Abstract
Background
Studies have shown that schizophrenia patients have motion perception deficit, which was thought to cause eye-tracking abnormality in schizophrenia. However, eye movement closely interacts with motion perception. The known eye-tracking difficulties in schizophrenia patients may interact with their motion perception.
Methods
Two speed discrimination experiments were conducted in a within-subject design. In experiment 1, the stimulus duration was 150 msec to minimize the chance of eye-tracking occurrence. In experiment 2, the duration was increased to 300 msec, increasing the possibility of eye movement intrusion. Regular eye-tracking performance was evaluated in a third experiment.
Results
At 150 msec, speed discrimination thresholds did not differ between schizophrenia patients (n = 38) and control subjects (n = 33). At 300 msec, patients had significantly higher thresholds than control subjects (p =.03). Furthermore, frequencies of eye tracking during the 300 msec stimulus were significantly correlated with speed discrimination in control subjects (p = .01) but not in patients, suggesting that eye-tracking initiation may benefit control subjects but not patients. The frequency of eye tracking during speed discrimination was not significantly related to regular eye-tracking performance.
Conclusions
Speed discrimination, per se, is not impaired in schizophrenia patients. The observed abnormality appears to be a consequence of impairment in generating or integrating the feedback information from eye movements. This study introduces a novel approach to motion perception studies and highlights the importance of concurrently measuring eye movements to understand interactions between these two systems; the results argue for a conceptual revision regarding motion perception abnormality in schizophrenia.
Keywords: Corollary discharge, efference copy, extraretinal, eye movement, eye tracking, frontal eye field, motion perception, MST, retinal, schizophrenia, smooth pursuit, speed discrimination, SPEM
Studies have found that motion perception is impaired in patients with schizophrenia and that higher motion perception thresholds are significantly correlated with poor eye tracking or smooth pursuit eye movement (SPEM) (1-4). These findings are generally interpreted as evidence of a motion-processing deficit causing the SPEM abnormality in schizophrenia. Many studies (3,5-10), including those from our own (11), have shown abnormal smooth pursuit onset in schizophrenia. Because response to initial target motion is a response to retinal motion before eye movement feedback occurs (12-14), abnormal initiation response suggests a motion perception impairment. However, this conflicts with studies that report normal or even faster onset latencies as well as accurate saccades and SPEMs in response to initial target motion in schizophrenia patients (10,14-18). Using unexpected target changes during smooth pursuit, we found that patients made more accurate response to unexpected target direction changes than healthy subjects, suggesting unimpaired motion processing during SPEM in schizophrenia patients (19). One possible explanation for the inconsistent findings on initiation responses could be the role of anticipation. We recently examined the effect of anticipation on pursuit initiation and found that when anticipation of pursuit onset was removed, schizophrenia patients and healthy subjects showed similar pursuit initiation; when anticipation was introduced, schizophrenia patients showed impaired pursuit onset (18). Based on these data, we hypothesize that motion processing per se is unimpaired and not the cause of eye-tracking abnormality observed in schizophrenia. If true, then how does one explain the findings of impaired speed discrimination in schizophrenia patients? Eye movements are known to closely interact with motion perception (20,21). A critical methodological weakness of previous motion perception studies in schizophrenia was that no attempts were made to monitor eye movements. One cannot rule out the possibility that the observed motion perception problem in schizophrenia may be a consequence rather than the source of the patients’ known SPEM difficulty.
Smooth pursuit eye movements occur frequently in motion perception tasks because humans have difficulty holding their eyes still when presented with moving images (22). Smooth pursuit eye movement is initiated in response to retinal motion, a moving image across the retina. The motor command to move the eye is considered a source of extraretinal motion information (21,23). The extraretinal motion signal is critical for normal motion perception (20,21). Data suggest that retinal motion and reafferent stimuli, i.e., the motion of the target image resulting from the eye movement, interact with the motor command to form an accurate motion perception (24,25). Traditional speed discrimination studies use motion of objects that are presented for a duration of 200 msec or longer, which is sufficient time to initiate SPEM and to generate a motor command that can be incorporated into the motion perception.
Schizophrenia patients have specific problems in processing extraretinal motion information (26-28). Their poor motion perception could be derived from deficits in extraretinal rather than retinal motion processing, if the normal process of integrating the oculomotor command into motion perception (29) is impaired. This hypothesis can be studied by simultaneously examining motion perception and eye movements.
A behavioral study that pinpoints whether the primary deficit is motion perception or SPEM has important implications for understanding the pathophysiology of schizophrenia, since these two visual functions are distinct in many brain areas. Motion perception involves the early visual pathway from lateral geniculate nucleus to primary visual cortex to middle temporal (MT) cortex, while SPEM pathway involves signals from this early visual pathway and also medio-superior temporal (MST), frontal, parietal, and cerebellar mechanisms (30-32). In this study, we hypothesized that speed discrimination deficit in schizophrenia patients described in previous studies may be affected by their eye-tracking difficulties. This hypothesis was tested in two contrasting experiments. In experiment 1, motion stimulus was presented for 150 msec duration, so the stimulus ended before the SPEM initiation, which generally occurs within 150 msec to 180 msec of target motion onset. Thus, the 150 msec condition should limit the influence of feedback information from eye movement during motion perception testing by minimizing the induction of eye movements (20). In consequence, we expected speed discrimination thresholds would not differ between schizophrenia patients and control subjects. In experiment 2, the motion stimulus was presented for 300 msec, intended to replicate the previous finding of speed discrimination deficit in schizophrenia patients. Eye movements were monitored in both experiments to determine whether or not individuals maintained fixation during motion perception testing and if they did not, to what extent eye movements contributed to the speed discrimination thresholds. A third experiment was performed to examine regular smooth pursuit and to determine whether subjects’ eye movements during speed discrimination are related to their capacity to pursue a moving target when they were instructed to do so.
