Abstract
Amblyopia is the most common cause of monocular visual loss in children, affecting 1.3% to 3.6% of children. Current treatments are effective in reducing the visual acuity deficit but many amblyopic individuals are left with residual visual acuity deficits, ocular motor abnormalities, deficient fine motor skills, and risk for recurrent amblyopia. Using a combination of psychophysical, electrophysiological, imaging, risk factor analysis, and fine motor skill assessment, the primary role of binocular dysfunction in the genesis of amblyopia and the constellation of visual and motor deficits that accompany the visual acuity deficit has been identified. These findings motivated us to evaluate a new, binocular approach to amblyopia treatment with the goals of reducing or eliminating residual and recurrent amblyopia and of improving the deficient ocular motor function and fine motor skills that accompany amblyopia.
Keywords: amblyopia, binocular vision, stereoacuity, treatment, anisometropia, strabismus
1. Introduction
1.1 Amblyopia
Amblyopia is diminished vision that results from inadequate visual experience during the first years of life. Typically, amblyopia is clinically defined as reduced visual acuity accompanied by one or more known amblyogenic factors, such as strabismus, anisometropia, high refractive error, and cataract. Amblyogenic factors interfere with normal development of the visual pathways during a critical period of maturation. The result is structural and functional impairment of the visual cortex, and impaired form vision.
Although amblyopia can be bilateral, it most commonly affects one eye of children with strabismus, anisometropia, or both. The prevalence of strabismus, anisometropia, and amblyopia has been reported in a number of recent population-based studies of preschool children and school children in the United States, the United Kingdom, Netherlands, Sweden, and Australia (Table 1).
Table 1.
Prevalence of strabismus, anisometropia, and amblyopia in recent (2001–2012) population-based studies of children.
| Site | N | Age | Strabismus | Anisometropia | Amblyopia | |
|---|---|---|---|---|---|---|
| BPEDS (Friedman et al., 2009; Giordano et al., 2009) | US | 2546 | 0.5–6 yr | 2.2% | 4.5% | 1.3% |
| MEPEDS (Multi-Ethnic Pediatric Eye Disease Study, 2008) | US | 6014 | 0.5–6 yr | 2.4% | 4.1% | 2.0% |
| NICHS (Donnelly et al., 2005) | UK | 1582 | 8–9 yr | 4.0% | - | 2.0% |
| ALSPAC (Williams et al., 2008) | UK | 7825 | 7 yr | 2.3% | - | 3.6% |
| RAMSES (Groenewoud et al., 2010) | N | 2964 | 7 yr | - | - | 3.4% |
| SCHC (Kvarnstrom et al., 2001) | S | 3126 | 10 yr | 3.1% | - | |
| SPEDS (Pai et al., 2012) | A | 1422 | 2.5–6 yr | - | - | 1.9% |
| SMS (Huynh et al., 2006; Robaei et al., 2006) | A | 1765 | 5–8 yr | 2.8% | 2.6% | 1.8% |
| MEAN | 2.8% | 3.8% | 2.4% |
US = United States, UK = United Kingdom, N=Netherlands, S=Sweden, A=Australia
Overall, results of the studies are consistent, with a mean prevalence of 2.8% for strabismus, 3.5% for anisometropia, and 2.4% for amblyopia. With 625 million children under the age of 5 years worldwide, more than 15 million may have amblyopia, and more than half of them will not be identified before they reach school age. (Wu and Hunter, 2006) Many affected children will suffer irreversible vision loss that could have been prevented. The consequences of not identifying and treating strabismus and amblyopia early include permanent visual impairment, adverse effects on school performance, poor fine motor skills, social interactions, and self-image.(Choong et al., 2004; Chua and Mitchell, 2004; Horwood et al., 2005; O’Connor et al., 2010b; O’Connor et al., 2009; Packwood et al., 1999; Rahi et al., 2006; Webber et al., 2008a, b) Importantly, permanent monocular visual impairment due to amblyopia is a risk factor for total blindness if the better eye is injured or if the fellow eye is affected by disease later in life.(Harrad and Williams, 2003)
The predominant theory is that amblyopia results when there is a mismatch between the images to each eye; one eye is favored while the information from the other eye is suppressed.(Harrad et al., 1996) Because amblyopia typically affects visual acuity in one eye, amblyopia is often considered to be a monocular disease. Thus, the mainstay of treatment for amblyopia has been patching of the normal fellow eye to improve the monocular function of the amblyopic eye. However, there are significant shortcomings to this approach to understanding and treating amblyopia. Our research studies on amblyopia have revealed that binocular dysfunction plays an important role in amblyopia and has laid the groundwork for a new approach for the development of more effective treatment.
1.2 Clinical Profile of Amblyopia
Two large studies have defined the clinical profile of amblyopic children.(Birch and Holmes, 2010; Pediatric Eye Disease Investigator Group, 2002a) Both studies included children with strabismic, anisometropic, or combined-mechanism amblyopia referred by multiple community- and university-based pediatric ophthalmologists. In the Pediatric Eye Disease Investigator Group (PEDIG) study, we assessed the clinical characteristics of 409 children age 3–6.9 years with moderate amblyopia who were enrolled in a multicenter randomized study of amblyopia treatment.(Pediatric Eye Disease Investigator Group, 2002a) Strabismus or anisometropia each accounted for about 40% of amblyopia, with just over 20% of amblyopia in children with both strabismus and anisometropia (Figure 1). Overall, mean refractive error in the amblyopic eye was +4.52 D and in the fellow eye was +2.83D, but fellow eye refractive error was higher in the strabismic children (+3.54 D) and lower in the anisometropic children (+1.95 D).
Figure 1.
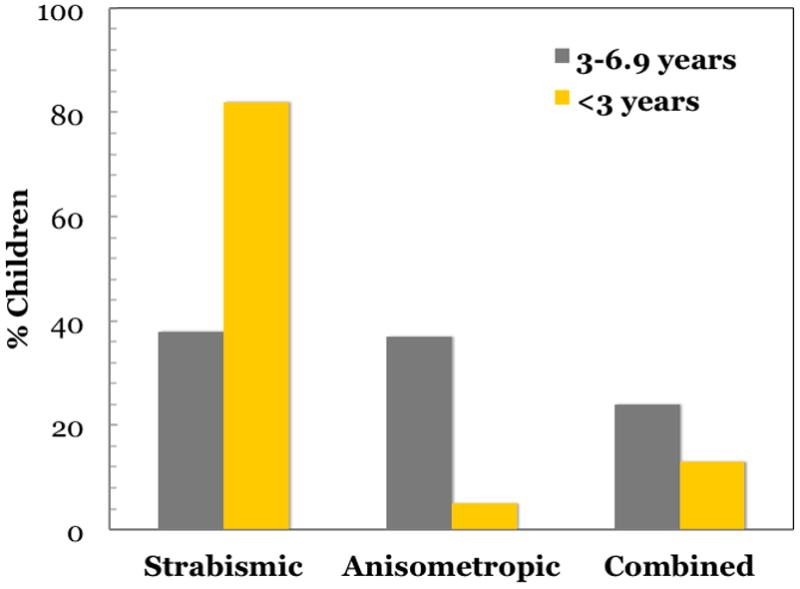
In the<3-year-old cohort,(Birch and Holmes, 2010) 82% of amblyopia was associated with strabismus, 5% with anisometropia, and 13% with combined mechanisms. This finding is strikingly different from the PEDIG 3- to 6-year-old cohort,(Pediatric Eye Disease Investigator Group, 2002a) where only 38% of amblyopia was associated with strabismus, 37% with anisometropia, and 24% with combined mechanism (p<0.001 for each of the 3 paired comparisons). Among children with strabismus in the <3-year-old cohort, 62% had infantile esotropia (onset ≤6 months of age), 22% had accommodative esotropia, 10% had acquired nonaccommodative esotropia (onset ≥7 months of age), and 6% had other types of strabismus.
In a second study, we examined the clinical profile of 250 amblyopic children less than 3 years old in the Dallas area (Birch and Holmes, 2010); 82% of amblyopia was associated with strabismus, 5% with anisometropia, and 13% with both (Figure 1), a strikingly different distribution of amblyogenic factors compared with the older cohort. Mean refractive error in the amblyopic eye was +2.63 D, substantially lower than in the older cohort. Fellow eye refractive error averaged +2.60 D, similar to the overall mean for the older cohort and, as with the older cohort, fellow eye refractive error was higher in the strabismic children (+2.59 D) and lower in the anisometropic children (+0.94D). Our studies, along with two additional studies from the UK (Shaw et al., 1988; Woodruff et al., 1994b), suggest that the factors responsible for amblyopia vary with age (Figure 2). Strabismus is the overwhelmingly the factor most associated with amblyopia during the first year. Anisometropia, either alone or in combination with strabismus, becomes equally prominent as a cause of amblyopia by the third year and, by the fifth year, is the causative factor in nearly two-thirds of amblyopic children.
Figure 2.
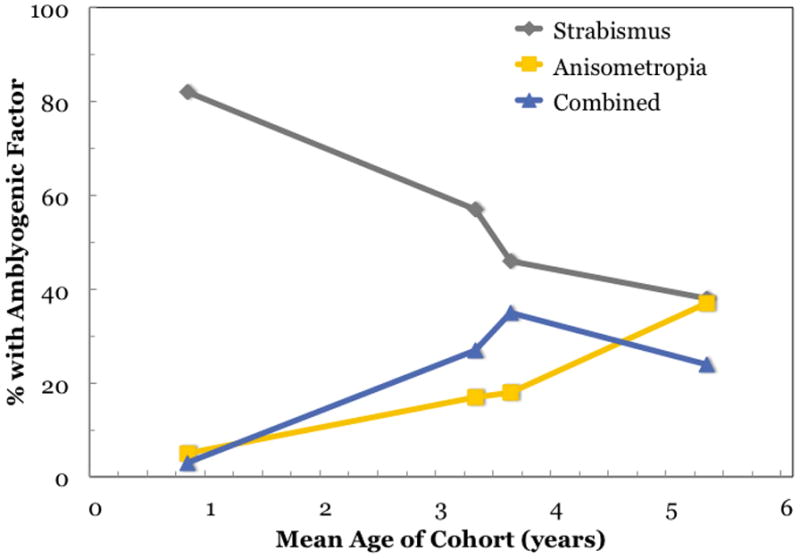
Strabismic amblyopia was diagnosed much more commonly than anisometropic or combined-mechanism amblyopia in children<3 years of age.(Birch and Holmes, 2010) This finding is in sharp contrast to the PEDIG report of approximately equal proportionsof patients with amblyopia attributable to strabismus and anisometropia, and approximately a quarter of the amblyopic patients exhibiting both.(Pediatric Eye Disease Investigator Group, 2002a) Two UK studies of amblyopic children, which included younger children than the PEDIG study, found the cause to be strabismus in a greater percentage than the PEDIG study (45%–57%), a lower percentage to be anisometropic (17%), and about the same percentage to be combined mechanism (27%–35%).(Shaw et al., 1988; Woodruff et al., 1994b) This summary of all 4 studies suggests that one source of the differences in the proportion of amblyopia attributable to strabismus may be age.
The low percentage of amblyopia attributable to anisometropia in the<3 year cohort is unlikely to be the result of marked under-referral of anisometropia in this age range. Overall, in the parent study from which the amblyopic children <3 years old were drawn, only 18% of the 67 of anisometropic children were diagnosed with amblyopia.(Birch and Holmes, 2010) In contrast, almost 50% of the 396 children with strabismus or strabismus with anisometropia were diagnosed with amblyopia. This suggests that anisometropia may develop later, and become an etiologic factor for amblyopia primarily after 3 years of age. Another alternative is that anisometropia may be present early but requires a longer duration than strabismus to cause amblyopia.
1.3 Amblyopia Treatment
Current treatments for amblyopia rely on depriving the healthy, fellow eye of vision to force use of the amblyopic eye. The assumption is that the primary deficit in amblyopia is a monocular visual acuity deficit caused by preference for fixation by the fellow eye. By depriving the fellow eye of vision, suppression of the amblyopic eye is eliminated and visual experience will promote development or recovery of visual acuity. Patching, atropine, and filters that penalize the fellow eye have been used to treat amblyopia for hundreds of years.(Loudon and Simonsz, 2005) Only in the last 15 years have randomized clinical trials been conducted to evaluate the effectiveness of amblyopia treatment and to begin to define optimal treatment protocols. The Pediatric Eye Disease Investigator Group (PEDIG) has conducted a series of randomized clinical trials of amblyopic treatment for children ages 3–17 years old. To date, our major results are:
A period of 16–22 weeks of treatment with optical correction alone leads to an improvement of ≥0.2 logMAR (2 lines) in both children with anisometropic amblyopia (Cotter et al., 2006) and children strabismic or combined mechanism amblyopia. (Cotter et al., 2012) In nearly one-third of amblyopic children treated with optical correction, amblyopia completed resolved. Similar results have been reported by the Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Cooperative Group, and the Randomized Occlusion Treatment of Amblyopia (ROTAS) Cooperative Group studies. (Stewart et al., 2011) Thus, refractive correction as the sole initial treatment for amblyopia is a viable option.
Patching is an effective treatment for amblyopia. After stable visual acuity was achieved with spectacle wear, 3- to 7-year-old amblyopic children who were randomized to patching treatment had further visual acuity improvement of 0.22 logMAR (2.2 lines).(Wallace et al., 2006)
For moderate amblyopia, a prescribed patching dose of 2 hr/day results in the same visual acuity outcome as a prescribed patching dose of 6 hr/day(Repka et al., 2003) and, for severe amblyopia, 6 hr/day yields similar results to 12 hr/day.(Holmes et al., 2003) Objective monitoring of compliance with prescribed patching in a similar UK study suggests that the similar visual acuity outcomes may have been due to lack of compliance with the higher prescribed doses; i.e., the two treatment groups may have actually received similar doses despite assignment to different doses.(Stewart et al., 2007b)
Atropine and patching result in similar improvements in visual acuity when used as the initial treatment of moderate amblyopia in children aged 3 - 6 years. (Pediatric Eye Disease Investigator Group, 2002b) Most children had ≥0.3 logMAR (3 lines) improvement in visual acuity and about 75% achieved 20/30 or better visual acuity within 6 months. (Pediatric Eye Disease Investigator Group, 2002b)
Among children with severe amblyopia (>0.7 logMAR), treatment on weekends with atropine led to an average improvement of 0.45 logMAR (4.5 lines). (Repka et al., 2009)
Treatment of amblyopia can be effective beyond 7 years of age, although the rate of response to treatment may be slower, may require a higher dose of patching, and the extent of recovery may be less complete.(Holmes et al., 2011; Pediatric Eye Disease Investigator Group, 2003, 2004)
The PEDIG results, along with results from MOTAS and ROTAS are summarized in Figure 3.
Figure 3.
