Abstract
This article reviews the treatment of hallucinations in schizophrenia. The first treatment option for hallucinations in schizophrenia is antipsychotic medication, which can induce a rapid decrease in severity. Only 8% of first-episode patients still experience mild to moderate hallucinations after continuing medication for 1 year. Olanzapine, amisulpride, ziprasidone, and quetiapine are equally effective against hallucinations, but haloperidol may be slightly inferior. If the drug of first choice provides inadequate improvement, it is probably best to switch medication after 2–4 weeks of treatment. Clozapine is the drug of choice for patients who are resistant to 2 antipsychotic agents. Blood levels should be above 350–450 μg/ml for maximal effect. For relapse prevention, medication should be continued in the same dose. Depot medication should be considered for all patients because nonadherence is high. Cognitive-behavioral therapy (CBT) can be applied as an augmentation to antipsychotic medication. The success of CBT depends on the reduction of catastrophic appraisals, thereby reducing the concurrent anxiety and distress. CBT aims at reducing the emotional distress associated with auditory hallucinations and develops new coping strategies. Transcranial magnetic stimulation (TMS) is capable of reducing the frequency and severity of auditory hallucinations. Several meta-analyses found significantly better symptom reduction for low-frequency repetitive TMS as compared with placebo. Consequently, TMS currently has the status of a potentially useful treatment method for auditory hallucinations, but only in combination with state of the art antipsychotic treatment. Electroconvulsive therapy (ECT) is considered a last resort for treatment-resistant psychosis. Although several studies showed clinical improvement, a specific reduction in hallucination severity has never been demonstrated.
Keywords: antipsychotics, cognitive, behavioral therapy, electroconvulsive therapy, pharmacotherapy, schizophrenia, transcranial magnetic stimulation
Introduction
Schizophrenia can be accompanied by hallucinations in any of the sensory modalities. In 70% of the cases they are auditory in nature, and in 50% of those cases visual hallucinations are also experienced at some point. Other types of hallucination are less prevalent. But whatever the sensory modality in which they are experienced, hallucinations can be such a burden that they require expert treatment. Treatment usually consists of psychoeducation, medication, psychosocial interventions, psychotherapy, and in some instances transcranial magnetic stimulation (TMS) or electroconvulsive therapy (ECT).
The present article will focus on medication, cognitive-behavioral therapy (CBT), TMS, and ECT. We will summarize the existing literature and offer recommendations for the treatment of hallucinations in schizophrenia.
Pharmacological Treatment of Hallucinations in Schizophrenia Spectrum Disorders
The only type of medication known to effectively reduce the frequency and severity of hallucinations in schizophrenia spectrum disorders is antipsychotic medication. So far, no clinical trials have been published that compare the efficacy of various antipsychotic drugs for the sole and specific indication of hallucinations. Therefore, we used the data from the European First-Episode Schizophrenia Trial (EUFEST) to assess the potential of 5 antipsychotic agents to reduce the severity of hallucinations.
The EUFEST study1 included 498 patients with a first-psychotic episode, who were randomized to receive haloperidol, olanzapine, amisulpride, quetiapine, or ziprasidone. The reduction in total symptom severity was virtually the same in all groups, around 60% after 12 months of treatment, but differences were observed in the discontinuation rate, which was higher for haloperidol and lower for amisulpride and olanzapine.1 We reanalyzed these data with item P3 (severity of hallucinations) of the Positive and Negative Syndrome Scale,2 as the primary outcome measure.
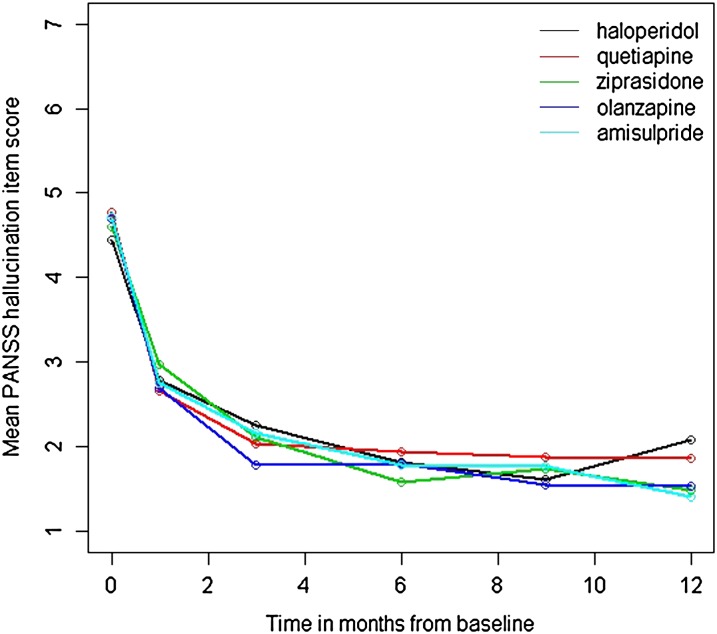
All subjects with a score of 3 or higher at baseline (indicating at least mild hallucinations) were included (N = 362; 73% of the total sample). Even though 54% of the patients discontinued treatment within 12 months, unbiased parameter estimates were obtained under the assumption of Missing at Random. A mean reduction in the severity of hallucinations from 4.4 at baseline, indicating marked to severe hallucinations, to a mean value of 2.5, indicating minimal to mild hallucinations, was found after 4 weeks. The severity of hallucinations continued to decline with prolonged treatment to mean values of around 1.5, reflecting the presence of absent to minimal hallucinations after 6 months of treatment (see figure 1). Likewise, the percentage of subjects with at least mild levels of hallucinations decreased strongly over time from 100% at baseline to 8% after 12 months of treatment. These findings indicate that hallucinations in patients with a psychotic disorder respond fairly well to antipsychotic treatment, showing a strong reduction in symptom severity in the first month.
Fig. 1.
Mean decrease in hallucination severity (item P3 of the Positive and Negative Syndrome Scale [PANSS]) in first-episode patients with a nonaffective psychotic disorder after 1, 3, 6, 9, and 12 months on antipsychotic medication.
