Abstract
Although caffeine can enhance cognitive function acutely, long-term effects of consumption of caffeine-containing beverages such as tea and coffee are uncertain. Data on 4,809 participants aged 65 and older from the Cardiovascular Health Study (CHS) were used to examine the relationship of consumption of tea and coffee, assessed by food frequency questionnaire, on change in cognitive function by gender. Cognitive performance was assessed using serial Modified Mini-Mental State (3MS) examinations, which were administered annually up to 9 times. Linear mixed models were used to estimate rates of change in standard 3MS scores and scores modeled using item response theory (IRT). Models were adjusted for age, education, smoking status, clinic site, diabetes, hypertension, stroke, coronary heart disease, depression score, and APOE genotype. Over the median 7.9 years of follow-up, participants who did not consume tea or coffee declined annually by an average of 1.30 points (women) and 1.11 points (men) on standard 3MS scores. In fully adjusted models using either standard or IRT 3MS scores, we found modestly reduced rates of cognitive decline for some, but not all, levels of coffee and tea consumption for women, with no consistent effect for men. Caffeine consumption was also associated with attenuation in cognitive decline in women. Dose-response relationships were not linear. These longitudinal analyses suggest a somewhat attenuated rate of cognitive decline among tea and coffee consumers compared to non-consumers in women but not in men. Whether this association is causal or due to unmeasured confounding requires further study.
Keywords: Caffeine, coffee, cognition, tea
INTRODUCTION
Experimental studies suggest that physical activity, social engagement, caloric restriction, and mental exercise may be effective in delaying cognitive decline, a major problem in our aging population [1]. Observational studies have suggested that tea and coffee possibly reduce cognitive decline. Among the seven published prospective studies examining the effect of tea, coffee, or caffeine consumption on cognitive decline (Table 1), findings have been inconclusive [2–8]. Tea has only been studied in one cohort of older Chinese adults, and consumption was inversely associated with cognitive decline [5]. Coffee has been studied in four longitudinal studies with no consistent associations observed [3, 5–7]. Among three prospective studies, caffeine was inversely associated with cognitive decline in two, but only among women [2, 4, 8].
Table 1.
Longitudinal studies of Tea, Coffee or Caffeine and cognitive status or cognitive decline
| Associations with cognitive decline |
||||||
|---|---|---|---|---|---|---|
| Country and Year [reference] |
N | Age (follow up duration) |
Tea | Coffee | Caffeine | Mean consumption |
| Netherlands 2003 [2] |
1376 | 24–81 (6 y) |
No effect | Mean caffeine consumption ranged from approximately 340–550 mg/day.a |
||
| Finland, Italy, Netherlands 2007 [3] |
676 | ‘Elderly’ (10 y) |
Less decline in 3 categories, no linear dose response |
Mean coffee consumption calculated to be 2.2 cups/day.b |
||
| France 2007 [4] | 7017 | ≥65 (4 y) |
Less decline in women only |
Mean caffeine consumption calculated to be 176 mg/day for men and 186 mg/day for women.c |
||
| Chinese living in Singapore 2008 [5] |
1438 | ≥551, (2 y) |
Lower prevalence of impairment with p for trend <0.001 Less decline with p for trend = 0.04 |
67%–70% drank less than 1 cup/week. 12%–15% drank more than 1 cup/week but less than 1 cup/day. 18%–19% drank≥1 cup/day.d |
||
| Finland, CAIDE Study 2009 [6] |
1409 | 50–72 (21 y) |
No effect, but very few tea drinkers |
65% reduction in risk of dementia with 3–5 cups/d, no linear dose response |
60.5% did not drink tea, and 80% of tea drinkers consumed 1, 2 cups per day. Mean coffee consumption calculated to be 4.3 cups/day.e |
|
| Finland, Twin Study 2009 [7] |
2606 | 46–52 (28 y) |
No effect | Mean coffee consumption for both men and women calculated to be 5.4 cups/day.f |
||
| Portugal 2010 [8] | 648 | ≥65 (5 y) |
Less decline in women only |
Caffeine intake medians were 32 mg/day for women and 33 to 52 mg/day for men.g |
||
Read from Fig. 1 of article.
Calculated from “Categories of daily coffee consumption” in Table 2 of article. Five cups assumed for category “>4 cups”.
Calculated from Table 1 of article. Categories calculated as: 0, 1 units = 50 mg; 1, 2 units = 150 mg; 2, 3 units = 250 mg; and >3 units = 350 mg.
Black or oolong tea consumptions read from Table 1 of article.
Calculated from Table 1 of article. Categories calculated as: 0–2 cups/day = 1 cup/day; 3–5 cups/day = 4 cups/day; and≥5 cups/day = 6 cups/day.
Calculated from Table 1 of article. Categories calculated as: 0–3 cups/day = 1.5 cups/day; 3.5–8 cups/day = 5.75 cups/day; and≥8 cups/day = 10 cups/day.
Read from Table 1 of article. Multiple medians are provided because cohort was divided into three categories.
If a true effect exists, it might be mediated by caffeine, one of the active ingredients common to both tea and coffee. As a widely used psychoactive agent, caffeine affects information processing speed, attention, and reaction time [9]. Caffeine consumption has been associated with enhanced acute cognitive performance in specific domains and in sleep-compromised individuals [10–14]. Caffeine is known to be an adenosine receptor antagonist in the brain. Cognitive effects of caffeine are believed to be a function of its ability to antagonize A1 adenosine receptors in the hippocampus and cortex. As endogenous adenosine inhibits long-term synaptic plasticity phenomena A1 adenosine receptor antagonists have been proposed as treatment for memory disorders. [15–17].
Tea is a weaker source of caffeine, with less than half the caffeine per cup found in caffeinated coffee [18]. Tea also has a number of substances other than caffeine that have been demonstrated to cross the blood-brain barrier. These substances include theanine, an amino acid found only in tea and mushrooms [19, 20]. Theanine may be a neurostimulant both independently [21, 22] and in combination with caffeine [23], and some evidence suggests that theanine and caffeine together may affect cognition more strongly than caffeine alone [14, 24, 25].
Both tea and coffee have been demonstrated to improve acute attention [26, 27], subjective alertness, and psychomotor performance [28, 29]. The study of longer-term effects of tea and coffee on cognition, and particularly on cognitive decline, presents many challenges including: testing demented elderly, missing values, loss to follow up, many potential confounding variables, and changes in exposure categorization for reasons that might be related to the outcomes of interest. In addition, commonly used measures of global cognition, such as the 100-point Modified Mini-Mental State (3MS) examination [30, 31], are characterized by uneven distributions of item difficulty across the ability spectrum. The use of standard total scores can result in bias in estimating rates of decline [32]. For example, a 5-point drop in standard 3MS scores for high-functioning individuals represents a much greater cognitive decline than a 5-point drop in individuals with moderate functioning. Few studies to date have taken the uneven distribution of item difficulty into account.
In this paper, we examine tea and coffee consumption in a large prospective study of older adults, the Cardiovascular Health Study (CHS), whose long follow-up makes it particularly valuable for this purpose. We used annual 3MS data, collected over up to 9 years (median 7.9), to determine cognitive decline as a function of baseline tea, coffee, and total caffeine consumption. Our hypothesis is that consumption of caffeinated beverages (tea and coffee) is associated with a slower rate of decline in cognitive functioning of older adults.
MATERIALS AND METHODS
Study population
CHS is a prospective, population-based longitudinal study of cardiovascular disease in participants aged 65 years and older. Details of the study design, sampling, and recruitment have been published [30, 33]. Briefly, 5,201 men and women were enrolled in 1989 and 1990 from a random sample of Medicare-eligible residents in four U.S. communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania. Participants underwent a comprehensive evaluation at baseline, including administration of food frequency and other questionnaires, physical examination, cognitive assessment with the 3MS, and laboratory testing. We did not include data from a supplemental cohort of 687 African-American men and women enrolled during 1992 and 1993 because a food frequency questionnaire was not administered to these individuals at baseline. Each center’s institutional review committee approved the study, and all participants provided informed written consent.
