Abstract
Background
Overweight individuals (body mass index (BMI) 25–29.9 kg/m2) are at higher risk for developing cardiovascular disease and hypertension when compared with lean individuals of normal weight (BMI 18.5–24.9 kg/m2). The purpose of this study was to test the hypothesis that exaggerated sympathetic nervous system responses to stressors may be one potential mechanism that predisposes overweight individuals to developing hypertension.
Methods
We compared heart rate (HR), blood pressure (BP), and muscle sympathetic nerve activity (MSNA) using microneurography, in normotensive overweight individuals compared with age-matched lean controls, at baseline and during two sympathoexcitatory maneuvers: cold pressor test (CPT), and static handgrip exercise (SHG 30%).
Results
During CPT, MSNA increased in both groups, but the magnitude of MSNA response was significantly greater (P = 0.03) in overweight (+18.1 ± 2.8 bursts/min) compared with lean controls (+10.8 ± 1.2 bursts/min). MSNA response to SHG at 30% maximum voluntary contraction (MVC) was similar between the two groups. There were no significant differences in systolic (SBP) or diastolic BP (DBP) responses or HR responses between the two groups during either maneuver.
Conclusions
Normotensive overweight individuals have an exaggerated MSNA response to the CPT. Augmented sympathetic reactivity to cold stress may contribute to increased risk of hypertension in overweight individuals.
Keywords: blood pressure, hypertension, overweight, physiological stress response, sympathetic nervous system
Overweight individuals (body mass index (BMI) 25–29.9 kg/m2) are at higher risk for developing hypertension and cardiovascular disease when compared with lean individuals of normal weight (BMI 18.5–24.9 kg/m2).1,2 The mechanisms underlying the increased risk have not been fully elucidated, but may include abnormalities of the sympathetic nervous system (SNS). Chronic baseline sympathetic overactivity, as well as greater sympathetic responses to stressful stimuli, are potential abnormalities that might predispose overweight individuals to developing hypertension and cardiovascular disease.
Heightened cardiovascular and sympathetic reactivity to stressful stimuli has been associated with the development of hypertension and cardiovascular disease.3–7 Increased cardiovascular reactivity to the cold pressor test (CPT), a known sympathoexcitatory stimulus,8 predicts the future development of hypertension,9 and may represent a preclinical manifestation of hypertension before elevations in peripheral arterial blood pressure (BP) are detected. Augmented sympathetic reactivity may be one possible mechanism underlying the increased risk of developing hypertension and cardiovascular disease in overweight individuals, especially because sympathetic activity is known to be chronically elevated in obesity (BMI ≥30 kg/m2),10–12 and likely contributes to the pathogenesis of obesity-related hypertension. It is less established if overweight individuals, with a lesser degree of excess weight than obese patients, also have heightened baseline SNS activity, and sympathetic responses to sympathoexcitatory stimuli have never before been studied in normotensive overweight individuals.
The purpose of this study was to test the hypothesis that normotensive overweight individuals have chronic baseline sympathetic overactivity, and exaggerated pressor and SNS responses to stressful stimuli. To test this hypothesis, we made hemodynamic measurements and direct measurements of muscle sympathetic nerve activity (MSNA) using microneurography at baseline and during two sympathoexcitatory maneuvers (CPT and static handgrip exercise (SHG)) in healthy overweight individuals, and lean age-matched controls.