These results should be encouraging for patients who suffer from hallucinations, and it might help them decide to start and continue pharmacotherapy. We investigated differences in the efficacy of the 5 antipsychotic agents by comparing the fit of a model in which the mean reduction in hallucinations is constrained to be equal across groups with the fit of a model in which reduction in hallucinations is allowed to be different across groups. Results indicated that there was no significant difference in efficacy between haloperidol, olanzapine, ziprasidone, quetiapine, and amisulpride in their potential to combat hallucinations (χ2(4) = 7.90, P = .095). Even though the difference between groups is not significant, we compared the steepness of the growth curves (shown in figure 1) as the difference between treatment arms could be considered a trend finding. This revealed that haloperidol showed less reduction in hallucinations compared with the other antipsychotic agents. The largest difference was found between haloperidol and olanzapine (χ2(1) = 6.93, P = .008) which is not significant after Bonferroni correction for the 15 comparisons which have been made (ie, testing at a type I error rate if .003). This finding should therefore be interpreted with caution.
With regards to their side effects, antipsychotics can be divided roughly into those predominantly inducing weight increase and sedation (ie, quetiapine, olanzapine, and clozapine) and those frequently associated with dystonia, parkinsonism, and akathisia (ie, all other antipsychotic drugs). The Patient Outcomes Research Team guidelines do not recommend olanzapine or clozapine as drugs of first choice because of the severe weight gain they may induce.3 Yet, a recent Finnish study found that the use of these 2 antipsychotics was associated with lower rates of rehospitalization, suggesting better efficacy and/or adherence over other antipsychotics.4
If remission is not obtained with the drug of first choice, a relatively quick switch is warranted. The exact moment for such a switch is still under discussion, but there is cumulating evidence that antipsychotic drugs require only little time (in the order of hours rather than days or weeks) to manifest their potential.5 This would imply that a switch can be considered after 2 or 4 weeks of treatment. A second antipsychotic is usually chosen from the group of drugs with a different receptor profile, although direct evidence to support this strategy is scarce.3 For those patients who even fail to respond to a second antipsychotic agent, clozapine is considered the drug of choice. The landmark trial by Kane et al6 demonstrated superior efficacy of clozapine for the subgroup of medication-resistant patients in comparison with any other antipsychotic agent, a finding that has consistently been replicated.7 In order to optimize clozapine therapy, various studies have evaluated the relationship between clozapine blood levels and therapeutic response. Blood levels above 350–450 μg/ml are associated with superior treatment results, not only for intractable hallucinations but also for negative symptoms, disorganized behavior, and thought disorder.7 Despite these unique qualities, clozapine has failed to gain the status of a drug of first or second choice as a result of its rare, but potentially severe side effects.
Maintenance Treatment
When successful, antipsychotic medication should be prescribed for patients with schizophrenia in an unaltered dose for at least 1 year.3 However, this does not imply that the treatment should be discontinued as soon as the year is over. As the propensity to hallucinate depends for the greater part on our genetic makeup, the vulnerability for hallucinations is life long. Therefore, as long as the side effects are tolerable, it is preferable not to discontinue the medication that has led to the initial improvement, not even after a prolonged psychosis-free period.
To prevent relapses, 2 strategies can be followed: continuous maintenance treatment with antipsychotic medication, or intermittent treatment, to be started as soon as any signs of potential relapse are detected. In a randomized study, maintenance treatment was found to be more effective than targeted intermittent treatment in preventing relapse, even in stable patients after their first year of maintenance treatment.8 There has been considerable discussion regarding the optimal dose for maintenance treatment. In an elegant study, Wang et al9 randomized 404 patients with schizophrenia in remission to 3 conditions: I. Initial optimal therapeutic dose continued throughout the study, II. Initial optimal therapeutic dose continued for 4 weeks and then reduced to 50%, and III. Initial optimal therapeutic dose continued for 6 months and then reduced to 50%. After 1 year, the relapse rates were 9.4% for group I, 30.5% for group II, and 19.5% for group III. These findings indicate that a dose reduction of 50% increases the risk for relapse 2- or 3-fold. Whether a dose reduction of less than 50% is as effective as continuation of the initial dose remains unclear. Current evidence thus suggests that continuous maintenance treatment with the initial dose used for symptom remission provides the lowest relapse rates.
Depot Medication
As relapses of hallucinatory episodes are most frequently associated with nonadherence to antipsychotic treatment,10 long-acting injectables (the so-called “depots”) constitute a valuable alternative for oral medication. Studies comparing short-acting oral and long-acting injectable antipsychotics found the latter to be superior in terms of relapse prevention and improvement of social functioning.11 In parallel, a large population–based study found in a pair-wise comparison between depot injections and their equivalent oral formulations, the risk of rehospitalization for patients receiving depot medications to be about one-third of that for patients receiving oral medications.4 Therefore, depot medication may be beneficial to prevent relapse in many patients and should be explained as an option for maintenance treatment to all patients.
Poor Response to Antipsychotic Medication
Although clozapine is considered the most effective antipsychotic agent for patients with refractory hallucinations, not all patients can achieve remission, even with adequate blood levels of clozapine.6 Treatment of these patients has remained a persistent public health problem because they often have a low quality of life. For these ultra-resistant patients, several treatment strategies are available, including psychotherapy, pharmacological augmentation, repetitive TMS (rTMS), and ECT. In clinical practice, clozapine is often augmented with lithium, sodium valproate, benzodiazepines, selective serotonin reuptake inhibitors, lamotrigine, risperidone, haloperidol, or aripiprazole. A recent meta-analysis quantitatively summarized all randomized controlled trials (RCTs) involving the pharmacological augmentation of clozapine.12 That review included 29 RCTs reporting on 15 different augmentation strategies prescribed to 1066 patients in total. Better improvement of total symptom severity—in comparison with placebo—was found for lamotrigine, sulpiride, citalopram, and the glutamatergic agonist CX516. However, the superiority of lamotrigine and topiramate turned out to depend on the inclusion of a single outlier, whereas the claim of superiority of sulpiride, citalopram, and CX516 was based on single RCTs. We must conclude, therefore, that pharmacological augmentation strategies in clozapine therapy are not (yet) supported by much convincing evidence from the literature.
