Abstract
Background
Neoadjuvant therapy has been investigated for localized and locally advanced pancreatic ductal adenocarcinoma (PDAC) but no standard of care exists. Combination cetuximab/gemcitabine/radiotherapy demonstrates encouraging preclinical activity in PDAC. We investigated cetuximab with twice-weekly gemcitabine and intensity-modulated radiotherapy (IMRT) as neoadjuvant therapy in patients with localized or locally advanced PDAC.
Experimental design
Treatment consisted of cetuximab load at 400 mg/m2 followed by cetuximab 250 mg/m2 weekly and gemcitabine 50 mg/m2 twice-weekly given concurrently with IMRT to 54 Gy. Following therapy, patients were considered for resection.
Results
Thirty-seven patients were enrolled with 33 assessable for response. Ten patients (30%) manifested partial response and 20 (61%) manifested stable disease by RECIST. Twenty-five patients (76%) underwent resection, including 18/23 previously borderline and 3/6 previously unresectable tumors. Twenty-three (92%) of these had negative surgical margins. Pathology revealed that 24% of resected tumors had grade III/IV tumor kill, including two pathological complete responses (8%). Median survival was 24.3 months in resected patients. Outcome did not vary by epidermal growth factor receptor status.
Conclusions
Neoadjuvant therapy with cetuximab/gemcitabine/IMRT is tolerable and active in PDAC. Margin-negative resection rates are high and some locally advanced tumors can be downstaged to allow for complete resection with encouraging survival. Pathological complete responses can occur. This combination warrants further investigation.
Keywords: cetuximab, gemcitabine, intensity-modulated radiotherapy, neoadjuvant therapy, pancreatic cancer
introduction
Pancreatic ductal adenocarcinoma (PDAC) is highly lethal with 5-year mortality of 95% [1]. Complete resection of localized disease results in long-term survival of ∼20% with patients dying to both local and metastatic recurrence [2]. Improved outcomes for patients with PDAC will depend on earlier detection, increased rate of complete resection, and reduced rates of both local and distant relapse.
The dismal survival seen in patients with locally advanced pancreas cancer has led to numerous neoadjuvant trials that have met with variable efficacy and toxicity. No standard approach yet exists. However, theoretical advantages of a neoadjuvant approach include (i) delivery of full dose treatment unimpeded by surgery and its recovery, (ii) ability to objectify tumor response to newer therapies, and (iii) downstaging of tumor to allow for a potentially curative surgical resection.
Gemcitabine has been shown in vitro to be a potent radiosensitizer [3]. Radiosensitization occurs at noncytotoxic concentrations and correlates with extent and duration of dATP depletion. We have previously established the maximum tolerated dose of twice-weekly gemcitabine as 50 mg/m2 when given concurrently with external beam radiotherapy in patients with PDAC [4]. This dosing is tolerable and effective and can allow for downstaging and complete surgical resection in some patients [4, 5].
The epidermal growth factor receptor (EGFR) is a member of the erb-B receptor tyrosine kinase family [6]. The EGFR signal transduction network plays an important role in multiple tumorigenic processes [6, 7]. EGFR is overexpressed in 30%–89% of PDACs and is associated with increased tumor size, advanced clinical stage, and decreased survival [8–11]. Cetuximab is a monoclonal antibody which blocks ligand binding to EGFR and stimulates EGFR internalization, effectively removing the receptor from cell surface interactions [12]. Cetuximab inhibits growth and metastasis of human PDAC, an effect potentiated by concomitant administration of gemcitabine [13, 14].
Evidence indicates that the EGFR-tyrosine kinase plays an important role in determining cellular response to ionizing radiation by activation of downstream signal transduction pathways. Cetuximab treatment of murine ovarian carcinoma cells transfected with human EGFR reverses cellular radioresistance [15]. Experiments with PDAC xenografts show enhanced inhibition of tumor cell growth with the addition of cetuximab to gemcitabine/radiotherapy [16]. It is not known, however, whether cetuximab therapy can improve outcome in PDAC in the context of neoadjuvant chemoradiotherapy.