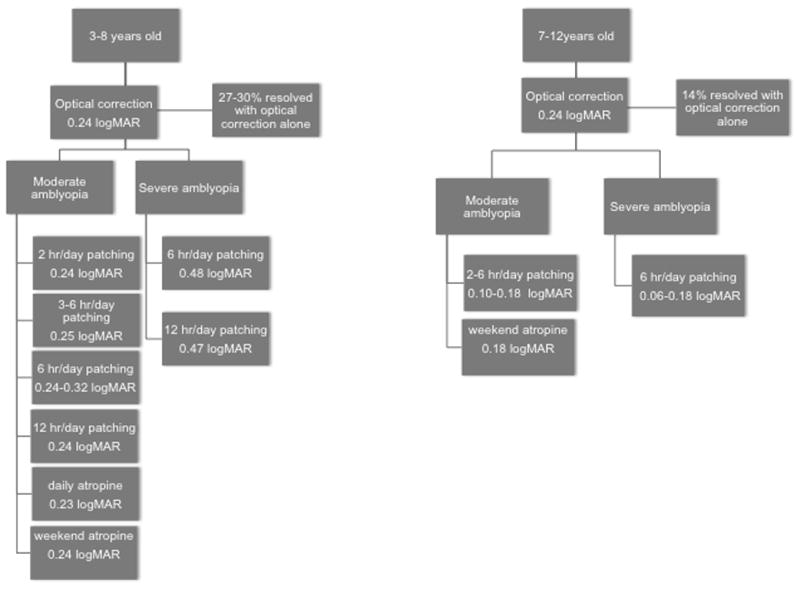
Summary of results from recent randomized clinical trials for the treatment of amblyopia conducted by the Pediatric Eye Disease Investigator Group (PEDIG) (Holmes et al., 2006; Quinn et al., 2004; Repka and Holmes, 2012), the Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Cooperative Group, (Stewart et al., 2002, 2005; Stewart et al., 2004a; Stewart et al., 2004b; Stewart et al., 2007a) and the Randomized Occlusion Treatment of Amblyopia (ROTAS) Cooperative Group.(Stewart et al., 2007b) Values represent mean improvement of visual acuity (logMAR) with 3–6 months of treatment. When more than one randomized clinical trial evaluated similar treatment plans, the range of means reported in the various studies is provided.
1.4 Failures of Amblyopia Treatment
Not all amblyopic children achieve normal visual acuity despite treatment. Although 73–90% have improvements in visual acuity with various treatment modalities alone or in combination,(Repka et al., 2003; Repka et al., 2004; Repka et al., 2005; Rutstein et al., 2010; Stewart et al., 2004b; Wallace et al., 2006) 15–50% fail to achieve normal visual acuity even after extended periods of treatment. (Birch and Stager, 2006; Birch et al., 2004; Repka et al., 2003; Repka et al., 2004; Repka et al., 2005; Rutstein et al., 2010; Stewart et al., 2004b; Wallace et al., 2006; Woodruff et al., 1994a) One possible explanation for the failure to achieve normal visual acuity is that the treatment was started too late.
Both experimental and clinical data support the hypothesis that there is an early sensitive period during which strabismus and anisometropia can lead to abnormal visual development, conventionally considered to be the first 7 years of life. As a consequence, there is also a widely held assumption that treatment during this early period is critical for obtaining optimal visual acuity outcome. A recent meta-analysis of 4 multi-center amblyopia treatment studies (Holmes et al., 2011) reported that 7- to 13-year-old children were significantly less responsive to amblyopia treatment than children less than 7 years old. However, the meta-analysis failed to find a significant difference in treatment response between the 3- and 4-year olds and the 5- and 6-years olds. On the one hand, these results appear to support the concept of a critical period for treatment that coincides with the critical period for visual development. On the other hand, poor response to treatment among older children could simply reflect poor compliance with treatment; none of these treatment studies included an objective measure of compliance with patching and glasses. In fact, we have observed approximately the same prevalence of unresolved amblyopia in children whose amblyopia treatment was initiated during the first year of life (Birch and Stager, 2006; Birch et al., 1990; Birch et al., 2004) as we and others have observed when treatment is not initiated until 3–6 years of age. (Birch and Stager, 2006; Birch et al., 2004; Repka et al., 2003; Repka et al., 2004; Repka et al., 2005; Rutstein et al., 2010; Stewart et al., 2004b; Wallace et al., 2006; Woodruff et al., 1994a) This suggests that delay in treatment is not the sole reason for failure to recover normal visual acuity.
An alternative explanation for failure to achieve normal visual acuity is lack of compliance with treatment. Using objective occlusion dose monitoring devices, it has been demonstrated that compliance varies widely among children treated for amblyopia and that visual acuity outcome is related to compliance. (Loudon et al., 2002, 2003; Stewart et al., 2004b; Stewart et al., 2007a, b) Randomized clinical trials have supported the use of educational and motivational material for the children and the parents to improve compliance.(Loudon et al., 2006; Tjiam et al., 2012) However, none of these studies has yet demonstrated that improved compliance reduces the proportion of children who fail to achieve normal visual acuity with amblyopia treatment.
A third possible reason for failure to achieve normal visual acuity with standard treatments for amblyopia is that current treatments are inadequate. In other words, perhaps more intensive treatment is needed as a “final push” to overcome residual amblyopia. Recently, the Pediatric Eye Disease Investigator Group conducted a multi-center randomized clinical trial for children with residual amblyopia do 0.2–0.5 logMAR who had stopped improving with 6 hours of prescribed daily patching or daily atropine.(Wallace et al., 2011a) We found that an intensive combined treatment of patching and atropine did not result in better visual acuity outcomes after 10 weeks compared with a control group in which treatment was gradually discontinued.
Finally, it has been suggested that children who fail to recover normal visual acuity with standard amblyopia treatments may have subtle retinal, optic nerve, or gaze control abnormalities that limit their potential for visual acuity recovery. (Giaschi et al., 1992; Lempert, 2000, 2003, 2004, 2008a, b) Abnormal macular thickness (Al-Haddad et al., 2011; Altintas et al., 2005; Dickmann et al., 2009; Huynh et al., 2009; Walker et al., 2011), optic disc dysversion (Lempert and Porter, 1998), optic nerve hypoplasia (Lempert, 2000), and gaze instability (Regan et al., 1992) have all been described in subsets of patients with amblyopia. To evaluate whether changes in the retina, optic nerve, or gaze control may limit some children’s response to amblyopia treatment, we evaluated each of these aspects of the visual system in 26 children with persistent residual strabismic, anisometropic, or combined mechanism amblyopia. We compared the retina, optic nerve, and gaze control characteristics of their amblyopic eyes with those of the fellow eyes, with age-matched nonamblyopic children who had strabismus or anisometropia (n-12), and with age-matched normal controls (n=48). (Subramanian et al., in press) All amblyopic children had been treated with glasses, patching, and/or atropine for 0.8–5 years, had a residual visual acuity deficit, and no improvement in visual acuity despite treatment and excellent compliance for at least 6 months prior to enrollment.
Images of the macula were obtained with the Spectralis (Heidelberg Engineering) spectral domain OCT (SD-OCT) using the eye-tracking feature (ART). Each child had one to three line scans centered on the optic disc to measure horizontal and vertical disc diameter, and one to three 3mm high-resolution peripapillary RNFL circular scans, and one to three high-resolution macular volume scans. Optic disc dysversion (tilt) was quantified by calculating the ratio of vertical to horizontal disc diameters. Optic nerve hypoplasia was quantified by calculating the area of the ellipse specified by the vertical and horizontal diameters. In order to assess possible sectoral hypoplasia,(Lee and Kee, 2009) RNFL thickness was automatically segmented using the Spectralis software that provided average thickness measurements for each of 6 RNFL sectors centered on the optic disc [temporal superior (TS), temporal (T), temporal inferior (TI), nasal inferior (NI), nasal (N), nasal superior (NS)] together with a global average (G). Total macular thickness and sectional volumes were automatically determined by software provided by Heidelberg Engineering for the Spectralis SD-OCT, using a modified ETDRS circle grid (center, middle and outer rings: 1, 2, and 3 mm). To assess gaze control, eye movements during attempted steady fixation in primary gaze (binocular and monocular) were recorded using an infrared eye tracker.
As shown in Figure 4, no abnormalities were apparent in macular structure, and there were no significant differences in macular thickness between the amblyopic and fellow eyes, nonamblyopic eyes, or normal control eyes. Nor were there significant differences among these groups in optic disc diameter or shape (dysversion), or in peripapillary RNFL thickness (Figure 4). Children with persistent amblyopia had eye movement abnormalities in both amblyopic and fellow eyes, including infantile nystagmus (13%), fusion maldevelopment nystagmus (47.8%), and saccadic oscillations (39.1%). Some non-amblyopic eyes of children with strabismus or anisometropia also had saccadic oscillations (44.4%) or fusion maldevelopment nystagmus (11.1%). Normal controls showed no gaze abnormalities. Thus, retinal and optic nerve abnormalities cannot explain persistent amblyopia. Gaze instabilities are typically bilateral, but may be a plausible explanation of persistent amblyopia in a subset of children who have asymmetric gaze instability (more instability in the amblyopic eye).
Figure 4.
Spectral domain optical coherence tomography (SD-OCT) scans of the amblyopic (top left) and non-amblyopic (top right) eyes of a child with persistent amblyopia.(Subramanian et al., in press) Overall (n=26), there were no significant differences in total macular thickness or in any of the ETDRS macular sectors (second panel) between amblyopic and fellow eyes (p>0.2 for all paired t-tests.) The third panel shows a sample SD-OCT scan of the RNFL obtained by SD-OCT and means for each RNFL sector; there were no significant differences in global RFNL thickness nor in any sector between amblyopic and fellow eyes (p>0.4 for all paired t-tests). The bottom panel illustrates the method used to quantify optic disc dysversion (Horiz:Vert disc diameter ratio). No significant differences were found for amblyopic vs. fellow eyes (H/Vambly= 1.15 ±0.18; H/Vfellow= 1.14±0.17; p=0.87). Hypoplasia (assessed by area of the optic disc, mm2) did not differ for amblyopic vs. fellow eyes (Areaambly= 1.88±0.44; Areafellow= 2.10±0.61; p=0.26).
Even among the 50–85% of children who do achieve normal visual acuity with amblyopia treatment, the risk for recurrence of amblyopia is high. In a series of prospective, longitudinal studies of infantile and accommodative esotropia conducted in our laboratory over the past 22 years,(Birch, 2003; Birch et al., 2005; Birch and Stager, 2006; Birch et al., 1990; Birch et al., 2004; Birch et al., 2010) 80% of esotropic children were treated for amblyopia at least once during the first 5 years of life; ≥ 60% had recurrent amblyopia that required retreatment during the 5-year follow-up. PEDIG reported that 25% of successfully treated amblyopic children experience a recurrence within the first year off treatment.(Holmes et al., 2004)
1.5 Insights from Treatment Failures
Residual and recurrent amblyopia remain unexplained. The studies reviewed in Sections 1.3 and 1.4 all have one important feature in common; amblyopia treatment was monocular. Yet the predominant theory is that amblyopia arises when there is a mismatch between the images to each eye. In other words, abnormal binocular experience is the primary cause. This prompted us to investigate the link between amblyopia and binocular function from a number of different approaches. Our investigations have evaluated the role of binocular dysfunction in the disproportionate loss of optotype visual acuity in strabismic amblyopia (Section 2.1), in the risk for persistent, residual amblyopia (Section 2.2), in asymmetric fusional suppression (Section 2.3), in the gaze instabilities associated with amblyopia (Section 2.4, and in the fine motor skill deficits that are present in amblyopic children and adults (Section 2.5). As evidence about the primary role of binocular dysfunction in amblyopia has accumulated, we have worked to develop approaches to amblyopia treatment (Section 3).
2. Amblyopia and Binocular Vision
2.1 Stereoacuity and Amblyopia
Strabismic and anisometropic amblyopia differ in the spectrum of associated visual deficits despite their common effect on visual acuity. Strabismic amblyopia is associated with disproportionately greater deficits in optotype visual acuity and vernier acuity compared with grating acuity, but anisometropic amblyopia is associated with proportional deficits in optotype, vernier, and grating acuity. (Levi and Klein, 1982; Levi and Klein, 1985) There are two hypotheses regarding the source of differences in the pattern of visual deficits between anisometropic and strabismic amblyopia. First, the differences may reflect fundamentally different pathophysiological processes (etiology hypothesis).(Levi, 1990; Levi and Carkeet, 1993) For example, sparse/irregular sampling may be associated with binocular competition between two discordant images in strabismus but not between the sharp versus defocused images in anisometropia. The second hypothesis is that the different constellations of spatial deficits in anisometropic and strabismic amblyopia reflect the degree of visual maturation present at the onset of amblyopia (effective age hypothesis);(Levi, 1990; Levi and Carkeet, 1993) that is, anisometropic amblyopia may arise at an age where visual maturation is more complete. The low prevalence of anisometropic amblyopic in children <3 years old (Section 1.2) is consistent with the effective age hypothesis. The effective age hypothesis predicts that visual functions that mature earliest will be less susceptible to disruption by abnormal visual experience. Much of the data from adult with amblyopia is consistent with both of these ideas but direct tests of the alternative hypotheses were not possible in adults because complete clinical histories are rarely available to precisely document etiology and the time course over which amblyopia developed.
We designed a study to overcome this limitation by evaluating vernier, resolution and recognition acuities of amblyopic children who were enrolled in a prospective study of visual maturation during infancy and early childhood, i.e. amblyopic children with known age of onset and etiology. (Birch and Swanson, 2000) We found that strabismic amblyopia, whether it had infantile onset or preschool age onset, was associated with disproportionately larger optotype and vernier acuity deficits compared to grating acuity deficits (Figure 5). Moreover, looking only at the subgroup of amblyopic children with infantile onset (Figure 5), we observed differences in the pattern of visual deficits. Children with infantile strabismic amblyopia had disproportionately larger optotype and vernier acuity deficits compared to grating acuity deficits but children with infantile anisometropic amblyopia had proportional deficits in optotype, vernier, and grating acuity. These data support the etiology hypothesis. Infantile anisometropic amblyopia results from blurred vision in one eye during the first years of life; in infant monkeys, experimentally induced blur leads to a selective loss of neurons tuned to high spatial frequencies. (Kiorpes et al., 1998; Kiorpes and McKee, 1999) On the other hand, infantile strabismus disrupts the binocular connections of cortical neurons and can lead to the development of a fixation preference for one eye and a constellation of monocular visual deficits in the non-preferred eye in which optotype and vernier acuity are especially compromised. (Kiorpes et al., 1998; Kiorpes and McKee, 1999) However, it is important to note that anisometropic amblyopia, whose eyes are aligned but who lack central binocular function, resemble those with strabismic amblyopia in their pattern of visual deficits. (Levi et al., 2011) Thus, it appears that the presence or absence of binocular function drives the pattern of visual deficits, not the presence or absence of strabismus.