Cognitive-Behavioral Therapy
CBT for Auditory Verbal Hallucinations
Auditory verbal hallucinations (AVH) occurring in the context of psychotic disorders can be characterized by 5 specific aspects: the content of the voices is personally meaningful, the voices have a more or less fixed identity, the relationship with the voices tends to be intimate, the experience has a significant impact on the patient’s life, and the experience has a compelling sense of reality.13 All these aspects are targeted in CBT.
The application of CBT is based on a cognitive model of auditory hallucinations.14 The classic cognitive-behavioral model hinges on the way hallucinations are appraised. Appraisals that tend to aggravate the severity of hallucinatory experiences are those that exaggerate their power, characterize them as omnipotent and omniscient, place their source in the external world, and endow them with malevolent intentions. CBT targets the ways in which the voices are being appraised. It puts the appraisals in perspective through a collaborative approach, in which other possible explanations for the origin and meaning of voices are considered together with the patient.
The behavioral part involves the testing of alternative ways to deal with particular situations as well as attempts to change the patient’s feelings about them. As soon as the patient starts to show any signs of doubt, it is time to encourage behavioral changes such as limiting the time spent with the voices, picking up routines of daily life again, and attempting to regain important social roles that lend meaning and fulfillment to life. Also, safety behaviors must be broken down in order to promote the experience that any anticipated disastrous consequences fail to happen.
CBT has some new developments, sometimes referred to as third wave therapies. We will also discuss compassionate mind training (CMT), imagery techniques such as competitive memory training (COMET), acceptance and commitment therapy (ACT), and the application of eye movement desensitization and reprocessing (EMDR) in people with psychosis, and posttraumatic stress disorder (PTSD).
The Effectiveness of CBT for Hallucinations
The effectiveness of CBT for hallucinations and other psychotic symptoms is well documented in several meta-analyses. A recent meta-analysis reported overall beneficial effects for the target symptom (33 studies; effect size 0.40) as well as significant effects for positive symptoms (32 studies), negative symptoms (23 studies), general functioning (15 studies), mood (13 studies), and social anxiety (2 studies) with effect sizes ranging from 0.35 to 0.44. Although 32 studies reported significant effects for positive symptoms, only 26 specifically targeted positive symptoms. One study aimed to reduce compliance with command hallucinations and reported an effect size of 1.1.15 Group therapy, however, has not yielded any clinically significant results.16 Involving family members in the cognitive treatment of hallucinations, on the other hand, turns out to be quite effective, with long-lasting results and effect sizes ranging from 0.51 to 0.60.17
The Effectiveness of Content-Specific CBT for Hallucinations
The specific content of auditory hallucinations has consequences for the emotional responses to them and may warrant various changes to the basic treatment protocol. We will discuss specific treatment approaches for command hallucinations, humiliating voices, critical voices, and voices associated with traumatic experiences and PTSD.
Command Hallucinations.
Threatening voices and command hallucinations can pose a serious threat to the patient and his environment. Fortunately, many patients resist dangerous and aggressive commands of the voices, but some will comply with the commands. By changing patients’ beliefs about the power of their voices, the risk that they will comply with command hallucinations can be reduced. Trower and colleagues18 tested the effectiveness of cognitive therapy for command hallucinations by randomizing 38 patients who had recently complied with their voices’ commands and had suffered the consequences. The control condition was treatment-as-usual, and the patients were followed-up after 6 and 12 months. In the cognitive-therapy group, the authors found large and significant reductions in compliant behavior, with an effect size of 1.1 (Cohen’s d). Improvements were recorded in the treatment group (but not in the control group) regarding the power attributed to the voices, the perceived need to comply, and the levels of concomitant distress and depression. No changes in the frequency, loudness, and content of the voices were recorded. The differences were still significant at 12 months follow-up.
Humiliating Voices.
Some voices may constantly humiliate the patient by telling him that he is a loser, that nobody cares for him, that he is incompetent, or that he would be better off dead. Patients who experience such humiliating voices also tend to feel depressed and helpless and to ruminate about what they say.19 They often agree with the content of their voices. COMET is based on the notion that a therapy is successful when it changes the hierarchy of relevant neural networks, and the order in which those networks are activated. A successful treatment reinforces the neural networks that are incompatible with so-called “negative” networks. When instead “positive” networks are activated over and over again, they may well move up in the hierarchy of networks and come to surpass the negative ones. COMET teaches the patient to reexperience personal memories that are incompatible with the dominant voices’ messages. Applied to hallucinations, COMET primarily reduces depression, with a medium to large effect size (Cohen’s d = 0.64). That effect is mediated by the improvement of self-esteem and the acceptance of the voices. The effect on the auditory hallucinations scale of the Psychotic Rating Scales (PSYRATS) was nonsignificant (P = .197; Cohen’s d = 0.30), while the effect on the cognitive interpretation subscale measuring the appraisal of the voices was significant (P = .009; Cohen’s d = 0.63).20
Critical Voices.
Critical voices are sometimes talking directly to the patient and sometimes to each other while discussing the patient or gossiping about him, thus constantly criticizing what the patients does or thinks. CMT targets shame and self-criticism, as well as the ensuing submissiveness and negative affect. With the aid of the 2-chair technique, a criticizing voice (or “inner bully”) can be interviewed, and the criticisms can then be compared with the patient’s personal needs and distresses. The patient is encouraged to respond toward himself with warmth and compassion rather than with criticism. Case-series reports are promising,21 and an RCT is underway.
Reperceptive or Memory-Based Hallucinations.