We evaluated the combination of weekly cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with PDAC. We hypothesized that this would result in enhanced antitumor activity and acceptable toxicity. Primary end point was objective response rate by RECIST criteria. Secondary end points included toxicity assessment, post-treatment resectability, pattern of failure, and survival.
patients and methods
eligibility
This was a single institution phase II trial, approved by the Dartmouth College Institutional Review Board. Eligibility included biopsy-proven PDAC, with measurable stage I, II, or III disease, and sufficient biopsy tissue available to perform EGFR assessment. Staging included high-resolution computed tomography (CT) scan of chest/abdomen/pelvis, pulmonary function testing, endoscopic ultrasound, and diagnostic laparoscopy. Inclusion parameters included age ≥ 18 years, Karnofsky Performace Score ≥ 70%, neutrophil count ≥ 1500/ul, platelets ≥ 100 000, creatinine ≤ 1.5× ULN, total bilirubin ≤ 1.5× ULN, aspartate transaminase ≤ 2.5× ULN. Elderly patients were not excluded. No prior tumor directed therapy was allowed. Endoscopic ultrasound was carried out within 35 days of starting therapy. Other staging procedures were completed within 28 days.
All subjects were evaluated at Multidisciplinary Gastrointestinal (GI) Tumor Board. Tumor resectability was based on CT as defined by the American Hepato-Pancreato-Biliary Association Convened Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer [17]. Resectable disease was defined as no evidence of superior mesenteric vein (SMV) or portal vein (PV) abutment, distortion, tumor thrombus, or venous encasement, and clear fat planes around celiac axis (CA), hepatic artery (HA), and superior mesenteric artery (SMA). Borderline resectable disease included tumors showing involvement of SMV/PV (including narrowing, encasement, or short-segment occlusion but with suitable vessel involvement, allowing for safe resection and reconstruction), gastroduodenal or HA involvement/encasement without extension to the CA, or abutment of SMA <180°. Unresectable disease included encasement of SMA, CA, or proximal HA.
Tumor was assessed for EGFR status by immunohistochemistry. Both EGFR(+) and EGFR(−) subjects were eligible for protocol, since it was not known, a priori, whether EGFR levels which were undetectable by immunostaining could still drive tumor progression and responsiveness to cetuximab. Formalin-fixed paraffin-embedded tissue sections were pretreated with Citra reagent and incubated with a 1:10 dilution of EGFR antisera supplied by Novocastra (Leica Microsystems Buffalo Grove, Illinois). Antigen was subsequently localized with standard avidin–biotin complex immunoperoxidase techniques. Positive cases were graded by pathologist as 1+, 2+, or 3+ depending on the intensity of staining. Additionally, pretreatment tumor tissue was tested for KRAS mutation analysis where sufficient sample existed. This was carried out by a series of allelic discrimination assays using real-time PCR.
treatment
A cetuximab load (400 mg/m2) was given i.v. over 2 h, 6–8 days before starting radiotherapy and gemcitabine. This was followed by weekly infusions (250 mg/m2) over 1 h for six additional doses. Gemcitabine (50 mg/m2) was administered as a 30-min i.v. infusion on a twice-weekly schedule (Mon/Thurs or Tue/Fri) for 12 doses. Chemotherapy dosing was based on actual body surface area. Gemcitabine was held on days when radiation was not delivered. Diphenhydramine (50 mg i.v.) was given before cetuximab. Premedication was otherwise at physician discretion. GI prophylaxis with a proton pump inhibitor was administered throughout radiation therapy and for an additional month thereafter.
Radiation was delivered using IMRT. Prescription dose was defined at the minimum dose to the primary planning target volume. Gross tumor volume (GTV) was defined by the physician as all known disease including imaging proven nodal disease. The primary planning target volume (PTV1) included the GTV with 2- to 3-cm margins in all directions as well as potential nodal involvement. Secondary planning target volume (PTV2) included the GTV with 1- to 1.5-cm margins on all sides including proven nodal involvement. Tertiary planning treatment volume (PTV3) included the area of the GTV adjacent to the vascular structures specifically the mesenteric and portal vessels with a 0.5-cm margin. The prescription dose delivered to PTV3 was 54 Gy in 28 fractions. Synchronously, PTV1 and PTV2 received 45 and 50.4 Gy, respectively. All fields were treated daily at five fractions per week. Quality assurance was verified with Matrixx, Chamber, and daily orthogonal films.