Methods
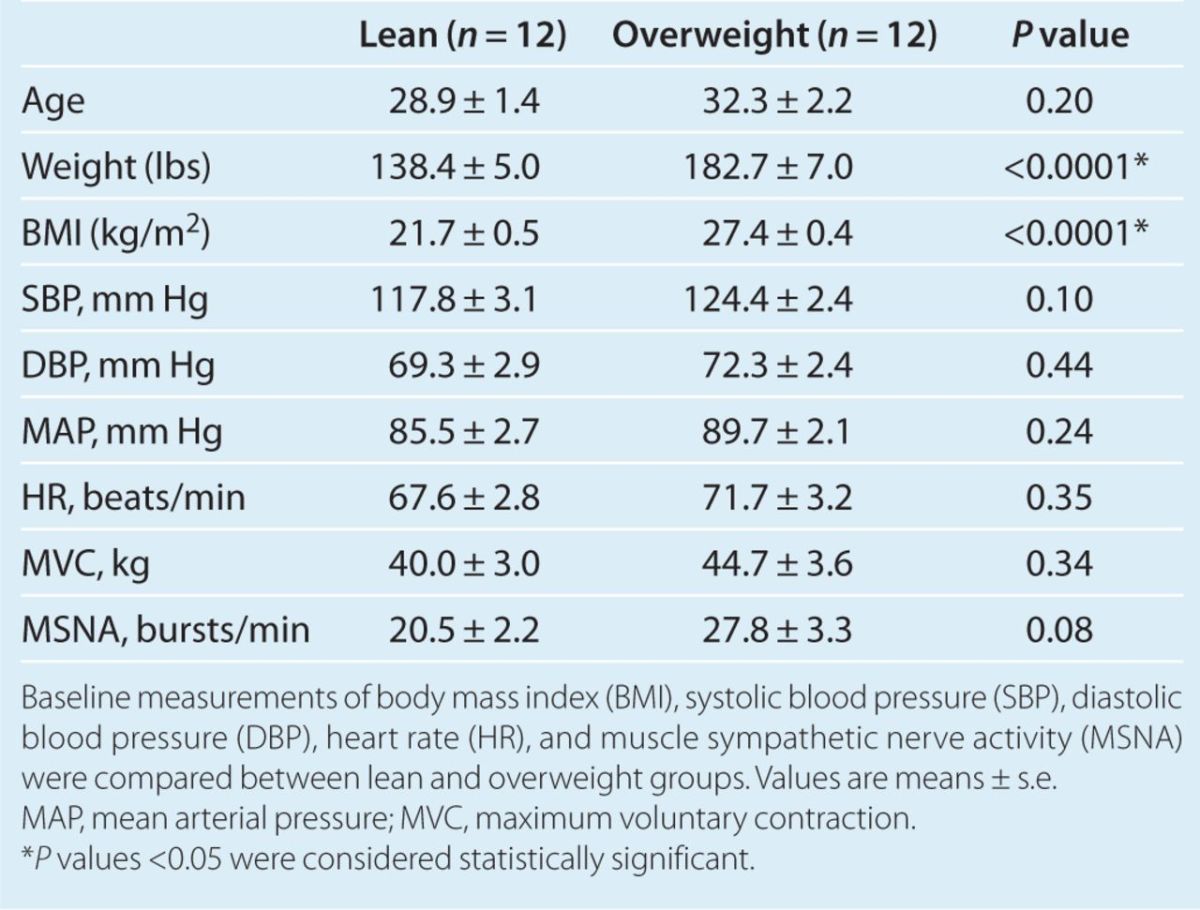
Study population. The study population consisted of 24 total participants (12 overweight and 12 age-matched lean controls). No obese individuals (BMI ≥30 kg/m2) were included in the study. All participants were healthy nonsmokers without evidence of hypertension, diabetes, heart or vascular disease (as determined by medical history and physical exam), and none of the participants were taking regular medications. Those that had laboratory data available had no evidence of abnormal fasting blood sugars, or any other laboratory abnormalities. Baseline noninvasive brachial arterial BP was <140/90 mm Hg in all study participants. Overweight (five females, seven males) and lean controls (four females, eight males) were well matched for age and gender (Table 1). All premenopausal females were studied during the follicular phase of the menstrual cycle. BMI ranged between 19.2 and 24.3 kg/m2 among lean controls, and between 25.0 and 29.6 kg/m2 among overweight participants. The study protocol was approved by the institutional review board of the Keck School of Medicine at the University of Southern California, and all participants signed an approved informed consent document.
Table 1.
Baseline characteristics and measurements
Measurements and procedures
BP. Baseline arterial BP was measured with an automated sphygmomanometer (Dinamap PRO Series; Critikon, Milwaukee, WI) while the participant was seated, after 5 min of quiet rest. The arm was supported at heart level, and an appropriately sized BP cuff with bladder encircling at least 80% of the upper arm was used. Each data point of BP was the mean of at least three consecutive readings. Each measurement was recorded by a single trained investigator.