Eleven to 52% of those diagnosed with schizophrenia also have comorbid PTSD.22 Victimization, especially in the context of sexual traumatization, results in a 10-fold likelihood to develop hallucinations and other psychotic symptoms.23 The hallucinations experienced by those patients can perhaps best be characterized as reexperiences of a trauma. Memory processes probably mediate such reperceptive hallucinations, whereas command hallucinations may be predominantly attributable to inner speech processes.24 Reperceptive hallucinations can be treated effectively with the aid of CBT, exposure, and EMDR. Mueser and colleagues25 carried out an RCT with CBT and found effect sizes of 0.59 for the reduction of the total symptom score. In an open trial with 20 patients, 12 patients ceased to fulfill the diagnostic criteria of PTSD after prolonged exposure.26 In an open trial with EMDR among 27 patients, within-group effect sizes of 1.16 were found for the total trauma symptoms score (Clinician Administered PTSD Scale), 0.85 for depression (Beck Depression Inventory), and 0.79 for anxiety (Beck Anxiety Inventory). Eight people were hearing voices and 5 of them stopped hearing voices after EMDR.22
Acceptance and Commitment Therapy
Yet another type of treatment is ACT. This is a third wave CBT and is a transdiagnostic therapy aimed (1) at the acceptance of symptoms such as voices as mental phenomena, (2) a reduction of the patient’s investment in his symptoms, and (3) an increased commitment to important real-life goals and activities. An RCT by Bach and Hayes27 unexpectedly found an increase in reported hallucinations, but this was associated with a 50% decrease in rehospitalization rate compared with treatment as usual.
TMS for Hallucinations in Schizophrenia
What is TMS?
TMS is a technique in which a strong pulse of electrical current is sent through a coil. When the coil is placed over a person’s skull, this induces a magnetic field pulse in a small brain area, depolarizing local neurons up to a depth of 2 cm. When TMS is applied repetitive, it is thought to induce longer-lasting effects as a result of long-term potentiation or depression at the neuronal level.28 TMS is noninvasive, has only few side effects, and is relatively safe. In the past, epileptic seizures have occurred when rTMS was applied at a relatively high frequency, for a long duration of time or at a high percentage of the individual motor threshold. However, since Wassermann29 developed specific safety guidelines, seizures have become extremely rare. Side effects such as headache, local discomfort due to direct stimulation of the facial musculature, and transient changes in the auditory threshold have been described. In order to prevent the latter, earplugs are recommended during rTMS treatment.
TMS for AVH
In 1999, Hoffman and colleagues30 started to explore rTMS for the treatment of AVH. When the coil was directed at the left temporoparietal cortex, they were able to ameliorate medication-resistant AVH. Since then, more studies on this subject have been published, mostly—although not exclusively—carried out among patients diagnosed with schizophrenia. The results of these studies have been summarized in 4 meta-analyses, which all conclude that rTMS has a moderate to good effect on AVH, with effect sizes ranging from 0.51 to 1.04.31–34 However, a note of caution may be in place here. When new treatment strategies are being introduced, the initial reports tend to feature relatively small sample sizes and favorable results, whereas small studies with negative findings do not tend to be published. With an increase of studies with larger sample sizes in a later phase, negative findings tend to become published as well. In other areas of research, such trends have led effect sizes to decrease per year of publication.35 As rTMS is a relatively young treatment method, future studies may well show less favorable results than the initial ones. Thus it is no surprise that in our latest meta-analysis, we indeed found a trend toward larger studies being published in recent years, yielding negative results.36 We therefore take into account that the initially reported positive effects may perhaps disappear when more studies with larger patient samples will be published. Nevertheless, the present state of evidence allows us to recommend low-frequency rTMS directed at the left temporoparietal area for the treatment of AVH, although always in combination with state of the art pharmacological treatment.
Alternative TMS Paradigms
Only few studies have examined the effects of low-frequency rTMS targeted at other brain regions than the left temporoparietal cortex. Lee and colleagues37 reported a reduction in the severity of AVH after rTMS directed at the right temporoparietal cortex, which was not replicated by others. rTMS directed at the left and right temporoparietal cortex38 and rTMS directed at foci with maximal hallucinatory activity, as indicated by functional MRI,39 were not superior to sham treatment. Furthermore, the effects of rTMS applied to either Broca’s area or to the left superior temporal gyrus were equal to those of sham treatment.39 No firm conclusions can be drawn due to the small number of studies, but there is currently no evidence to suggest that locations other than the left temporoparietal cortex are suitable options for the treatment of auditory verbal hallucinations with rTMS.
In the majority of studies, rTMS was applied with a frequency of 1 Hz. But high-frequency rTMS has also been studied as a treatment option for AVH, yielding a strong clinical response with a frequency of 20 hertz. However, the patient sample was small, and no control condition was included, which precludes any firm conclusions regarding this type of treatment too.40 A recent study from the Utrecht group assessed 20-Hz stimulation and 1-Hz-stimulation in a double-blind head-to-head comparison, and found no differences between the 2 treatment arms (De Weijer, A.D., Meijering, A.L., Bloemendaal M., et al., unpublished data). In a RCT, the effects of low-frequency rTMS preceded by 5 minutes of 6-Hz rTMS (priming) was compared with low-frequency rTMS, and again no differences could be revealed between the 2 conditions (Slotema, C.W., Blom, J.D., De Weijer, A.D., et al., unpublished data). Two case reports described relief from chronic intractable auditory hallucinations after bilateral and continuous theta-burst-TMS, respectively.41,42 However, before any large sham-controlled RCTs with favorable results becomes available, we will not be able to recommend high-frequency or theta-burst stimulations for the treatment of AVH.
The majority of studies have been performed with the aid of a figure-of-eight coil. H-coils, which have the shape of a helmet, are designed to maximize the electrical current induced in deep brain tissues by their ability to summate separate magnetic fields projected into the skull from several points around its periphery. In an open-label study, 8 patients were treated with deep-brain rTMS using such an H-coil, which resulted in a significant reduction in the severity of AVH.43 However, this study also lacked a control group. As a consequence, its results need to be replicated in randomized, placebo-controlled double-blind studies before any conclusions can be drawn.
Electroconvulsive Therapy for Hallucinations in Schizophrenia
Another augmentation strategy for the treatment of intractable hallucinations occurring in the context of psychosis is electroconvulsive therapy (ECT). Introduced as a treatment method with highly promising results during the 1930s and subsequently discarded during the 1970s, ECT is now a well-established psychiatric treatment method. It nevertheless continues to be the most stigmatized therapeutic in psychiatry, although for a limited number of indications (notably catatonia and severe depression), it can be extremely effective and potentially life saving.
What is ECT?