Patients were evaluated weekly while on treatment. Adverse events were graded according to NCI-CTCAE Version 3.0. Cetuximab was discontinued for grade 3 or 4 infusion reactions and held for any other grade 3 or 4 toxic effects. Gemcitabine was held for grade 3-4 nonhematologic toxicity, for absolute neutrophil count < 1.0 × 109/l, or platelets <50 x 109/l. Radiation was held at the discretion of the Radiation Oncologist for grade 3 or higher toxicity. Treatment resumed when toxicity resolved to grade 2 or less.
One month following therapy, patients were restaged with CT scan of chest/abdomen/pelvis. Response assessment was carried out by RECIST 1.0 criteria [18]. Patients deemed to be candidates for surgical resection were offered laparotomy ∼6 to 10 weeks after completion of neoadjuvant therapy. Those who underwent resection were also treated with intraoperative radiation therapy (IORT), as was the standard practice at our institution. IORT was delivered with energy of 300 KV out of a fluence rate of 10 mA. The delivered dose was 10 Gy to the target in a single fraction, prescribed to the surface.
pathological evaluation
Surgical specimens were evaluated according to a standardized College of American Pathologists pancreas (exocrine) protocol based on the American Joint Committee on Cancer/International Union against Cancer TNM classification, Sixth Edition. The bile duct, pancreatic neck, and SMA margins were inked by the surgeon ex vivo and sent to pathology. Pancreatic neck and bile duct margins were evaluated en face. SMA margin was cut perpendicular to the inked surface and examined with radial sections. Representative sections of gastric and duodenal margins were also submitted. Residual tumor was serially sectioned and submitted in its entirety for microscopic analysis. Additionally, surgical specimens were graded for percentage of tumor kill according to Evans et al. [19].
No adjuvant therapy was given following resection. Following therapy, patients were seen every 3 months for 2 years and then every 6 months for a total of 5 years. Restaging CT scans of chest/abdomen/pelvis were carried out every 6 months. For patients with relapse or progression following therapy, treatment was at physician discretion.
statistical analysis
Initial enrollment was planned for 48 patients, with 24 EGFR(+) and 24 EGFR(−) cases. With a total sample size of 24 patients, the 95% confidence interval for the response rate would have a half width no greater than ±20%. The actual half width was dependent on observed response rate. Exact methods based on binomial distribution were used to compute the 95% confidence interval for the response rate. Toxic events were summarized according to type and grade. This was done for all patients receiving any treatment. Survival curves were estimated using the product–limit method.
results
Thirty-seven patients were enrolled between January 2005 and August 2008. Patient characteristics are shown in Table 1. The relatively low percentage of EGFR(−) tumors coupled with the observation of little difference in response or toxicity between the two groups led us to halt accrual after enrollment of 24 assessable EGFR(+) cases. Three patients (8%) were removed from trial and replaced after experiencing infusion reactions to the loading dose of cetuximab. None of these was life threatening. One patient died on therapy as described below. Overall, 33 patients (89%) were assessable for response. Of these patients, 4 (12%) presented with resectable tumor, 23 (70%) with borderline resectable tumor, and 6 (18%) with unresectable tumor.
Table 1.
Patient characteristics
| Enrolled patients | 37 |
| Mean age (years) | 64 (range 39-82) |
| Sex | M = 43%/F = 57% |
| EGFR(+) tumor | 28 (76%) |
EGFR, epidermal growth factor receptor; F, female; M, male.
dosing and toxicity
Of 33 patients assessable for response, all received full-dose radiotherapy and mean of 6.3 doses of cetuximab (range 5–7) and 10.5 doses of gemcitabine (range 6–12). All 37 patients were assessable for toxicity. Grade 3 and higher toxic effects are shown in Table 2. The most commonly encountered toxic effects were cytopenias, nausea/vomiting, and cholangitis due to biliary stent occlusion. Sixteen patients (43%) were hospitalized while on therapy. Two patients experienced acute cerebrovascular accidents (CVA), each at the time of admission for cholangitis due to biliary stent occlusion. This was a fatal event in one patient, aged 81 years. Autopsy revealed severe atherosclerotic disease and evidence of acute and remote CVAs. Additionally, portal vein thromboses were a late event in four patients who underwent pancreaticoduodenectomy at a mean of 21 months post-surgery (range 5–35 months). These were treated with portal vein recanalization.
Table 2.