Figure 5.
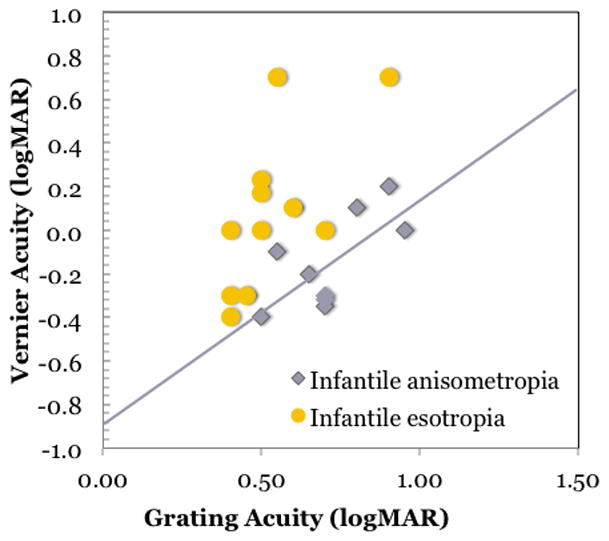
Grating acuity and vernier acuity at 6–9 years of age for 20 amblyopic children enrolled in a prospective study at the time of diagnosis during infancy.(Birch and Swanson, 2000) The solid line shows the predicted relationship if comparable deficits were present for both vernier and grating acuity so that the normal grating:vernier acuity ratio of 8:1 was maintained. Data from amblyopic children with infantile anisometropia fall near the prediction line. Data from amblyopic children with infantile esotropia fall above the prediction line, i.e., there is a disproportionally larger loss in vernier acuity.
We tested the hypothesis that regardless of the etiology of amblyopia, those who have nil stereoacuity experience a disproportionate loss in optotype acuity, and this loss exceeds what is seen in amblyopia with preserved stereopsis.(Bosworth and Birch, 2003) Optotype acuity, Teller grating acuity, and stereoacuity were evaluated in 106 children with moderate amblyopia (0.3 – 0.7 logMAR) due to anisometropia, strabismus, or both. Overall, amblyopic children had 2 times worse optotype acuity than grating acuity. Regardless of age at onset or etiology, amblyopic children with nil stereoacuity had large optotype-grating acuity discrepancies (Figure 6), Our data suggest that the residual monocular sampling mosaic provides adequate information for grating detection but is inadequate to represent the local spatial relationships needed for optotypes. These data support the hypothesized link between impaired binocular function and the neural undersampling/disarray associated with amblyopia.
Figure 6.
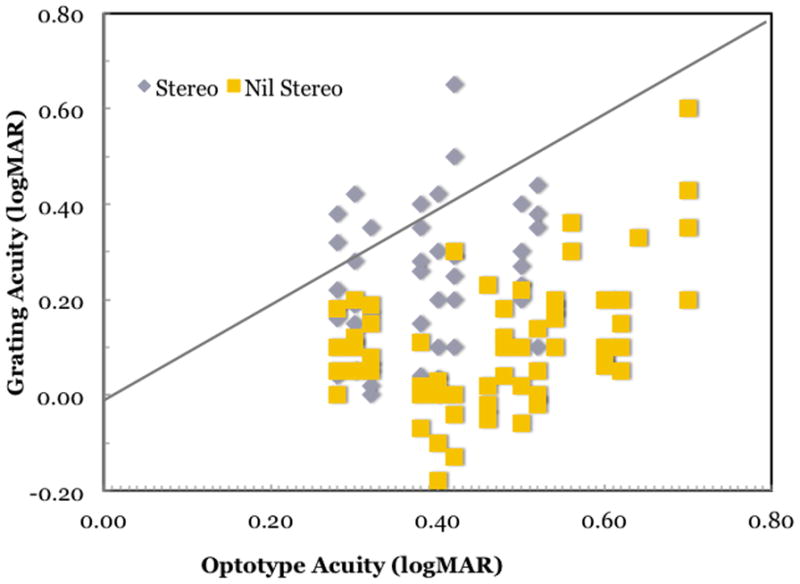
Grating and optotype acuities for 106 children with mild-to-moderate amblyopia.(Bosworth and Birch, 2003) Amblyopic children with preserved stereopsis (grey symbols) fall near the 1:1 line (gray line), consistent with similar loss of grating acuity and optotype acuity. Amblyopic children with nil stereoacuity (yellow symbols) fall to the right of the 1:1 line, consistent with similar greater loss optotype acuity of optotype acuity than grating acuity. The mean optotype grating acuity difference was 0.13 ± 0.13 logMAR for amblyopic children with preserved stereopsis and 0.22 ± 0.16 logMAR for amblyopic children with nil stereoacuity (F1,104 = 10.4; p = 0.0016).
2.2 Risk Factors for Persistent Amblyopia
Independent support for a strong link between binocular dysfunction and amblyopia has come from our studies of persistent residual amblyopia. To evaluate potential risk factors for persistent amblyopia,(Birch et al., 2007) we enrolled 130 consecutive children with infantile esotropia or accommodative esotropia in a prospective, longitudinal study at the time of their initial diagnosis, and conducted regular follow-up visits to assess visual acuity and stereoacuity through visual maturity (mean age = 9.5 years). On the basis of the longitudinal data set, the children were classified as: never amblyopic, recovered (treated for amblyopia and currently 0.1 logMAR or better visual acuity and 0.1 logMAR or less interocular difference in visual acuity), or persistent amblyopic (lengthy and/or repeated attempts to treat amblyopia with one or more treatment modalities but visual acuity remained 0.2 logMAR or poorer and 0.2 logMAR or more interocular difference in visual acuity persisted). Four risk factors for amblyopia were evaluated: age of onset of esotropia, delay in referral to an ophthalmologist, age at surgery, and initial refractive error.
Early onset of esotropia might be a risk factor for persistent amblyopia because it may represent more severe disease (Tychsen, 2005) or because early onset may result in a longer duration of abnormal visual experience (Birch, 2003; Birch et al., 2000; Tychsen, 2005, 2007; Tychsen et al., 2004) The incidence of amblyopia was high in children diagnosed with esotropia; 80% of the children with infantile esotropia and 74% of the children with accommodative esotropia developed amblyopia. In neither the infantile esotropia cohort nor the accommodative esotropia cohort, was there an association between age at onset of esotropia and the prevalence of persistent amblyopia (Figure 7).
Figure 7.
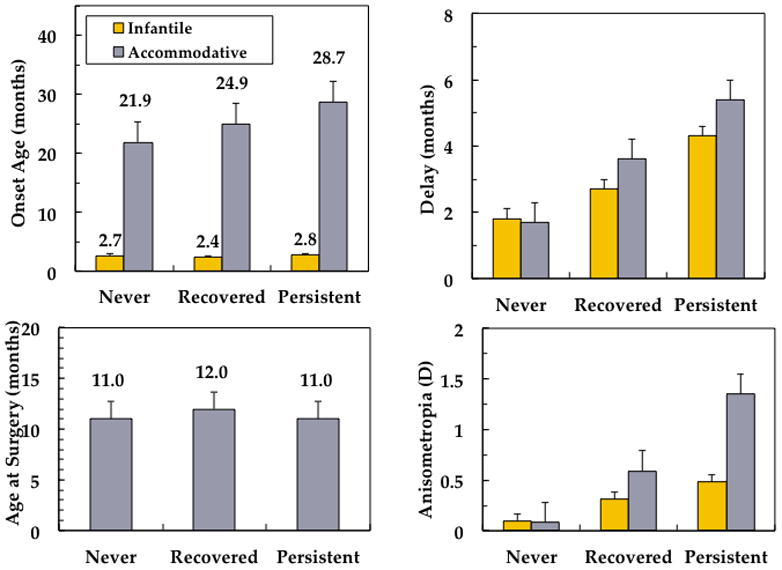
Risk factors for amblyopia in 130 consecutive children with infantile esotropia or accommodative esotropia enrolled in a prospective longitudinal study at the time of initial diagnosis with follow-up to a mean age of 9.5 years.(Birch et al., 2007) No significant differences between nonamblyopic, recovered amblyopic, and persistent amblyopic groups were found for age of onset (upper left) or age at surgery (lower left). Delay in referral for treatment was associated with risk for amblyopia and persistent amblyopia (F = 12.6, p<0.001). Anisometropia ≥1.00 D on the initial visit was also associated with elevated risk for amblyopia and persistent amblyopia (F = 11.1, p=0.002).
Delay in referral was evaluated as a risk factor for persistent amblyopia because it is known that a shorter duration of esotropia optimizes stereoacuity outcomes (Birch, 2003; Birch et al., 2000; Birch et al., 2005; Fawcett et al., 2000; Fawcett and Birch, 2003) and stereopsis may reduce the risk for moderate to severe amblyopia. (Bosworth and Birch, 2003; McKee et al., 2003) Among children who were referred for treatment within 3 months of onset of esotropia, only 12% of the infantile esotropia cohort and 14% of the accommodative esotropia cohort developed persistent amblyopia. Among those who were not referred for treatment for more than 3 months post-onset, 42% of the infantile esotropia cohort and 56% of the accommodative esotropia cohort developed persistent amblyopia; i.e., a 3.5–4 times elevated risk for persistent amblyopia was associated with delayed referral (Figure 7). Within the infantile esotropia cohort, we were also able to evaluate whether age at surgery was a risk factor for persistent amblyopia (surgical treatment in the accommodative esotropia cohort was rare). Very early surgery, by 6 months of age, may preserve stereopsis in children with infantile esotropia. (Birch et al., 1998; Birch et al., 2000; Ing, 1991; Ing and Okino, 2002; Wright et al., 1994) Nonetheless, we found no significant differences in age at surgery among those who never had amblyopia, those who recovered, and those who developed persistent amblyopia (Figure 7).
Initial refractive error was evaluated as a risk factor for persistent amblyopia because amblyopia may be more prevalent in children with moderate to high hyperopia (American Academy of Ophthalmology, 2011) and because spherical anisometropia of 1.00 D or more had been shown previously to be associated with increased risk for amblyopia in hyperopic children with no strabismus (Weakley, 1999, 2001) Neither the infantile esotropia cohort nor the accommodative esotropia cohort exhibited an association between initial spherical equivalent refractive error and the prevalence of persistent amblyopia. On the other hand, initial anisometropia was a strong risk factor for persistent amblyopia. Among children with less than 1.00 D initial anisometropia, only 12% of the infantile esotropia cohort and 16% of the accommodative esotropia cohort developed persistent amblyopia. Among those with 1.00 D or more initial anisometropia, 39% of the infantile esotropia cohort and 50% of the accommodative esotropia cohort developed persistent amblyopia; i.e., a 3.1–3.3 times elevated risk for persistent amblyopia was associated when anisometropia of at least 1.00 D was present at the initial visit (Figure 7).
In a related study, we determined whether nil stereoacuity was associated with risk for poor response to amblyopia treatment.(Bosworth and Birch, 2003) Seventy-one children were enrolled in a prospective study of amblyopia treatment when they were 4–6 years old. At enrollment 66% had nil stereoacuity; following two years of treatment, 55% remained amblyopic. In contrast, among children who had measureable stereoacuity, only 25% remained amblyopic. Thus the risk for persistent amblyopia was 2.2 times greater among children with nil stereoacuity.
Taken together, these data demonstrate that risk for persistent amblyopia is elevated in infantile esotropia and accommodative esotropia if there is a long delay between onset and treatment and by anisometropia. The finding that the same two risk factors greatly elevate the risk for abnormal stereoacuity and persistent amblyopia supports the hypothesis that there is a link between binocular function and persistent amblyopia. In individuals with preserved binocular function, the fellow eye may maintain a binocular grid of fine receptive fields so that input to the weaker eye is not severely undersampled. Treatment plans designed to preserve stereoacuity may help to minimize the additional loss in optotype acuity associated with neural undersampling.
2.3 Fusional Suppression
When information from the two eyes is combined and binocular disparity is used to decode depth, monocular position information is lost. “Fusional suppression” is the term coined by McKee and Harrad (McKee and Harrad, 1993) to describe the suppression of monocular position information in the presence of a standing non-zero binocular disparity. They were able to measure the suppression of monocular position information in terms of diminished visual evoked potential (VEP) amplitude in response to vernier offsets when a standing non-zero disparity was introduced. We used this paradigm to investigate the link between the fusional suppression that occurs in normal binocular vision and the suppression of the amblyopic eye in accommodative esotropia.(Fu et al., 2006) We chose to study children with accommodative esotropia have a wide range of stereoacuity, from normal to nil, which makes it easier to observe the relationships among stereoacuity, fusional suppression, and amblyopia.
The stimulus paradigm was to record VEPs in response to oscillating vernier positional offsets of a stripe pattern presented to one eye (Figure 8). What was varied between the test two conditions was the pattern in the other eye, which could either have 0 min binocular disparity or 5 min binocular disparity. Since it was a standing (static) disparity, it could only influence VEP amplitude through its indirect effect on the vernier positional response generated by the other eye; i.e., fusional suppression. In a cohort of 37 children with accommodative esotropia, we found that all 8 children with normal stereoacuity and 10 children with deficient stereoacuity had normal fusion suppression (see Figure 9 for a representative set of data from a normal control child). Amplitudes of responses to vernier offsets were diminished in the presence of a standing binocular disparity. The vernier responses were similar in each eye and the degree of fusional suppression was similar regardless of which eye viewed the vernier stimulus and which eye viewed the static disparity stimulus. Children with accommodative ET and nil stereoacuity but no amblyopia (n=11) showed little if any fusional suppression; VEP amplitudes were similar whether or not a standing binocular disparity was present (not shown). Results from the 8 amblyopic children had a distinct pattern; fusional suppression was noted when the standing disparity was presented to the fellow eye but no suppression was observed when the standing disparity was presented to the amblyopic eye (Figure 9). These data from children with accommodative esotropia support an association between asymmetric fusional suppression and amblyopia. Moreover, the evidence that suppression is present and, at least in part, involves the same mechanisms responsible for fusion in normal binocular vision suggests that restoration of binocular function may be possible in amblyopia.
Figure 8.