During ECT, an electrical current is passed briefly through the brain via electrodes attached to the scalp to induce a generalized seizure. ECT is performed under general anesthesia; muscle relaxants are administered to prevent body spasms. The ECT electrodes can either be placed on both sides of the head (bilateral placement) or on one side only (unilateral placement). Unilateral placement is usually over the nondominant half of the brain, with the aim to reduce cognitive side effects. However, bilateral electrode placement tends to yield a faster improvement. The amount of current required to induce a seizure (called the seizure threshold) can vary largely among individuals and may increase during the course of treatment. Cognitive impairments, especially memory problems, can occur immediately after the administration of ECT as well as afterward. However, pretreatment functioning levels tend to be reached within the first months following treatment.44
Although ECT has been used in clinical practice since the 1930s, there is still no generally accepted hypothesis explaining its mechanism of action. In rat models, ECT (contrary to antidepressants) can induce mossy fiber sprouting, and there is growing evidence that it impacts brain-derived neurotrophic factors capable of inducing neuroproliferation.45
ECT for Hallucinations
There is no consistency whether persistent hallucinations in psychosis should be considered a valid indication for ECT. Recently, the National Institute of Clinical Excellence (NICE) concluded that “the current state of the evidence does not allow the general use of ECT in the management of schizophrenia to be recommended.”46 Understandably, only a low number of studies have assessed the effects of ECT in a double-blind sham-controlled design. Tharyan and Adams47 published a systematic meta-analysis of double-blind randomized studies comparing ECT and antipsychotic medication to sham and medication. They included 10 RCTs with a total of 392 patients. The relative risk for clinical improvement was 0.78 in favor of real ECT, a significant finding.
However, it should be noted that none of the above-mentioned studies provided any details on the reaction of hallucinations to ECT specifically. As a consequence, the reported clinical improvement in all those studies is not necessarily attributable to a reduction in the severity of psychosis. In fact, we were unable to retrieve a single study demonstrating a specific relief of hallucinations in medication-resistant schizophrenia caused by ECT. As a consequence, we must conclude that ECT as an augmentation to antipsychotic medication is capable of improving the clinical status of some patients with medication-resistant psychosis, but this may be attributable to other symptoms (mood, motor retardation, agitation, and catatonia) than the core psychotic symptoms. The effect of ECT on hallucinations per se is as yet unclear and might well be low.
Conclusions
Hallucinations in individuals diagnosed with schizophrenia are usually treated with antipsychotic medication. Antipsychotic medication is capable of inducing a rapid decrease in hallucination severity, and only 8% of the first-episode patients go on to experience mild, moderate, or severe hallucinations when they continue their medication as prescribed during 1 year. Olanzapine, sulpride ziprasidone, and quetiapine appear to be equally effective against hallucinations, but haloperidol may not be the agent of first choice. If the drug of first choice does not provide adequate symptom reduction, it is probably best to switch to another antipsychotic agent at a relatively early stage (ie, after 2 to 4 wk). Clozapine dosed to induce blood levels above 350–450 μg/ml, is the drug of choice for patients who are resistant to 2 other antipsychotic agents. For the sake of relapse prevention, the same drug that induced remission should be continued, preferably in the same dose. Depot medication should be considered for all schizophrenia patients, as nonadherence is high among individuals thus diagnosed, as is the chance of relapse.
CBT can be applied as an augmentation to antipsychotic medication for all psychotic patients who experience hallucinations. The success of CBT depends on the reduction of catastrophic appraisals, thereby reducing concurrent anxiety and distress. CBT teaches the patient to ignore the voices and focus on future plans and aims that will increase their quality of life. It should be noted, however, that CBT does not generally reduce the frequency of hallucinations.
TMS, on the other hand, is capable of actually reducing the frequency and severity of auditory hallucinations. Several meta-analyses found significantly better symptom reduction when low-frequency rTMS was applied to the left temporoparietal area (as compared with placebo), with only transient and mild side effects. Yet, larger studies with lower effect sizes have recently been published, and future meta-analyses may not be able to replicate the positive main effects initially reported. As a consequence, TMS currently has the status of a potentially useful treatment method for auditory hallucinations, but only in combination with state of the art antipsychotic treatment.
Several guidelines mention electroconvulsive therapy as a last resort for treatment-resistant psychosis in schizophrenia patients. Although several double-blind sham-controlled studies showed improvement in overall symptom severity as compared with sham treatment, a specific reduction in hallucination severity has never been described at group level.
The Treatment of Hallucinations in Other Disorders
Hallucinations occur in the context of many different disorders and syndromes. Therefore, the choice for a specific type of treatment does not only depend on the type of hallucination and its consequences for daily functioning but also on the underlying disorder. It may be difficult, however, to determine what the underlying disorder is, as hallucinations in for example borderline-personality disorders,48 psychotic depression, or temporal lobe epilepsy49 may be indistinguishable from schizophrenic hallucinations on the phenomenological level. Accompanying symptoms, such as epileptic insults, parkinsonian motor symptoms, vision or hearing loss, are the most reliable anchors for differential diagnosis.50 Some individuals who hallucinate only sporadically may be merely concerned that their experiences are a sign of mental disease, without being troubled by the hallucinations themselves. For others, the burden of their hallucinations may not outweigh the side effects of treatment. As a consequence, treatment may not be necessary in all cases. In this paragraph, a few disorders that frequently present with hallucinations will be discussed together with the specific treatment options.