MSNA. Multiunit postganglionic sympathetic nerve activity directed to muscle (MSNA) was recorded directly from the peroneal nerve by microneurography, as previously described.13 A tungsten microelectrode (tip diameter 5–15µm) (Department of Bioengineering, University of Iowa, Iowa City, IA) was inserted into the nerve, and a reference microelectrode was inserted subcutaneously 1–2 cm from the recording electrode. The signals were preamplified (gain 1,000), amplified (gain 50–100), filtered (700–2,000 Hz) (Nerve Traffic Analyzer, Model 662C-3; Department of Bioengineering, University of Iowa), rectified, and integrated (time constant 0.1 s) to obtain a mean voltage display of sympathetic nerve activity that was recorded by the Chart 5 Program (PowerLab 16sp; ADInstruments, Colorado Springs, CO). Lead II of the electrocardiogram was recorded simultaneously with the neurogram. All MSNA recordings met previously established criteria.13–15 Sympathetic bursts were identified by visual inspection of nerve bursts by a single investigator without knowledge of the participant's weight. MSNA was expressed as burst frequency (bursts/min).
CPT. A subset of lean (n = 8) and overweight (n = 8) participants underwent CPT, a sympathoexcitatory maneuver that is known to evoke an increase in MSNA.8 CPT was done by submerging the participant's hand up to the wrist in ice-cold water for 1 min. The temperature of the water was ~0–1 °C. The participant was asked to rate the degree of pain during the maneuver on a six-point pain scale, ranging from 0 (no pain) to 5 (severe pain). Participants were instructed to maintain normal breathing patterns and avoid inadvertent Valsalva.
Moderate SHG. Moderate SHG is known to elicit increases in MSNA.16 The participant was asked to squeeze a hand dynamometer (Stoelting, Wood Dale, IL) with maximal force. The highest force attained from three attempts was considered the maximum voluntary contraction (MVC). Moderate SHG was performed by squeezing the hand dynamometer at 30% of MVC in a sustained manner for 3 min. The participant was instructed to avoid inadvertent Valsalva, and to maintain normal breathing patterns.
Experimental protocol. All participants were studied in the early afternoon, after abstaining from food for 4 h, and exercise, caffeine, and alcohol for at least 12 h. All participants were nonsmokers. The study room was quiet, semidark, and temperate (~21 °C). Participants were placed in a supine position and fitted with a BP cuff on the upper arm for intermittent automatic BP monitoring, and electrocardiogram patch electrodes for continuous heart rate (HR) recordings. Baseline BP and HR were measured after 5 min of rest. The leg was positioned for microneurography, and the tungsten microelectrode was inserted and manipulated to obtain a satisfactory nerve recording. After 10 min of rest, baseline sympathetic nerve activity to the muscle was recorded for 10 min. The participant then underwent two sympathoexcitatory stresses: (i) CPT for 1 min, and (ii) SHG at 30% MVC for 3 min. The order of the two maneuvers was random. MSNA and HR were recorded continuously, and BP was checked intermittently during the maneuvers; 30 min of rest was given between the two maneuvers.
Statistical analysis. Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, NC). Baseline characteristics and measurements were compared using independent t-test. Differences between the two groups in the change in MSNA and hemodynamics from baseline during CPT and static handgrip were analyzed using two-way analysis of variance with repeated measures. All values were reported as means ± s.e.m. P values <0.05 were considered statistically significant.
Results
Baseline measurements
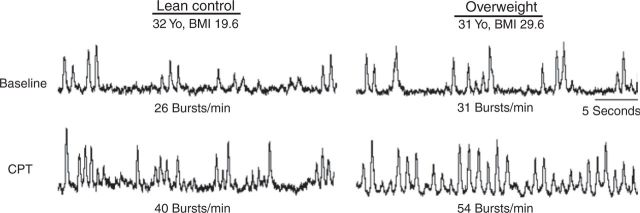
The mean BMI was 27.4 ± 0.4 kg/m2 in the overweight group, and 21.7 ± 0.5 kg/m2 in the lean group (Table 1). Overweight participants were on average +20.1 kg (44.3 pounds) heavier than their lean counterparts. None of the participants in either group had obstructive sleep apnea, or any other breathing abnormalities, as determined by review of the medical record and clinical history. There were no significant differences in race between the two groups. The control group included: 6 Caucasian, 3 Hispanic, and 3 Asian. The overweight group included: 7 Caucasian, 4 Hispanic, and 1 Asian. We obtained family history data on eight controls and eight overweight participants. Five of eight controls had a positive family history of hypertension, and six of eight overweight participants had a positive family history. There were no significant differences in family history of hypertension between the two groups. There were no significant differences in baseline systolic BP (SBP), diastolic BP (DBP), mean arterial pressure, HR, and MSNA between the overweight and control groups, although there was a nonsignificant trend toward higher baseline SBP and MSNA in the overweight group. Figure 1 depicts MSNA microneurograms at baseline and during CPT in a representative lean control and overweight participant.