Grade 3 or higher toxic effects (No. of patients)
| Toxicity name | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|
| Leukopenia/Neutropenia | 21 | 4 | |
| Nausea/Vomiting | 9 | ||
| Cholangitis/Stent block | 8 | ||
| Thrombocytopenia | 8 | 4 | |
| Fatigue | 5 | 1 | |
| Gastritis/Gastrointestinal bleed | 5 | 1 | |
| Pain | 4 | ||
| Anorexia | 3 | ||
| Cetuximab reaction | 3 | ||
| Rash | 3 | ||
| Elevated liver functions | 3 | ||
| Deep vein thrombosis | 2 | ||
| Dehydration | 2 | ||
| Diarrhea | 2 | ||
| Hypokalemia | 2 | 1 | |
| Hypophosphatemia | 2 | ||
| Urinary tract infection | 2 | ||
| Anemia | 1 | ||
| Cellulitis | 1 | ||
| Constipation/Obstipation | 1 | ||
| Hyperglycemia | 1 | ||
| Ischemic stroke | 1 | 1 | |
| Pulmonary emboli | 1 | ||
| Syncope | 1 |
response
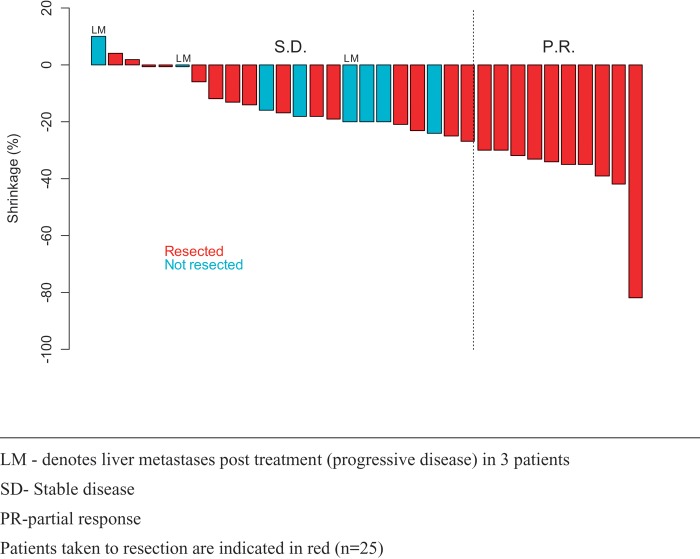
See Figure 1. Ten patients (30%) met RECIST criteria for partial response. Twenty patients (61%) had stable disease, with most of these manifesting a measurable degree of tumor shrinkage. Three patients (9%) had evidence of progressive disease in the liver with stable disease in the pancreas. Figure 2 illustrates CT images in two patients with response to treatment. Response rate was 33% for EGFR(−) tumors (3/9 patients) and 29% for EGFR(+) tumors (7/24 patients) (P = 1.0).
Figure 1.
Tumor response to therapy (N = 33). Patients taken to resection are indicated in red (n = 25). LM denotes liver metastases posttreatment (progressive disease) in three patients. PR, partial response; SD, stable disease.
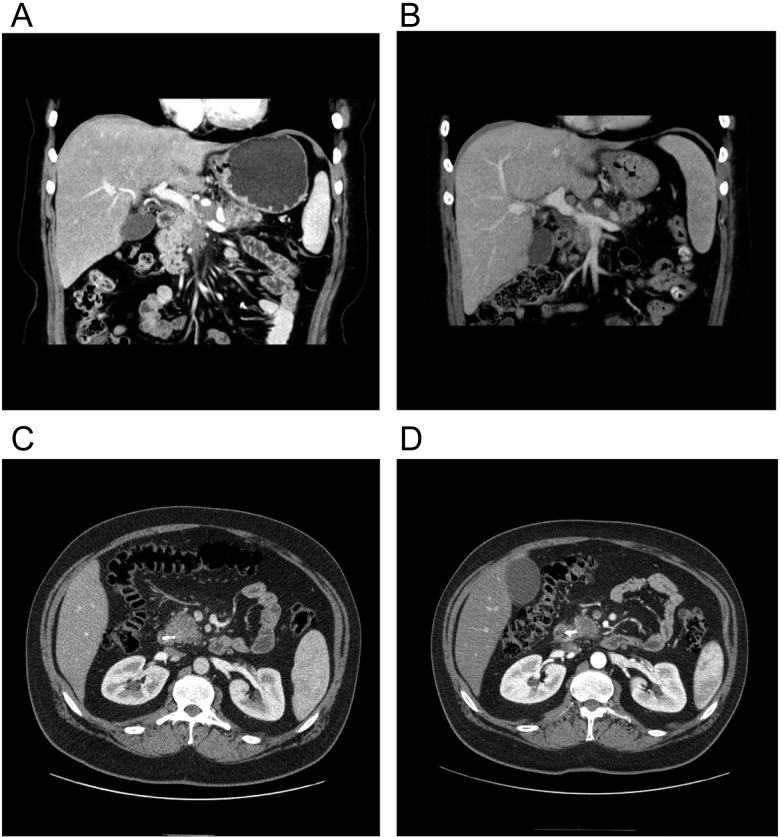
Figure 2.
