The renin-angiotensin system (RAS) is a coordinated hormonal cascade critical to the control of renal sodium (Na+) excretion and blood pressure (BP) (1). Angiotensin II (Ang II), the principal RAS effector peptide, binds to two distinct receptors, the Ang type-1 receptor (AT1R) and the Ang type-2 receptor (AT2R) with high affinity (1,2). The vast majority of actions of Ang II are transmitted via AT1Rs, including cellular dedifferentiation and proliferation; vasoconstriction, reduction of vascular compliance, cardiac contractility, increased renal tubule sodium (Na+) reabsorption; aldosterone, vasopressin and endothelin secretion; salt appetite; thirst and activation of the sympathetic nervous system (1,2). In contrast, AT2Rs generally oppose the actions of Ang II via AT1Rs under most circumstances (1–4).
Another major regulatory system in cardiovascular and renal physiology is the peripheral dopaminergic system. Dopamine is mainly synthesized in renal proximal tubule cells (RPTCs) via the decarboxylation of L-dihydroxyphenylalanine (L-DOPA) that has been filtered at the glomerulus and transported into the RPTC across the apical brush border (5). Synthesized DA exits the cell mainly across the apical plasma membrane into the lumen where it can bind to and activate specific DA D1-like receptors (D1-likeR: D1 and D5) (5,6). D1-like receptor activation induces vasodilation and inhibits renal Na+ reabsorption, actions which also oppose those of Ang II via AT1Rs.
The purpose of this brief review is to summarize some of the key findings leading to our present concepts of AT2Rs and D1-like receptors that oppose the actions of Ang II via AT1Rs within the kidney.
Evidence for an independent functional intrarenal RAS
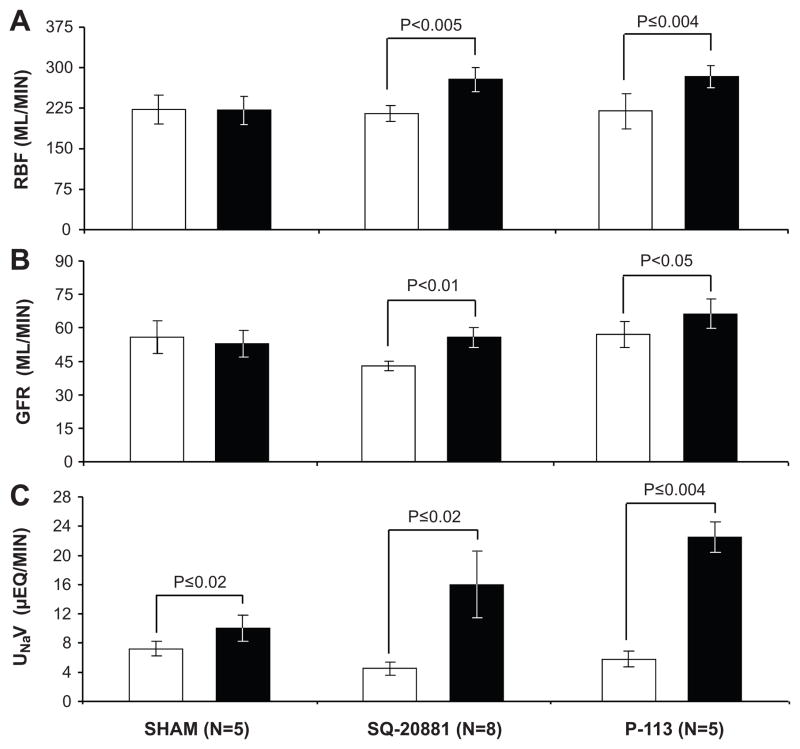
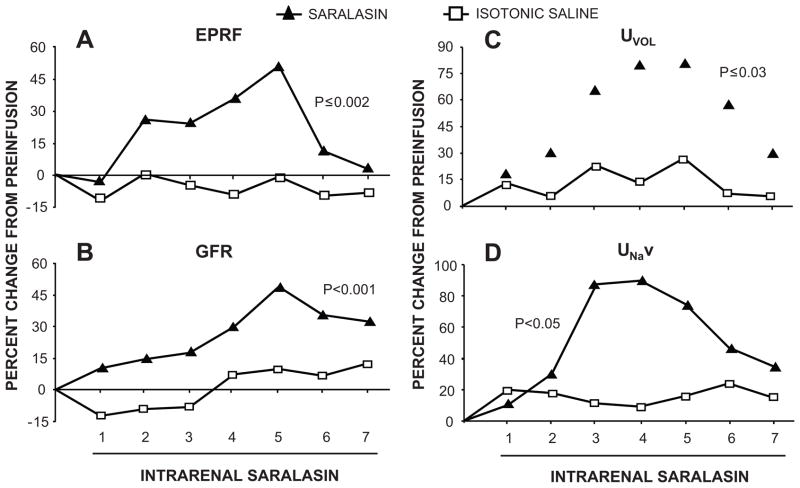
Although renin was identified in the brain and the adrenal cortex in the late 1960s and early 1970s, the intrarenal RAS was the first independent functional tissue RAS to be described (7–9). The initial observations were from in vivo studies which demonstrated that intrarenal inhibition of the RAS with angiotensin converting enzyme (ACE) inhibitors or Ang receptor blockers, at infusion rates that did not alter systemic BP during the experimental period, increased renal plasma flow, glomerular filtration rate and Na+ and water excretion (7) (Figure 1). These results were later confirmed by more rigorous approaches showing that small intrarenal doses of Ang receptor blocker, while not altering pressor responses to systemically