Methods and Materials
Subjects
Written informed consent was obtained from each subject. Subjects were 18 to 55 years of age, with no neurological or ophthalmologic conditions, substance dependence within the past 6 months, or current substance abuse. We tested 38 schizophrenia patients and 33 normal control subjects. All patients were clinically stable, with a diagnosis of schizophrenia based on the Structured Clinical Interview for DSM-IV (SCID-IV). Three patients were on first-generation antipsychotic medications and the rest of the patients were on second-generation antipsychotic medications. Clinical symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS). Control subjects were recruited by local media advertisements. Control subjects had no DSM-IV Axis I psychotic disorders, no cluster A personality diagnoses, and no family history of psychotic illness.
Experimental Procedures
Setting and Stimulus
Stimuli were displayed on a 22-in flat screen monitor set to 60 Hz at 1280 × 1024 pixels, placed at a viewing distance of 60 cm in a room with illuminance of 2 lux. The target was a vertical sinusoidal grating with a spatial frequency of .5 cycles per degree. Slower spatial frequency (e.g., .5–1.0 cycles per degree) is related to the magnocellular function and schizophrenia patients are thought to be impaired in this function (33), but this interpretation is not without dispute (34). The grating was presented in a circular disk 19 visual degrees in diameter. The luminance of the monitor was calibrated using the LaCie blue eye vision calibrator (LaCie, Hillsboro, Oregon). The average luminance of the display was 24 candelas (cd)/m2. The contrast of the grating was .15. The grating setting replicated that used by Chen et al. (3), with the exception of a blurred edge to reduce edge effects.
Psychophysical Procedures
A two-alternative forced choice (2AFC) parameter estimation by sequential testing (PEST) adaptive procedure was used to determine the speed discrimination thresholds (35,36). A trial consisted of a grating moving at a test speed followed by a grating moving in the same direction 500 msec later but at a reference speed (Figure 1). A fixation crosshair was always present. The subjects were instructed to maintain fixation. Subjects indicated whether the first grating (test speed) was moving faster or slower than the second grating (reference speed) by pressing response buttons. The next trial started randomly anywhere from 1 sec to 2 sec after a response. The gratings moved in either direction, randomly determined trial to trial, to make the onset of the test gratings unpredictable. The duration of the stimuli was either 150 msec (experiment 1) or 300 msec (experiment 2). Each experiment was tested using three reference speeds: 3.8°/sec, 10°/sec, and 18.7°/sec. Each block of tasks had 26 trials with stimuli of the same reference speed and duration. Two PEST procedures were run per block, with 13 trials from each PEST procedure randomly interlaced. The procedures had an initial test speed 40% faster or slower than the reference speed. Based on the subject’s response, the adaptive procedure (35) determined the next test speed to be presented. Subjects performed a total of six blocks (3 speeds × 2 durations). The orders of blocks were randomized across subjects by means of a Latin Square table. Speed discrimination threshold was expressed as Weber fraction ΔV/V (|final test speed − reference speed|/reference speed). Each subject was given one block of practice trials.
Figure 1.
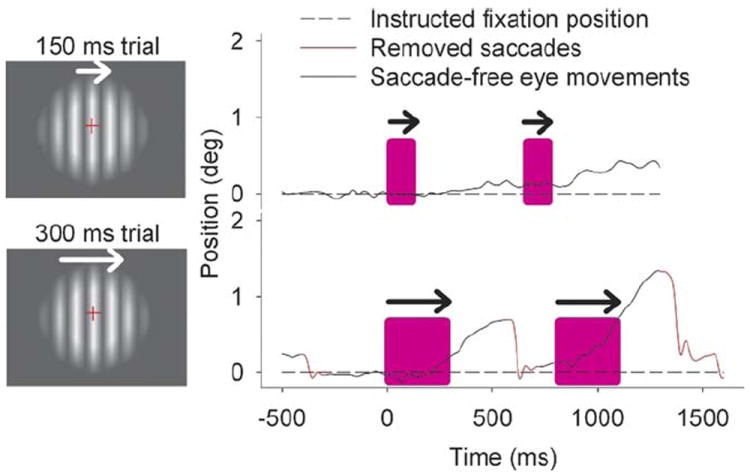
An illustration of typical eye movements occurring during motion perception experiments. Top graphs: an example of the first experiment where moving gratings were presented at 150 msec. The first color bar indicates the movement of the testing grating, which varies in speed from trial to trial based on the PEST algorithm and also varies in direction of movement, thus making the presentation unpredictable. The second color bar indicates the movement of the reference grating, which is always presented at the same speed across a block (reference speed at 10°/sec in this recording), is always in the same direction and time interval from the first grating, and is predictable. Note that the participant was able to fixate on the crosshair during the moving gratings. Some SPEM in the direction of the grating still occurred, but these occurred after the window of target viewing. Bottom graphs: an example of the second experiment where moving gratings were presented at 300 msec (reference speed 10°/sec). Note the initiation of pursuit eye movements during the testing grating presentation (with a latency of about 180 msec). The interstimulus duration was always 500 msec from the end of first stimulus to the beginning of the second stimulus of a pair. PEST, parameter estimation by sequential testing; SPEM, smooth pursuit eye movement.