Figure 1. Microneurograms at baseline and during cold pressor test (CPT). Representative microneurograms in a 32-year-old (Yo) lean control and 31-Yo overweight participants at baseline and during the CPT. BMI, body mass index.
Moderate SHG
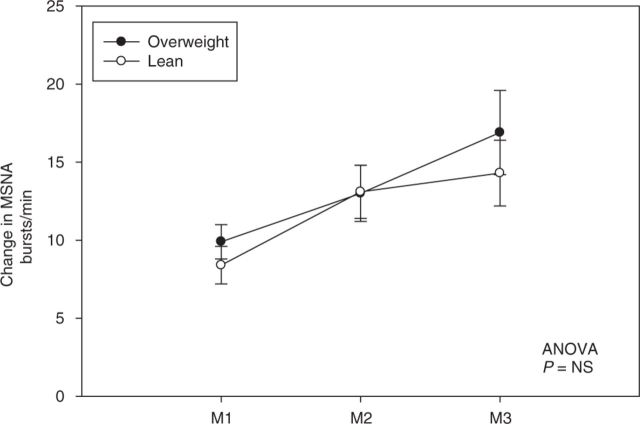
MVC was similar between the two groups (Table 1). During 3 min of SHG at 30% MVC, both lean and overweight participants had increases in SBP, DBP, and HR. There was no statistically significant difference in the BP and HR responses during SHG 30% between the two groups; however, there was a trend toward a blunted SBP and HR response during SHG 30% among the overweight individuals (Table 2). MSNA increased during each minute of SHG 30% in both overweight and lean controls; however, there was no difference in magnitude of MSNA response between the two groups (P = not significant, Figure 2).
Table 2.
Hemodynamic changes during static handgrip exercise
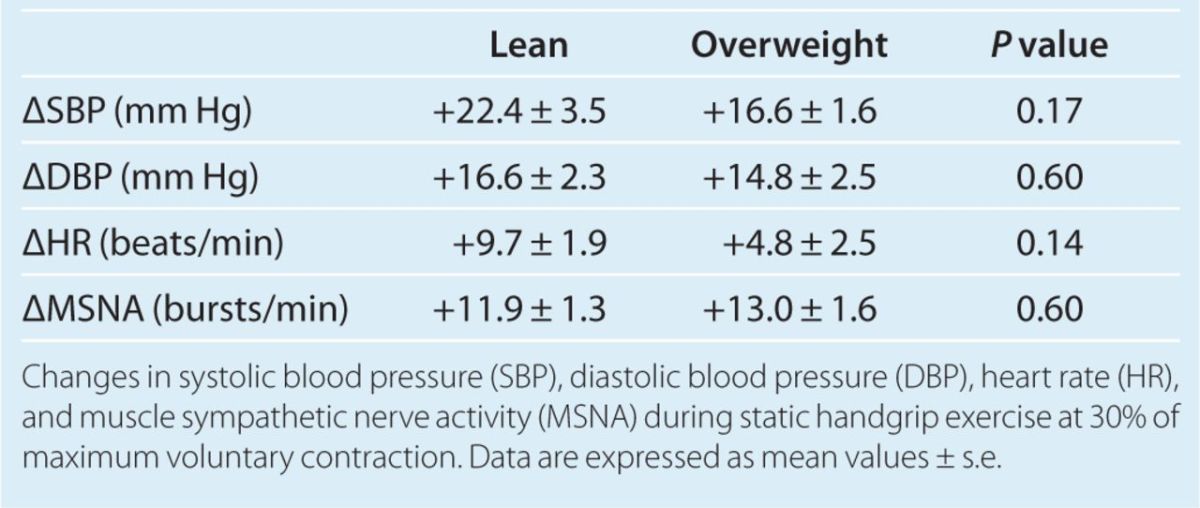
Figure 2. Change in muscle sympathetic nerve activity (MSNA) during moderate static handgrip exercise. Change in MSNA during each minute (M) of static handgrip exercise at 30% maximum voluntary contraction. Values are mean ± s.e. ANOVA, analysis of variance; NS, not significant.