CT Images in representative responding patients. (A) Pretreatment scan shows extensive mass encircling, and nearly obstructing PV. (B) Posttreatment study shows regression of tumor with widely patent PV. Patient went on to complete resection. (C) Pretreatment CT showing pancreatic head mass. (D) Posttreatment CT shows response of tumor in pancreatic head and retraction from superior mesenteric vein. Patient went on to complete resection. CT, computed tomography; PV, portal vein.
Pretreatment tumor biopsy specimens were analyzed for KRAS mutations. Sufficient tissue was present in 26 of the assessable patients. Eighteen of these (69%) exhibited mutations at codon 12 or 13. Response rates for KRAS mutated and wild-type tumors were 33% and 13%, respectively (P = 0.37).
surgical resection and pathological evaluation
Of the 33 patients who completed therapy on trial, 26 (79%) were judged candidates for surgical resection. One refused surgery and 25 patients were taken to laparotomy. Pancreaticoduodenectomy was carried out in 22 patients and distal pancreatectomy in the remaining three. This included 4/4 patients presenting with resectable tumor, 18/23 previously borderline tumors, and 3/6 previously unresectable tumors. Margin-negative resections were achieved in 23 patients (92%). One patient had a microscopic positive vascular groove margin, and the second had microscopic disease at proximal pancreatic and SMA margins. Tumor specimens yielded pronounced treatment effect, with grade IIa (destruction of 10%–50% of tumor cells) or higher effect in all specimens. Six specimens (24%) had grade III (<10% viable tumor cells) or IV (no viable tumor cells), including two specimens (8%) with confirmed pathological complete response.
outcome
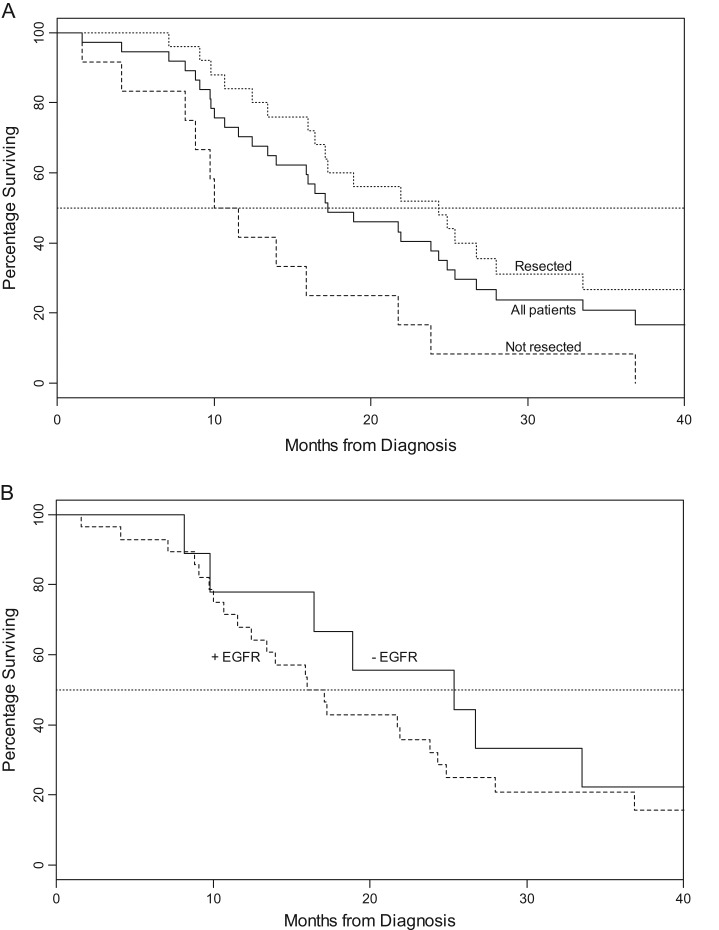
See Figure 3. Intent-to-treat analysis yielded a median survival of 17.3 months. Median survival was 24.3 months for patients who underwent surgical resection versus 10.0 months for those who did not. Survival did not vary by EGFR status. Twenty-six patients died from recurrent/progressive PDAC, two patients from pneumonia/sepsis, one from traumatic subdural bleed (not therapy related), and one from CVA as described. Of the patients who underwent resection (n = 25), 2 experienced local recurrence only (8%), 1 local and distant recurrence (4%), and 17 (68%) distant disease recurrence. At 48-month median follow-up, seven patients were alive and four without evidence of disease. Of the seven, two presented with resectable and five with borderline tumor Two patients remained disease free >5 years from enrollment. One each presented with resectable and borderline disease.