Dietary assessment
Usual dietary intake was assessed at baseline using a picture-sort version of the National Cancer Institute food frequency questionnaire [34]. Participants were asked to indicate how often, on average, they had consumed specific foods and beverages during the past year, including tea, coffee, regular soda, and diet soda. Choices were provided as ordinal categories: “Never or less than 5 times per year” (referred to as “non-consumers”), “5–10 times per year”, “1–3 times per month”, “1–4 times per week”, and “almost every day”. We estimated total weekly caffeine consumption for participants by multiplying their reported frequency of tea and coffee consumption by the respective estimates of caffeine content: 30 mg caffeine per cup of tea and 85 mg caffeine per cup of coffee [35] and summing the caffeine amounts from tea and coffee. We assumed a serving size of 1 cup and estimated consumption in cups per week based on the ordinal responses as follows: Never or less than 5 times per year: 0 cups per week; 5–10 times per year: 0.14 cups per week; 1–3 times per month: 0.46 cups per week; 1–4 times per week: 2.5 cups per week; and almost every day: 5 cups per week. As the questionnaire did not distinguish between caffeinated and non-caffeinated sodas, calculation of total caffeine included only tea and coffee sources. Dietary intake, including coffee, tea, caffeinated, and decaffeinated soda, was assessed again in 1995–1996 using Willet’s food frequency questionnaire [36]. We used data from the second dietary assessment to estimate the relative stability of tea and coffee consumption over 6 years, the numbers of cups consumed per day at that latter time point and the frequency of intake of caffeinated sodas in the CHS population.
Cognitive assessments
Cognitive function was measured during annual in-person visits from 1990–1999 using the modified Mini-Mental State (3MS) examination [31]. Beginning in 1996, participants who did not attend the in-person visit were contacted by phone and asked to complete the Telephone Interview for Cognitive Status (TICS), a brief 11-item telephone interview designed to identify cognitive impairment without relying on a face-to-face interaction [37]. Alternatively, a proxy was administered the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), a 26-item questionnaire designed to assess changes in cognitive performance compared to 10 years prior [38].
Statistical analysis
We excluded from the analysis, participants who were missing information on tea or coffee consumption (n = 61) or were missing scores for cognitive function at the first follow-up visit in 1990–1991 (n = 331), which served as baseline for this analysis. The final analysis sample included 4,809 participants: 2,722 women and 2,077 men.
As with other commonly used global cognitive tests, standard 3MS test items do not have difficulty levels evenly distributed across the ability spectrum [32]. Prior analyses using CHS data have shown that this uneven distribution of item difficulties in the standard 3MS can result in biased estimates of rates of decline if standard scores are used [32]. To minimize this bias, we used Item Response Theory (IRT) to score standard 3MS data, resulting in a metric with linear scaling properties [39, 40]. We used both standard 3MS scores and IRT 3MS scores in analyses. Analyses using the standard 3MS scores provide a sense of the magnitude of change on the original scale and allow comparability with other research. Analyses using the IRT 3MS scores provide a more statistically robust estimate of the associations evaluated.
To generate the IRT scores, we used Samejima’s graded response model [41, 42] to estimate item parameters from the baseline time point with Parscale software [43]. We then treated those item parameters as fixed to obtain cognitive ability estimates from subsequent time points. For the values presented in Fig. 1, we transformed Parscale’s output such that the mean at baseline was 100 and the standard deviation was 15, as we have done in the past [39]. Results from models fit on 3MS scores transformed using log (101-3MS) were very similar to those from models using the IRT 3MS scores and are not presented.
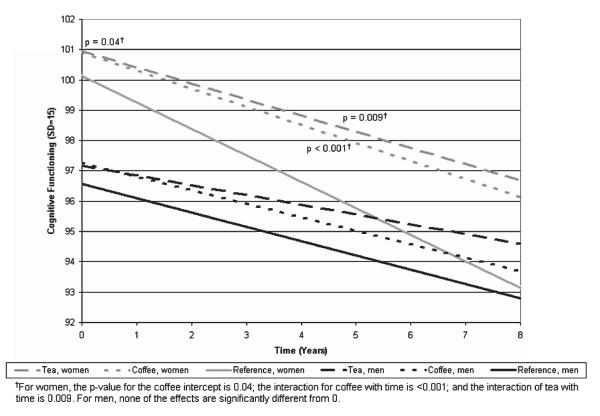
Fig. 1.
Relationship between tea and coffee consumption versus non-consumption and cognition and cognitive decline by gender. Tea and coffee estimates are from a fully adjusted model using IRT 3MS score and including both tea and coffee consumers, regardless of the level of consumption (≥5 cups per year). Non-consumers of tea and coffee comprise the reference group (<5 cups per year). IRT 3MS scores are scaled such that the mean score at baseline is 100 and the standard deviation is 15. The approximately 1 point difference between tea or coffee consumers at baseline thus represents 1/15 of the standard deviation.
Previous research within the CHS cohort has indicated that participants experiencing greater cognitive decline were less likely to participate in the in-person visits [44]. To minimize the influence of this type of non-participation on the assessment of cognitive function over time, we incorporated data from the two alternative cognitive assessments administered by telephone, the TICS and IQCODE. For the standard 3MS scores, we used a regression model based on the TICS and IQCODE, a method previously developed and validated in the CHS cohort [44]. A total of 1,141 (3%) 3MS scores were imputed among participants with TICS and IQCODE scores, but without standard 3MS scores, at one or more annual assessments during this period. For the IRT 3MS scores, we co-calibrated the TICS with the standard 3MS using CHS data when both tests were present (332 person-occasions). We used 3MS item parameters from the baseline with 3MS and TICS item responses to obtain item parameters for TICS items on the same scale as 3MS [32]. We then used these TICS item parameters to obtain cognitive ability estimates from time points when only TICS data were available.
We used linear mixed models to examine the association between baseline beverage consumption and the change in cognitive test scores over time using both the observed and imputed standard 3MS scores, and the IRT 3MS combined with TICS scores. One model included both tea and coffee with the referent group being non-consumers of both beverages. Another model included quintiles of caffeine with the referent group being those in the lowest quintile. Participants who had only a baseline cognitive evaluation (n = 175) contributed to estimation of intercepts and were retained. All models were adjusted for age at baseline (continuous), gender, race (African American, non-African American), educational attainment (<high school, high school graduate or some college, ≥college graduate), field center, diabetes, hypertension, current smoking, history of stroke, history of coronary heart disease (CHD), Center for Epidemiologic Studies Depression Scale (CES-D) score [45], and APOEε4 allele carriership. Coefficients of the main effect beverage terms can be interpreted as the average point difference in cognitive test scores associated with consumption of the beverage compared with the reference group (never or <5 cups/year) at baseline. The coefficients of the beverage-by-time interaction terms can be interpreted as the average annual difference in slopes (rates of change in cognitive test scores) between the beverage consumers and the reference group.
We obtained regression results from a similar model in which we grouped together all levels of coffee and all levels of tea consumption of 5 cups or more per year because a linear trend by level of consumption was lacking for both beverages. We used these results to predict IRT 3MS scores at baseline and 8 years after baseline, just over the median duration of follow-up in the sample, separately for men and women and for tea and coffee. For each gender, we plotted these results using Excel separately for non-consumers, tea consumers, and coffee consumers. Analyses were conducted using Stata [46]; Parscale was used to calculate 3MS IRT scores [43].