Eye Movement Monitoring During Speed Discrimination
Eye data were collected using an EyeLink II eye tracker (SR Research Ltd., Toronto, Canada) sampling at 500 Hz. The subject’s head was stabilized using a chin rest. A calibration sequence (−5°, 0°, +5°) routine was inserted every five trials for offline calibration. Eye data were low-pass filtered at 20 Hz and differentiated into velocity and acceleration records. Analysis of the oculomotor data was automated using in-house algorithms developed in Matlab (MathWorks, Inc. Natick, Massachusetts). The algorithm identified saccades based on velocity (>35°/sec) and acceleration (>600°/sec2) criteria. After saccade removal, eye movement occurrence was determined as follows: if the average speed within the 150 msec to 400 msec after the test stimulus onset exceeded a threshold of 1°/sec in the direction of the stimulus, the trial was considered as having an eye movement occurrence. Eye movements during the reference stimulus were also scored but not considered as a primary measure because eye movements here are in part anticipatory rather than based only on retinal motion. This is because the presentation of reference gratings was not the source of variation in stimulus speed (always the same speed in a block) and was entirely predictable (always the same direction as the first grating and the same interval). We also measured a number of eye movement characteristics (test grating only) in trials with eye movements, including pursuit initiation latency, initiation mean and peak velocity and acceleration within the first 100 msec of pursuit, and the peak velocity within the first 500 msec after initiation.
Smooth Pursuit Initiation
A third experiment was performed to obtain smooth pursuit measures. Briefly, pursuit initiation was triggered using a step-ramp procedure, which is known to minimize saccadic responses during the initiation (37), followed by a 2- to 4-cycle of horizontal eye movement at a constant speed (10.0 °/sec or 18.7°/sec targets). Each speed was tested for 12 trials. Subjects were instructed to follow the target with their eyes as closely as possible. We obtained the pursuit initiation latency and mean initiation acceleration within the first 100 msec of pursuit (for detailed methods, see 11). Closed-loop pursuit gain during constant target movement was also obtained. Data were scored blind to the subject and group identity.
Statistical Analysis
The overall hypothesis was that abnormal speed discrimination thresholds (Weber fraction) occur only in the 300 msec condition and not in 150 msec condition in schizophrenia patients. It was tested using repeated measures analysis of variance (ANOVA) where Weber fraction was the dependent measure, stimulus duration (150 msec and 300 msec) and speed (3.8°/sec, 10.0°/sec, and 18.7°/sec) were within-subjects factors, and diagnosis was the between-subject factor. Significant interactions were examined by separate repeated measures ANOVA for each stimulus duration and post hoc simple effect analysis on each stimulus speed. Frequency of eye movements (number of trials with eye movement divided by 26 trials in a block) was similarly evaluated where duration, speed, and stimulus (test and reference grating) were the repeated measures. Relationships between eye movement frequency and Weber fraction and between dependent measures and clinical characteristics were examined using Pearson’s correlations.
Results
Speed Discrimination
Schizophrenia patients and control subjects were not significantly different in age [mean ± SD: 40.3 ± 10.3 vs. 40.1 ± 11.2; F(1,70) = .01, p = .93], race (Caucasian:African American:Asian American: 18:19:1 vs. 19:11:3; χ2 = 2.82, p = .24), or gender (female:male: 11:27 vs. 11:22; χ2 = .16, p = .80). Repeated measure ANOVA showed no significant main effect of diagnosis [F(1,69) = 2.63, p = .11] and a significant three-way stimulus duration × speed × diagnosis interaction [F(2,69) = 2.95, p = .05]. At 150 msec, there was no significant effect of diagnosis [F(1,69) = .55, p = .46] or speed by diagnosis interaction [F(1,69) = .13, p = .29] (Figure 2A). At 300 msec, there was a significant effect of diagnosis [F(1,69) = 5.15, p = .03] and a trend of speed by diagnosis interaction [F(1,69) = 3.30, p = .07]. Schizophrenia patients had higher speed discrimination thresholds at target speeds of 18.7°/sec [F(1,69) = 6.54, p = .01] and 10.0°/sec [F(1,69) = 5.07, p = .03] but not at 3.8°/sec [F(1,69) = .11, p = .74] compared with control subjects (Figure 2B).
Figure 2.
