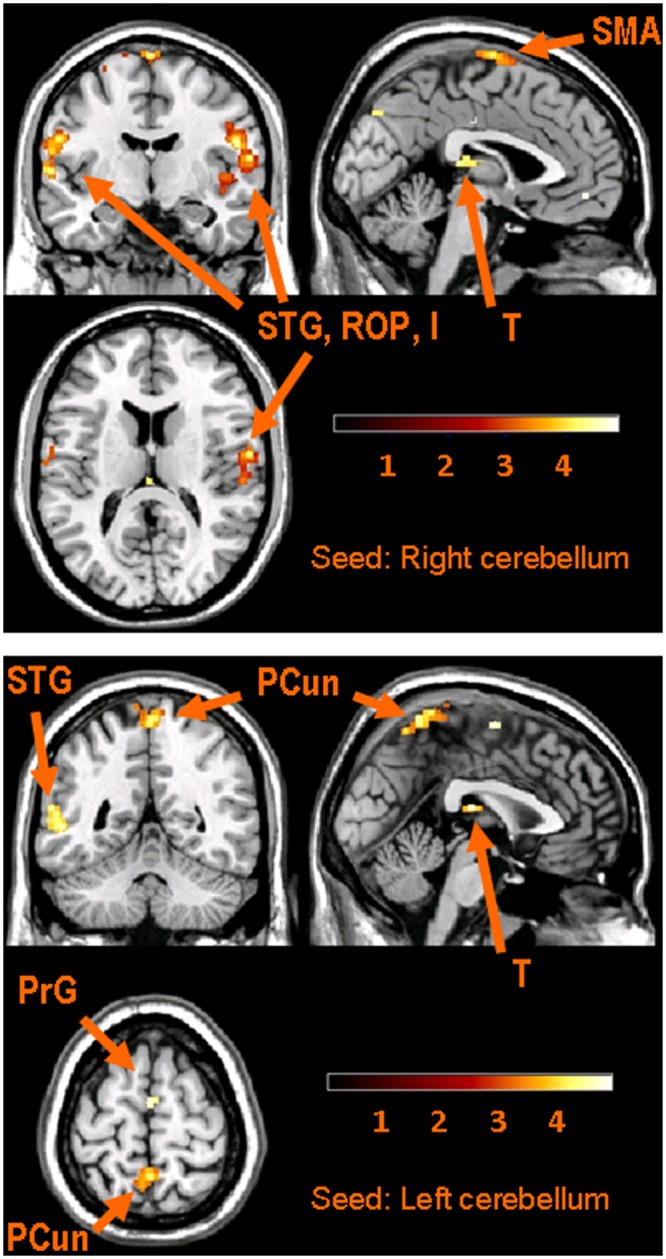
Images of the connectivity maps for the seeds in the cerebellum. Upper panel: Results for right posterior cerebellar seed. Three sections are shown in the upper panel: top left, coronal (y = -8); top right, sagittal (x = -2); and bottom left, axial (z = 20). Lower panel: Results for left posterior cerebellar seed. The 3 sections in the lower panel are: top left, coronal (y = -50); top right, sagittal (x = 2); and bottom left, axial (z = 65). Brain-section orientations in both panels are the same as those used in Fig. 2. Color-coded bars display z-scores; results are based on voxel-level, p < 0.001, uncorrected. Key: Pre-central gyrus (PrG); precuneus (PCun); Rolandic operculum (ROP); superior temporal gyrus (STG); supplementary motor area (SMA); thalamus (T). The labeled structures indicate where the peak values occurred; however, several clusters expand into other areas that are not named in the Fig. (see text and Table).
Results
Fig. 1 shows data for one participant. Activity in the left cortex represents BOLD signal within the spherical seed area only. Activity in the right cortex represents BOLD signal from the entire region in the right motor cortex that demonstrated significant functional connectivity with the left seed. Although Fig. 1 shows the entire 6-minute run, functional connectivity results are based upon the correlated activity unique to the chew-block time periods only.