CPT
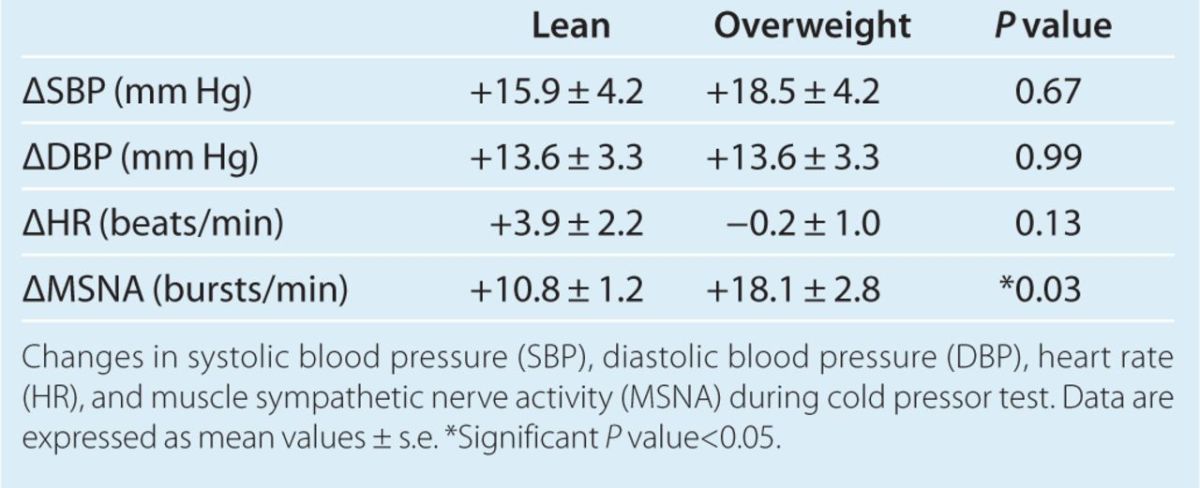
MSNA increased in both lean and overweight participants during CPT. However, the magnitude of the MSNA response was significantly greater in overweight participants compared with lean controls (Figure 3). Mean increase in MSNA during CPT was 18.1 ± 2.8 bursts/min in overweight, compared with an increase of 10.8 ± 1.2 bursts/min in lean controls (P = 0.03). In addition, analysis of MSNA during 30 s of recovery time immediately following the 60 s of CPT in a subset of participants (seven controls, and five overweight) revealed that MSNA remained significantly more elevated from baseline levels during recovery in the overweight group vs. controls (+19.5 ± 3.2 vs. +6.7 ± 2.4 bursts/min, P = 0.01). CPT evoked increases in SBP and DBP in both groups, which was not significantly different between the groups (Table 3). There was also no significant difference in HR response during CPT between the groups. Pain perception during CPT on the pain-rating scale was similar between the two groups. Among controls, pain perception was 3-moderate (1), 4-moderately severe (4), and severe (3). Among the overweight group, pain perception was 3-moderate (2), 4-moderately severe (4), and severe (2).
Figure 3. Muscle sympathetic nerve activity (MSNA) at baseline and during the cold pressor test (CPT) in lean vs. overweight participants. Open circles represent individual data points. Closed circles represent mean values ± s.e. *Significant P value <0.05 for difference between groups.
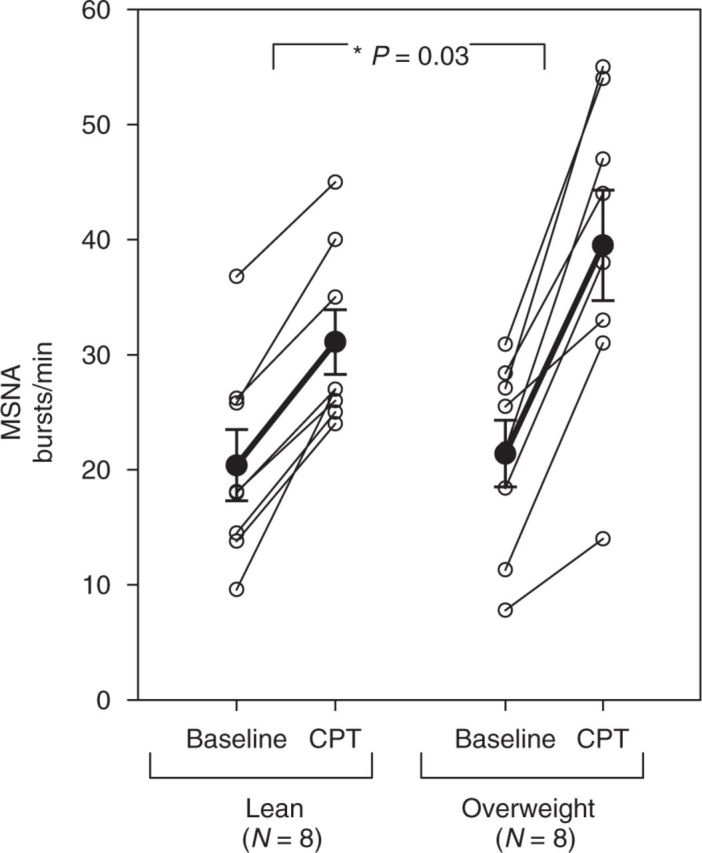
Table 3.