RESULTS
For men and women combined, participants were fairly evenly distributed across the five categories of tea consumption with: 23.5%, less than 5 times per year; 11.1%, 5–10 times per year; 18.5%, 1–3 times per month; 22.2%, 1–4 times per week; and 24.6 %, 5 or more times per week. Coffee consumption was more frequent than tea consumption with 43.1% consuming coffee 5 or more times per week. We were able to determine stability of consumption by examining coffee and tea consumption in a subset of cohort survivors 6 years after their baseline measurements. We found that 73% of those in the lowest category of tea consumption at baseline remained non-consumers of tea 6 years later, as did 77% of those in the lowest category of coffee consumption. At the other extreme, 73% of those reporting tea consumption and 78% of those reporting coffee consumption more than once a week at baseline maintained this level of consumption at the later interview. This latter assessment had more details on caffeinated beverage consumption than the initial assessment and showed that only 5% of the participants consumed caffeinated sodas on a daily basis. From the latter interview where more information on number of cups was collected, we calculated a mean consumption of 0.95 cups of coffee per day and 0.57 cups of tea per day.
The distribution of socio-demographic characteristics by category of tea and coffee consumption is presented in Table 2a for women and Table 2b for men. The mean age of consumers of both tea and coffee was highest at the lowest level of consumption. Higher proportions of men drank coffee and lower proportions drank tea. Tea consumption was associated with higher education and less cigarette smoking. Individuals with a history of cardiovascular disease or stroke were less likely to be high consumers of coffee. Many aspects of diet were associated with tea and coffee consumption. Generally healthier eating habits (such as higher cereal and vegetable consumption) were noted among tea drinkers and less healthy habits noted among coffee drinkers. The mean scores for the standard 3MS and IRT 3MS are presented in the last two rows of Tables 2a and 2b. The unadjusted mean score for the standard 3MS among all CHS participants at baseline was 90.5 and was positively associated with consumption of both tea (ptrend = 0.001) and coffee (ptrend < 0.001).
Table 2a.
Demographic, medical, lifestyle and nutrition characteristics of female CHS participants at baseline (n = 2.722), according to usual consumption of Tea and Coffee
| Women Characteristic |
Tea consumption at baseline |
Coffee consumption at baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <5/yr N = 580 (21.1%) |
5–10/yr N = 289 (10.6%) |
1–3/mo N = 503 (18.4%) |
1–4/wk N = 618 (22.6%) |
≥5/wk N = 742 (27.2%) |
<5/yr N = 1, 019 (37.4%) |
5–10/yr N = 169 (6.2%) |
1–3/mo N = 178 (6.5%) |
1–4/wk N = 208 (7.6%) |
≥5/wk N = 1.148 (42.2%) |
|
| Age, mean (SD), y | 72.7 (5.4) | 72.0 (5.3) | 71.7 (5.0) | 72.2 (5.0) | 72.2 (5.3) | 72.7 (5.4) | 71.1 (5.0) | 71.3 (4.6) | 72.3 (5.5) | 72.0(5.1) |
| Black, % | 4.5 | 7.6 | 4.4 | 5.3 | 3.4 | 5.6 | 3.0 | 7.3 | 4.3 | 3.8 |
| <High school education, % |
33.6 | 26.6 | 19.5 | 23.5 | 24.8 | 28.6 | 15.5 | 23.0 | 20.8 | 25.9 |
| Current smoker, % | 14.5 | 14.5 | 13.7 | 9.6 | 11.1 | 9.1 | 7.7 | 7.9 | 9.6 | 16.9 |
| Body mass index, mean (SD), kg/m2 |
26.6 (5.2) | 26.3 (5.1) | 26.3 (5.1) | 26.8 (5.0) | 26.1 (4.8) | 26.8 (5.3) | 26.4 (4.9) | 26.7 (5.5) | 26.1 (4.4) | 26.2 (4.8) |
| History of CHD, % | 16.2 | 15.9 | 13.1 | 12.3 | 15.0 | 18.1 | 8.3 | 18.0 | 15.4 | 11.3 |
| History of stroke, % | 2.8 | 1.7 | 2.0 | 1.9 | 2.7 | 2.9 | 1.2 | 1.7 | 3.4 | 1.9 |
| Diabetes, % | 16.7 | 10.1 | 12.8 | 10.2 | 12.0 | 14.8 | 10.1 | 15.3 | 13.0 | 10.4 |
| Hypertension, % | 59.5 | 51.7 | 53.8 | 58.6 | 58.7 | 59.9 | 52.7 | 54.2 | 57.5 | 55.7 |
| Physical activity, median (IQR), kcal/day |
1005 (296–2160) |
1054 (375–2252) |
1118 (475–2469) |
1014 (394–2151) |
1010 (397–2270) |
405 (0–1198) |
490 (90–1238) |
476 (126–1294) |
453 (94–1213) |
525 (82–1175) |
| CES–D score, median (IQR) |
4(1–7) | 4 (1–7) | 4 (1–6) | 4 (2–8) | 4 (2–7) | 4 (2–7) | 4 (2–7) | 4 (2–7) | 4 (2–7) | 4 (1–7) |
| ApoE4 carrier, % | 25.8 | 21.8 | 23.1 | 24.2 | 27.6 | 26.1 | 25.2 | 27.6 | 19.8 | 24.5 |
| Fair or poor self–reported health |
26.4 | 22.2 | 23.4 | 21.1 | 21.2 | 26.0 | 16.6 | 25.8 | 19.3 | 21.1 |
| Vegetables, mean (SD), servings/d |
2.6 (1.5) | 2.5 (1.2) | 2.6 (1.3) | 2.6 (1.4) | 2.7 (1.4) | 2.6 (1.4) | 2.7 (1.3) | 2.7 (1.3) | 2.7 (1.4) | 2.6 (1.4) |
| Cereal fiber, mean (SD), g/d |
4.2 (2.8) | 3.9 (2.8) | 4.4 (2.5) | 4.3 (2.6) | 4.4 (2.8) | 4.4 (2.7) | 4.3 (2.8) | 4.5 (2.5) | 4.1 (2.6) | 4.2 (2.7) |
| Saturated fat, mean (SD), % calories |
11.3 (3.3) | 11.2 (3.0) | 11.4 (2.9) | 11.5 (2.9) | 11.6 (3.0) | 11.0 (3.1) | 10.6 (2.8) | 11.5 (2.9) | 11.6 (3.1) | 11.9 (2.9) |
| Total energy intake, mean (SD), kcal/d |
1652 (674) | 1628 (572) | 1671 (606) | 1730 (623) | 1848 (702) | 1686 (643) | 1684 (638) | 1747 (600) | 1687 (649) | 1767 (672) |
| Baseline cognitive scores* |
||||||||||
| Standard 3MS, mean (SD) | 90.3 (8.8) | 91.0 (8.3) | 92.5 (7.3) | 91.7 (6.9) | 91.2 (7.8) | 90.6 (8.2) | 92.9 (6.6) | 91.7 (10.6) | 91.4 (7.0) | 91.8 (7.2) |
| IRT 3MS, mean (SD) | 101.25 (15.1) | 102.67 (15.2) | 105.65 (14.7) | 103.94 (14.3) | 103.63 (14.6) | 101.95 (15.3) | 105.53 (14.6) | 105.80 (13.2) | 103.53 (14.4) | 103.16 (14.1) |
At 1990–91 visit.
Abbreviations: SD = standard deviation, IQR = interquartile range, CHD = coronary heart disease, IRT = Item response theory, 3MS = Modified Mini–Mental State Examination, CES–D score = Center for Epidemiologic Studies Depression Scale.
Table 2b.