administered Ang II, induced marked increases in renal hemodynamic and tubular function (8,9) (Figure 2). Later, it was demonstrated that the mRNAs and proteins for all of the system components (renin, angiotensinogen (Agt), ACE and AT1Rs) are localized in a site-specific manner within the kidney and that intrarenal formation of Ang II occurs independently of renal uptake of the peptide (1).
Figure 1.
Evidence for a functional intrarenal renin-angiotensin system in uninephrectomized conscious dogs. Panel A: Renal blood flow (RBF); Panel B: glomerular filtration rate (GFR); Panel C: Urinary Na+ excretion (UNaV) in response to intrarenal arterial administration of ACE inhibitor SQ-20881 (2 μg/kg/min) or Ang receptor blocker P-113 (saralasin; 2 μg/kg/min). Control vehicle infusion, white bars; experimental agent infusion, black bars. Sham data include vehicle infusion only. Data are expressed as mean ± 1 SE. Adapted from Kimbrough HM et al. Circ Res. 1977;40:174–178.
Figure 2.
Validation of an independent functional intrarenal renin-angiotensin system in uninephrectomized conscious dogs. Estimated renal plasma flow (ERPF; Panel A), glomerular filtration rate (GFR; Panel B), urine flow rate (UVOL; Panel C) and urinary Na+ excretion (UNaV; Panel D) in response to low-dose intrarenal arterial infusion of Ang receptor blocker saralasin (0.07 μg/kg/min). Numbers on abscissa represent 20 min clearance periods. Adapted from Levens NR et al. Endocrinology. 1983:112:43–49 with permission.
Additional evidence for a separate renal tissue RAS came from studies showing that intrarenal Ang II levels were elevated in the nanomolar range in renal interstitial fluid compared to picomolar concentrations in plasma, that intrarenal Ang II concentrations were markedly increased compared to plasma levels in response to Na+ restriction and that this response was blocked with intrarenal renin inhibition (10). Cellular studies demonstrated that Agt, Ang I and Ang II could be co-localized with renin in proximal tubule and juxtaglomerular cells, that Ang peptides were released from these cells and that the release was regulated (11). Further functional studies demonstrated that combined intrarenal RAS blockade with low doses of ACE inhibitor, AT1R blocker and renin inhibitor, while confined to the kidney, augmented major increases in renal function that were blocked with concurrent intrarenal Ang II administration (12). Altogether, these studies provided strong support for the existence of an independent functional intrarenal RAS.