Hemodynamic changes during cold pressor test
Discussion
The major new finding of this study is that MSNA reactivity to the CPT is augmented in normotensive overweight individuals compared with lean controls. Increased sympathetic reactivity to stress may in part explain the propensity toward developing hypertension in overweight individuals, and may represent a preclinical marker for those at risk for cardiovascular disease.
The majority of the US population is now obese or overweight, and at increased risk for the multitude of obesity-related health problems, including hypertension and heart disease.17 Obesity is associated with an increased risk of cardiovascular disease1 and premature mortality.18 SNS overactivity is thought to be an important component in the pathogenesis of hypertension and heart disease in obesity.10,11,19–21 Studies using microneurography to directly measure sympathetic nerve traffic have revealed that MSNA is elevated in obesity.11,12,20–22 In obese patients, chronic MSNA overactivity persists, even without hypertension12,23 or obstructive sleep apnea,10 and obesity further augments the chronic MSNA activation seen in hypertension12,24 and heart failure.25,26
Overweight individuals, with a lesser degree of excess weight than obese patients, are also at increased risk of hypertension2 and cardiovascular mortality1; however, it is less well established whether SNS overactivity is also a feature of overweight individuals without hypertension. Abate et al. previously showed that BMI correlated positively with MSNA in white men, white women, and black women, but not in black men.27 Lambert et al. observed that obese and overweight men had higher MSNA than lean men.28 In another study, MSNA correlated positively with percent body fat in both men and women.29 In our study, we excluded obese individuals, and concentrated on overweight as a separate experimental cohort. Although there was no significant difference in baseline MSNA, we observed that the magnitude of MSNA response to cold stress was significantly higher in overweight participants compared with lean controls.
MSNA reactivity to CPT has never been reported in overweight, but was previously tested in obese women. In that study, although the overall levels of MSNA during CPT were higher in obese women (due to elevated baseline MSNA), the absolute change in MSNA during CPT was the same in obese and lean women.20 Correspondingly, the magnitude of MSNA response to other sympathoexcitatory stimuli including mental stress and SHG have been found to be similar between obese and lean individuals.20,30 In contrast to the prior finding in obesity, we observed greater absolute increases in MSNA with CPT in overweight individuals.
Increased cardiovascular reactivity to CPT in normotensive individuals has been shown to predict the future development of hypertension.9 CPT is a potent stimulus for central SNS outflow, and the increase in MSNA correlates directly with increases in BP, implicating that CPT-mediated pressor responses are largely mediated by SNS-driven peripheral vasoconstriction.8 Young normotensive individuals with exaggerated pressor responses during CPT were at substantially higher risk for developing hypertension later in life.31 In addition, populations with an increased propensity for developing hypertension, including African-Americans,32 patients with a positive family history of hypertension,4 and prehypertensive individuals,3 have exaggerated increases in MSNA during CPT, suggesting that abnormal SNS reactivity to CPT may be a preclinical marker for increased risk of hypertension. Overweight individuals are also at increased risk of developing hypertension,2 and heightened MSNA reactivity to stress and chronic baseline SNS overactivity may be a possible mechanistic link.
We observed significantly greater MSNA responses during CPT in overweight individuals, without concomitant augmented increases in BP and HR. The dissociation between the MSNA response and BP/HR responses during stress are unclear, but may include differences in the generation of concomitant vasodilatory substances, differences in the vasoconstrictive response for a given release of norepinephrine (NE) at sympathetic nerve endings, other vasoconstrictive mediators such as endothelin, variation in nitric oxide bioavailability due to the differential generation of reactive oxygen species, as well as differences in parasympathetic innervation of the heart that also governs HR control during stress. One potential mediator of the hemodynamic response during CPT is the nucleoside adenosine (Ado). Ado levels are increased systemically during CPT,33 likely due to local generation from ischemic tissues, as well as dephosphorylation of adenosine triphosphate that is co-released with NE from sympathetic nerve endings. Ado acts directly as a vasodilator, and also modulates NE release from nerve terminals by acting on inhibitory A1 receptors. Whether differences in Ado release, or other vasoactive substances, contribute to the dissociation between the pressor and MSNA response during CPT in overweight individuals remains to be tested.