Figure 3.
Survival graphs. (A) Survival by resection status. (B) Survival by tumor epidermal growth factor receptor (EGFR) status (P = 0.437).
discussion
Our study demonstrates that gemcitabine and radiotherapy can be successfully combined with an EGFR inhibitor and result in a significant antitumor effect in patients with PDAC. Despite a high percentage of patients presenting with locally advanced disease, most were subsequently able to undergo complete resection of tumor. It is interesting to note that radiographic response was highly predictive of subsequent surgical resection but that many patients who manifested minor responses were also able to undergo surgery. This most often reflected sufficient shrinkage of disease to render a radiographic (and surgical) margin between tumor and vasculature. As such, posttreatment resectability of the tumor by CT criteria is probably a more important end point clinically than is response by RECIST criteria.
As preclinical data suggest, the combination of EGFR inhibition with gemcitabine and radiotherapy may act synergistically to overcome mechanisms of resistance in these tumor cells in vivo. Particularly intriguing is the grade III–IV tumor kill seen in one-quarter of resected specimens. Pathological complete response is the ultimate goal of neoadjuvant therapy and has been associated with improved survival in both esophageal and rectal cancer [20, 21]. Whether this survival benefit will be observed in pancreatic cancer remains to be seen but is a critical area of inquiry.
Tumor EGFR overexpression did not seem to affect response to therapy or survival in our trial. Several potential explanations exist for this apparent lack of correlation between EGFR levels and response. PDACs frequently express relatively high levels of the EGFR-related receptors (ErbB-2/HER2 and ErbB-3/HER3) as well as EGF and related ligands. [22]. As a consequence of receptor heterodimerization with HER2 and/or HER3, it is conceivable that even low levels of EGFR confer a growth advantage to pancreatic cancer cells. Most PDACs harbor a mutated KRAS gene, which may enhance signaling downstream of EGFR. Lastly, cross-talk mechanisms that trans-activate EGFR, such as integrin-dependent interactions [23], may also lead to increased pathway activation, which renders the cancer sensitive to therapy.
Subjects experienced frequent but manageable toxicity on our protocol. Neutropenia and thrombocytopenia were common but reversible in most cases. Biliary stent obstructions with resulting cholangitis were the most common cause of admission and treatment delay. This could be partially abrogated in the future by the routine use of metal stents, which have been shown to have a lower risk of obstruction compared with plastic stents [24]. Patients tolerated surgery without any pattern of worsened postoperative difficulties, although prominent peritumoral scarring/fibrosis were noted in some patients, which made resection more technically challenging. Importantly, no postoperative deaths occurred. Median survival of patients undergoing resection exceeded 24 months despite a cohort largely composed of locally advanced tumors. This compares favorably with single institution data on resectable disease patients who underwent surgery followed by adjuvant therapy [25, 26].
There is a rapidly growing body of data that supports a neoadjuvant approach in the management of PDAC. A retrospective review from Duke University showed that neoadjuvant chemoradiotherapy did not increase morbidity or mortality of subsequent pancreaticoduodenectomy and was associated with a marked reduction in risk of pancreatic duct leak [27]. Japanese data on resection from 68 patients treated with neoadjuvant chemoradiotherapy yielded an increased rate of clear surgical margins and decreased the rate of lymph node positivity, resulting in improved survival compared with surgery alone [28]. We have previously published evidence that neoadjuvant therapy significantly reduces local relapse rates when compared with ‘standard’ adjuvant treatment [29]. Recent meta-analysis of >100 trials of neoadjuvant therapy in PDAC revealed that approximately one-third of patients presenting with locally advanced disease can undergo successful resection following neoadjuvant therapy with survival rates equivalent to those presenting with localized disease [30].