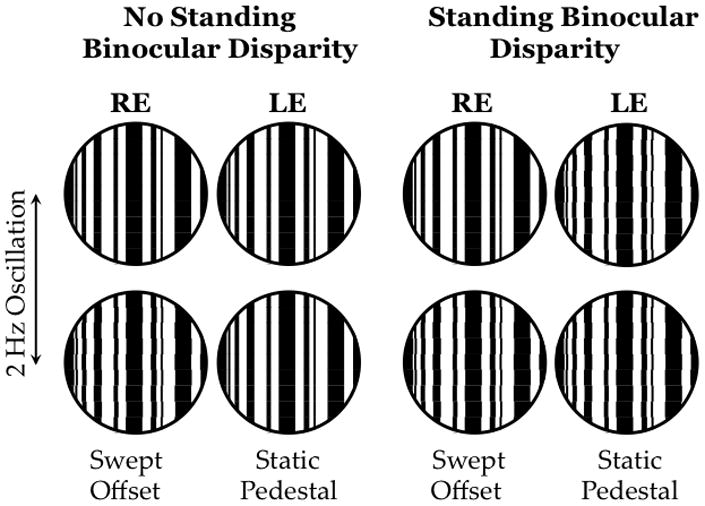
Schematic of the stimuli used to assess fusional suppression.(Fu et al., 2006) Stimuli were dichoptic (anaglyphic) multi-bar vernier targets. When there was no standing binocular disparity present (left), 5 static segments interspersed with 4 oscillating segments that aligned and misaligned at 2 Hz. The magnitude of the misalignment offset was swept in 7 steps from 10 to 1 min over each 7 sec trial while the other eye viewed a static multi-bar target with 0 min standing binocular disparity. The VEP was elicited by the making and breaking of co-linearity in the eye that viewed the vernier offsets. The standing disparity condition was similar except that the other eye viewed static multi-bar target with 5 min standing disparity. As in the other condition, the VEP was elicited by the making and breaking of co-linearity in the eye that viewed the vernier offsets; any difference in VEP amplitude between the two conditions is due to a suppressive effect of the static binocular disparity on the monocular vernier position response.
Figure 9.
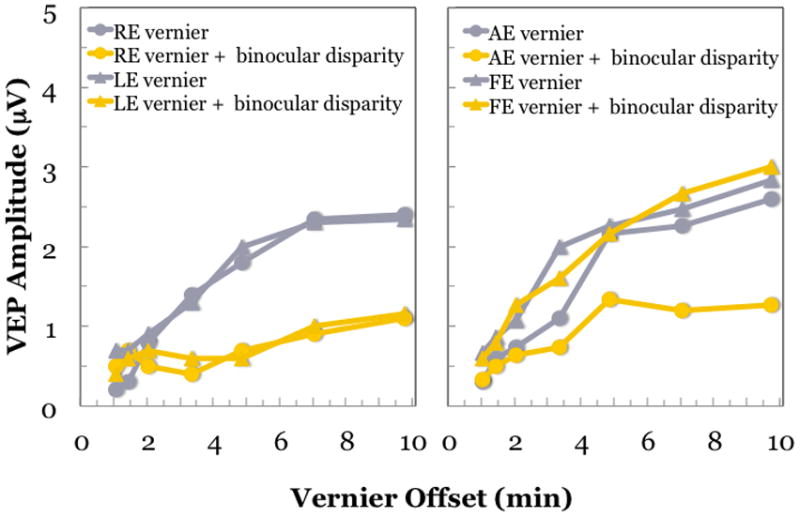
VEP responses to monocular oscillating vernier offsets recorded from a child with normal vision (left) and an amblyopic child (right) with 0 or 5 min standing binocular disparity.(Fu et al., 2006) For the child with normal vision, vernier responses are similar for each eye (grey symbols). The addition of a standing binocular disparity results in decreased VEP amplitude, consistent with fusional suppression. The amount of suppression is similar regardless of which eye views the vernier target and which views the static, disparate stimulus (yellow symbols). For the amblyopic child (right), the amblyopic eye has slightly, but consistently lower, amplitude vernier responses than the fellow eye. When the standing disparity is introduced to the fellow eye, fusion suppression is observed (yellow circles). However, when the standing disparity is introduced in the amblyopic eye, no fusional suppression is observed (yellow triangles). Fusion suppression is asymmetric in the amblyopic child.
2.4 Gaze Instability, Binocular Vision, and Amblyopia
Abnormal visual experience during infancy or early childhood that interferes with binocular fusion is invariably associated with gaze instability, most often fusion maldevelopment nystagmus syndrome (FMNS).(Tychsen et al., 2010) FMNS is a commonly associated with strabismus and amblyopia. It is characterized by a conjugate horizontal slow-phase nasalward drift of eye position, with temporalward corrective flicks. Because the slow drift is always nasalward, a switch in fixation eye results in an immediate reversal of the direction of the drift. This feature makes it easily distinguishable from infantile nystagmus (INS). Studies of non-human primates demonstrated that FMNS can result from the loss of binocular connections in the striate cortex that occurs in experimental models of strabismus and anisometropia. In a series of elegant experiments, Tychsen and colleagues (Tychsen et al., 2010) have demonstrated that the prevalence and severity of FMNS increases as the period of decorrelated abnormal binocular experience is lengthened, with a duration in primates that is equivalent to 3 months in humans resulting in 100% prevalence. Tychsen et al (Tychsen et al., 2010) propose that the binocular maldevelopment of the striate cortex is passed on to downstream extrastriate regions of cerebral cortex that drive conjugate gaze, including medial superior temporal area and that unbalanced monocular activity may result in one hemisphere becoming more active than the other, resulting in nasalward drift bias that typifies FMNS.
We evaluated the link between binocular vision, gaze instability, and amblyopia in a group of 33 children ≥5 years of age with hyperopic anisometropia.(Birch et al., 2012) Hyperopic anisometropia places the child at risk for abnormal stereoacuity and microstrabimsus, which is noted clinically as a temporalward “flick” as each assumes fixation on cover testing. The dominant hypothesis is that abnormal sensory experience due to anisometropia leads to foveal suppression, which in turn leads to microstrabimsus.(American Academy of Ophthalmology, 2011) We proposed an alternative hypothesis; namely, that disruption of bifoveal fusion by anisometropia has a direct effect on ocular motor function. Fixation stability was measured using the Nidek MP-1 microperimeter, which combines a non-mydriatic infrared fundus camera to obtain real time retinal images and a liquid crystal display (LCD) to present a fixation target. The fixation target was a 1° radius red circular ring, displayed in the center of the LCD screen,. Children were provided a chin rest and forehead rest and were instructed to fixate steadily at the center of the ring. Initially, a reference fundus image with 1 pixel (0.1 deg) resolution was captured, and a high contrast retinal landmark in the frozen image was identified by the examiner. During the 30 sec test period, software calculated shifts in horizontal and vertical eye position between the reference image and the real-time fundus image at 25 Hz. A scattergraph of the fixation points was displayed and fixation stability was quantified as the area of the 95% bivariate contour ellipse (BCEA); i.e. an ellipse that provided the best fit to the boundary of 95% of the 750 fixation points acquired during the 30 sec test interval. Stereoacuity was measured using the Preschool Randot Stereoacuity Test. Examples of the fixation stability data obtained from 3 children with hyperopic anisometropia are shown in Figure 10.
Figure 10.

Examples of fixation stability obtained from 33 5- to 9-year-old children with hyperopic anisometropia using the Nidek MP-1 microperimeter.(Birch et al., 2012) On each image, the small blue dots mark the location of the 750 fixation points acquired during the 30 sec test period. The Nidek MP-1 calculated three ellipses to describe the areas that enclose 68%, 95%, and 99% of the fixation points; the 95% BCEA ellipse is highlighted in yellow. On the left is a child with steady fixation; only small amplitude drifts and microsaccades were present and nearly all of the fixation points fell inside of the 1 deg fixation circle shown in red (BCEA = 0.8 deg2). In the center is a more typical anisometropic child, who shows moderate fixation instability (BCEA = 5.9 deg2). On the right, a child with substantial fixation instability is shown (BCEA = 10.8 deg2).
All children with normal stereoacuity had normal fixation stability (Figure 11). Children with abnormal stereoacuity had unstable fixation, and children with nil stereoacuity had the most instability (Figure 11). Although there was moderate variability among children, there was a strong correlation between stereoacuity and fixation instability (BCEA area); rs=0.54, p=0.002. We were also able to export the raw eye movement data to analyze waveforms and categorize eye movements as normal (with or without square-wave oscillations), FMNS, or INS (Figure 12).
Figure 11.
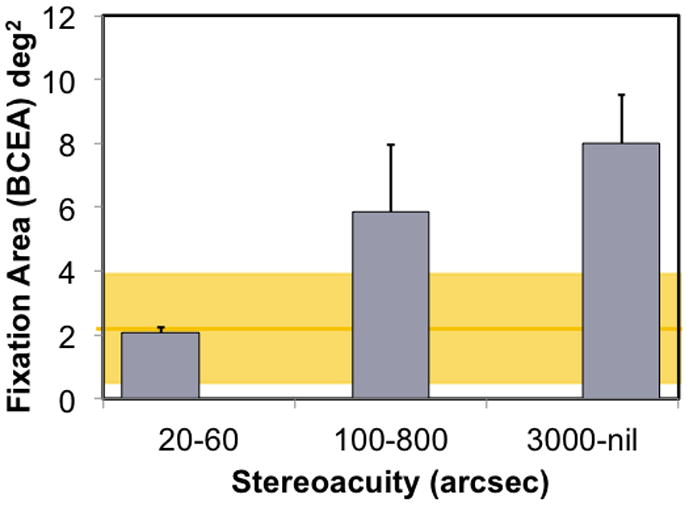
Fixation instability during 30 sec of attempted steady fixation for 33 children with hyperopic anisometropia.(Birch et al., 2012) Age-matched normal controls had a mean BCEA = 2.1 ± 0.9 deg2 (yellow line), with a 95% tolerance range of 0.2 to 3.8 deg2 (yellow shaded area). Anisometropic children with normal stereoacuity (bifoveal; 20–60 arcsecs) had normal fixation stability. Children with reduced stereoacuity of 100–800 arcsecs (monofixation) had significantly more fixation instability than normal (p=0.008) and those with nil stereoacuity were significantly more unstable than those with normal stereoacuity and those with reduced stereoacuity ( p<0.001 for both comparisons).
Figure 12.
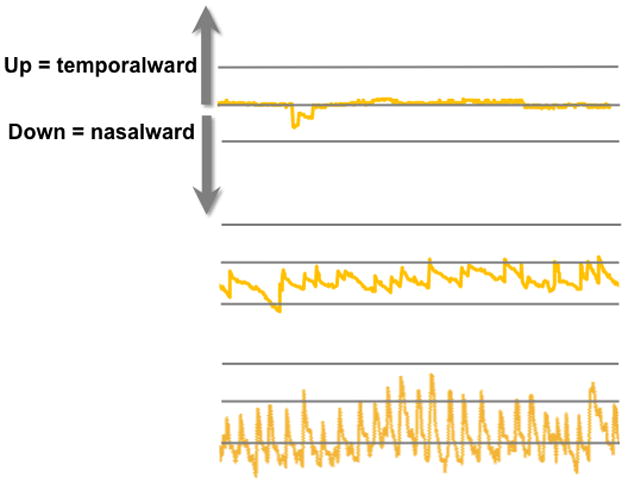
Eye position records derived from the Nidek MP-1 for the 3 anisometropic children shown in Figure 10.(Birch et al., 2012) The top trace shows eye position for the child with normal fixation stability (Figure 10, left); overall, fixation is accurate, with only microsaccades and a brief saccadic oscillation. The middle trace shows the typical anisometropic child (Figure 10, middle); it shows the classic waveform of FMNS with slow drifts nasalward, and rapid re-fixating temporalward saccades. The bottom trace shows the anisometropic child with extreme fixation instability (Figure 10, right); high frequency, large amplitude FMNS is evident.
None of the children with normal stereoacuity had FMNS waveforms, 67% of children with abnormal stereoacuity had FMNS waveforms, and nearly all of the children with nil stereoacuity had FMNS waveforms (Figure 13). Thus, the temporalward flick that is seen during cover testing in children with hyperopic anisometropia is likely evidence of a direct effect of disruption of bifoveal fusion by anisometropia on ocular motor function; i.e., the decorrelation of retinal images caused by anisometropia results in FMNS. Note that this FMNS is often very small in amplitude, making it difficult to appreciate clinically except with close observation, like during cover testing. More importantly, in most children with anisometropia, we observed FMNS only in intermittent, small amplitude bursts, so it may not always be obvious during a brief clinical examination. Our data are consistent with the model of FMNS proposed by Tychsen, Wong, and colleagues in which correlated visual experience is necessary for the development of stereopsis and balanced gaze.(Tychsen et al., 2010) Decorrelated visual experience, due to anisometropia can lead to stereoacuity deficits and the gaze asymmetry of FMNS, a disruption of ocular motor development. We propose that the temporalward re-foveating FMNS “flicks” may mimic microstrabismus during cover testing but are actually an ocular motor abnormality that directly results from binocularly decorrelated visual experience. If this hypothesis is correct, our proposal that binocular dysfunction plays a primary role in amblyopia leads us to also expect a relationship between amblyopia and fixation instability.
Figure 13.

Prevalence of FMNS among 33children with hyperopic anisometropia divided into 3 groups based on their stereoacuity. (Birch et al., 2012)
We investigated the relationship between fixation instability and amblyopic visual acuity deficits. Carpineto et al. (Carpineto et al., 2007) reported that fixation instability was present in some children with microstrabimsus and amblyopia. More recently, Gonzalez et al. (Gonzalez et al., 2012) reported significantly larger fixation areas (more instability) in amblyopic eyes of 13 adults compared with fellow eyes and control eye. We quantified fixation stability in 89 amblyopic and nonamblyopic children with strabismus, anisometropia, or both and an age-matched normal control group using the Nidek MP-1 microperimeter to acquire eye position and BCEA to quantify fixation instability.(Subramanian et al., 2012) During attempted steady fixation with their amblyopic eyes, children had 3–5X greater fixation instability (larger BCEA areas) than when fixing with their fellow eyes and compared with normal controls fixing monocularly (Figure 14).
Figure 14.
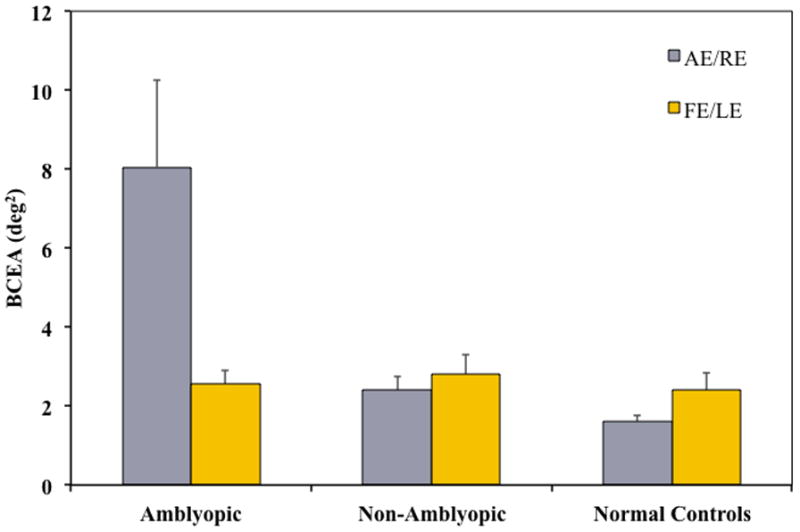
Mean fixation areas (95% BCEA) for amblyopic, non-amblyopic and normal control children.(Subramanian et al., 2012) Compared with children with strabismus, anisometropia, or both who were nonamblyopic, amblyopic children had significantly greater fixation instability; mean BCEA for amblyopic eyes was significantly larger than for non-amblyopic right or left eyes (p ≤ 0.02). Mean BCEA when fixating with the amblyopic eye was significantly larger than for normal controls’ right or left eyes (p ≤ 0.01) left eyes. Amblyopic eyes also had significantly more fixation instability than their fellow eyes (p = 0.01). Fixation stability of fellow eyes did not differ significantly from right or left eyes of normal controls.