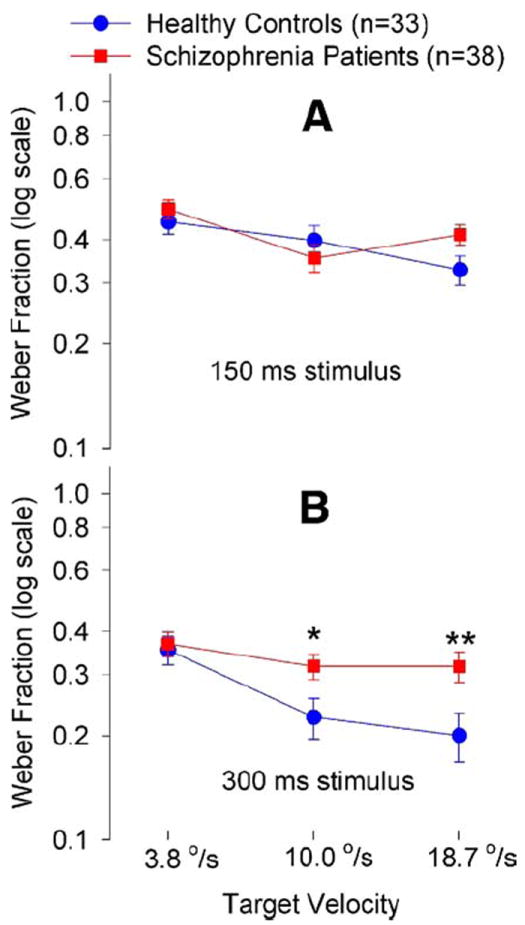
Speed discrimination thresholds (mean ± SE) for target duration at 150 msec (A) and 300 msec (B). *p = .03; **p = .01, were based on post hoc ANOVA comparing the measures between the two groups. ANOVA, analysis of variance.
Eye movements occurred in both presentation durations despite explicit instruction to fixate (Figure 3) but much less common with the 150 msec presentation. There were significant four-way diagnosis × speed × duration × stimulus (p = .03) and two-way diagnosis × duration (p = .008) interactions and no other significant diagnosis related main effect or interaction. Post hoc analyses of the significant interactions showed that control subjects and patients were not significantly different in frequency of eye movements at the 150 msec conditions in test [F(1,69) = 1.23, p = .27] or reference (p = .39) stimulus (Figure 3A and 3C). At 300 msec, control subjects had significantly more eye movement in test (p = .02) and reference (p = .04) stimulus compared with patients (Figure 3B and 3D). There were significant effects of duration (p < .001) and stimulus (p < .001), owing to significantly more eye movement occurrences in the 300 msec compared with 150 msec duration, and more eye movements during reference compared with test stimulus (Figure 3).
Figure 3.
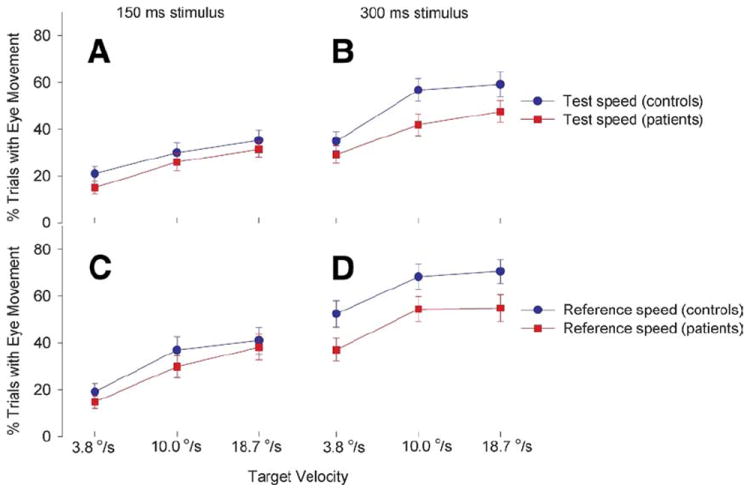
Eye movement frequency (mean ± SE) during motion perception test and reference gratings. There were significantly less eye movement occurrences during the 150 msec condition (A, B) compared with the 300 msec condition (C, D). Control subjects made significantly more eye movements during the 300 msec conditions but not in the 150 ms conditions compared with schizophrenia patients.
We also replicated previous findings of impaired motion perception in schizophrenia patients using the 300 msec target, but there were substantial eye movements during this condition.
Eye Movements During Impaired Speed Discrimination
Exploratory analyses were performed to assess why schizophrenia patients have worse speed discrimination during 300 msec 10.0°/sec and 18.7°/sec presentations, where there were more frequent occurrences of pursuit initiation. Did pursuit initiation differentially affect (benefit) motion discrimination in control subjects compared with patients? To address this question, we examined the correlations between the frequencies of eye movements and Weber fraction. There were no significant correlations in schizophrenia patients, but there were negative correlations in the control subjects, significant at the 10.0°/sec test speed (p = .01; Figure 4), suggesting that more eye movements were associated with better speed discrimination thresholds in the control subjects but not in the patients. No significant correlations were found in reference stimuli (Figure 4).
Figure 4.
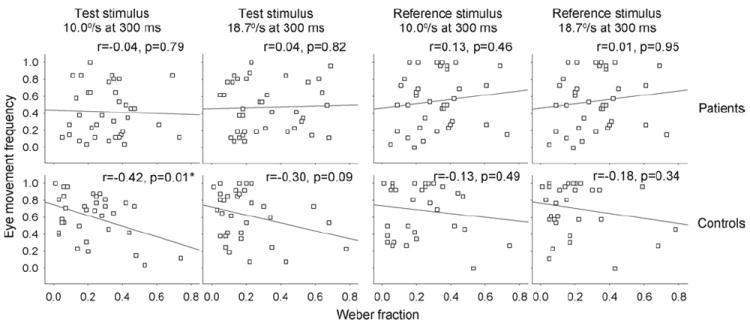
Eye movement frequencies versus speed discrimination thresholds at 300 msec stimulus condition, where schizophrenia patients showed impaired speed discrimination thresholds. There were no significant correlations in schizophrenia patients in the 10.0°/sec or 18.7°/sec test speeds (first stimulus). There were negative correlations in the control subjects, significant at the 10.0°/sec test speed, suggesting that more eye movements were associated with better speed discrimination thresholds in the control subjects but not in the patients. There were no significant correlations in the reference speeds (second stimulus) in either group, even though there were more eye movements here.