EGFR inhibition has been advocated as a method to enhance the therapeutic index of gemcitabine and radiotherapy in PDAC [31]. Both cetuximab and erlotinib increase the efficacy of gemcitabine/radiotherapy in human pancreatic cancer cell lines, supporting the integration of EGFR inhibitors into clinical trials [32]. EGFR inhibitors administered with gemcitabine/radiotherapy inhibit phosphorylation of EGFR in a time-dependent fashion and result in reduction of pAKT (S473), a pro-survival molecule downstream of EGFR. The addition of EGFR inhibition in mouse models did not result in increased weight loss in study subjects, suggesting that antitumor efficacy was increased without additional toxicity to normal tissue.
Our study suffers limitations common to single institutional trials, namely small patient numbers and selection bias. Our cohort of subjects is too small to adequately assess the effect of tumor EGFR and KRAS status on outcome. Most patients who underwent resection subsequently relapsed with metastatic disease, underlying the ongoing need for more active systemic agents in this disease. Promising results with FOLFIRINOX in metastatic pancreatic cancer suggest that incorporating this regimen into a combined modality approach might improve relapse rates [33].
The activity of this neoadjuvant regimen and the high rate of resection following therapy lend credence to preclinical models suggesting benefit for EGFR inhibition in combination with gemcitabine/radiotherapy. For patients presenting with nonmetastatic PDAC, our regimen exhibits substantial activity with the possibility of downsizing/downstaging of tumor to allow for subsequent complete resection in some patients with locally advanced disease. The high rate of margin-negative resection and encouraging median survival suggest that a larger trial of this regimen is warranted.
funding
This trial was supported by a grant from Bristol-Myers Squibb Oncology. This trial was supported in part by Cancer Center Support (CORE) Grant P30 CA023108-34 (Israel) PI. JMP received grant support for this study through Bristol-Myers Squibb Oncology/Investigator Initiated Trial Mechanism. Between 2004 and 2006, JMP was a member of the Bristol-Meyers Squibb Speakers Bureau.
disclosure
The authors have declared no conflicts of interest.
acknowledgements
From the Gastrointestinal Oncology Program, Dartmouth-Hitchcock Medical Center/Norris Cotton Cancer Center. All work associated with this paper is original, accurate, and has been approved by the authors. All authors actively contributed to the concept, writing, patient recruitment, data gathering, analysis, and/or preparation of this trial and manuscript. This work was fully approved and monitored by the Dartmouth College Committee for the Protection of Human Subjects, The Clinical Cancer Review Committee of Dartmouth-Hitchcock Medical Center, and the Data and Safety Monitoring Committee of the Norris Cotton Cancer Center.