There was a statistically significant positive correlation between visual acuity and BCEA (r = 0.58; p < 0.0001) for amblyopic eyes; amblyopic eyes with poorer visual acuity had greater fixation instability and, thus, larger and wider bivariate contour ellipse areas. Categorical severity of fixation instability has been previously reported to be associated with depth of amblyopia (Carpineto et al 2007). On the other hand, BCEA in 13 adults with amblyopia was not correlated with amblyopic eye visual acuity, although interocular differences in visual acuity were correlated with BCEA. (Gonzalez et al., 2012) Additionally, we found that amblyopic eyes had highly elliptical BCEAs, with a much longer horizontal than vertical axis, consistent with abnormal development of the ocular motor system. Fixation instability along both the horizontal and vertical axes was about 1.5–2X larger for amblyopic eyes than normal control eyes. This finding is consistent reports of predominantly horizontal square-wave oscillations and FMNS in amblyopic children. (Felius et al., 2011; Felius et al., 2012)
2.5 Motor Skills in Amblyopia
Deficits in fine motor skills have been widely reported to be present in amblyopic individuals.(Grant and Moseley, 2011) Most of these studies aimed to evaluate the utility and value of rehabilitation of the amblyopic eye as a “spare eye” and so relied on testing amblyopic individuals when they are forced use the amblyopic eye alone. However, if amblyopia is primarily the result of binocular dysfunction, we would also expect to see such deficits in binocular task performance. We evaluated the effects of amblyopia on motor skills under binocular viewing conditions (O’Connor et al., 2010a, b); specifically we evaluated performance on the Purdue pegboard (number of pegs placed in 30 seconds) and bead threading (time to thread a set number of large and small beads in 143 individuals, 47 of whom had manifest strabismus and 30 of whom had amblyopia. (21 strabismic amblyopia; 9 anisometropic amblyopia). Performance on the fine motor skills tasks was related to stereoacuity, with subjects with normal stereoacuity performing best on all tests (Figure 15). Individuals with amblyopia were significantly slower on both bead tasks than were those without amblyopia. However, when the statistical analysis was repeated with stereoacuity as a covariate, the difference between amblyopic and nonamblyopic individuals on the bead task was no longer statistically significant. This result is in agreement with a previous report that fine motor skill performance was related to the level of stereoacuity but not to the presence of unilateral vision impairment in 3- to 4-year-old children. (Grant et al., 2007) Webber et al.(Webber et al., 2008a) have also reported that both the amblyopia and stereoacuity deficits were associated with reduced performance on fine motor skill tasks. Taken together, these results confirm that children with binocular dysfunction have impaired fine motor skills and that this impairment is more closely related to the severity of binocular dysfunction than to the severity of amblyopia.
Figure 15.
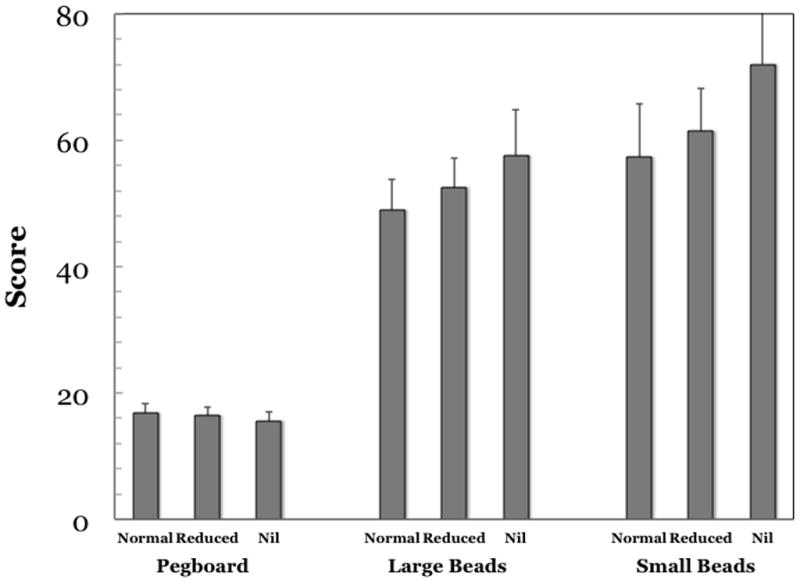
Performance on fine motor tasks by amblyopic and nonamblyopic individuals with three levels of binocular function.(O’Connor et al., 2010a, b) There was a significant difference in performance on the Purdue Pegboard and the large and small bead tasks (p< 0.03 in all cases). Post-hoc analyses demonstrated that those with normal stereoacuity performed significantly better than those with nil stereoacuity. When comparing those with reduced stereoacuity with those with normal stereoacuity, individuals with reduced stereoacuity were quicker than those with nil stereoacuity. Analysis of covariance between individuals with and without amblyopia found that amblyopia was associated with slower completion of both bead tasks (p< 0.04) but, when the analysis was repeated with stereoacuity as a covariate the difference in speed on the bead tasks was no longer statistically significant.
3. Binocular Treatment of Amblyopia
As noted above, current treatments for amblyopia are not sufficient to achieve normal visual acuity for 15–50% of amblyopic children. Even when normal visual acuity is achieved, amblyopia often recurs. Older children and adults with persistent amblyopia are rarely treated because the predominant mindset is that decorrelated binocular visual experience and habitual suppression of the amblyopic eye has caused permanent loss of binocular cells and changed the functional profile of cortical cells responsive to the amblyopic eye. However, the evidence discussed in Section 2 suggests, although there is binocular dysfunction, the capacity for binocular interaction is not absent. Morever, interocular suppression of the amblyopic eye may be preventing binocular interaction. Taken together, these data suggest that there is a need for a new, binocular approach to amblyopia treatment. The goal is to reduce the prevalence of residual amblyopia and prevent amblyopia recurrence.
3.1 Perceptual Learning
One new approach is perceptual learning. The effect of repeated practice on perceptual performance has become a focus of amblyopia treatment, particularly for adults and older children who have abandoned traditional approaches to treatment. It is clear that normal controls in the research laboratory who repeatedly practice on a demanding visual task achieve significant and durable improvement in their visual performance on that specific task.(Levi, 2012) More relevant, is the evidence that amblyopic individuals who practice a perceptual task improve not only on that specific task, but also have generalized improvement when tested with other perceptual tasks.(Levi, 2012)
Perceptual learning as a treatment for adult amblyopia has typically been conducted with the fellow eye patched. The visual task for training is designed to engage the amblyopic individual in making fine discriminations and to provide repeated exposure over many hours. It has been suggested that perceptual learning is simply an extension of the traditional effects of patching therapy, where the amblyopic individual must accomplish many visual discriminations in everyday life. The argument that perceptual learning succeeds where everyday experience fails in amblyopic adults is that the less plastic brain of the adult requires “attention and action using the amblyopic eye, supervised with feedback” in order to provide effective treatment.(Levi, 2012) Even with 40–50 hours of perceptual learning, most adults achieve only 0.1–0.2 logMAR improvements in visual acuity (1–2 lines).(Levi, 2012) The limited improvement in visual acuity, the dedicated time required for treatment, and the boredom/compliance issues associated with repetition of a perceptual task, have limited the application of perceptual learning as a routine treatment for amblyopia. To overcome these limitations, research has turned toward the evaluation of video games as a potential treatment for amblyopia in adults. The rationale is that action video games, in particular, engage visual attention and require demanding visual discriminations in a similar way to the more standard perceptual learning paradigms, but is able to sustain prolonged participation because of the story lines, varied visual tasks, and rewards provided by the games for making correct discriminations. A recent evaluation of an of-the-shelf action game (Medal of Honor: Pacific Assault) found that just 20 hours of play with the fellow eye patched resulted in a mean improvement of 0.15 logMAR and that additional small gains in visual acuity continued in some amblyopic individuals through 80 hours of play (Figure 16).(Li et al., 2011) Interestingly, though, similar gains in visual acuity were experienced by amblyopic adults playing a non-action video game (Sim City) or performing a simple perceptual learning grating acuity task while patched. Patching alone was not sufficient to improve visual acuity (Figure 16). Of course, the idea of visual stimulation as a part of amblyopia is not new. For many years, eye care professionals have placed an emphasis on the child performing “near activities while patched”; i.e., the role of active, attentive vision in amblyopia recovery has been appreciated for many years.
Figure 16.
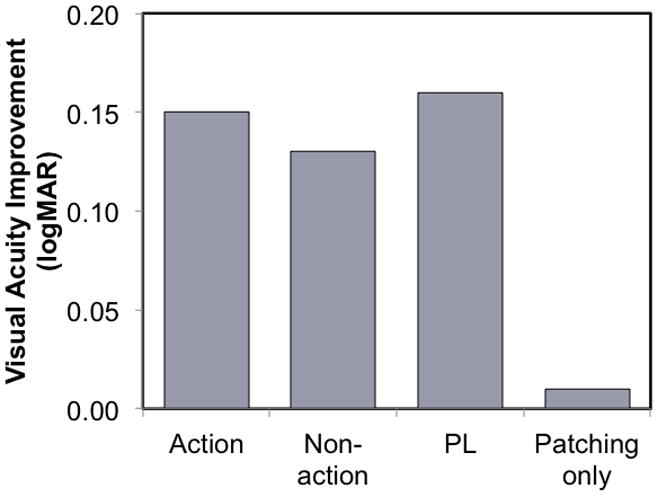
Improvement in visual acuity with patching in 18 amblyopic adults while playing an action video game or a non-action video game for 20 hours. Also shown is the visual acuity improvement achieved with 20 hours of patching while practicing a grating acuity task (perceptual learning; PL) and with patching alone for 20 hours. (Li et al., 2011)
3.2 Treating Binocular Dysfunction Amblyopia
In most studies to date, perceptual learning has been applied monocularly with the same aim as patching therapy, to improve visual acuity of the amblyopic eye. Some improvements in stereoacuity have been found to accompany the amblyopic eye visual acuity improvement,(Levi, 2012) similar to the modest gains in stereoacuity that have been reported to accompany successful patching therapy. (Wallace et al., 2011b) However, a growing appreciation of the role of decorrelation of binocular stimulation in suppression of the amblyopic eye has motivated a reformulation of amblyopia treatment. Decorrelated binocular experience may not only compromise binocular function but also contribute to or cause the monocular deficits. In other words, we have come to view binocular dysfunction as the primary deficit and the monocular visual acuity deficit as secondary. In this framework, patching, with or without perceptual learning treatment, treats the secondary monocular visual deficits but does not directly address the primary binocular deficit. We wanted to find a new approach to treatment for amblyopia that would not only improve visual acuity of the amblyopic eye but also provide repeated, correlated binocular visual experience. Unless the binocular dysfunction is treated, abnormal binocular vision may result in residual amblyopia, and may trigger recurrence of amblyopia.
Reducing contrast to the fellow eye to equate the visibility between amblyopic and fellow eyes can allow binocular contrast summation, (Baker et al., 2007) and suprathreshold binocular interactions in motion coherence and orientation discrimination tasks (Mansouri et al., 2008) to occur in at least some amblyopic individuals. This suggests that the absence of binocular interactions in amblyopia reported in the literature may have been due to the imbalance in the monocular signals rather than to an absence of capacity for binocular interaction. Hess and colleagues (Hess et al., 2010) reported improvement in visual acuity and binocular vision following several weeks of practice with a dichoptic motion coherence task presented via video gaming goggles. For the initial practice session, the contrast of the randomly moving dots in the fellow eye was reduced to about 10% while the amblyopic eye coherent dots were presented at 100% contrast. This allowed the amblyopic eye to “break-through” and observers were able to experience binocular vision. As the 10 amblyopic adults began to experience binocular vision, the contrast in the fellow eye was gradually increased during 3 or 4 sessions per week for 3 to 5 weeks until the two eyes worked together with more evenly matched contrasts. In addition to improvements in binocular combination, visual acuity improved 0.1 to 0.7 logMAR (Figure 17) and stereoacuity improved in 6 of the 10 adults. Knox et al (Knox et al., 2012) used the same device to administer one week of dichoptic treatment but used a more interesting video game task (Tetris). As with the motion coherence task, the contrast of the fellow eye image was reduced to allow binocular vision to occur. Five 1-hour practice sessions during a one week were provided. The 14 amblyopic children (5–11 years old) has a median improvement of 0.09 logMAR in visual acuity (about 1 line) and 9 of the 14 also had improvements in stereoacuity (Figure 17). Most recently To and colleagues (Hess et al., 2012; To et al., 2011) moved the dichoptic Tetris game to an iPod platform to allow for more comfortable play and play at home. They evaluated the effects of dichoptic Tetris practice (with low contrast to the fellow eye) in 10 amblyopic adults and found found a median visual acuity improvement of 0.2 logMAR after 10–19 one hour sessions (Figure 17); 7 of the 10 adults also had improvements in stereoacuity. Taken together, these data support the hypothesis that a binocular approach to treatment of amblyopia can be successful. Repeated experience with dichoptic perceptual tasks led to modest improvements in monocular visual acuity and, in some cases, improved stereopsis.
Figure 17.
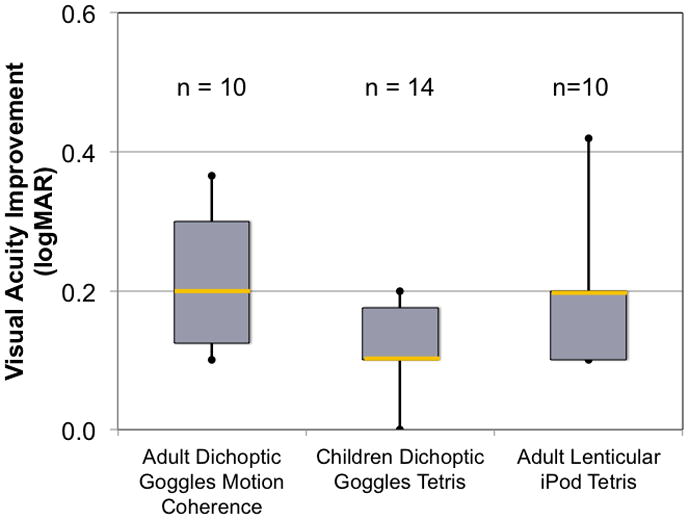
Visual acuity improvement following 5–20 hours of dichoptic training to amblyopic individuals using video game goggles to make motion coherence judgments (Hess et al., 2010) or play Tetris (Knox et al., 2012) or using a lenticular overlay on an iPod to play Tetris.(Hess et al., 2012; To et al., 2011) Despite the pilot studies having relatively short durations of dichoptic training and small cohorts, all 3 studies showed significant improvement in visual acuity and stereoacuity.