Motor Cortex Seed Results
There were significant bilateral functional connections between motor cortices (Table, Fig. 2). These were large clusters with peak values in the pre- and post-central gyri. These clusters extended from somatosensory and motor cortices into premotor and supplementary motor areas (SMA) (Fig. 2). Motor cortical seeds also showed bilateral functional connections with the cingulate cortex, cuneus, precuneus, and posterior cerebellar lobes (Table).
Cerebellar Seed Results
Both cerebellar hemispheres showed functional connectivity maps with each other (Table). The cerebellar seeds also showed ipsilateral functional connections with the superior temporal gyrus, pre- and post-central gyri. One of the left cerebellar seeds showed functional connectivity with the contralateral pre- and post-central gyri. One of the right cerebellar seeds showed functional connectivity with pre- and post-central gyri, insula, and SMA (Table, Fig. 3). Cerebellar connectivity also involved the cingulate cortex, middle and inferior temporal gyri, and superior parietal cortex. Voxel-level analysis revealed connectivity with the precuneus and thalamus as well (Fig. 3, uncorrected p < 0.001).
Discussion
Previously, we identified bilateral activations in the motor cortex associated with chewing gum (Quintero et al., 2012) and used the coordinates of the peak voxels as seeds in this study. The motor cortices were functionally interconnected, and the interconnectivity also involved the post-central gyri (Table, Fig. 2). Transcranial magnetic stimulation of the motor cortex elicits bilateral contraction of the masseter and digastric muscle in humans (Nordstrom, 2007). Animal studies have revealed connections between the 2 motor cortices involved in mastication (Hiraba and Sato, 2004). Primate studies demonstrated relationships between primary somatosensory and motor cortices, and evidence suggests that sensorimotor cortices play a role in the control of orofacial movements such as chewing (Avivi-Arber et al., 2011). These studies corroborate our findings of bilateral sensorimotor cortical connectivity during chewing.
Fig. 1, top, demonstrates the variation in activation levels during the MRI trials. fcMRI studies exploit significant positive correlations through time, between variation in the activity in a seed region vs. variation in other regions, to construct functional connectivity maps. This investigation sampled ‘snapshots’ of brain activity every 2.5 sec for ten 25-second-duration chewing blocks per participant. Given 29 participants, our results are based upon correlations involving 2,900 brain images. Although we did not monitor tongue or jaw movement kinematics, evidence suggests that sensorimotor cortical neurons are involved with specific jaw movements, e.g., opening vs. closing, in association with specific tongue movements, e.g., protrusion vs. retraction, during chewing (Yao et al., 2002). We hypothesize that variation in glossomandibular movements may be significantly related to the variations in activation levels observed in the regions identified in the connectivity maps (Figs. 2, 3, Table).
Our participants chewed gum in a supine position and on the right side only. Some of our results could reflect functional connectivity required for chewing to be adapted to this orientation and for a commanded task to be performed. Sensorimotor cortical neurons may be involved in adaptations to altered oral states or motor behaviors (Avivi-Arber et al., 2011). Additionally, activity in the SMA may be related to motor planning and the execution of learned tasks (Wong et al., 2011). We observed functional connectivity involving sensorimotor cortices and SMA, which suggests that our study’s gum-chewing task required adaptation and learning.
The Table shows large connectivity clusters between the motor cortex seeds and the contralateral cortices. These clusters extend into the SMA, superior temporal gyrus, insula, and sensorimotor cortices, all areas previously described as playing a role in chewing movements, specifically in coupling sensory and motor output to address variation in food hardness (Takahashi et al., 2007). That these regions were functionally connected during the present study suggests the existence of continuous sensorimotor coupling during the chewing of a gum bolus, which remained relatively stable in terms of mass and consistency.
As in a previous fMRI study of mastication (Onozuka et al., 2002), we found evidence for cerebellar involvement in chewing. The present study demonstrated evidence for functional connections between the cerebellum and sensorimotor and cingulate cortices during mastication. In primates, descending cortical neurons synapse in the pons, and the post-synaptic neurons project to the cerebellum via the cerebellar peduncle (Brodal, 1978; Kelly and Strick, 2003). These cerebellar projections originate in SMA, motor, cingulat, and somatosensory cortices (Glickstein et al., 1985).