Less Eye Movement Does Not Mean Impaired Ability to Follow Moving Targets
It is possible that those subjects who made none to minimal pursuit eye movements had problems with SPEM initiation or motion perception, in which case we would see a correlation between eye movement frequencies during speed discrimination testing and regular pursuit initiation latency and/or initiation acceleration. Schizophrenia patients did not show significant impairment in pursuit initiation in the SPEM experiment compared with control subjects (Table 1). In patients, there was no significant correlation between eye movement frequency during test grating and either latency or acceleration (all r ≤ |.24|, all p ≥ .15). In control subjects, there was a nominally significant correlation between eye movement frequency and latency at 18.7°/sec (r = −.35, p = .05). However, this would not be significant if corrected for multiple comparisons. Otherwise, there was no significant correlation between eye movement frequency and latency or acceleration in control subjects (all r ≤ |.35|, all p ≥ .05). These findings do not support that frequency of initiating eye movements during motion perception testing is related to subjects’ performance during instructed eye-tracking initiation.
Table 1.
Comparisons of Pursuit Initiation and Closed-Loop Gain During Regular Smooth Pursuit Task
| Target Speed | Measures | Control Subjects | Patients | F Value | p Value |
|---|---|---|---|---|---|
| 10.0°/sec | Initiation latency (msec) | 181.5 ± 3.2 | 191.0 ± 4.4 | .68 | .41 |
| Initiation acceleration (°/sec2) | 65.5 ± 3.2 | 68.5 ± 3.3 | .25 | .62 | |
| Closed loop-gain | .83 ± .02 | .79 ± .02 | .86 | .36 | |
| 18.7°/sec | Initiation latency (msec) | 168.0 ± 3.2 | 182.6 ± 4.2 | 2.34 | .13 |
| Initiation acceleration (°/sec2) | 79.5 ± 3.7 | 78.2 ± 3.6 | .13 | .72 | |
| Closed-loop gain | .85 ± .02 | .77 ± .03 | 4.25 | .04 |
Schizophrenia patients were not significantly impaired in either initiation latency or initiation acceleration measures but impaired in closed-loop gain atthe higher target velocity (mean ± SE).
There was a significant correlation between initiation acceleration (during typical SPEM task) and Weber fraction at 18.7°/sec in schizophrenia patients (n = 37, r = −.50, p = .001) but not in healthy control subjects (n = 33, p = −.17, r = .34), replicating previous findings (1-4).
For closed loop gain, patients showed reduced gain compared with control subjects, significant at 18.7°/sec (Table 1). There was no significant correlation between closed loop gain and Weber fraction in the 150 msec duration in either 10.0°/sec (r = −.22, p = .10) or 18.7°/sec (r = −.17, p = .16) target speeds. In the 300 msec duration, the correlation was not significant at 10.0°/sec (r = −.10, p = .40) but significant at 18.7°/sec (r = −.34, p = .004). These correlations were from the combined group (n = 70). We did not find any significant correlation when patients and control subjects were analyzed separately.
Eye Movement Characteristics During Speed Discrimination
There was a nominally significant difference in average acceleration at 18.7°/sec (p = .02, not significant after correction for 12 comparisons using Bonferroni correction p < .004). Otherwise, we did not find significant group differences in the range of eye movement parameters during test grating (Table 2).
Table 2.
Comparisons of Eye Movement Characteristics (Test Grating, Mean ± SE) During Speed Discrimination in 10.0°/sec and 18.7°/sec Targets Presented at 300 msec Duration When Patients and Control Subjects Were Different in Their Speed Discrimination Thresholds
| Target Speed | Eye Movements Parameter | Control Subjects | Patients | F Value | p Value |
|---|---|---|---|---|---|
| 10°/sec | Initiation latency (msec) | 160.6 ± 5.50 | 154.3 ± 4.30 | .83 | .36 |
| Initiation peak speed (°/sec) | 3.77 ± .19 | 3.63 ± .18 | .27 | .60 | |
| Initiation average speed (°/sec) | 1.48 ± .10 | 1.22 ± .10 | 3.22 | .08 | |
| Initiation peak acceleration (°/sec2) | 192.43 ± 9.60 | 195.02 ± 13.40 | .02 | .88 | |
| Initiation average acceleration (°/sec2) | 32.34 ± 3.84 | 23.49 ± 3.97 | 2.53 | .12 | |
| Peak pursuit speed (°/sec) | 4.17 ± 1.17 | 3.81 ± 1.08 | 1.75 | .19 | |
| 18°/sec | Initiation latency (msec) | 146.7 ± 6.50 | 160.8 ± 4.50 | 3.27 | .08 |
| Initiation peak speed (°/sec) | 4.12 ± .26 | 3.91 ± .20 | .43 | .51 | |
| Initiation average speed (°/sec) | 1.61 ± .16 | 1.38 ± .11 | 1.50 | .22 | |
| Initiation peak acceleration (°/sec2) | 214.33 ± 8.84 | 206.29 ± 12.44 | .27 | .61 | |
| Initiation average acceleration (°/sec2) | 34.85 ± 3.95 | 23.17 ± 2.96 | 5.76 | .02 | |
| Peak pursuit speed (°/sec) | 4.50 ± 1.25 | 4.10 ± 1.42 | 1.60 | .21 |
F and p values are statistics of ANOVA comparing the measure between the two groups. Please refer to the Methods and Materials section for the description of the measures.