references
- 1.Horner MJ, Ries LAG, Krapcho M, et al., editors. Adenocarcinoma of the Pancreas, in SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 2.Staley CA, Lee JE, Cleary KR, et al. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg. 1996;171:118–124. doi: 10.1016/S0002-9610(99)80085-3. discussion 124–125. [DOI] [PubMed] [Google Scholar]
- 3.Shewach DS, Lawrence TS. Radiosensitization of human tumor cells by gemcitabine in vitro. Semin Oncol. 1995;22:68–71. [PubMed] [Google Scholar]
- 4.Pipas JM, Mitchell SE, Barth RJ, et al. Phase I study of twice-weekly gemcitabine and concomitant external-beam radiotherapy in patients with adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2001;50:1317–1322. doi: 10.1016/s0360-3016(01)01580-2. [DOI] [PubMed] [Google Scholar]
- 5.Pipas JM, Barth RJ, Zaki B, et al. Docetaxel/Gemcitabine followed by gemcitabine and external beam radiotherapy in patients with pancreatic adenocarcinoma. Ann Surg Oncol. 2005;12:995–1004. doi: 10.1245/ASO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn J, Baird A, Fan Z, et al. Growth factors and their receptors in epithelial malignancies. In: Mendelsohn J, Howley PM, Israel MA, Liotta LA, editors. The Molecular Basis of Cancer. Philadelphia, PA: W.B. Saunders Company; 2001. pp. 137–144. [Google Scholar]
- 7.Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs. 1999;17:259–269. doi: 10.1023/a:1006384521198. [DOI] [PubMed] [Google Scholar]
- 8.Uegaki K, Nio Y, Inoue Y. Clinicopathological significance of epidermal growth factor and its receptor in human pancreatic cancer. Anticancer Res. 1997;17:3841–3847. [PubMed] [Google Scholar]
- 9.Korc M, Chandrasekar B, Yamanaka Y, et al. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with increases in the levels of epidermal growth factor and transforming growth factor alpha. J. Clin Invest. 1992;90:1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbruzzese JL, Rosenberg A, Xiong Q, et al. “Phase II Study of Anti-Epidermal Growth Factor Receptor (EGFR) Antibody Cetuximab (IMC-C225) in Combination with Gemcitabine in Patients with Advanced Pancreatic Cancer”. Proceedings of American Society of Clinical Oncology. 2001;20:518. [Google Scholar]
- 11.Yamanaka Y, Friess H, Kobrin MS, et al. Coexpression of epidermal growth factor receptor and its ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–569. [PubMed] [Google Scholar]
- 12.Waksal HW. Role of an anti-epidermal growth factor receptor in treating cancer. Cancer Metastasis Rev. 1999;18:427–436. doi: 10.1023/a:1006302101468. [DOI] [PubMed] [Google Scholar]
- 13.Bruns CJ, Harbison MT, Davis DW, et al. Epidermal growth factor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 14.Overholser JP, Prewett MC, Hooper AT, et al. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89:74–82. [PubMed] [Google Scholar]
- 15.Liang K, Ang KK, Milas L, et al. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246–254. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- 16.Buchsbaum DJ, Bonner JA, Grizzle WE, et al. Treatment of pancreatic cancer xenografts with erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. Int J Radiat Oncol Biol Phys. 2002;54:1180–1193. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- 17.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cance Inst. 2000;92:205–211. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Evans DB, Rich TA, Byrd D, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 20.Berger AC, Farma J, Scott W, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Maas M, Nelemans PJ, Vanentini V, et al. Long-term outcome in patients with a pathologic complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 22.Preis M, Korc M. Kinase signaling pathways as targets for intervention in pancreatic cancer. Cancer Biol Ther. 2010;9:1–10. doi: 10.4161/cbt.9.10.11534. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MA, Ginsberg MH. Network and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 24.Boulay BR, Gardner TB, Gordon SR. Occlusion rate and complications of plastic biliary stent placement in patients undergoing neoadjuvant chemoradiotherapy for pancreatic cancer with malignant biliary obstruction. J Clini Gastroenterol. 2010;44:452–455. doi: 10.1097/MCG.0b013e3181d2ef06. [DOI] [PubMed] [Google Scholar]
- 25.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based chemotherapy and radiation after pancreaticoduodonectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at The Johns Hopkins Hospital. J Clin Oncol. 2008;21:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic Experience (1975-2005) J Clin Oncol. 2008;21:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 27.Cheng TY, Sheth K, White RR, et al. Effect of neoadjuvant chemoradiation on operative mortality and morbidity for pancreaticoduodenectomy. Ann Surg Oncol. 2006;13:66–74. doi: 10.1245/ASO.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Cheng TY, Sheth K, White RR, et al. Surgical results after preoperative chemoradiation therapy for patients with pancreatic cancer. Pancreas. 2009;38:282–288. doi: 10.1097/MPA.0b013e31819438c3. [DOI] [PubMed] [Google Scholar]
- 29.Greer SE, Pipas JM, Sutton JE, et al. Effect of neoadjuvant therapy on local recurrence after resection of pancreatic adenocarcinoma. J Am Coll Surg. 2008;206:451–457. doi: 10.1016/j.jamcollsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Gillen S, Schuster T, Meyer zum Buschenfelde C, et al. Preoperative/Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan MA, Parsels LA, Maybaum J, et al. Improving gemcitabine-mediated radiosensitization using molecularly targeted therapy: a review. Clin Cancer Res. 2008;14:6744–6750. doi: 10.1158/1078-0432.CCR-08-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan MA, Parsels LA, Kollar LE, et al. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]