To date, this approach largely has been limited to the laboratory, with the use of video game goggles and the need for administration of practice sessions by trained staff, and limited to adults and school-age children. We wanted to extend binocular treatment to the preschool age range, to be used as an adjunct treatment along with patching (at separate times of day) to reduce residual amblyopia and to reduce risk for recurrence of amblyopia. The extension to an iPod platform and lenticular display was a step toward incorporating dichoptic training into clinical settings for adults and school-age children. However, it is difficult to extend the lenticular iPod to preschool children because the iPod requires fine motor skills and the lenticular display requires precise and steady head position to provide dichoptic stimulation. We worked with Hess and To to adapt the dichoptic Tetris game for use on the larger iPad format, which is more compatible with the immature fine motor skills of young children, and anaglyphic dichoptic separation, which is not dependent on head position. We are currently studying the effects of anaglyphic dichoptic training on amblyopia and risk for recurrence in 3- to 5-, 6- to 8-, and 9- to 11-year old children with strabismic, anisometropic, or combined mechanism amblyopia.(Birch et al., 2013) Many of the children we are studying were enrolled in our prospective longitudinal study of visual development at time of initial diagnosis of strabismus or anisometropia. For these children we have detailed histories of visual acuity, treatment regimens, and response to treatment. An example of a child’s visual acuity history and visual acuity improvement with the anaglyphic dichoptic training are shown in Figure 18. While still preliminary, our results from 23 children are consistent with the data from the published laboratory studies of small cohorts of amblyopic adults and school-age children. Many of the children have improved stereoacuity after only 15–20 hours of dichoptic practice, including some who achieve coarse stereopsis for the first time. In addition, there is an improvement in in visual acuity of 0.1–0.4 logMAR. That rehabilitation of binocular interaction is accompanied by improved monocular visual acuity supports the hypothesis that binocular dysfunction plays a pivotal role in amblyopia, an appreciation of which is not currently reflected in clinical practice.
Figure 18.
Visual acuity data from a child with anisometropic amblyopia before and after dichoptic training.(Birch et al., 2013) On the intitial visit at 4.4 years old, best corrected visual acuity for the amblyopic eye was 0.9 logMAR. Treatment with glasses, patching, atropine, and Bangerter filter resulted in improvement to 0.5–0.6 logMAR. Slight regression to 0.6–0.7 logMAR occurred, and visual acuity remained in this range for the next 3 years with continued spectacle wear. At 9.9 years, the child enrolled in the dichoptic treatment study and showed 0.2 logMAR improvement to visual acuity of 0.4 logMAR over the next 8 weeks. Three months after discontinuing dichoptic treatment, visual acuity for the amblyopic eye remained at 0.4 logMAR. Visual acuity of the fellow eye remained at 0.1 to 0.0 logMAR from age 7.4 years to 10.4 years.
4. Future Directions
As summarized above, there is accumulating evidence that amblyopia and binocular function are linked and several noteworthy clues that binocular dysfunction is primary and monocular visual acuity loss is secondary. This new understanding of amblyopia provides a foundation for a broadened scope of research and for crafting more effective treatments. The last 15 years has seen an explosion of randomized clinical trials to evaluate treatments for children with amblyopia, and a shift from treatment based on consensus to evidence-based treatment. Nonetheless, there remain many unanswered questions about amblyopia screening, diagnosis, and treatment.
4.1 Screening for Amblyopia
Screening for amblyopia is a part of the regular well-child visit recommended by the American Academy of Pediatrics. Screening by a pediatrician or family care practitioner can identify amblyopia at a time when treatment is most effective. Screening improves vision outcomes. In a population-based, randomized longitudinal study, repeated early screening resulted in a 60% decreased prevalence of amblyopia and improved visual acuity outcome at age 7 years compared with surveillance only until school entry.(Williams and Harrad, 2006; Williams et al., 2001; Williams et al., 2008) Amblyopia can be treated more effectively when treated early; the same study found a 70% lower prevalence of residual amblyopia after treatment when therapy was initiated before age 3 years. (Williams et al., 2001; Williams et al., 2002, 2003) However, pediatricians and family care practitioners experience considerable difficulties in screening for amblyopia.(Tingley, 2007) Most primary care providers in the United States still use visual acuity charts as their primary approach to vision screening for amblyopia.(Wall et al., 2002) Unfortunately, reading letters or numbers on a visual acuity chart requires a verbal, cooperative child and is not routinely successful in children under 5 years old, too late for the most effective therapy. When the testing is conducted earlier, nonstandard symbols or chart formats are used and often they are not presented in the linear or crowded formats needed to detect mild amblyopia. Table 2 summarizes recent studies that document how these difficulties impact the vision screening provided by primary care physicians to preschool children.
Table 2.
Reported success rate for vision screening of preschool children by pediatricians and family care practitioners.
| Study | 2-year-olds | 3-year-olds | 4-year-olds | 5-year-olds |
|---|---|---|---|---|
| Pediatric Research in Office Settings (Wasserman et al., 1992) | 0% | 38% | 71% | 81% |
| Kemper & Clark (Kemper and Clark, 2006) | 0% | 38% | 56% | 73% |
| Wall et al (Wall et al., 2002) | 0% | 37% | 79% | 91% |
| Marsh-Tootle et al (Marsh-Tootle et al., 2008) | 0% | 12% | 25% | 48% |
| Kemper et al (Kemper et al., 2011) | 0% | 43% | 64% | 73% |
| Range | 0% | 12–43% | 25–79% | 48–91% |
| Mean | 0% | 34% | 59% | 73% |
Automated devices, like the SureSight® and PlusOptix® have been developed to try to improve upon visual acuity testing, by providing a simple approach to screening for refractive error risk factors, rather than screening for amblyopia directly. These devices are attractive because they are designed for use by pediatric nurses, pediatric medical assistants, and even lay people. However, these automated devices miss many children with amblyopia and many normal children are sent to the ophthalmologist for an unnecessary eye examination, further increasing the cost of health care. For example, in the combined population-based cohorts of 2- to 6-year old children from the NIH-sponsored Multi-Ethnic Pediatric Eye Study (MEPEDS) and the Baltimore Pediatric Eye Study (BPEDS) (N=5710), only 14% of children with ≥1.00 D anisometropia had amblyopia and only 60% of children with ≥2.00 D anisometropia had amblyopia;(Cotter et al., 2011) i.e., screening for anisometropia and will have low specificity for amblyopia. In addition, amblyopia risk factor analysis failed to identify hyperopia a significant risk factor for unilateral amblyopia.(Tarczy-Hornoch et al., 2011) Because refraction screening devices cannot directly detect strabismus or amblyopia, many “at risk” children are referred for comprehensive ophthalmic examinations at considerable cost in time and money to the family. Once these “at risk” children are referred, they often are “monitored” with regularly scheduled ophthalmic examinations for years, even though the data suggest that only 9%–18% of the children “at risk” will ever develop amblyopia.
Recently, Hunter and colleagues developed an alternative approach to screening for amblyopia, a binocular birefringence scanner called the “Pediatric Vision Scanner” or “PVS”.(Hunter et al., 2004; Loudon et al., 2011; Nassif et al., 2004; Nassif et al., 2006) Simultaneous retinal birefringence scans of both eyes detect whether fixation of a target is foveal (central), steady, and maintained by identifying the unique polarization signal created by the radially arranged Henle fibers (photoreceptor axons) that emanate from the fovea.(Hunter et al., 2004; Loudon et al., 2011; Nassif et al., 2004) In a pilot study of the PVS, Loudon et al.(Loudon et al., 2011) reported that all normal control children had at least 4 of 5 scans consistent with bifoveal fixation, and that 62 of 64 amblyopic children failed to exhibit birefringence (97% sensitivity to amblyopia). The PVS was able to detect amblyopia even in the 22 children with anisometropic amblyopia and no strabismus (20/22; sensitivity = 91%) and birefringence was normal in children with anisometropia without amblyopia (11/12; specificity = 92%). We have independently confirmed these PVS results in anisometropic children with no strabismus.(Yanni et al., 2012) Moreover, these data are consistent with the association between fixation instability and amblyopia in both anisometropic and strabismic individuals (Section 2.4).(Birch et al., 2012; Subramanian et al., 2012) Taken together, the pilot data suggest that binocular birefringence screening could provide more accurate identification of children who need ophthalmological intervention.
4.2 Diagnosis of Amblyopia
Diagnosis of amblyopia relies on the measurement of visual acuity. Reduced best corrected visual acuity in presence of an amblyogenic factor (usually strabismus or anisometropia) with no other ocular or visual cortical abnormality is currently the critical component of amblyopia diagnosis. However, most children <3 years old and some older children cannot complete a visual acuity test. Instead, the clinical diagnosis of amblyopia in children <3 years old must rely on fixation preference.
Recently, fixation preference has been disparaged as a method for assessing amblyopia. The basis for questioning the value of fixation preference is found in two large (n=9970) population-based screening studies, MEPEDS and BPEDS.(Cotter et al., 2009; Friedman et al., 2008) Both included primarily normal children; only 44 had ≥2 lines interocular difference in visual acuity. With such a small sample of primarily mild amblyopia, it is not surprising that they reported low sensitivity of fixation preference. Only a handful had strabismus, so most fixation preference testing relied on the more difficult induced tropia (prism) test. Another limitation was that fixation preference was tested without optical correction while visual acuity was tested with optical correction. Given these limitations, it is not surprising that they reported low specificity. Data collected from clinical cohorts support much higher sensitivity and specificity for detection amblyopia by fixation preference in cohorts of children with strabismus (sensitivity 73%–93%; specificity 78%–97%).(Birch and Holmes, 2010; Kothari et al., 2009; Procianoy and Procianoy, 2010; Sener et al., 2002; Wright et al., 1986) As noted in Section 1.2, 95% of amblyopic children <3 years old have strabismus,(Birch and Holmes, 2010) so we anticipate high sensitivity and specificity of fixation preference in this age range where visual acuity cannot be tested with standard optotypes. Whether small changes in fixation preference can be used to track treatment response remains an open question. Given the scarcity of evidence for treatment effects and risks of amblyopia treatment (e.g., reverse amblyopia) in the <3 year age range, it is imperative to answer this question before we can begin to build an evidence base for treatment of amblyopia in children <3 years old.
4.3 Optimizing Treatment for Amblyopia
The cornerstone of amblyopia treatment is patching the “fellow” eye to force use of the amblyopic eye. Recent randomized clinical trials have provided and extensive knowledge base for treatment of amblyopia in children over 3 years old.(Holmes et al., 2006; Repka and Holmes, 2012; Stewart et al., 2011) Nonetheless, the high prevalence of persistent residual amblyopia and recurrent amblyopia underscore the need for more effective treatments. Our current focus is to provide regular binocular experience to treat binocular dysfunction by adjusting the contrast of dichoptic images to balance sensitivity differences between the amblyopic and fellow eyes and allow binocular interaction (Section 3.2). However, it is improbable that sensitivity differences are the only limiting factor. Other approaches to treating binocular dysfunction, tailored to the specific constellation of deficits present in the amblyopic individual may be more effective. In addition, it may prove impossible to fully restore binocular interaction. Such an outcome would shift the emphasis toward amblyopia prevention. We know, for example, that early surgery for infantile esotropia can preserve binocular function and reduce the risk for persistent amblyopia.(Birch and Stager, 2006; Birch et al., 2004) Clinical pearls, such as the reduced risk for recurrent amblyopia when patching therapy is tapered rather simply halted when the child attains equal visual acuities, may also point to new insights for the design of effective binocular treatments. Clearly, there is a need for a broader understanding of the effects of decorrelated binocular visual experience on the developing sensory and motor systems, the role of suppression in various forms of amblyopia, and the contribution of binocular dysfunction to residual and recurrent esotropia.
5. Summary
Amblyopia is widely viewed as a monocular disorder and treatments for amblyopia, many of which have been used for centuries, have traditionally focused on forcing use of the amblyopic eye to recover monocular visual acuity. Although this approach has had substantial success, and is supported by recent evidence from randomized clinical trials of patching and atropine treatment, many amblyopic individuals are left with residual visual acuity deficits, ocular motor abnormalities, deficient fine motor skills, and high risk for recurrent amblyopia. Converging evidence points to the pivotal role of decorrelated binocular experience in the genesis of amblyopia and the associated residual deficits. These findings suggest that a new treatment approach designed to treat the binocular dysfunction as the primary deficit in amblyopia may be needed.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Eye Institute (EY05236 and EY022313), Thrasher Research Fund, Pearle Vision Foundation, One Sight Foundation, Knights Templar, and Fight for Sight. The research depended on many important contributions by past and present post-doctoral fellows, the Dallas-area pediatric ophthalmologists who referred patients to the research projects and collaborated in data collection and analysis, research assistants, and interns. This research relied on the many contributions of the families of amblyopic children who have participated in these studies.
Footnotes
The research was conducted at the Retina Foundation of the Southwest.
Please note that the Retina Foundation of the Southwest will be moving to new building late in 2012 or early in 2013.