ANOVA, analysis of variance.
Correlations with Clinical and Demographic Information
The total BPRS scores were 30.7 ± 11.2 in the patients. There were no significant correlations between BPRS total scores and frequency of eye movement in any speed/duration conditions (all r ≤ .20 in absolute values, all p ≥ .28). There was a nominally significant correlation between BPRS score and Weber threshold at 3.8°/sec at 150 msec stimulus (n = 37, r = .36, p = .05, uncorrected). For BPRS subscales, the only nominally significant correlations were between Weber threshold at 3.8°/sec at 300 msec stimulus and thought disorder (r = .41, p = .02) and psychosis (r = .37, p = .03). None were significant after correction for multiple comparisons. In 24 schizophrenia patients whom we measured IQ (94.8 ± 15.5), we found a significant correlation between IQ and Weber threshold at 10.0°/sec at 150 msec stimulus (r = −.65, p = .001) but not at 300 msec condition (r = −.04, p = .84 at 10.0°/sec; r = −.31, p = .14 at 18.7°/sec). Age was significantly correlated to Weber threshold in all three velocities at 150 msec (r = .24–.27, p = .02–.04) but not 300 msec stimulus (all r ≤ .19, all p ≥ .11).
Discussion
This study examined speed discrimination thresholds in schizophrenia patients after accounting for eye movements. The first experiment presented the motion stimulus for a duration that was shorter than the response latency of the smooth pursuit system, thus preventing the integration of oculomotor commands into motion processing to derive a motion percept. Data from this experiment showed that when there is insufficient time for oculomotor feedback to influence perception, schizophrenia patients perform similarly to healthy control subjects in speed discrimination.
The simultaneous recording of eye movements during motion perception testing is a novel approach to understanding the interaction of these two functions in schizophrenia, a disorder with known impairments in both functions. Using this approach, the patients did show evidence of abnormal speed discrimination during the presentation of target motion in the second experiment, during 300 msec 10.0°/sec and 18.7°/sec presentations. However, there were frequent eye movements in both control subjects and patients in this experiment. Exploratory correlation analyses suggested that control subjects who made more frequent pursuit eye movements during the test stimulus tended to have better speed discrimination (significant at 10.0°/sec). This effect was not present during the reference stimulus. In comparison, schizophrenia patients showed no such correlation in either test or reference stimulus, suggesting that patients may have failed to benefit from pursuit initiation during motion perception. The stronger beneficial effect of eye movements from the test stimulus in control subjects was expected because the first stimulus had unexpected onset; eye movements that occurred during the first stimulus were likely based on retinal motion. Eye movements occurring at the second stimulus were likely influenced by anticipation in addition to motion perception because the target onset and direction were predictable; therefore, these eye movements might be less beneficial to speed discrimination.
For humans, particularly subjects not trained to maintain fixation, it is difficult not to initiate eye movements in the presence of a dominant moving object in the visual field. Thus, one may argue that subjects who were able to follow the instructions and keep eyes fixated during the motion perception task were able to do so because of poor motion processing ability. We ruled out this possibility because there was no significant correlation between eye movement frequency during speed discrimination testing and initiation latency or acceleration during normal smooth pursuit.
How do eye movements affect motion perception? One possibility is through an efference copy. Eye movements generate a copy of the oculomotor command, sometimes called the efference copy, which contributes to the extraretinal motion signal used for the subsequent pursuit (20,21). The oculomotor command is relayed to the motion sensors via corollary discharge during the late motion processing in the medio-superior temporal cortex (23). The MST integrates the corollary discharge to generate an accurate motion percept (29,38,39). Although the source of the efference copy of the pursuit command is unknown, recent data suggest that the pathway projecting from the superior colliculus to the frontal eye fields mediate efference copy of saccadic eye movements (40). Smooth pursuit eye movement during speed discrimination testing generates an efference copy of the eye movement command, which is used in computing the perceived target speed (29,38,39). Impaired smooth pursuit can lead to impaired analysis of moving objects (41). Conversely, SPEMs improve perception of coherent motions in normal control subjects (42). Pursuit-induced extraretinal signal is hypothesized to be the source of this enhancement (42). Studies have shown that schizophrenia patients and their relatives have a dysfunction in extraretinal motion processing (26,28). The data reported here suggest that a potential mechanism of motion perception deficit in schizophrenia may be secondary to impairments in generating, connecting, and/or integrating SPEM-induced extraretinal motion signals to render accurate motion perception. However, since patients made less eye movements in the 300 msec conditions, another hypothesis is that they might be in part “penalized” for more strictly following the instruction to fixate. This may apply to the current and previous motion perception studies in schizophrenia.