Hallucinations and other psychotic symptoms are common in patients with Parkinson’s disease (PD), with lifetime prevalence rates of up to 80%.51 In Lewy body dementia, a condition closely associated with PD, these numbers are even higher, especially for visual hallucinations. Auditory hallucinations are present in up to 20%.52 Hallucinations can have substantial psychosocial effects and historically constitute the main reason to place patients in nursing homes.53 The pathophysiology of psychosis in PD and Lewy body dementia involves a complex interplay of extrinsic and disease-related factors, including central dopaminergic overactivity and an imbalance of dopaminergic and cholinergic neurotransmission, dysfunction of the visual pathways, alterations of brainstem sleep-wake and dream regulation, and impaired attentional focus.53 The most important extrinsic factor, however, is medication. The treatment of hallucinations in PD involves patient-initiated coping strategies, a reduction of antiparkinson medication, augmentation with low-dose atypical neuroleptics, and, potentially, with cholinesterase inhibitors. Eng and Welty54 conducted a review of 13 studies on antipsychotic treatment for PD patients, all involving clozapine and quetiapine. They concluded that patients with PD may benefit from long-term clozapine therapy, whereas the results of the quetiapine studies were conflicting. Various open-label studies and one double-blind placebo-controlled trial involving 188 hallucinating PD patients are in support of the efficacy of rivastigmine. Thus, while the use of cholinesterase inhibitors, especially rivastigmine, appears to be a promising treatment for hallucinations in PD, evidence-based studies support only the use of clozapine.54
In Alzheimer’s disease (AD), the occurrence of psychosis in 30% to 50% of the cases has serious consequences for both patients and caregivers,55 especially since the optimal type of treatment is still elusive. Interventions that optimize environmental and interpersonal factors should be attempted in all cases, although their overall effectiveness and applicability are not entirely clear. Cholinesterase inhibitors such as donepezil may have a beneficial effect on hallucinations, while showing a relatively mild side effect profile.56 In a similar vein, memantine has been shown to be more effective than placebo treatment without causing any disturbing side effects. The Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer's Disease (CATIE-AD) study included 421 AD outpatients with psychosis and agitated and/or aggressive behavior. The patients were randomized to obtain masked flexible-dose treatment with olanzapine, quetiapine, risperidone, or placebo for up to 36 weeks. As regards the effects on psychotic symptoms, risperidone appeared to be superior to the other 2 drugs and placebo.57 Although antipsychotic medication can have a positive effect on hallucinations in dementia, several reports issue warnings against the excess risk of morbidity and even death associated with its use in older patients. As a consequence, it is strongly advised not to consider antipsychotic drugs as the first choice for treatment of psychotic symptoms in dementia. Extrapyramidal symptoms and arrhythmias due to QTc prolongation are well-known complications of the use of conventional antipsychotic agents, while cerebrovascular events appear to occur more frequently in association with atypical as well as conventional antipsychotics in comparison with placebo treatment. Nevertheless, a trial of these agents may be indicated when the severity of symptoms is extreme or when symptoms fail to respond to other types of medication or to nonpharmacological interventions.
Delirium is an acute neuropsychiatric syndrome, by definition due to organic disease, which is characterized by psychotic symptoms such as hallucinations and delusions in the presence of decreased attention, fluctuating consciousness, and other cognitive dysfunctions. It is very common in patients admitted to intensive care units, with a reported cross-sectional frequency of 32%, and a marked association with poor prognosis and increased mortality.58 The only causal treatment of delirium is the improvement of somatic health. The symptomatic treatment of hallucinations and other symptoms of delirium should commence with measures aimed at improving the patient’s circadian rhythm and orientation. Pharmacological treatment should preferably consist of haloperidol or olanzapine, as recommended by the latest NICE guidelines.46 Although benzodiazepines are widely applied for the treatment of delirium, they are recommended only for delirium tremens (ie, alcohol abstinence delirium). Cholinesterase inhibitors are not recommendable, as demonstrated by an RCT with rivastigmine in delirious patients admitted to an intensive care unit. This trial was terminated at an early stage because of significant higher mortality and an increased duration of delirium in comparison with the control group.59
The reported cross-sectional incidence of hallucinations and other psychotic symptoms in epilepsy is 3.3%, and in temporal lobe epilepsy as high as 14%.60 Hallucinations can occur shortly before (aura), during (ictal), or after an epileptic seizure, but frequently occur independently of any motor seizures. Ictal hallucinosis is considered relatively rare. Postictal hallucinations comprise some 25% of the hallucinatory episodes in epileptic patients. As post and interictal psychotic episodes resemble those in patients diagnosed with schizophrenia, they are also designated as “schizophrenia-like psychoses of epilepsy.” The treatment of ictal as well as post and interictal hallucinations should primarily consist of minimizing any medication capable of mediating these symptoms. Various antiepileptic drugs, such as phenobarbital, zonisamide, levetiracetam, and gabapentin, are known for their potential to induce hallucinations.62 In such cases, dose reduction or switching to another antiepileptic drug may lead to a relatively quick cessation of hallucinations. When antiepileptic drugs cannot be reduced or traded or when such an intervention is unsuccessful, antipsychotic medication is the next therapeutic step. Clozapine and chlorpromazine should be avoided because of their epileptogenic properties.61 Antipsychotics such as quetiapine, risperidone, and haloperidol are usually well tolerated.
Visually impaired patients may experience complex visual hallucinations, a condition known as the Charles Bonnet syndrome. Likewise, individuals with progressive hearing loss may develop auditory hallucinations consisting of music, voices, or other sounds. It is believed that such hallucinations are actually release phenomena due to a deafferentation of the visual or auditory association areas of the cerebral cortex, a process capable of yielding so-called “phantom percepts.”62 Cognitive defects and social isolation may act as additional risk factors. Release hallucinations generally affect the elderly; women more frequently than men. Patients who comprehend their unrealistic nature tend to be affected less severely by them, although they may still be distressed by the fear of imminent insanity. Reassurance and an explanation that the visions or auditory percepts do not imply any kind of mental illness may have a powerful therapeutic effect.63 Further therapeutic measures are not always necessary because release hallucinations may cease either spontaneously or upon the termination of social isolation. If warranted and possible, the treatment of first choice is the restoration of sight or hearing, for example by carrying out a cataract operation, cleaning the meatus externus or applying hearing aids. In addition, one may consider the optimization of visual or auditory stimuli. When interventions such as these are unsuccessful, pharmacological treatment may be considered, although the pros do not always outweigh the cons of side effects. Antipsychotics, antiepileptics, and cholinesterase inhibitors have been reported to be effective in case reports and open-label case series. There are currently no randomized trials on the efficacy of those types of medication in patients with release hallucinations. If pharmacological treatment is considered necessary, low-dose quetiapine or lamotrigine may be a good choice as it is usually tolerated well in elderly populations.63 rTMS has also been applied to this type of release hallucinations in the auditory domain, but results are inconclusive.64
Funding
This work was supported by 2 grants of Dr Sommer: NWO/ZonMW (Dutch Scientific Research Organization) Clinical Fellowship, number 40-00703-97-270 and NWO/ZonMW Innovation Impulse (VIDI) number 017.106.301.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of the study.