Demographic, medical, lifestyle and nutrition characteristics of male CHS participants at baseline (n = 2.077), according to usual consumption of Tea and Coffee
| Men Characteristic |
Tea consumption at baseline |
Coffee consumption at baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <5/yr N = 551 (26.5%) |
5–10/yr N = 244 (11.7%) |
1–3/mo N = 387 (18.6%) |
1–4/wk N = 452 (21.8%) |
≥5/wk N = 443 (21.3%) |
<5/yr N = 637 (30.8%) |
5–10/yr N = 139 (6.7%) |
1–3/mo N = 152 (7.4%) |
1–4/wk N = 225 (10.9%) |
≥5/wk N = 915 (44.2%) |
|
| Age, mean (SD), y | 74.0 (6.0) | 72.8 (5.4) | 72.8 (5.6) | 72.8 (5.2) | 73.0 (5.6) | 73.7 (5.9) | 73.3 (5.5) | 72.9 (5.2) | 73.1 (5.5) | 72.8 (5.5) |
| Black, % | 3.8 | 3.7 | 3.9 | 4.4 | 3.8 | 6.4 | 2.9 | 5.9 | 1.3 | 2.7 |
| <High school education, % |
33.9 | 23.9 | 23.8 | 24.4 | 26.0 | 29.7 | 26.6 | 23.0 | 27.0 | 26.0 |
| Current smoker, % | 9.1 | 8.2 | 11.7 | 9.5 | 10.4 | 6.9 | 5.8 | 8.0 | 8.0 | 13.2 |
| Body mass index, mean (SD), kg/m2 |
26.3 (3.8) | 26.8 (3.7) | 26.4 (3.6) | 26.7 (3.7) | 26.1 (3.7) | 26.4 (3.9) | 26.5 (3.8) | 26.1 (3.5) | 26.6 (3.5) | 26.4 (3.6) |
| History of CHD, % | 24.7 | 25.0 | 22.5 | 25.0 | 28.0 | 28.4 | 29.5 | 27.0 | 25.3 | 22.0 |
| History of stroke, % | 6.0 | 5.7 | 2.1 | 7.1 | 4.1 | 7.1 | 5.8 | 3.3 | 6.7 | 3.5 |
| Diabetes, % | 19.1 | 16.4 | 16.6 | 17.7 | 18.4 | 18.4 | 15.8 | 14.6 | 18.7 | 18.2 |
| Hypertension, % | 56.6 | 54.9 | 54.5 | 54.0 | 51.6 | 52.8 | 59.7 | 49.3 | 55.1 | 55.4 |
| Physical activity, median (IQR), kcal/day |
1350 (544–2771) |
1238 (540–2948) |
1470 (589–2943) |
1375 (555–3116) |
1365 (540–2790) |
1050 (368–2415) |
1334 (405–2715) |
1185 (473–2484) |
1260 (585–2786) |
1065 (375–2520) |
| CES–D score, median (IQR) |
3 (1–5) | 2 (1–5) | 3 (1–5) | 3 (1–5) | 3 (1–5) | 3 (1–5) | 3 (1–5) | 3 (1–5) | 2 (1–6) | 3 (1–5) |
| APOEε4 carrier, % | 24.6 | 23.6 | 26.7 | 23.4 | 23.8 | 25.0 | 23.0 | 23.9 | 26.7 | 23.7 |
| Fair or poor self–reported health |
23.5 | 19.7 | 17.9 | 21.1 | 19.4 | 23.9 | 20.9 | 20.5 | 19.2 | 18.8 |
| Vegetables, mean (SD), servings/d |
2.2 (1.4) | 2.3 (1.3) | 2.3 (1.2) | 2.3 (1.3) | 2.5 (1.4) | 2.3 (1.3) | 2.4 (1.3) | 2.4 (1.2) | 2.4 (1.5) | 2.2 (1.3) |
| Cereal fiber, mean (SD), g/d |
3.8 (2.7) | 4.1 (2.6) | 4.1 (2.6) | 4.3 (2.6) | 4.2 (2.6) | 4.1 (2.6) | 4.5 (2.5) | 4.4 (2.6) | 4.4 (2.7) | 3.9 (2.6) |
| Saturated fat, mean (SD), % calories |
12.4 (3.4) | 12.2 (2.8) | 12.2 (3.0) | 12.5 (2.9) | 12.8 (3.0) | 12.0 (3.3) | 12.1 (2.9) | 12.4 (2.8) | 12.6 (2.9) | 12.9 (3.0) |
| Total energy intake, mean (SD), kcal/d |
1844 (722) | 1803 (552) | 1853 (628) | 2034 (721) | 2077 (772) | 1843 (699) | 1903 (610) | 2037 (704) | 2024 (754) | 1957 (707) |
| Baseline cognitive scores* |
||||||||||
| Standard 3MS, mean (SD) | 88.3 (9.5) | 91.0 (9.4) | 90.7 (7.3) | 89.6 (8.6) | 89.5 (8.6) | 88.6 (9.6) | 89.3 (10.8) | 90.6 (8.9) | 89.2 (10.7) | 90.3 (8.0) |
| IRT 3MS, mean (SD) | 98.78 (15.3) | 102.77 (15.2) | 101.86 (15.1) | 102.17 (15.1) | 100.61 (14.4) | 99.94 (15.5) | 102.41 (15.2) | 102.44 (14.8) | 100.93 (15.8) | 101.30 (14.5) |
At 1990–91 visit.
Abbreviations: SD = standard deviation, IQR = interquartile range, CHD = coronary heart disease, IRT = Item response theory, 3MS = Modified Mini–Mental State Examination, CES–D score = Center for Epidemiologic Studies Depression Scale.
The relationships between consumption of tea and both caffeinated and decaffeinated coffee are presented in Appendix 1. Of those who reported drinking tea 5 or more times per week, 42.8% also drank coffee at least 5 times per week. Conversely, of those who reported drinking coffee 5 or more times per week, 24.4% also drank tea at least 5 times per week. 49.4% of the non-tea drinkers also did not drink coffee, and 33.6% of the non-coffee drinkers did not drink tea either. At the other extreme, 38.0% of those who drank 5 or more cups per week of tea never drank coffee. However, as can be seen in the appendix, the distribution of use varied enough to model each effect separately. As might be expected, heavy consumers of decaffeinated coffee were more likely to drink tea heavily and less likely to drink caffeinated coffee. These mixed drinking patterns are reflected in total caffeine intakes from tea and coffee combined, which had a median of 363 mg/week among the heavy tea drinkers and 439 mg/week among the heavy coffee drinkers. These findings were in contrast to median intakes of 12 mg/week and 14 mg/week, respectively, among those who professed to drinking tea and coffee rarely.
Table 3 shows results from four different regression models, with separate models for women and men, each with standard or IRT 3MS scores as the dependent variable. The first numeric column of the table shows results from a model for women with standard 3MS scores where women who drank less than 5 cups of tea and coffee per year formed the reference group. Sections I and II of results show the adjusted mean difference in the intercept (model estimated baseline) for total 3MS score associated with each level of tea and coffee consumption. Women who drank 5–10 cups of tea per year were 0.26 standard 3MS points lower at baseline than women with less than 5 cups per year on average, after adjustment’ (95% confidence interval –1.30 to 0.78). There was no consistent pattern of effect on the model estimated intercept associated with higher levels of tea consumption. For all but the highest level of coffee consumption, confidence intervals included 0, indicating no significant effect of coffee consumption on the adjusted baseline scores for women.
Table 3.