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Haddad CE, Mollayess GM, Cherfan CG, Jaafar DF, Bashshur ZF. Retinal nerve fibre layer and macular thickness in amblyopia as measured by spectral-domain optical coherence tomography. Br J Ophthalmol. 2011;95:1696–1699. doi: 10.1136/bjo.2010.195081. [DOI] [PubMed] [Google Scholar]
- Altintas O, Yuksel N, Ozkan B, Caglar Y. Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in patients with strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2005;42:216–221. doi: 10.3928/01913913-20050701-03. [DOI] [PubMed] [Google Scholar]
- American Academy of Ophthalmology. Pediatric ophthalmology and strabismus. American Academy of Ophthalmology; San Francisco, CA: 2011. [Google Scholar]
- Baker DH, Meese TS, Mansouri B, Hess RF. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci. 2007;48:5332–5338. doi: 10.1167/iovs.07-0194. [DOI] [PubMed] [Google Scholar]
- Birch E, Stager D, Wright K, Beck R. The natural history of infantile esotropia during the first six months of life. Pediatric Eye Disease Investigator Group. J AAPOS. 1998;2:325–328. doi: 10.1016/s1091-8531(98)90026-x. discussion 329. [DOI] [PubMed] [Google Scholar]
- Birch EE. Marshall Parks lecture. Binocular sensory outcomes in accommodative ET. J AAPOS. 2003;7:369–373. doi: 10.1016/j.jaapos.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Birch EE, Fawcett S, Stager DR. Why does early surgical alignment improve stereoacuity outcomes in infantile esotropia? J AAPOS. 2000;4:10–14. doi: 10.1016/s1091-8531(00)90005-3. [DOI] [PubMed] [Google Scholar]
- Birch EE, Fawcett SL, Morale SE, Weakley DR, Jr, Wheaton DH. Risk factors for accommodative esotropia among hypermetropic children. Invest Ophthalmol Vis Sci. 2005;46:526–529. doi: 10.1167/iovs.04-0618. [DOI] [PubMed] [Google Scholar]
- Birch EE, Holmes JM. The clinical profile of amblyopia in children younger than 3 years of age. J AAPOS. 2010;14:494–497. doi: 10.1016/j.jaapos.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Berry P, Everett ME. Prospective assessment of acuity and stereopsis in amblyopic infantile esotropes following early surgery. Invest Ophthalmol Vis Sci. 1990;31:758–765. [PubMed] [Google Scholar]
- Birch EE, Stager DR., Sr Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS. 2006;10:409–413. doi: 10.1016/j.jaapos.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Sr, Berry P, Leffler J. Stereopsis and long-term stability of alignment in esotropia. J AAPOS. 2004;8:146–150. doi: 10.1016/j.jaapos.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Sr, Wang J, O’Connor A. Longitudinal changes in refractive error of children with infantile esotropia. Eye (Lond) 2010;24:1814–1821. doi: 10.1038/eye.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Sr, Stager DR, Jr, Berry PM. Risk factors for esotropic amblyopia. Investigative Ophthalmology & Visual Science. 2007;48:e1108. [Google Scholar]
- Birch EE, Subramanian V, Jost S, Jost R, Stager DR, Sr, Stager DR, Jr, Dao L. Binocular iPad treatment for amblyopia in children. Journal of AAPOS. 2013;17:e2. [Google Scholar]
- Birch EE, Subramanian V, Weakley DR. Is the “flick in anisometropia a sign of microstrabismus or fixation instability? Journal of AAPOS. 2012;16:e2. doi: 10.1016/j.jaapos.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Swanson WH. Hyperacuity deficits in anisometropic and strabismic amblyopes with known ages of onset. Vision Res. 2000;40:1035–1040. doi: 10.1016/s0042-6989(00)00011-0. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Birch EE. Binocular function and optotype-grating acuity discrepancies in amblyopic children. Investigative Ophthalmology & Visual Science. 2003;44:e3183. [Google Scholar]
- Carpineto P, Ciancaglini M, Nubile M, Di Marzio G, Toto L, Di Antonio L, Mastropasqua L. Fixation patterns evaluation by means of MP-1 microperimeter in microstrabismic children treated for unilateral amblyopia. Eur J Ophthalmol. 2007;17:885–890. doi: 10.1177/112067210701700603. [DOI] [PubMed] [Google Scholar]
- Choong YF, Lukman H, Martin S, Laws DE. Childhood amblyopia treatment: psychosocial implications for patients and primary carers. Eye (Lond) 2004;18:369–375. doi: 10.1038/sj.eye.6700647. [DOI] [PubMed] [Google Scholar]
- Chua B, Mitchell P. Consequences of amblyopia on education, occupation, and long term vision loss. Br J Ophthalmol. 2004;88:1119–1121. doi: 10.1136/bjo.2004.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Edwards AR, Wallace DK, Beck RW, Arnold RW, Astle WF, Barnhardt CN, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia M, Repka MX, Sala NA, Silbert DI, Weise KK. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113:895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Foster NC, Holmes JM, Melia BM, Wallace DK, Repka MX, Tamkins SM, Kraker RT, Beck RW, Hoover DL, Crouch ER, 3rd, Miller AM, Morse CL, Suh DW. Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology. 2012;119:150–158. doi: 10.1016/j.ophtha.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Tarczy-Hornoch K, Song E, Lin J, Borchert M, Azen SP, Varma R. Fixation preference and visual acuity testing in a population-based cohort of preschool children with amblyopia risk factors. Ophthalmology. 2009;116:145–153. doi: 10.1016/j.ophtha.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Varma R, Tarczy-Hornoch K, McKean-Cowdin R, Lin J, Wen G, Wei J, Borchert M, Azen SP, Torres M, Tielsch JM, Friedman DS, Repka MX, Katz J, Ibironke J, Giordano L. Risk factors associated with childhood strabismus: the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118:2251–2261. doi: 10.1016/j.ophtha.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann A, Petroni S, Salerni A, Dell’Omo R, Balestrazzi E. Unilateral amblyopia: An optical coherence tomography study. J AAPOS. 2009;13:148–150. doi: 10.1016/j.jaapos.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiol. 2005;12:243–250. doi: 10.1080/09286580590967772. [DOI] [PubMed] [Google Scholar]
- Fawcett S, Leffler J, Birch EE. Factors influencing stereoacuity in accommodative esotropia. J AAPOS. 2000;4:15–20. doi: 10.1016/s1091-8531(00)90006-5. [DOI] [PubMed] [Google Scholar]
- Fawcett SL, Birch EE. Risk factors for abnormal binocular vision after successful alignment of accommodative esotropia. J AAPOS. 2003;7:256–262. doi: 10.1016/s1091-8531(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Felius J, Hertle RW, Wang J, Subramanian V, Jost R, Berry PM, Birch EE. Ocular motor analysis of monocular visual preference in children with amblyopia. Investigative Ophthalmology & Visual Science. 2011;52:e4690. [Google Scholar]
- Felius J, Jost R, Stager DR., Sr Interocular asymmetries in square-wave oscillations in children with amblyopia. Investigative Ophthalmology & Visual Science. 2012;53:e3898. [Google Scholar]
- Friedman DS, Katz J, Repka MX, Giordano L, Ibironke J, Hawse P, Tielsch JM. Lack of concordance between fixation preference and HOTV optotype visual acuity in preschool children: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2008;115:1796–1799. doi: 10.1016/j.ophtha.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, Tielsch JM. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:2128–2134. e2121–2122. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu VL, Norcia AM, Birch EE, et al. Fusional suppression in accommodative and infantile esotropia. Investigative Ophthalmology & Visual Science. 2006;47:e2450. [Google Scholar]
- Giaschi DE, Regan D, Kraft SP, Hong XH. Defective processing of motion-defined form in the fellow eye of patients with unilateral amblyopia. Invest Ophthalmol Vis Sci. 1992;33:2483–2489. [PubMed] [Google Scholar]
- Giordano L, Friedman DS, Repka MX, Katz J, Ibironke J, Hawes P, Tielsch JM. Prevalence of refractive error among preschool children in an urban population: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:739–746. 746 e731–734. doi: 10.1016/j.ophtha.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest Ophthalmol Vis Sci. 2012;53:5386–5394. doi: 10.1167/iovs.12-9941. [DOI] [PubMed] [Google Scholar]
- Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci. 2007;48:1139–1148. doi: 10.1167/iovs.06-0976. [DOI] [PubMed] [Google Scholar]
- Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus. 2011;19:119–128. doi: 10.3109/09273972.2011.600423. [DOI] [PubMed] [Google Scholar]
- Groenewoud JH, Tjiam AM, Lantau VK, Hoogeveen WC, de Faber JT, Juttmann RE, de Koning HJ, Simonsz HJ. Rotterdam AMblyopia screening effectiveness study: detection and causes of amblyopia in a large birth cohort. Invest Ophthalmol Vis Sci. 2010;51:3476–3484. doi: 10.1167/iovs.08-3352. [DOI] [PubMed] [Google Scholar]
- Harrad R, Sengpiel F, Blakemore C. Physiology of suppression in strabismic amblyopia. Br J Ophthalmol. 1996;80:373–377. doi: 10.1136/bjo.80.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad R, Williams C. Risk, causes and outcomes of visual impairment after loss of vision in the non-amblyopic eye, a population-based study, by J. S. Rahi, S. Logan, C. Timms, I. Russel-Eggitt, and D. Taylor. Lancet 360:597–602, 2002. Surv Ophthalmol. 2003;48:235. [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neurosci. 2010;28:793–802. doi: 10.3233/RNN-2010-0550. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Black JM, Maehara G, Zhang P, Bobier WR, To L, Cooperstock J. An iPod treatment of amblyopia: An updated binocular approach. Optometry. (2012) 2012 Feb; http://viewer.zmags.com/publication/95f199fd-/95f199fd/28. [PubMed]
- Holmes JM, Beck RW, Kraker RT, Astle WF, Birch EE, Cole SR, Cotter SA, Donahue S, Everett DF, Hertle RW, Keech RV, Paysse E, Quinn GF, Repka MX, Scheiman MM. Risk of amblyopia recurrence after cessation of treatment. J AAPOS. 2004;8:420–428. doi: 10.1016/S1091853104001612. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Kraker RT, Beck RW, Birch EE, Cotter SA, Everett DF, Hertle RW, Quinn GE, Repka MX, Scheiman MM, Wallace DK. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–2087. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, Frazier MG, Hertle RW, Repka MX, Quinn GE, Weise KK. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol. 2011;129:1451–1457. doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Repka MX, Kraker RT, Clarke MP. The treatment of amblyopia. Strabismus. 2006;14:37–42. doi: 10.1080/09273970500536227. [DOI] [PubMed] [Google Scholar]
- Horwood J, Waylen A, Herrick D, Williams C, Wolke D. Common visual defects and peer victimization in children. Invest Ophthalmol Vis Sci. 2005;46:1177–1181. doi: 10.1167/iovs.04-0597. [DOI] [PubMed] [Google Scholar]
- Hunter DG, Nassif DS, Piskun NV, Winsor R, Gramatikov BI, Guyton DL. Pediatric Vision Screener 1: instrument design and operation. J Biomed Opt. 2004;9:1363–1368. doi: 10.1117/1.1805560. [DOI] [PubMed] [Google Scholar]
- Huynh SC, Samarawickrama C, Wang XY, Rochtchina E, Wong TY, Gole GA, Rose KA, Mitchell P. Macular and nerve fiber layer thickness in amblyopia: the Sydney Childhood Eye Study. Ophthalmology. 2009;116:1604–1609. doi: 10.1016/j.ophtha.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Huynh SC, Wang XY, Ip J, Robaei D, Kifley A, Rose KA, Mitchell P. Prevalence and associations of anisometropia and aniso-astigmatism in a population based sample of 6 year old children. Br J Ophthalmol. 2006;90:597–601. doi: 10.1136/bjo.2005.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing MR. Early surgery for essential infantile esotropia. J Pediatr Ophthalmol Strabismus. 1991;28:119. doi: 10.3928/0191-3913-19910301-15. [DOI] [PubMed] [Google Scholar]
- Ing MR, Okino LM. Outcome study of stereopsis in relation to duration of misalignment in congenital esotropia. J AAPOS. 2002;6:3–8. doi: 10.1067/mpa.2002.120172. [DOI] [PubMed] [Google Scholar]
- Kemper AR, Clark SJ. Preschool vision screening in pediatric practices. Clin Pediatr (Phila) 2006;45:263–266. doi: 10.1177/000992280604500309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper AR, Wallace DK, Patel N, Crews JE. Preschool vision testing by health providers in the United States: findings from the 2006–2007 Medical Expenditure Panel Survey. J AAPOS. 2011;15:480–483. doi: 10.1016/j.jaapos.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998;18:6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999;9:480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012;53:817–824. doi: 10.1167/iovs.11-8219. [DOI] [PubMed] [Google Scholar]
- Kothari M, Bhaskare A, Mete D, Toshniwal S, Doshi P, Kaul S. Evaluation of central, steady, maintained fixation grading for predicting inter-eye visual acuity difference to diagnose and treat amblyopia in strabismic patients. Indian J Ophthalmol. 2009;57:281–284. doi: 10.4103/0301-4738.53052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvarnstrom G, Jakobsson P, Lennerstrand G. Visual screening of Swedish children: an ophthalmological evaluation. Acta Ophthalmol Scand. 2001;79:240–244. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kee C. Optical coherence tomography and Heidelberg retina tomography for superior segmental optic hypoplasia. Br J Ophthalmol. 2009;93:1468–1473. doi: 10.1136/bjo.2009.157776. [DOI] [PubMed] [Google Scholar]
- Lempert P. Optic nerve hypoplasia and small eyes in presumed amblyopia. J AAPOS. 2000;4:258–266. doi: 10.1067/mpa.2000.106963. [DOI] [PubMed] [Google Scholar]
- Lempert P. Axial length-disc area ratio in esotropic amblyopia. Arch Ophthalmol. 2003;121:821–824. doi: 10.1001/archopht.121.6.821. [DOI] [PubMed] [Google Scholar]
- Lempert P. The axial length/disc area ratio in anisometropic hyperopic amblyopia: a hypothesis for decreased unilateral vision associated with hyperopic anisometropia. Ophthalmology. 2004;111:304–308. doi: 10.1016/j.ophtha.2003.05.020. [DOI] [PubMed] [Google Scholar]
- Lempert P. Optic disc area and retinal area in amblyopia. Semin Ophthalmol. 2008a;23:302–306. doi: 10.1080/08820530802505997. [DOI] [PubMed] [Google Scholar]
- Lempert P. Retinal area and optic disc rim area in amblyopic, fellow, and normal hyperopic eyes: a hypothesis for decreased acuity in amblyopia. Ophthalmology. 2008b;115:2259–2261. doi: 10.1016/j.ophtha.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Lempert P, Porter L. Dysversion of the optic disc and axial length measurements in a presumed amblyopic population. J AAPOS. 1998;2:207–213. doi: 10.1016/s1091-8531(98)90054-4. [DOI] [PubMed] [Google Scholar]
- Levi D. Visual acuity in strabismic and anisometropic amblyopia. Ophthalmololgy Clinics of North America. 1990;3:289–301. [Google Scholar]
- Levi DM. Prentice award lecture 2011: removing the brakes on plasticity in the amblyopic brain. Optom Vis Sci. 2012;89:827–838. doi: 10.1097/OPX.0b013e318257a187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Carkeet A. Amblyopia: A consquence of abnormal visual development. In: Simons K, editor. Early Visual Development: Normal and Abnormal. Oxford University Press; New York: 1993. pp. 391–405. [Google Scholar]
- Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Res. 1985;25:979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Res. 2011;51:48–57. doi: 10.1016/j.visres.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 2011;9:e1001135. doi: 10.1371/journal.pbio.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon SE, Fronius M, Looman CW, Awan M, Simonsz B, van der Maas PJ, Simonsz HJ. Predictors and a remedy for noncompliance with amblyopia therapy in children measured with the occlusion dose monitor. Invest Ophthalmol Vis Sci. 2006;47:4393–4400. doi: 10.1167/iovs.05-1428. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Polling JR, Simonsz HJ. A preliminary report about the relation between visual acuity increase and compliance in patching therapy for amblyopia. Strabismus. 2002;10:79–82. doi: 10.1076/stra.10.2.79.8143. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Polling JR, Simonsz HJ. Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefes Arch Clin Exp Ophthalmol. 2003;241:176–180. doi: 10.1007/s00417-002-0570-z. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Rook CA, Nassif DS, Piskun NV, Hunter DG. Rapid, high-accuracy detection of strabismus and amblyopia using the pediatric vision scanner. Invest Ophthalmol Vis Sci. 2011;52:5043–5048. doi: 10.1167/iovs.11-7503. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Simonsz HJ. The history of the treatment of amblyopia. Strabismus. 2005;13:93–106. doi: 10.1080/09273970590949818. [DOI] [PubMed] [Google Scholar]
- Mansouri B, Thompson B, Hess RF. Measurement of suprathreshold binocular interactions in amblyopia. Vision Res. 2008;48:2775–2784. doi: 10.1016/j.visres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Marsh-Tootle WL, Wall TC, Tootle JS, Person SD, Kristofco RE. Quantitative pediatric vision screening in primary care settings in Alabama. Optom Vis Sci. 2008;85:849–856. doi: 10.1097/OPX.0b013e318185282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SP, Harrad RA. Fusional suppression in normal and stereoanomalous observers. Vision Res. 1993;33:1645–1658. doi: 10.1016/0042-6989(93)90030-z. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Multi-Ethnic Pediatric Eye Disease Study. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months the multi-ethnic pediatric eye disease study. Ophthalmology. 2008;115:1229–1236. e1221. doi: 10.1016/j.ophtha.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif DS, Piskun NV, Gramatikov BI, Guyton DL, Hunter DG. Pediatric Vision Screener 2: pilot study in adults. J Biomed Opt. 2004;9:1369–1374. doi: 10.1117/1.1805561. [DOI] [PubMed] [Google Scholar]
- Nassif DS, Piskun NV, Hunter DG. The Pediatric Vision Screener III: detection of strabismus in children. Arch Ophthalmol. 2006;124:509–513. doi: 10.1001/archopht.124.4.509. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H. The functional significance of stereopsis. Invest Ophthalmol Vis Sci. 2010a;51:2019–2023. doi: 10.1167/iovs.09-4434. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci. 2010b;87:942–947. doi: 10.1097/OPX.0b013e3181fd132e. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Spencer R. Factors affecting development of motor skills in extremely low birth weight children. Strabismus. 2009;17:20–23. doi: 10.1080/09273970802679006. [DOI] [PubMed] [Google Scholar]
- Packwood EA, Cruz OA, Rychwalski PJ, Keech RV. The psychosocial effects of amblyopia study. J AAPOS. 1999;3:15–17. doi: 10.1016/s1091-8531(99)70089-3. [DOI] [PubMed] [Google Scholar]
- Pai AS, Rose KA, Leone JF, Sharbini S, Burlutsky G, Varma R, Wong TY, Mitchell P. Amblyopia prevalence and risk factors in Australian preschool children. Ophthalmology. 2012;119:138–144. doi: 10.1016/j.ophtha.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. The clinical profile of moderate amblyopia in children younger than 7 years. Arch Ophthalmol. 2002a;120:281–287. [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A randomized trial of atropine vs. patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002b;120:268–278. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A comparison of atropine and patching treatments for moderate amblyopia by patient age, cause of amblyopia, depth of amblyopia, and other factors. Ophthalmology. 2003;110:1632–1637. doi: 10.1016/S0161-6420(03)00500-1. discussion 1637–1638. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A prospective, pilot study of treatment of amblyopia in children 10 to <18 years old. Am J Ophthalmol. 2004;137:581–583. doi: 10.1016/j.ajo.2003.08.043. [DOI] [PubMed] [Google Scholar]
- Procianoy L, Procianoy E. The accuracy of binocular fixation preference for the diagnosis of strabismic amblyopia. J AAPOS. 2010;14:205–210. doi: 10.1016/j.jaapos.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Beck RW, Holmes JM, Repka MX. Recent advances in the treatment of amblyopia. Pediatrics. 2004;113:1800–1802. doi: 10.1542/peds.113.6.1800. [DOI] [PubMed] [Google Scholar]
- Rahi JS, Cumberland PM, Peckham CS. Does amblyopia affect educational, health, and social outcomes? Findings from 1958 British birth cohort. BMJ. 2006;332:820–825. doi: 10.1136/bmj.38751.597963.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan D, Giaschi DE, Kraft SP, Kothe AC. Method for identifying amblyopes whose reduced line acuity is caused by defective selection and/or control of gaze. Ophthalmic Physiol Opt. 1992;12:425–432. [PubMed] [Google Scholar]
- Repka MX, Beck RW, Holmes JM, Birch EE, Chandler DL, Cotter SA, Hertle RW, Kraker RT, Moke PS, Quinn GE, Scheiman MM. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121:603–611. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- Repka MX, Cotter SA, Beck RW, Kraker RT, Birch EE, Everett DF, Hertle RW, Holmes JM, Quinn GE, Sala NA, Scheiman MM, Stager DR, Sr, Wallace DK. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111:2076–2085. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Repka MX, Holmes JM. Lessons from the amblyopia treatment studies. Ophthalmology. 2012;119:657–658. doi: 10.1016/j.ophtha.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Beck RW, Birch E, Cotter SA, Holmes JM, Hertle RW, Hoover DL, Klimek DL, Marsh-Tootle W, Scheiman MM, Suh DW, Weakley DR. Treatment of severe amblyopia with weekend atropine: results from 2 randomized clinical trials. J AAPOS. 2009;13:258–263. doi: 10.1016/j.jaapos.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Wallace DK, Beck RW, Kraker RT, Birch EE, Cotter SA, Donahue S, Everett DF, Hertle RW, Holmes JM, Quinn GE, Scheiman MM, Weakley DR. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2005;123:149–157. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- Robaei D, Huynh SC, Kifley A, Mitchell P. Correctable and non-correctable visual impairment in a population-based sample of 12-year-old Australian children. Am J Ophthalmol. 2006;142:112–118. doi: 10.1016/j.ajo.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Rutstein RP, Quinn GE, Lazar EL, Beck RW, Bonsall DJ, Cotter SA, Crouch ER, Holmes JM, Hoover DL, Leske DA, Lorenzana IJ, Repka MX, Suh DW. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology. 2010;117:998–1004. e1006. doi: 10.1016/j.ophtha.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener EC, Mocan MC, Gedik S, Ergin A, Sanac AS. The reliability of grading the fixation preference test for the assessment of interocular visual acuity differences in patients with strabismus. J AAPOS. 2002;6:191–194. doi: 10.1067/mpa.2002.122364. [DOI] [PubMed] [Google Scholar]
- Shaw DE, Fielder AR, Minshull C, Rosenthal AR. Amblyopia--factors influencing age of presentation. Lancet. 1988;2:207–209. doi: 10.1016/s0140-6736(88)92301-x. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Fielder AR, Stephens DA, Moseley MJ. Design of the Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Br J Ophthalmol. 2002;86:915–919. doi: 10.1136/bjo.86.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Fielder AR, Stephens DA, Moseley MJ. Treatment of unilateral amblyopia: factors influencing visual outcome. Invest Ophthalmol Vis Sci. 2005;46:3152–3160. doi: 10.1167/iovs.05-0357. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder AR. Amblyopia therapy: an update. Strabismus. 2011;19:91–98. doi: 10.3109/09273972.2011.600421. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder AR, Stephens DA. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol. 2004a;88:1552–1556. doi: 10.1136/bjo.2004.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Invest Ophthalmol Vis Sci. 2004b;45:3048–3054. doi: 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ. Modeling dose-response in amblyopia: toward a child-specific treatment plan. Invest Ophthalmol Vis Sci. 2007a;48:2589–2594. doi: 10.1167/iovs.05-1243. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ. Objectively monitored patching regimens for treatment of amblyopia: randomised trial. BMJ. 2007b;335:707. doi: 10.1136/bmj.39301.460150.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Wang J, Hertle RW, Felius J, Birch EE. Eye Movement, optic nerve, and macular characteristics of children with persistent amblyopia. in press. [Google Scholar]
- Subramanian V, Jost R, Birch EE. A quantitative study of fixation stability in amblyopia. Journal of AAPOS. 2012;15:e30. doi: 10.1167/iovs.12-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczy-Hornoch K, Varma R, Cotter SA, McKean-Cowdin R, Lin JH, Borchert MS, Torres M, Wen G, Azen SP, Tielsch JM, Friedman DS, Repka MX, Katz J, Ibironke J, Giordano L. Risk factors for decreased visual acuity in preschool children: the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118:2262–2273. doi: 10.1016/j.ophtha.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley DH. Vision screening essentials: screening today for eye disorders in the pediatric patient. Pediatr Rev. 2007;28:54–61. doi: 10.1542/pir.28-2-54. [DOI] [PubMed] [Google Scholar]
- Tjiam AM, Holtslag G, Van Minderhout HM, Simonsz-Toth B, Vermeulen-Jong MH, Borsboom GJ, Loudon SE, Simonsz HJ. Randomised comparison of three tools for improving compliance with occlusion therapy: an educational cartoon story, a reward calendar, and an information leaflet for parents. Graefes Arch Clin Exp Ophthalmol. 2012 doi: 10.1007/s00417-012-2107-4. [DOI] [PubMed] [Google Scholar]
- To L, Thompson B, Blum JR, Maehara G, Hess RF, Cooperstock JR. A game platform for treatment of amblyopia. IEEE Trans Neural Syst Rehabil Eng. 2011;19:280–289. doi: 10.1109/TNSRE.2011.2115255. [DOI] [PubMed] [Google Scholar]
- Tychsen L. Can ophthalmologists repair the brain in infantile esotropia? Early surgery, stereopsis, monofixation syndrome, and the legacy of Marshall Parks. J AAPOS. 2005;9:510–521. doi: 10.1016/j.jaapos.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Tychsen L. Causing and curing infantile esotropia in primates: the role of decorrelated binocular input (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:564–593. [PMC free article] [PubMed] [Google Scholar]
- Tychsen L, Richards M, Wong A, Foeller P, Bradley D, Burkhalter A. The neural mechanism for latent (fusion maldevelopment) nystagmus. J Neuroophthalmol. 2010;30:276–283. doi: 10.1097/WNO.0b013e3181dfa9ca. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Wong AM, Foeller P, Bradley D. Early versus delayed repair of infantile strabismus in macaque monkeys: II. Effects on motion visually evoked responses. Invest Ophthalmol Vis Sci. 2004;45:821–827. doi: 10.1167/iovs.03-0564. [DOI] [PubMed] [Google Scholar]
- Walker RA, Rubab S, Voll AR, Erraguntla V, Murphy PH. Macular and peripapillary retinal nerve fibre layer thickness in adults with amblyopia. Can J Ophthalmol. 2011;46:425–427. doi: 10.1016/j.jcjo.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Wall TC, Marsh-Tootle W, Evans HH, Fargason CA, Ashworth CS, Hardin JM. Compliance with vision-screening guidelines among a national sample of pediatricians. Ambul Pediatr. 2002;2:449–455. doi: 10.1367/1539-4409(2002)002<0449:cwvsga>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Edwards AR, Cotter SA, Beck RW, Arnold RW, Astle WF, Barnhardt CN, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia M, Repka MX, Sala NA, Silbert DI, Weise KK. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113:904–912. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DK, Kraker RT, Beck RW, Cotter SA, Davis PL, Holmes JM, Repka MX, Suh DW. Randomized trial to evaluate combined patching and atropine for residual amblyopia. Arch Ophthalmol. 2011a;129:960–962. doi: 10.1001/archophthalmol.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DK, Lazar EL, Melia M, Birch EE, Holmes JM, Hopkins KB, Kraker RT, Kulp MT, Pang Y, Repka MX, Tamkins SM, Weise KK. Stereoacuity in children with anisometropic amblyopia. J AAPOS. 2011b;15:455–461. doi: 10.1016/j.jaapos.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman RC, Croft CA, Brotherton SE. Preschool vision screening in pediatric practice: a study from the Pediatric Research in Office Settings (PROS) Network. American Academy of Pediatrics. Pediatrics. 1992;89:834–838. [PubMed] [Google Scholar]
- Weakley DR. The association between anisometropia, amblyopia, and binocularity in the absence of strabismus. Trans Am Ophthalmol Soc. 1999;97:987–1021. [PMC free article] [PubMed] [Google Scholar]
- Weakley DR., Jr The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108:163–171. doi: 10.1016/s0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci. 2008a;49:594–603. doi: 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optom Vis Sci. 2008b;85:1074–1081. doi: 10.1097/OPX.0b013e31818b9911. [DOI] [PubMed] [Google Scholar]
- Williams C, Harrad R. Amblyopia: contemporary clinical issues. Strabismus. 2006;14:43–50. doi: 10.1080/09273970500538090. [DOI] [PubMed] [Google Scholar]
- Williams C, Harrad RA, Harvey I, Sparrow JM. Screening for amblyopia in preschool children: results of a population-based, randomised controlled trial. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Ophthalmic Epidemiol. 2001;8:279–295. doi: 10.1080/09286586.2001.11644257. [DOI] [PubMed] [Google Scholar]
- Williams C, Northstone K, Harrad RA, Sparrow JM, Harvey I. Amblyopia treatment outcomes after screening before or at age 3 years: follow up from randomised trial. BMJ. 2002;324:1549. doi: 10.1136/bmj.324.7353.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Northstone K, Harrad RA, Sparrow JM, Harvey I. Amblyopia treatment outcomes after preschool screening v school entry screening: observational data from a prospective cohort study. Br J Ophthalmol. 2003;87:988–993. doi: 10.1136/bjo.87.8.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008;92:959–964. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Hiscox F, Thompson JR, Smith LK. Factors affecting the outcome of children treated for amblyopia. Eye (Lond) 1994a;8 (Pt 6):627–631. doi: 10.1038/eye.1994.157. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Hiscox F, Thompson JR, Smith LK. The presentation of children with amblyopia. Eye (Lond) 1994b;8 ( Pt 6):623–626. doi: 10.1038/eye.1994.156. [DOI] [PubMed] [Google Scholar]
- Wright KW, Edelman PM, McVey JH, Terry AP, Lin M. High-grade stereo acuity after early surgery for congenital esotropia. Arch Ophthalmol. 1994;112:913–919. doi: 10.1001/archopht.1994.01090190061022. [DOI] [PubMed] [Google Scholar]
- Wright KW, Edelman PM, Walonker F, Yiu S. Reliability of fixation preference testing in diagnosing amblyopia. Arch Ophthalmol. 1986;104:549–553. doi: 10.1001/archopht.1986.01050160105023. [DOI] [PubMed] [Google Scholar]
- Wu C, Hunter DG. Amblyopia: diagnostic and therapeutic options. Am J Ophthalmol. 2006;141:175–184. doi: 10.1016/j.ajo.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Yanni SE, Jost R, Beauchamp CL, Stager DR, Sr, Birch EE. Objective detection of amblyopia and strabismus … not just risk factors. Journal of AAPOS. 2012;16:e9. doi: 10.1001/jamaophthalmol.2014.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