The cerebellum plays a feed-forward role in planning motor output to match known environmental cues, e.g., if the weight of a lifted object is known, the cerebellum plans motor output to match the weight (reviewed in Manto et al., 2012). Regarding oral function, cerebellar ablation in guinea pigs decreases both chewing-cycle frequency and frequency variability (Byrd and Luschei, 1980). In our study, the cerebellum may have been involved in semi-automating chewing rhythmicity and bite force, based on relatively predictable physical properties of the gum.
The cerebellum also coordinates time-locked sequential transitions in motor behavior, and it rapidly updates movements based on proprioceptive input (Manto et al., 2012). Increasing food hardness leads to increased cerebellar activity during chewing (Takahashi et al., 2007). In our study, the cerebellum may have been involved with coordinating glossomandibular movement timing, based on variation in the position and shape of the gum bolus, the need to swallow, etc.
There were also connections between the motor cortex and both the precuneus and cuneus (Table). These areas have not been described as being involved in mastication. These areas appear to increase in activity during upper limb and eye movements (Wenderoth et al., 2005; Bédard and Sanes, 2009). The precuneus increases activation secondary to electrical stimulation of the dentition (Ettlin et al., 2009), to pin-prick stimulation to the mental nerve region (Abrahamsen et al., 2010), and to hypertonic saline injections into the masseter muscle (Kupers et al., 2004). The precuneus is one of the least explored regions of the cerebral cortex because of its hidden location and because few focal lesions occur here (Cavanna and Trimble, 2006). A recent review provides some evidence for its roles in episodic memory retrieval and self-processing operations, including first-person perspective-taking and an experience of agency (Cavanna and Trimble, 2006). With this evidence in mind, we hypothesize that the precuneus could be involved with the association between movement and sensory inputs. Furthermore, given that chewing is usually spontaneous and automatic, precuneate activity in the present study may reflect both sensorimotor and self-processing operations unique to the experimental conditions, e.g., “I see that I am supposed to chew now, so that is what I’ll do.”
Study weaknesses included not accounting for chewing side preference. Lateralization of brain function may be related to chewing side preference (reviewed in Avivi-Arber et al., 2011). If this is the case, connectivity asymmetries would be lost in the averaging process. Also, since we did not monitor jaw and tongue kinematics, we could not confirm that participants were performing tasks correctly. Kinematic studies in the MRI environment are challenging, which is the main reason for our not monitoring oral-function parameters. However, all participants were debriefed to determine if there were problems performing the tasks in the scanner.
Finally, fcMRI methods are relatively new, and reliability studies should be further developed. Evidence suggests that fcMRI reliability is strongest when only statistically strong and positive correlations, averaged across large numbers of participants, are reported (Van Dijk et al., 2010). All of these criteria were a part of our study design to improve the strength of the findings.
Acknowledgments
This work is based on a thesis submitted by Dr. Andres Quintero to the graduate faculty, University of Michigan, in partial fulfillment of the requirements for the PhD degree. We acknowledge Keith Newnham’s technical assistance with the MRI scanner.