Beta estimates and 95% confidence intervals from linear mixed models* of change in modified mini-mental state (3MS) examination scores over an 8-year period associated with Tea and Coffee consumption at baseline, by gender
| Women |
Men |
||||
|---|---|---|---|---|---|
| Sectionf | Standard 3MS | IRT 3MS‡ | Standard 3MS | IRT 3MS‡ | |
| I | Tea§ | ||||
| <5 x/yr | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | |
| 5–10 x/yr | −0.26 [−1.30, 0.78] | −0.32 [−1.80, 1.17] | 0.56 [−0.68, 1.80] | 0.71 [−0.98, 2.40] | |
| 1–3 x/mo | 1.04 [0.15, 1.92] | 1.65 [0.45, 3.00] | 0.87 [−0.20, 1.95] | 1.14 [−0.32, 2.55] | |
| 1–4 x/wk | 0.51 [−0.32, 1.33] | 0.66 [−0.53, 1.80] | 0.31 [−0.72, 1.34] | 0.84 [−0.56, 2.25] | |
| ≥ 5x/wk | 0.62 [−0.18, 1.41] | 0.81 [−0.33, 1.95] | 0.34 [−0.68, 1.37] | 0.01 [−1.38, 1.41] | |
| p-value | 0.07 | 0.04 | 0.61 | 0.45 | |
| II | Coffee§ | ||||
| <5 x/yr | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | |
| 5–10 x/yr | 0.67 [−0.51, 1.85] | 0.69 [−1.01, 2.40] | −0.34 [−1.81, 1.12] | 0.20 [−1.80, 2.25] | |
| 1–3 x/mo | 0.19 [−0.95, 1.34] | 1.01 [−0.63, 2.70] | 0.39 [−1.07, 1.84] | 0.86 [−1.13, 2.85] | |
| 1–4 x/wk | 0.32 [−0.77, 1.41] | 0.17 [−1.40, 1.65] | −0.96 [−2.21,0.29] | −0.50 [−2.25, 1.19] | |
| ≥5 x/wk | 0.72 [0.10, 1.35] | 0.99 [0.11, 1.95] | 0.63 [−0.22, 1.48] | 0.95 [−0.21, 2.10] | |
| p-value | 0.23 | 0.24 | 0.08 | 0.32 | |
| III | Annual change in tea & coffee drinkers of <5 x/yr |
−1.30 [−1.53, −1.07] p < 0.001 |
−0.87 [−1.07, −0.68] p < 0.001 |
−1.11 [−1.38, −0.84] p < 0.001 |
−0.47 [−0.69, −0.24] p < 0.001 |
| IV | Tea × Time† | ||||
| 5–10 x/yr | 0.23 [−0.14,0.60] | 0.20 [−0.13, 0.51] | 0.25 [−0.15,0.64] | 0.18 [−0.15, 0.51] | |
| 1–3 x/mo | 0.44 [0.13,0.75] | 0.27 [0.00, 0.54] | 0.30 [−0.04, 0.65] | 0.13 [−0.17, 0.42] | |
| 1–4 x/wk | 0.53 [0.24, 0.82] | 0.38 [0.12, 0.62] | 0.40 [0.07, 0.73] | 0.20 [−0.08, 0.48] | |
| ≥5 x/wk | 0.29 [0.01,0.57] | 0.21 [−0.03, 0.45] | 0.38 [0.04, 0.71] | 0.10 [−0.18, 0.38] | |
| p-value | 0.007 | 0.07 | 0.12 | 0.67 | |
| V | Coffee × Time† | ||||
| 5–10 x/yr | 0.37 [−0.05, 0.78] | 0.44 [0.07, 0.80] | −0.13 [−0.61,0.34] | 0.01 [−0.39, 0.42] | |
| 1–3 x/mo | 0.53 [0.12, 0.93] | 0.54 [0.20, 0.89] | −0.09 [−0.56, 0.38] | 0.01 [−0.39, 0.39] | |
| 1–4 x/wk | 0.15 [−0.23,0.54] | 0.14 [−0.20, 0.48] | −0.02 [−0.42, 0.38] | 0.01 [−0.33, 0.35] | |
| ≥5 x/wk | 0.31 [0.10, 0.53] | 0.32 [0.13, 0.51] | 0.14 [−0.13,0.40] | 0.03 [−0.20, 0.26] | |
| p-value | 0.02 | 0.002 | 0.63 | 0.99 | |
All four models are adjusted for age, race, educational attainment, field center, history of stroke, history of CHD, diabetes, hypertension, current smoking, depression score, and APOEε4. Each numerical column refers to a different model.
Coefficients estimate the difference in annual change in cognitive scores compared to the reference group.
IRT 3MS are the item response theory 3MS scores.
Coefficients estimate the average difference in baseline cognitive scores compared to the reference group.
Sections are noted to aid within model interpretations of these estimates in relation to beverage, model estimated baseline and adjusted mean values of cognitive decline (see full description in results section).
The subsequent sections show the effects of consumption on the estimated rate of cognitive change. Section III shows the adjusted mean rate of decline for the reference group (women who drank less than 5 cups of tea and coffee per year) to be 1.30 standard 3MS points per year (95% confidence interval –1.07 to 1.53). Sections IV and V show the differences from this reference rate of decline associated with increasing intensities of tea and coffee consumption. Thus, women who drank 5–10 cups of tea per year had model adjusted average rates of decline that were 0.23 standard 3MS points per year better than the reference category. Compared to the reference group, whose decline is 1.30 points per year; this exposure group declined by 0.23 fewer points per year, or 1.07 points per year. The 95% confidence interval for this group ranged from 0.14 points per year worse (i.e., 1.30 + 0.14 = 1.44 points decline per year) to 0.60 points per year better (i.e., 1.30 – 0.60 = 0.70 points decline per year). For the highest three levels of tea consumption, confidence intervals excluded 0, indicating a significant beneficial association of tea on the rate of decline in standard 3MS scores. Each of the groups defined by greater coffee consumption had a point estimate suggesting a beneficial effect of coffee on the rate of decline in standard 3MS scores, but two of the confidence intervals included 0, suggesting no significant effect.
The second numeric column in Table 3 shows the results of a model with IRT 3MS scores for women. The pattern of findings for the effects of tea consumption (Section I) and the effects of coffee consumption (Section II) on the adjusted baseline scores estimated from the model was similar to that seen with standard 3MS scores. Section III of this column shows the adjusted mean rate of decline for the reference group as 0.87 points per year, where 15 points represents 1 standard deviation of the IRT scores at baseline. This value suggests that on average it would take 17 years to decline a full standard deviation. Sections IV and V show the effects of increasing levels of tea and coffee consumption on rates of decline. In each case, the point estimates were positive, indicating possible beneficial effects, but confidence intervals for the lowest and highest groups included 0, indicating no significant effect of tea exposure on rates of cognitive decline for those two groups. For coffee the point estimates are positive, indicating possible beneficial effects, but the confidence interval for the 1–4 cups per week group included 0, indicating no significant effect.
The next set of columns in Table 3 shows results of these models for men. Point estimates in the top section of the table suggest small mean differences in the model intercept, but all four confidence intervals include 0, suggesting no significant effect of tea on estimated intercepts for men. Point estimates in Section II of the table show a confusing pattern with some positive signs and some negative signs, and again all confidence intervals include 0, suggesting no significant effect of coffee on estimated intercepts for men. Section III shows the model’s estimated rate of decline for the reference group for men was 1.11 points per year (95% confidence interval, 0.84 to 1.38 points per year). The point estimates for tea consumers in Section IV are all greater than 0, indicating possible beneficial effects, but the confidence intervals include 0 for the second and third groups, indicating no significant effect. For coffee in Section V, point estimates had positive or negative signs, indicating no consistent effect across groups defined by intensity of exposure, and all of the confidence intervals include 0, indicating no significant effect.
The final column in Table 3 in the model with IRT 3MS scores for men shows a lack of significant effect of any level of tea or coffee consumption on model-based intercepts (Section I and II) or on the estimated rate of cognitive decline (Section IV and V).
As we did not see a dose-response effect for coffee or tea consumption, we performed an additional regression model with men and women together using IRT 3MS scores as the dependent variable. In this model, we grouped together any exposure to coffee more than 5 cups per year and any exposure to tea more than 5 cups per year. We provide a plot of model-estimated trajectories of cognitive functioning in Fig. 1. Women in the reference group with less than 5 cups of tea and coffee (solid gray line) had a model based intercept right around 100, while men in the reference group (solid black line) had an intercept between 96 and 97, a difference of about 3.5 points, or about one quarter of the standard deviation for the entire cohort at baseline (15 points). The rate of decline for the reference group for women was higher than for any other group. Coffee (dotted gray line) and tea (dashed gray line) exposures were associated with slower rates of decline for women, while there was minimal effect of coffee (dotted black line) and tea (dashed black line) for men.