References
- 1.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;29:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 2.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANNS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- 5.Agid O, Remington G, Kapur S, Arenovich T, Zipursky RB. Early use of clozapine for poorly responding first-episode psychosis. J Clin Psychopharmacol. 2007;27:369–373. doi: 10.1097/jcp.0b013e3180d0a6d4. [DOI] [PubMed] [Google Scholar]
- 6.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;5:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 7.McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15–18. [PubMed] [Google Scholar]
- 8.Gaebel W, Riesbeck M, Wölwer W, et al. Relapse prevention in first-episode schizophrenia-maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German research network on schizophrenia. J Clin Psychiatry. 2011;72:205–218. doi: 10.4088/JCP.09m05459yel. [DOI] [PubMed] [Google Scholar]
- 9.Wang CY, Xiang YT, Cai ZJ, et al. Risperidone Maintenance Treatment in Schizophrenia (RMTS) investigators. Risperidone maintenance treatment in schizophrenia: a randomized, controlled trial. Am J Psychiatry. 2010;167:676–685. doi: 10.1176/appi.ajp.2009.09030358. [DOI] [PubMed] [Google Scholar]
- 10.Patel MX, de Zoysa N, Bernadt M, David AS. A cross-sectional study of patients' perspectives on adherence to antipsychotic medication: depot versus oral. J Clin Psychiatry. 2008;69:1548–1556. doi: 10.4088/jcp.v69n1004. [DOI] [PubMed] [Google Scholar]
- 11.Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia–a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127:83–92. doi: 10.1016/j.schres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Sommer IE, Begemann MJ, Temmerman A, Leucht S. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. [published online ahead of print March 21, 2011] Schizophr Bull. doi: 10.1093/schbul/sbr004. doi:10.1093/schbul/sbr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beavan V. Towards a definition of “hearing voices”: a phenomenological approach. Psychosis. 2011;3:63–73. [Google Scholar]
- 14.Chadwick P, Birchwood M. The omnipotence of voices. A cognitive approach to auditory hallucinations. Br J Psychiatry. 1994;164:190–201. doi: 10.1192/bjp.164.2.190. [DOI] [PubMed] [Google Scholar]
- 15.Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. doi: 10.1093/schbul/sbm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wykes T, Hayward P, Thomas N, et al. What are the effects of group cognitive behaviour therapy for voices? A randomised control trial. Schizophr Res. 2005;77:201–210. doi: 10.1016/j.schres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Jenner JA, Nienhuis FJ, van de Willige G, Wiersma D. “Hitting” voices of schizophrenia patients may lastingly reduce persistent auditory hallucinations and their burden: 18-Month outcome of a randomized controlled trial. Can J Psychiatry. 2006;51:169–177. doi: 10.1177/070674370605100307. [DOI] [PubMed] [Google Scholar]
- 18.Trower P, Birchwood M, Meaden A, Byrne S, Nelson A, Ross K. Cognitive therapy for command hallucinations: randomised controlled trial. Br J Psychiatry. 2004;184:312–320. doi: 10.1192/bjp.184.4.312. [DOI] [PubMed] [Google Scholar]
- 19.Badcock JC, Paulik G, Maybery MT. The role of emotion regulation in auditory hallucinations. Psychiatry Res. 2011;185:303–308. doi: 10.1016/j.psychres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Van der Gaag M, van Oosterhout B, Daalman K, Sommer I, Korrelboom K. Initial evaluation of the effects of competitive memory training (COMET) on depression in schizophrenia-spectrum patients with persistent auditory verbal hallucinations: a randomised controlled trial. Br J Clin Psychol. In press doi: 10.1111/j.2044-8260.2011.02025.x. [DOI] [PubMed] [Google Scholar]
- 21.Mayhew SL, Gilbert P. Compassionate mind training with people who hear malevolent voices: a case series report. Clin Psychol Psychother. 2008;15:113–138. doi: 10.1002/cpp.566. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berg DPG, van der Gaag M. Treating trauma in psychosis with EMDR: a pilot study. J Behav Ther Exp Psychiatry. In press doi: 10.1016/j.jbtep.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Bebbington P, Jonas S, Kuipers E, et al. Childhood sexual abuse and psychosis: data from a cross-sectional national psychiatric survey in England. Br J Psychiatry. 2011;199:29–37. doi: 10.1192/bjp.bp.110.083642. [DOI] [PubMed] [Google Scholar]
- 24.Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull. 2010;36:566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueser KT, Rosenberg SD, Xie H, et al. A randomized controlled trial of cognitive-behavioral treatment for posttraumatic stress disorder in severe mental illness. J Consult Clin Psychol. 2008;76:259–271. doi: 10.1037/0022-006X.76.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frueh BC, Grubaugh AL, Cusack KJ, et al. Exposure-based cognitive-behavioral treatment of PTSD in adults with schizophrenia or schizoaffective disorder: a pilot study. J Anxiety Disord. 2009;23:665–675. doi: 10.1016/j.janxdis.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bach P, Hayes SC. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: a randomized controlled trial. J Consult Clin Psychol. 2002;70:1129–1139. doi: 10.1037//0022-006x.70.5.1129. [DOI] [PubMed] [Google Scholar]
- 28.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 29.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1996;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman RE, Boutros NN, Berman RM, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol Psychiatry. 1999;46:130–132. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- 31.Aleman A, Sommer IEC, Kahn RS. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68:416–421. doi: 10.4088/jcp.v68n0310. [DOI] [PubMed] [Google Scholar]