Although overweight individuals had exaggerated increases in MSNA during CPT in this study, we did not observe differences in MSNA or pressor responses during SHG between the groups. Both CPT and SHG 30% are potent stimulators of central SNS output.8,16 In prior studies, normal controls, as well as hypertensive humans and patients with renal failure, had equivocal increases in CPT compared to 3 min of SHG 30%34,35; thus, both maneuvers have comparable capacity to elicit a sympathoexcitatory response in normal humans, and certain disease groups. Whether SHG 30% is less effective at producing sympathoexcitation specifically in obese or overweight individuals, thereby contributing to the differential effects on MSNA response observed between SHG and CPT, is unclear. During SHG 30%, reflex activation of SNS activity is largely mediated by stimulation of metaboreceptors that are sensitized by metabolic by-products generated during exercise.36 Prior studies have demonstrated that obese humans have a blunted metaboreceptor-mediated response in SNS activity during exercise.30 In this study, although there were no significant differences in MSNA response during SHG between overweight and lean individuals, there was a trend towards blunted BP and HR response during SHG among the overweight group. Whether metaboreceptors are also blunted in overweight humans remains to be tested, but may be one possible explanation for a lack of exaggerated SNS response during SHG in overweight individuals.
We recognize several limitations to our study. First, we assessed SNS activity using microneurography, which allowed for precise real-time measurement of SNS changes, but limited our measurements to SNS activity directed to muscle (MSNA). However, MSNA correlates with multiple indexes of SNS activity in humans, including plasma NE37 and whole body, cardiac, and renal NE spillover.38,39 Second, the study population was fairly young, and findings may not be generalizable to older populations, or those with comorbid conditions. Third, only 1 min of CPT was administered. Although this was enough time to observe significant differences in MSNA response, the lack of significant difference in BP response during CPT may have been due to the shorter duration of CPT. Fourth, there are potential limitations in using SHG 30% as the comparator stimulus to CPT, given that metaboreflex-mediated SNS responses during exercise have been found to be blunted in obese women.30 However, although MSNA responses during posthandgrip circulatory arrest during which metaboreceptors are isolated were blunted, the MSNA response during SHG 30% was robust and not blunted in obesity. Finally, the sympathoexcitatory stimuli were limited to CPT and SHG 30% in this study. It is unknown whether overweight individuals have augmented SNS responses to other sympathoexcitatory stimuli. Our pilot data should be followed up in future studies that evaluate other sympathoexcitatory stimuli such as mental stress, lower body negative pressure, or baroreflex unloading, as well as ambulatory BP monitoring and metabolic studies.
In conclusion, this study demonstrates that healthy overweight individuals without hypertension have an augmented MSNA response during the CPT. These abnormalities in autonomic responses during stress may precede the development of overt clinical disease in overweight individuals. These findings highlight the importance of recognizing not only obese, but also overweight individuals as an at-risk population. Whether CPT may be a clinically useful adjunct to predict the future risk of hypertension, and whether weight loss improves SNS reactivity during CPT in overweight individuals, remains to be tested.
Acknowledgments
J.P. is supported by National Institutes of Health grant K23HL098744, Amgen Nephrology Junior Faculty Award, and the Atlanta Research and Education Foundation.
Disclosure
The authors declared no conflict of interest.