In Table 4, we show results for regression models that evaluated the effects of total caffeine exposure on cognitive functioning. In the left column, we show results for women with standard 3MS scores. Compared to the group with the lowest caffeine consumption, other groups had modest differences at baseline, though the confidence intervals for only the top two quintiles excluded 0, indicating a significant effect. The first quintile, the quintile with the lowest caffeine consumption, declined by an average of 1.21 points per year (95% confidence interval from 1.00 to 1.43 points decline per year). Rates of decline were attenuated for all of the other caffeine consumption groups, and all confidence intervals excluded 0. The next column shows results for the IRT 3MS scores. As for the standard 3MS scores, point estimates suggest a modest beneficial effect of higher levels of caffeine consumption on the intercepts, though this effect was only significant for the highest two groups. Also in parallel with findings for standard 3MS scores, rates of decline were somewhat attenuated for all groups defined by greater caffeine consumption, and again all confidence intervals excluded 0.
Table 4.
Beta estimates and 95% confidence intervals from linear mixed model* of change in Modified Mini-Mental State (3MS) examination scores over an 8-year period associated with total caffeine consumption from Tea and Coffee at baseline, by gender
| Women |
Men |
|||
|---|---|---|---|---|
| Standard 3MS | IRT 3MS | Standard 3MS | IRT 3MS | |
| Quintile 1‡ | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Quintile 2‡ | 0.68 [−0.11, 1.47] | 1.10 [−0.03, 2.25] | −0.05 [−1.13, 1.03] | 0.15 [−1.31, 1.65] |
| Quintile 3‡ | 0.78 [−0.09, 1.65] | 1.04 [−0.21,2.25] | −0.11 [−1.17,0.95] | 0.07 [−1.37, 1.50] |
| Quintile 4‡ | 1.22 [0.27, 2.16] | 1.80 [0.39, 3.15] | 1.13 [−0.04,2.31] | 1.65 [0.08, 3.30] |
| Quintile 5‡ | 1.01 [0.17, 1.85] | 1.41 [0.21, 2.55] | 0.74 [−0.39, 1.87] | 1.02 [−0.51, 2.55] |
| p-value | 0.08 | 0.08 | 0.13 | 0.16 |
| Annual change in Quintile 1 (Ref.) | −1.21 [−1.43,−1.00] p < 0.001 |
− 0.80 [−0.99,−0.62] p < 0.001 |
−1.14 [−1.39,−0.88] p < 0.001 |
−0.51 [−0.72,−0.30] p < 0.001 |
| Quintile 2 × Time† | 0.55 [0.27, 0.83] | 0.45 [0.20, 0.69] | 0.40 [0.06, 0.75] | 0.24 [−0.06, 0.53] |
| Quintile 3 × Time† | 0.42 [0.11, 0.73] | 0.38 [0.11, 0.65] | 0.23 [−0.12, 0.57] | 0.12 [−0.17, 0.41] |
| Quintile 4 × Time† | 0.64 [0.30, 0.97] | 0.53 [0.23, 0.81] | 0.50 [0.13,0.88] | 0.32 [0.01, 0.63] |
| Quintile 5 × Time† | 0.53 [0.24, 0.83] | 0.48 [0.23, 0.74] | 0.55 [0.19,0.91] | 0.17 [−0.14, 0.47] |
| p-value | 0.0003 | 0.0006 | 0.02 | 0.31 |
Models are adjusted for age, race, educational attainment, field center, history oi stroke, history oi cnu, diaDetes, hypertension, current smoking, depression score, and APOEε4.
Coefficients estimate the difference in annual change in cognitive scores compared to the reference group.
Categories of caffeine consumption (mg/wk) are as follows: Quintile 1: <16.2; Quintile 2:16.3–150.1; Quintile 3:150.2–425.1; Quintile 4:425.1–438.9; Quintile 5: ≥438.9. Joint quintiles for both genders were applied. The distributions of these cutoffs by gender resulted in the following gender specific distributions: Quintile 1:20.8% women, 20.5% men; Quintile 2:26.2% women, 22.1% men; Quintile 3:18.5% women, 23.1% men; Quintile 4:14.1% women, 15.5% men; Quintile 5:20.4% women, 18.8% men.
The third column in Table 4 shows results for caffeine consumption for men with standard 3MS scores as the dependent variable. There was no consistent pattern of effect of higher levels of caffeine consumption on the intercept. Point estimates for the effect of higher levels of caffeine consumption on the slope suggested a modest beneficial effect, but the confidence interval for the third quintile included 0, indicating no significant effect. The final column in Table 4 shows results for caffeine consumption with IRT 3MS scores as the dependent variable. Unlike the standard 3MS scores, point estimates for the intercepts for the IRT scores suggested a modest beneficial effect of higher levels of caffeine consumption (all values greater than 0). Like the standard 3MS results, confidence intervals included 0 for the effects on the baseline, though for the fourth quintile the confidence interval excluded 0. The last section of results shows the effects of increasing levels of caffeine consumption on rates of decline. All of the point estimates were positive, suggesting a modest beneficial effect of caffeine consumption, but three of the four confidence intervals included 0, indicating no statistically significant effect, with the exception of the fourth quintile.
To control for the possibility that caffeine intake may just be a marker of some other behavior, we assessed confounding by other dietary factors by including additional variables in the models, such as vegetable, meat, and cereal fiber intake. Additional adjustment for these variables made virtually no difference to the estimates of the effects of tea, coffee, or caffeine consumption. Alcohol consumption was not related to tea or coffee consumption in a graded fashion in women, nor was it related to tea consumption in men. Alcohol did show a weak positive trend with coffee consumption in men, but addition of the alcohol covariate to multivariable models had no appreciable effect on the beverage estimates.
DISCUSSION
In this study, we found modestly reduced rates of cognitive decline over a median follow up of 7.9 years for some, but not all, levels of coffee and tea consumption for women. This reduction in cognitive decline was not linearly related to frequency of consumption. There was no consistent association between coffee or tea consumption and the rate of cognitive decline for men. Caffeine consumption calculated as the sum of tea and coffee consumed, with the coffee consumption weighted more heavily, was also associated with slower cognitive decline among the women but not men.
Evidence from four cross-sectional studies on tea consumption and cognitive performance suggest better performance among the tea consumers compared to non-consumers [5, 47–49]. Global cognition scores in a community-dwelling, non-demented elderly population in Dublin were positively correlated with tea intake [47]. Among 2,031 participants in the population-based cross-sectional Hordaland Health Study in Norway, those who consumed tea had significantly better mean test scores and lower prevalence of poor cognitive performance than those who did not, and the relationship to consumption approximated linearity [49]. In a meta analysis of caffeine and cognitive decline conducted by Santos et al., the total effect estimate from three publications was insignificant at 0.98 (CI 0.87–1.11). However, the estimated in each of the three populations included were lower for men than for women [50].
The study of tea and health in western cultures is not characterized by high levels of tea consumption and may be biased by an unhealthy tea drinker effect, whereby coffee consumers switch to tea at the onset of health problems in a classic example of reverse causality. This bias could suppress the magnitude of any beneficial effect of tea. Findings from Asian studies are less likely to suffer such a tea drinker bias. One Asian study of tea and cognition reported less cognitive impairment among former tea drinkers in a study of 90–108 year olds living in western China [5]. In Japan, among adults over 70 years, those drinking 2 or more cups a day of green tea were less likely to show impairment [48]. Longitudinal studies with long follow up periods are also less susceptible to this form of bias.
Our study is the second largest longitudinal study—the largest from a western culture—to suggest a reduction in cognitive decline among tea drinkers. The largest study included Chinese men and women drinking black and oolong tea, followed for 1, 2 years in the Singapore Longitudinal Ageing Studies cohort [5]. A third longitudinal study showed no association, but this finding was dismissed by the investigators because the low prevalence of tea drinking led to lack of power to detect a tea-specific effect [6]. These cross-sectional findings on cognitive status and longitudinal findings on cognitive decline are consistent and supportive of a positive association between tea and cognitive function. In the CHS, tea consumption was much less frequent than in the Singapore Longitudinal Ageing Studies cohort and the CHS baseline questionnaire truncated consumption at the upper levels of 5 or more cups per week. With this categorization of intake, no dose response relationship was found in CHS.