The pattern of speed discrimination impairments, i.e., deficits at 10.0°/sec and 18.7°/sec but not at 3.8°/sec, replicated findings by Chen et al.(2), who showed similar impairment at 10.0°/sec and 16.0°/sec but not at 3.8°/sec stimulus in schizophrenia patients. However, the results of the study challenge the previous conclusion on the speed discrimination deficit leading to SPEM problems in schizophrenia. The presentations of the motion stimuli were 200 msec to 250 msec (43) to 300 msec to 4000 msec (1,2,4) in previous studies, durations that are long enough to allow SPEM. Most of the previous studies also found a correlation between direction or speed discrimination thresholds and pursuit gain only in schizophrenia patients (1-4). Our experiment replicated the correlation between initiation acceleration and speed discrimination only in schizophrenia patients. In previous studies, the significant correlations were deemed as supportive evidence for the conclusion that the motion perception deficit leads to a SPEM deficit in schizophrenia. However, there are some problems with this interpretation. Impaired motion perception does not necessarily lead to abnormal smooth pursuit (31). Data presented here suggest that eye movements may also affect motion perception: when eye movements were limited, there were no between-group differences in speed discrimination. Several studies have shown a lack of significant impairment in schizophrenia patients in the pursuit initiation response (10,14-18,44). Both motion perception and SPEM impairments were observed at a later time course in the respective tasks, when performance could be affected by eye-tracking induced motor commands.
We also observed that patients showed reduced closed loop gain compared with control subjects, significant at 18.7°/sec, and a correlation between speed discrimination and closed loop pursuit gain at 300 msec (18.7°/sec) but not at 150 msec presentation. This correlation by itself does not imply a causal relationship: it can imply motion perception effects on pursuit or the opposite. However, the combined observations of unimpaired motion perception in 150 msec and impaired closed loop eye tracking in schizophrenia patients, the lack of significant correlation between motion perception and pursuit gain when eye movements are minimized (at 150 msec), and a significant correlation emerging when eye movements are often (at 300 msec) do not support that impaired motion perception causes eye-tracking abnormality but provides additional support to the findings of the primary analysis that occurrence of eye movements may determine patient-control subject difference in motion perception.
Self-generated actions can potentially distort the perception of the outside world, if the reafferent stimuli are left unchecked. Normally, this extraretinal motion information due to self-generated action modulates sensory processing to maintain high fidelity of the perceptual experience. Schizophrenia patients showed significantly impaired extraretinal motion processing (26-28). In the presence of impaired processing of this extraretinal information, one would expect distorted perception. Data reported here suggest that abnormal motion perception observed in schizophrenia may not primarily be a retinal motion processing problem, nor a problem of the eye movement itself, but a result of impaired processing or integration of oculomotor feedback signals into a motion percept.
Acknowledgments
Support was received from National Institute of Mental Health (NIMH) Grants MH-67014, MH-77852, MH-68580, MH-79172, and MH-70644; General Clinical Research Center Grant M01-RR16500; and the VA Capitol Health Care Network (VISN 5) Mental Illness Research, Education, and Clinical Center (MIRECC).
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci U S A. 1999;96:4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 4.Slaghuis WL, Holthouse T, Hawkes A, Bruno R. Eye movement and visual motion perception in schizophrenia II: Global coherent motion as a function of target velocity and stimulus density. Exp Brain Res. 2007;182:415–426. doi: 10.1007/s00221-007-1003-3. [DOI] [PubMed] [Google Scholar]
- 5.Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: Abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31:79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 6.Clementz BA, Reid SA, McDowell JE, Cadenhead KS. Abnormality of smooth pursuit eye movement initiation: Specificity to the schizophrenia spectrum? Psychophysiology. 1995;32:130–134. doi: 10.1111/j.1469-8986.1995.tb03304.x. [DOI] [PubMed] [Google Scholar]
- 7.Farber RH, Clementz BA, Swerdlow NR. Characteristics of open- and closed-loop smooth pursuit responses among obsessive-compulsive disorder, schizophrenia, and nonpsychiatric individuals. Psychophysiology. 1997;34:157–162. doi: 10.1111/j.1469-8986.1997.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 8.Radant AD, Claypoole K, Wingerson DK, Cowley DS, Roy-Byrne PP. Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol Psychiatry. 1997;42:797–805. doi: 10.1016/s0006-3223(96)00464-7. [DOI] [PubMed] [Google Scholar]
- 9.Ross RG, Hommer D, Radant A, Roath M, Freedman R. Early expression of smooth-pursuit eye movement abnormalities in children of schizophrenic parents. J Am Acad Child Adolesc Psychiatry. 1996;35:941–949. doi: 10.1097/00004583-199607000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, et al. Eye tracking abnormalities in schizophrenia: Evidence for dysfunction in the frontal eye fields. Biol Psychiatry. 1998;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 11.Hong LE, Avila M, Adami H, Elliott A, Thaker GK. Components of the smooth pursuit function in deficit and nondeficit schizophrenia. Schizophr Res. 2003;63:39–48. doi: 10.1016/s0920-9964(02)00388-2. [DOI] [PubMed] [Google Scholar]