- 32.Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108:11–24. doi: 10.1016/j.schres.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slotema CW, Blom JD, Hoek HW, Sommer IEC. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation? A meta-analysis of the efficacy of rTMS for psychiatric disorders. J Clin Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 34.Tranulis C, Sepehry AA, Galinowski A, Stip E. Should we treat auditory hallucinations with repetitive transcranial magnetic stimulation? A meta-analysis. Can J Psychiatry. 2008;53:577–586. doi: 10.1177/070674370805300904. [DOI] [PubMed] [Google Scholar]
- 35.Emerson GB, Warme WJ, Wolf FM, et al. Testing for the presence of positive-outcome bias in peer review: a randomized controlled trial. Arch Intern Med. 2010;170:1934–1939. doi: 10.1001/archinternmed.2010.406. [DOI] [PubMed] [Google Scholar]
- 36.Slotema CW, Blom JD, De Weijer AD, et al. Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry. 2011;69:450–456. doi: 10.1016/j.biopsych.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 37.Lee S-H, Kim W, Chung Y-C, et al. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376:177–181. doi: 10.1016/j.neulet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Vercammen A, Knegtering H, Bruggeman R, et al. Effects of bilateral repetitive transcranial magnetic stimulation on treatment resistant auditory-verbal hallucinations in schizophrenia: a randomized controlled trial. Schizophr Res. 2009;114:172–179. doi: 10.1016/j.schres.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Schönfeldt-Lecuona C, Grön G, Walter H, et al. Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15:1669–1673. doi: 10.1097/01.wnr.0000126504.89983.ec. [DOI] [PubMed] [Google Scholar]
- 40.Montagne-Larmurier A, Etard O, Razafimandimby A, Morello R, Dollfus S. Two-day treatment of auditory hallucinations by high frequency rTMS guided by cerebral imaging: a 6 month follow-up pilot study. Schizophr Res. 2009;113:77–83. doi: 10.1016/j.schres.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Eberle M-C, Wildgruber D, Wasserka B, Fallgatter AJ, Plewnia C. Relief from chronic intractable auditory hallucinations after long-term bilateral theta burst stimulation. Am J Psychiatry. 2010;167:1410. doi: 10.1176/appi.ajp.2010.10070988. [DOI] [PubMed] [Google Scholar]
- 42.Poulet E, Brunelin J, Ben Makhlouf W, D'Amato T, Saoud M. A case report of cTBS for the treatment of auditory hallucinations in a patient with schizophrenia. Brain Stimul. 2009;2:118–119. doi: 10.1016/j.brs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg O, Roth Y, Kotler M, Zangen A, Dannon P. Deep transcranial magnetic stimulation for the treatment of auditory hallucinations: a preliminary open-label study. Ann Gen Psychiatry. 2011;10:3. doi: 10.1186/1744-859X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68:568–577. doi: 10.1016/j.biopsych.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Grønli O, Stensland GØ, Wynn R, Olstad R. Neurotrophic factors in serum following ECT: a pilot study. World J Biol Psychiatry. 2009;10:295–301. doi: 10.3109/15622970701586323. [DOI] [PubMed] [Google Scholar]
- 46.Young J, Murthy L, Westby M, Akunne A, O'Mahony R Guideline Development Group. Diagnosis, prevention, and management of delirium: summary of NICE guidance. Br Med J. 2010;341:c3704. doi: 10.1136/bmj.c3704. [DOI] [PubMed] [Google Scholar]
- 47.Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia. Cochrane Database Syst Rev. 2005;18:CD000076. doi: 10.1002/14651858.CD000076.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Slotema CW, Daalman K, Blom JD, Diederen KM, Hoek HW, Sommer IEC. Auditory verbal hallucinations in patients with borderline personality disorder are similar to those in schizophrenia. Psychol Med. In press doi: 10.1017/S0033291712000165. [DOI] [PubMed] [Google Scholar]
- 49.Korsnes MS, Hugdahl K, Nygård M, Bjørnaes H. An fMRI study of auditory hallucinations in patients with epilepsy. Epilepsia. 2010;51:610–617. doi: 10.1111/j.1528-1167.2009.02338.x. [DOI] [PubMed] [Google Scholar]
- 50.Sommer IE, Koops S, Blom JD. Comparison of auditory hallucinations across different disorders and syndromes. Neuropsychiatry. 2012;2:1–12. [Google Scholar]
- 51.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 52.Fénelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci. 2010;15:12–17. doi: 10.1016/j.jns.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Diederich NJ, Fénelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5:331–342. doi: 10.1038/nrneurol.2009.62. [DOI] [PubMed] [Google Scholar]
- 54.Eng ML, Welty TE. Management of hallucinations and psychosis in Parkinson's disease. Am J Geriatr Pharmacother. 2010;8:316–330. doi: 10.1016/j.amjopharm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:1026–1035. doi: 10.1097/JGP.0b013e3181d6b68d. [DOI] [PubMed] [Google Scholar]
- 56.Wynn ZJ, Cummings JL. Cholinesterase inhibitor therapies and neuropsychiatric manifestations of Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:100–108. doi: 10.1159/000074281. [DOI] [PubMed] [Google Scholar]
- 57.Sultzer DL, Davis SM, Tariot PN, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165:844–854. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salluh JI, Soares M, Teles JM, et al. Decca (Delirium Epidemiology In Critical Care) Study GroupDelirium epidemiology in Critical Care (DECCA): an international study. Critical Care. 2010;14:R210. doi: 10.1186/cc9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–1837. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- 60.Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2–20. doi: 10.1111/j.1528-1157.1999.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 61.Alper KR, Barry JJ, Balabanov AJ. Treatment of psychosis, aggression, and irritability in patients with epilepsy. Epilepsy Behav. 2002;3:13–18. doi: 10.1016/s1525-5069(02)00500-5. [DOI] [PubMed] [Google Scholar]
- 62.Menon GJ, Rahman I, Menon SJ, Dutton GN. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003;48:58–72. doi: 10.1016/s0039-6257(02)00414-9. [DOI] [PubMed] [Google Scholar]
- 63.Rossom RC, Rector TS, Lederle FA, Dysken MW. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc. 2010;58:1027–1034. doi: 10.1111/j.1532-5415.2010.02873.x. [DOI] [PubMed] [Google Scholar]
- 64.Meng Z, Liu S, Zheng Y, Phillips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev. 2011;(10) doi: 10.1002/14651858.CD007946.pub2. CD007946. [DOI] [PubMed] [Google Scholar]