References
- 1.Yan LL, Daviglus ML, Liu K, Stamler J, Wang R, Pirzada A, Garside DB, Dyer AR, Van Horn L, Liao Y, Fries JF, Greenland P. Midlife body mass index and hospitalization and mortality in older age. JAMA 2006;295:190–198 [DOI] [PubMed] [Google Scholar]
- 2.Garrison RJ, Kannel WB, Stokes J. 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 1987;16:235–251 [DOI] [PubMed] [Google Scholar]
- 3.Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand 1991;141:157–165 [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA, Mutinga ML. Race, family history of hypertension, and sympathetic response to cold pressor testing. Blood Press 1997;6:209–213 [DOI] [PubMed] [Google Scholar]
- 5.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension 1993;22:479–485 [DOI] [PubMed] [Google Scholar]
- 6.Matthews KA, Salomon K, Brady SS, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosom Med 2003;65:410–415 [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens 2005;23:529–536 [DOI] [PubMed] [Google Scholar]
- 8.Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 1987;9:429–436 [DOI] [PubMed] [Google Scholar]
- 9.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med 2003;65:46–62 [DOI] [PubMed] [Google Scholar]
- 10.Grassi G, Facchini A, Trevano FQ, Dell'Oro R, Arenare F, Tana F, Bolla G, Monzani A, Robuschi M, Mancia G. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension 2005;46:321–325 [DOI] [PubMed] [Google Scholar]
- 11.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 2002;106:2533–2536 [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 2000;36:538–542 [DOI] [PubMed] [Google Scholar]
- 13.Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 2006;117:2357–2384 [DOI] [PubMed] [Google Scholar]
- 14.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 1972;84:65–81 [DOI] [PubMed] [Google Scholar]
- 15.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 1972;84:82–94 [DOI] [PubMed] [Google Scholar]
- 16.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 1985;57:461–469 [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 18.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 2003;289:187–193 [DOI] [PubMed] [Google Scholar]
- 19.Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension 1995;25:834–838 [DOI] [PubMed] [Google Scholar]
- 20.Kuniyoshi FH, Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Gowdak MM, Barretto AC, Halpern A, Villares SM, Lima EG, Negrão CE. Abnormal neurovascular control during sympathoexcitation in obesity. Obes Res 2003;11:1411–1419 [DOI] [PubMed] [Google Scholar]
- 21.Eikelis N, Esler M. The neurobiology of human obesity. Exp Physiol 2005;90:673–682 [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Dell'Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens 2004;22:2363–2369 [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, Cattaneo BM, Seravalle G, Colombo M, Cavagnini F, Mancia G. Obesity and the sympathetic nervous system. Blood Press Suppl 1996;1:43–46 [PubMed] [Google Scholar]
- 24.Huggett RJ, Burns J, Mackintosh AF, Mary DA. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension 2004;44:847–852 [DOI] [PubMed] [Google Scholar]
- 25.Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell'Oro R, Bolla G, Mancia G. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension 2007;49:535–541 [DOI] [PubMed] [Google Scholar]
- 26.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension 2003;42:873–877 [DOI] [PubMed] [Google Scholar]
- 27.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG. Overweight and sympathetic overactivity in black Americans. Hypertension 2001;38:379–383 [DOI] [PubMed] [Google Scholar]
- 28.Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, Schlaich M, Lambert G. Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens 2007;25:1411–1419 [DOI] [PubMed] [Google Scholar]
- 29.Jones PP, Snitker S, Skinner JS, Ravussin E. Gender differences in muscle sympathetic nerve activity: effect of body fat distribution. Am J Physiol 1996;270:E363–E366 [DOI] [PubMed] [Google Scholar]
- 30.Negrão CE, Trombetta IC, Batalha LT, Ribeiro MM, Rondon MU, Tinucci T, Forjaz CL, Barretto AC, Halpern A, Villares SM. Muscle metaboreflex control is diminished in normotensive obese women. Am J Physiol Heart Circ Physiol 2001;281:H469–H475 [DOI] [PubMed] [Google Scholar]
- 31.Wood DL, Sheps SG, Elveback LR, Schirger A. Cold pressor test as a predictor of hypertension. Hypertension 1984;6:301–306 [DOI] [PubMed] [Google Scholar]
- 32.Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension 1993;22:801–805 [DOI] [PubMed] [Google Scholar]
- 33.Pasini FL, Capecchi PL, Colafati M, Randisi P, Puccetti L, Di Perri T. Systemic adenosine increase during cold pressor test is dependent on sympathetic activation. Clin Exp Pharmacol Physiol 1999;26:774–778 [DOI] [PubMed] [Google Scholar]
- 34.Park J, Campese VM, Middlekauff HR. Exercise pressor reflex in humans with end-stage renal disease. Am J Physiol Regul Integr Comp Physiol 2008;295:R1188–R1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 2010;299:H1318–H1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 1991;19:313–349 [PubMed] [Google Scholar]
- 37.Seals DR, Victor RG, Mark AL. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J Appl Physiol 1988;65:940–944 [DOI] [PubMed] [Google Scholar]
- 38.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol (Lond) 1996;491 (Pt 3):881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol (Lond) 1992;453:45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]