- 12.Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris EJ, Lisberger SG. Different responses to small visual errors during initiation and maintenance of smooth-pursuit eye movements in monkeys. J Neurophysiol. 1987;58:1351–1369. doi: 10.1152/jn.1987.58.6.1351. [DOI] [PubMed] [Google Scholar]
- 14.Gellman RS, Carl JR. Motion processing for saccadic eye movements in humans. Exp Brain Res. 1991;84:660–667. doi: 10.1007/BF00230979. [DOI] [PubMed] [Google Scholar]
- 15.Clementz BA, Reid SA, McDowell JE, Cadenhead KS. Abnormality of smooth pursuit eye movement initiation: Specificity to the schizophrenia spectrum? Saccades to moving targets in schizophrenia: Evidence for normal posterior cortex functioning. Psychophysiology. 1996;33:650–654. [Google Scholar]
- 16.Keller E, Johnsen SD. Velocity prediction in corrective saccades during smooth-pursuit eye movements in monkey. Exp Brain Res. 1990;80:525–531. doi: 10.1007/BF00227993. [DOI] [PubMed] [Google Scholar]
- 17.Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biol Psychiatry. 2005;57:145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Avila MT, Hong LE, Moates A, Turano KA, Thaker GK. Role of anticipation in schizophrenia-related pursuit initiation deficits. J Neurophysiol. 2006;95:593–601. doi: 10.1152/jn.00369.2005. [DOI] [PubMed] [Google Scholar]
- 19.Hong LE, Avila MT, Thaker GK. Response to unexpected target changes during sustained visual tracking in schizophrenic patients. Exp Brain Res. 2005;165:125–131. doi: 10.1007/s00221-005-2276-z. [DOI] [PubMed] [Google Scholar]
- 20.von Helmholtz H. Treatise on Physiological Optics. New York: Dover; 1962. [Google Scholar]
- 21.Haarmeier T, Thier P, Repnow M, Petersen D. False perception of motion in a patient who cannot compensate for eye movements. Nature. 1997;389:849–852. doi: 10.1038/39872. [DOI] [PubMed] [Google Scholar]
- 22.Murphy BJ, Kowler E, Steinman RM. Slow oculomotor control in the presence of moving backgrounds. Vision Res. 1975;15:1263–1268. doi: 10.1016/0042-6989(75)90172-8. [DOI] [PubMed] [Google Scholar]
- 23.Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 24.Poulet JF, Hedwig B. The cellular basis of a corollary discharge. Science. 2006;311:518–522. doi: 10.1126/science.1120847. [DOI] [PubMed] [Google Scholar]
- 25.Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci. 2007;30:14–21. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, et al. Smooth pursuit eye movements to extraretinal motion signals: Deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 27.Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR. Smooth pursuit eye movements to extraretinal motion signals: Deficits in patients with schizophrenia. Psychiatry Res. 1999;88:209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 28.Hong LE, Turano KA, O’neill H, Hao L, Wonodi I, McMahon RP, et al. Refining the predictive pursuit endophenotype in schizophrenia. Biol Psychiatry. 2008;63:458–464. doi: 10.1016/j.biopsych.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turano KA, Massof RW. Nonlinear contribution of eye velocity to motion perception. Vision Res. 2001;41:385–395. doi: 10.1016/s0042-6989(00)00255-8. [DOI] [PubMed] [Google Scholar]
- 30.Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: Neuronal mechanisms and pathways. Neurosci Res. 1991;11:79–107. doi: 10.1016/0168-0102(91)90048-4. [DOI] [PubMed] [Google Scholar]
- 31.Barton JJ, Simpson T, Kiriakopoulos E, Stewart C, Crawley A, Guthrie B, et al. Functional MRI of lateral occipitotemporal cortex during pursuit and motion perception. Ann Neurol. 1996;40:387–398. doi: 10.1002/ana.410400308. [DOI] [PubMed] [Google Scholar]
- 32.Culham J, He S, Dukelow S, Verstraten FA. Visual motion and the human brain: What has neuroimaging told us? Acta Psychol (Amst) 2001;107:69–94. doi: 10.1016/s0001-6918(01)00022-1. [DOI] [PubMed] [Google Scholar]
- 33.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrenia. Biol Psychiatry. 2004;55:834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Pentland A. Maximum likelihood estimation: The best PEST. Percept Psychophys. 1980;28:377–379. doi: 10.3758/bf03204398. [DOI] [PubMed] [Google Scholar]
- 36.Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63:1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- 37.Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman TC, Banks MS. Perceived head-centric speed is affected by both extra-retinal and retinal errors. Vision Res. 1998;38:941–945. doi: 10.1016/s0042-6989(97)00395-7. [DOI] [PubMed] [Google Scholar]
- 39.Turano KA, Heidenreich SM. Eye movements affect the perceived speed of visual motion. Vision Res. 1999;39:1177–1187. doi: 10.1016/s0042-6989(98)00174-6. [DOI] [PubMed] [Google Scholar]
- 40.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 41.Haarmeier T, Thier P. Impaired analysis of moving objects due to deficient smooth pursuit eye movements. Brain. 1999;122(Pt 8):1495–1505. doi: 10.1093/brain/122.8.1495. [DOI] [PubMed] [Google Scholar]
- 42.Greenlee MW, Schira MM, Kimmig H. Coherent motion pops out during smooth pursuit. Neuroreport. 2002;13:1313–1316. doi: 10.1097/00001756-200207190-00020. [DOI] [PubMed] [Google Scholar]
- 43.Clementz BA, McDowell JE, Dobkins KR. Compromised speed discrimination among schizophrenia patients when viewing smooth pursuit targets. Schizophr Res. 2007;95:61–64. doi: 10.1016/j.schres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CE, Thaker GK, Ross DE, Moran MJ. Accuracies of saccades to moving targets during pursuit initiation and maintenance. Exp Brain Res. 1997;113:371–377. doi: 10.1007/BF02450336. [DOI] [PubMed] [Google Scholar]