As for coffee, four longitudinal studies have examined its relationship to cognitive decline with inconsistent evidence of an association (Table 1). Two of the studies showed no relationship [5, 7] while the other two showed isolated statistically significant findings but without demonstrating a dose-response relationship [3, 6]. The CHS cohort is considerably larger than the other four cohorts. With the exception of the Singapore study, all were traditionally regular coffee drinking populations. In CHS, an association was found between coffee drinking and somewhat attenuated rates of cognitive decline in women.
The strengths of this study include its long follow up (up to 9 years), large size, and repeated measures of cognitive function using the 3MS examination and IRT 3MS scores. The CHS is a well-established multicenter cohort of high quality with minimal loss to follow-up. Known risk factors for cognitive decline were measured and included in the mixed models to minimize confounding. The study of cognitive decline is plagued by the bias in response from survivors and those least affected by decline. This problem was at least partly addressed by imputation of missing standard 3MS scores from TICS and IQCODE data and by the use of mixed models to model cognitive decline.
Nonetheless, the study has limitations. Chief among these, the assessment of beverage consumption at baseline did not allow for a finer degree of examination of consumption. The assessment was based on frequencies of consumption rather than cups consumed and was truncated at the frequency of greater than 5 times a week. A greater precision to assess those drinking multiple cups per day would have been desirable. This prohibits direct comparisons with doses of coffee, tea and total caffeine intakes estimated from other studies of cognitive decline. The low ceilings of intake reporting options result in conservative assessments of intake that are likely to underestimate the true population consumption as influenced by heavy daily consumers.
Another concern is that caffeine is also underestimated. The questionnaires did not allow differentiation of caffeinated sodas at baseline. Therefore, this source of caffeine was not added to the total weekly caffeine consumption, and contributions from chocolate and other caffeinated sources were not included. Assessment at a later time period revealed that only 5% of the CHS population reported drinking caffeinated sodas on a daily basis however suggest that this source; caffeinated sodas, might not contribute greatly to total caffeine intake in this population at that time.
Secondly, tea consumption at the daily level was relatively infrequent in this population, which was characterized by 15% more subjects consuming coffee regularly (5 or more times per week) than tea, and average consumptions of coffee, that were estimated to be 40% greater than tea. As tea and coffee consumption are generally inversely related, both were included in the same analytic model.
Thirdly, as with all dietary exposures, measurement error is inevitable. Individuals poorly remember their usual consumption of foods and beverages. This concern is in addition to the potential effect of unmeasured confounders and residual confounding that is inevitable in studies of this nature.
One final limitation is that consumption behavior was only considered at baseline. Ideally, repeat measures of consumption would be used to characterize individuals. Baseline values are only useful if there is a relative stability in consumption over time among elder individuals, as was suggested in CHS by an assessment of beverage consumption six years after the baseline assessment.
Surprising in this analysis, as mirrored in other epidemiologic studies, is the relatively consistent effect for women at a very low frequency of consumption, and the lack of a linear dose-response relationship. For coffee, neither the background literature nor these CHS findings point to a consistent linear effect. For tea, the other published cohort in a tea drinking population shows a linear effect not seen in the CHS. The lack of a linear dose-response relationship suggests that some other factor or factors associated with consumption and non-consumption of these beverages may explain the associations found in this study.
Tea or coffee consumption may somewhat attenuate the rate of cognitive decline in women. Our results confirm the finding in other populations, of an association between caffeine consumption and cognition among women. Our longitudinal tea findings are the first in a western tea drinking population. Examination of tea in other populations with heavier tea drinking deserves further study. The lack of a linear dose-response relationship in this and other longitudinal studies and the lack of any finding in men remain a concern. Whether these findings reflect underlying measurement error in the exposure assessment, fluctuations in behavior, a true biological threshold, or basic differences between consumers and non-consumers requires further investigation. The mounting evidence of a possible protective role of tea, coffee, or caffeine against neurodegenerative disorders, and the apparent lack of risk suggest the next steps to definitely confirm or dismiss this possibility would be a clinical trial of factorial design
ACKNOWLEDGMENTS
This research was supported in part by Unilever, in part by contract numbers N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number HL080295 from the National Heart, Lung, and Blood Institute and grant number AG-023269 from the National Institute on Aging, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through AG-15928, AG-20098, and AG-027058 from the National Institute on Aging, HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. We also gratefully thank Jasmine Yaxun Chen for her support in the preparation of this manuscript.
Appendix 1.
Consumption of Tea, Coffee, decaffeinated Coffee, and estimated total Caffeine intake at baseline
| Characteristic | Tea consumption at baseline, cups | Caffeinated Coffee consumption at baseline, cups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Never N = 1.131 |
5–10/yr N = 533 |
1–3/mo N = 890 |
1–4/wk N = 1.070 |
≥5/wk N = 1.185 |
Never N = 1.656 |
5–10/yr N = 308 |
1–3/mo N = 330 |
1–4/wk N = 433 |
≥5/wk N = 2.063 |
|
| Tea, % | ||||||||||
| Never | 33.6 | 12.3 | 12.7 | 17.8 | 20.1 | |||||
| 5–10 times/yr | 7.3 | 19.8 | 13.3 | 8.8 | 13.0 | |||||
| 1–3 times/mo | 12.7 | 19.2 | 28.8 | 20.1 | 21.1 | |||||
| 1–4 times/wk | 19.3 | 25.3 | 26.1 | 32.1 | 21.4 | |||||
| ≥5 times/wk | 27.0 | 23.4 | 19.1 | 21.3 | 24.4 | |||||
| Coffee, caffeinated, % | ||||||||||
| Never | 49.4 | 22.7 | 23.8 | 30.1 | 38.0 | |||||
| 5–10 times/yr | 3.4 | 11.4 | 6.7 | 7.3 | 6.1 | |||||
| 1–3 times/mo | 3.7 | 8.3 | 10.7 | 8.1 | 5.4 | |||||
| 1–4 times/wk | 6.8 | 7.1 | 9.8 | 13.1 | 7.8 | |||||
| ≥5 times/wk | 36.7 | 50.5 | 49.0 | 41.5 | 42.8 | |||||
| Coffee, decaffeinated, % | ||||||||||
| Never | 44.2 | 36.9 | 36.5 | 31.5 | 39.9 | 31.4 | 11.7 | 7.3 | 22.9 | 55.4 |
| 5–10 times/yr | 4.3 | 11.2 | 7.6 | 7.0 | 6.0 | 3.2 | 5.9 | 4.0 | 5.8 | 10.1 |
| 1–3 times/mo | 5.6 | 7.2 | 12.4 | 7.7 | 8.2 | 4.9 | 7.8 | 15.2 | 6.5 | 10.0 |
| 1–4 times/wk | 8.9 | 9.6 | 12.4 | 15.1 | 8.7 | 10.5 | 13.4 | 14.6 | 22.7 | 8.0 |
| ≥5 times/wk | 37.1 | 35.2 | 31.1 | 38.8 | 37.1 | 50.0 | 61.2 | 59.0 | 42.1 | 16.5 |
| Total caffeine from coffee & tea, median, mg/wk |
12 | 429 | 226 | 288 | 363 | 14 | 26 | 53 | 288 | 439 |
| Total caffeine from coffee & tea, mean, mg/wk |
172 | 238 | 248 | 283 | 351 | 57 | 69 | 92 | 272 | 481 |
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=936).