Footnotes
This project was supported by the NIDCR (USPHS Research Grant DE-018528 to GEG).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abrahamsen R, Dietz M, Lodahl S, Roepstorff A, Zachariae R, Østergaard L, et al. (2010). Effect of hypnotic pain modulation on brain activity in patients with temporomandibular disorder pain. Pain 151:825-833. [DOI] [PubMed] [Google Scholar]
- Avivi-Arber L, Martin R, Lee J-C, Sessle BJ. (2011). Face sensorimotor cortex and its neuroplasticity related to orofacial sensorimotor functions. Arch Oral Biol 56:1440-1465. [DOI] [PubMed] [Google Scholar]
- Bédard P, Sanes JN. (2009). Gaze and hand position effects on finger-movement-related human brain activation. J Neurophysiol 101:834-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P. (1978). The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain 101:251-283. [DOI] [PubMed] [Google Scholar]
- Byrd KE, Luschei ES. (1980). Cerebellar ablation and mastication in the guinea pig ( Cavia porcellus). Brain Res 197:577-581. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(Pt 3):564-583. [DOI] [PubMed] [Google Scholar]
- DaSilva A, Becerra L, Makris N, Strassman A, Gonzalez R, Geatrakis N, et al. (2002). Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22:8183-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. (1992). Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6:301-355. [PubMed] [Google Scholar]
- Ettlin DA, Brügger M, Keller T, Luechinger R, Jäncke L, Palla S, et al. (2009). Interindividual differences in the perception of dental stimulation and related brain activity. Eur J Oral Sci 117:27-33. [DOI] [PubMed] [Google Scholar]
- Glickstein M, May JG, Mercier BE. (1985). Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol 235:343-359. [DOI] [PubMed] [Google Scholar]
- Glover GH, Thomason ME. (2004). Improved combination of spiral-in/out images for BOLD fMRI. Magn Reson Med 51:863-868. [DOI] [PubMed] [Google Scholar]
- Hiraba H, Sato T. (2004). Cortical control of mastication in the cat: properties of mastication-related neurons in motor and masticatory cortices. Somatosens Mot Res 21:217-227. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PR, Gibson AR, Houk JC. (1986). Functional and anatomic differentiation between parvicellular and magnocellular regions of red nucleus in the monkey. Brain Res 364:124-136. [DOI] [PubMed] [Google Scholar]
- Kupers RC, Svensson P, Jensen TS. (2004). Central representation of muscle pain and mechanical hyperesthesia in the orofacial region: a positron emission tomography study. Pain 108:284-293. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. (2006). Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 21:167-174. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A, Westberg KG, Scott G. (1998). Brainstem mechanisms underlying feeding behaviors. Curr Opin Neurobiol 8:718-724. [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SN, Gerwig M, et al. (2012). Consensus paper: Roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum 11:457-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Kato T, Hidaka O, Matsuo R, Inoue T, Iwata K, et al. (2001). Neuronal activity in the putamen and the globus pallidus of rabbit during mastication. Neurosci Res 39:11-19. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Pendse G, Morris S, Aiello-Lammens M, Becerra L, Borsook D. (2009). Segmentally arranged somatotopy within the face representation of human primary somatosensory cortex. Hum Brain Mapp 30:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA. (2007). Insights into the bilateral cortical control of human masticatory muscles revealed by transcranial magnetic stimulation. Arch Oral Biol 52:338-342. [DOI] [PubMed] [Google Scholar]
- Olsson KA, Landgren S, Westberg KG. (1986). Location of, and peripheral convergence on, the interneuron in the disynaptic path from the coronal gyrus of the cerebral cortex to the trigeminal motoneurons in the cat. Exp Brain Res 65:83-97. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, et al. (2002). Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res 81:743-746. [DOI] [PubMed] [Google Scholar]
- Quintero A, Ichesco E, Myers C, Schutt R, Gerstner G. (2012). Brain activity and human unilateral chewing: an fMRI study. J Dent Res 92:136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland EK, Nakamura Y, Clemente CD. (1967). The role of the lower brain stem in cortically induced inhibition of somatic reflexes in the cat. Brain Res 6:164-180. [DOI] [PubMed] [Google Scholar]
- Sesay M, Tanaka A, Ueno Y, Lecaroz P, De Beaufort DG. (2000). Assessment of regional cerebral blood flow by xenon-enhanced computed tomography during mastication in humans. Keio J Med 49(Suppl 1):A125-A128. [PubMed] [Google Scholar]
- Takahashi T, Miyamoto T, Terao A, Yokoyama A. (2007). Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience 145:791-794. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. (2005). The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci 22:235-246. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125-141. [DOI] [PubMed] [Google Scholar]
- Wong D, Dzemidzic M, Talavage TM, Romito LM, Byrd KE. (2011). Motor control of jaw movements: an fMRI study of parafunctional clench and grind behavior. Brain Res 1383:206-217. [DOI] [PubMed] [Google Scholar]
- Yao D, Yamamura K, Narita N, Martin RE, Murray GM, Sessle BJ. (2002). Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol 87:2531-2541. [DOI] [PubMed] [Google Scholar]