REFERENCES
- [1].Daffner KR. Promoting successful cognitive aging: A comprehensive review. J Alzheimers Dis. 2010;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van Boxtel MP, Schmitt JA, Bosma H, Jolles J. The effects of habitual caffeine use on cognitive change: A longitudinal perspective. Pharmacol Biochem Behav. 2003;75:921–927. doi: 10.1016/s0091-3057(03)00171-0. [DOI] [PubMed] [Google Scholar]
- [3].van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur J Clin Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- [4].Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: A prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- [5].Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr. 2008;88:224–231. doi: 10.1093/ajcn/88.1.224. [DOI] [PubMed] [Google Scholar]
- [6].Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- [7].Laitala VS, Kaprio J, Koskenvuo M, Raiha I, Rinne JO, Silventoinen K. Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr. 2009;90:640–646. doi: 10.3945/ajcn.2009.27660. [DOI] [PubMed] [Google Scholar]
- [8].Santos C, Lunet N, Azevedo A, de Mendonca A, Ritchie K, Barros H. Caffeine intake is associated with a lower risk of cognitive decline: A cohort study from Portugal. J Alzheimers Dis. 2010;20(Suppl 1):S175–S185. doi: 10.3233/JAD-2010-091303. [DOI] [PubMed] [Google Scholar]
- [9].Ruxton CHS. The impact of caffeine on mood, cognitive function, performance and hydration: A review of benefits and risks. Nutrition Bulletin. 2008;33:15–25. [Google Scholar]
- [10].Heckman MA, Weil J, Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010;75:R77–R87. doi: 10.1111/j.1750-3841.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- [11].Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- [12].Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land. Psychopharmacology (Berl) 2002;164:250–261. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- [13].Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20(Suppl 1):S85–S94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- [14].Einother SJ, Martens VE, Rycroft JA, De Bruin EA. L-theanine and caffeine improve task switching but not intersensory attention or subjective alertness. Appetite. 2010;54:406–409. doi: 10.1016/j.appet.2010.01.003. [DOI] [PubMed] [Google Scholar]
- [15].Cauli O, Morelli M. Caffeine and the dopaminergic system. Behav Pharmacol. 2005;16:63–77. doi: 10.1097/00008877-200503000-00001. [DOI] [PubMed] [Google Scholar]
- [16].Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell Mol Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ribeiro JA, Sebastiao AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- [18].Chin JM, Merves ML, Goldberger BA, Sampson-Cone A, Cone EJ. Caffeine content of brewed teas. J Anal Toxicol. 2008;32:702–704. doi: 10.1093/jat/32.8.702. [DOI] [PubMed] [Google Scholar]
- [19].Casimir J, Jadot J, Renard M. Separation and characterization of N-ethyl-gamma-glutamine from Xerocomus badius. Biochim Biophys Acta. 1960;39:462–468. doi: 10.1016/0006-3002(60)90199-2. [DOI] [PubMed] [Google Scholar]
- [20].Tsushida T, Takeo T. Occurrence of theanine in camellia japonica and camellia sasanqua seedlings. Agric Biol Chem. 1984;48:2861–2862. [Google Scholar]
- [21].Gomez-Ramirez M, Higgins BA, Rycroft JA, Owen GN, Mahoney J, Shpaner M, Foxe JJ. The deployment of intersensory selective attention: A high-density electrical mapping study of the effects of theanine. Clin Neuropharmacol. 2007;30:25–38. doi: 10.1097/01.WNF.0000240940.13876.17. [DOI] [PubMed] [Google Scholar]
- [22].Gomez-Ramirez M, Kelly SP, Montesi JL, Foxe JJ. The effects of L-theanine on alpha-band oscillatory brain activity during a visuo-spatial attention task. Brain Topogr. 2009;22:44–51. doi: 10.1007/s10548-008-0068-z. [DOI] [PubMed] [Google Scholar]
- [23].Kelly SP, Gomez-Ramirez M, Montesi JL, Foxe JJ. L-theanine and caffeine in combination affect human cognition as evidenced by oscillatory alpha-band activity and attention task performance. J Nutr. 2008;138:1572S–1577S. doi: 10.1093/jn/138.8.1572S. [DOI] [PubMed] [Google Scholar]
- [24].Giesbrecht T, Rycroft JA, Rowson MJ, De Bruin EA. The combination of L-theanine and caffeine improves cognitive performance and increases subjective alertness. Nutr Neurosci. 2010;13:283–290. doi: 10.1179/147683010X12611460764840. [DOI] [PubMed] [Google Scholar]
- [25].Owen GN, Parnell H, De Bruin EA, Rycroft JA. The combined effects of L-theanine and caffeine on cognitive performance and mood. Nutr Neurosci. 2008;11:193–198. doi: 10.1179/147683008X301513. [DOI] [PubMed] [Google Scholar]
- [26].Quinlan PT, Lane J, Moore KL, Aspen J, Rycroft JA, O’Brien DC. The acute physiological and mood effects of tea and coffee: The role of caffeine level. Pharmacol Biochem Behav. 2000;66:19–28. doi: 10.1016/s0091-3057(00)00192-1. [DOI] [PubMed] [Google Scholar]
- [27].De Bruin EA, Rowson MJ, Van Buren L, Rycroft JA, Owen GN. Black tea improves attention and self-reported alertness. Appetite. 2011;56:235–240. doi: 10.1016/j.appet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- [28].Hindmarch I, Quinlan PT, Moore KL, Parkin C. The effects of black tea and other beverages on aspects of cognition and psychomotor performance. Psychopharmacology (Berl) 1998;139:230–238. doi: 10.1007/s002130050709. [DOI] [PubMed] [Google Scholar]
- [29].Hindmarch I, Rigney U, Stanley N, Quinlan P, Rycroft J, Lane J. A naturalistic investigation of the effects of day-long consumption of tea, coffee and water on alertness, sleep onset and sleep quality. Psychopharmacology (Berl) 2000;149:203–216. doi: 10.1007/s002130000383. [DOI] [PubMed] [Google Scholar]
- [30].Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- [31].Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- [32].Crane PK, Narasimhalu K, Gibbons LE, Mungas DM, Haneuse S, Larson EB, Kuller L, Hall K, van Belle G. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61:1018–1027.e9. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- [34].Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr. 1997;65:1123S–1129S. doi: 10.1093/ajcn/65.4.1123S. [DOI] [PubMed] [Google Scholar]
- [35].Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- [36].Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- [37].Brandt J, Spencer M, McSorley P, Folstein MF. Semantic activation and implicit memory in Alzheimer disease. Alzheimer Dis Assoc Disord. 1988;2:112–119. doi: 10.1097/00002093-198802020-00003. [DOI] [PubMed] [Google Scholar]
- [38].Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- [39].Crane PK, Gruhl JC, Erosheva EA, Gibbons LE, McCurry SM, Rhoads K, Nguyen V, Arani K, Masaki K, White L. Use of spoken and written Japanese did not protect Japanese-American men from cognitive decline in late life. J Gerontol B Psychol Sci Soc Sci. 2010;65:654–666. doi: 10.1093/geronb/gbq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. J Am Med Assoc. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Samejima F. Estimation of latent ability using a response pattern of graded scores. 1969. Psychometrika Monograph No. 17.
- [42].Samejima F. Graded response model. In: van der Linden WJ, Hambleton RK, editors. Handbook of modern item response theory. Springer; NY: 1997. pp. 85–100. [Google Scholar]
- [43].Muraki E, Bock D. PARSCALE for Windows. Scientific Software International; Chicago: 2003. [Google Scholar]
- [44].Arnold AM, Newman AB, Dermond N, Haan M, Fitzpatrick A. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. The Cardiovascular Health Study Neuroepidemiology. 2009;33:55–65. doi: 10.1159/000215830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- [46].StataCorp . Stata Statistical Software: Release 11. Stat-aCorp LP; College Station, TX: 2009. [Google Scholar]
- [47].Chin AV, Robinson DJ, O’Connell H, Hamilton F, Bruce I, Coen R, Walsh B, Coakley D, Molloy A, Scott J, Lawlor BA, Cunningham CJ. Vascular biomarkers of cognitive performance in a community-based elderly population: The Dublin Healthy Ageing study. Age Ageing. 2008;37:559–564. doi: 10.1093/ageing/afn144. [DOI] [PubMed] [Google Scholar]
- [48].Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- [49].Nurk E, Refsum H, Drevon CA, Tell GS, Nygaard HA, Engedal K, Smith AD. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009;139:120–127. doi: 10.3945/jn.108.095182. [DOI] [PubMed] [Google Scholar]
- [50].Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: Systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187–204. doi: 10.3233/JAD-2010-091387. [DOI] [PubMed] [Google Scholar]