Definitive molecular evidence for an independent intrarenal RAS and its importance in the control of BP was obtained using a transgenic mouse model over-expressing Agt either in the kidney or the systemic circulation (13). Expression of Agt selectively within the kidney induced chronic hypertension independently of the endocrine RAS (13). Within the kidney, there is now substantial evidence for a separate intratubular RAS in which Ang II formation is auto-amplified by Ang II-induced up-regulation of Agt, creating a positive feedback loop that may play a role in renal tissue damage and hypertension (14). Current studies are also providing evidence for intracellular RASs that are independently functioning in a specific subcellular compartment. Such subcellular RASs have recently been described both within nuclei and mitochondria (15, 16).
AT2R Expression and Cell Signaling Pathways
The AT2R is a 7-transmembrane G-protein-coupled receptor, encoded on the X -chromosome, with only 34% amino acid sequence homology with the AT1R (2). AT2Rs are expressed ubiquitously at very high levels in the fetus, but decline precipitously in the neonatal period in most, but not all tissues. Although the expression of AT2Rs is substantially lower than that of AT1Rs in the adult, AT2R mRNA and protein can be easily detected in the adult kidney, adrenal cortex, heart and vasculature and predominates over AT1Rs in the uterus, ovary, adrenal medulla and in discrete areas of the brain (17–20). Within the kidney, AT2Rs are expressed predominantly in RPTCs and glomeruli (18, 19).
The cell signaling mechanisms of AT2Rs differ substantially from those of AT1Rs. AT2R activation initiated by binding of Ang II to the receptor in the plasma membrane triggers G protein coupling of Giα2 and Giα3 via the third intracellular loop of the receptor. G protein coupling initiates the activation of phosphotyrosine phosphatases, which dephosphorylate and inactivate mitogen-activated protein (MAP) kinases including extracellular-regulated kinase (ERK)-1 and ERK-2. Phosphotyrosine phosphatase activation can also occur through a non-G protein-coupled mechanism. MAP kinase inhibition via AT2Rs opposes MAP kinase activation as a result of AT1R activation. The opposing action of AT1Rs and AT2Rs on MAP kinases is considered a fundamental signaling mechanism for receptor-receptor interactions (1–4).
AT2Rs can also activate the phospholipase A2 pathway leading to arachidonic acid release and long-term AT2R activation can also increase the biosynthesis of ceremides, which can stimulate stress kinases and caspases to induce apoptosis (4).
Vascular AT2R Actions and Mechanisms
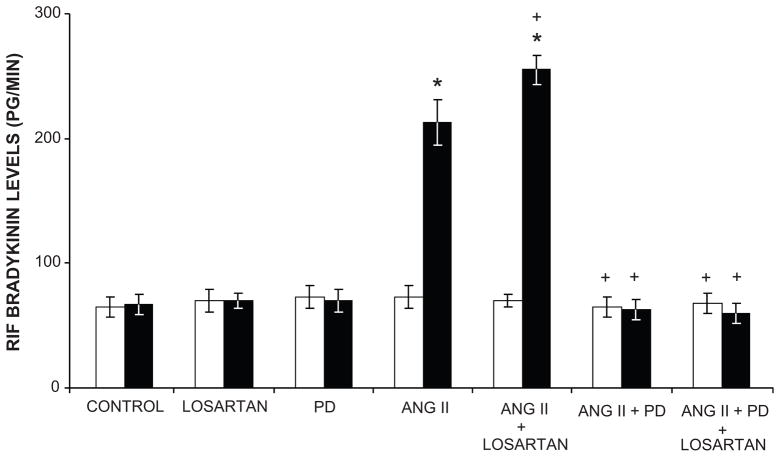
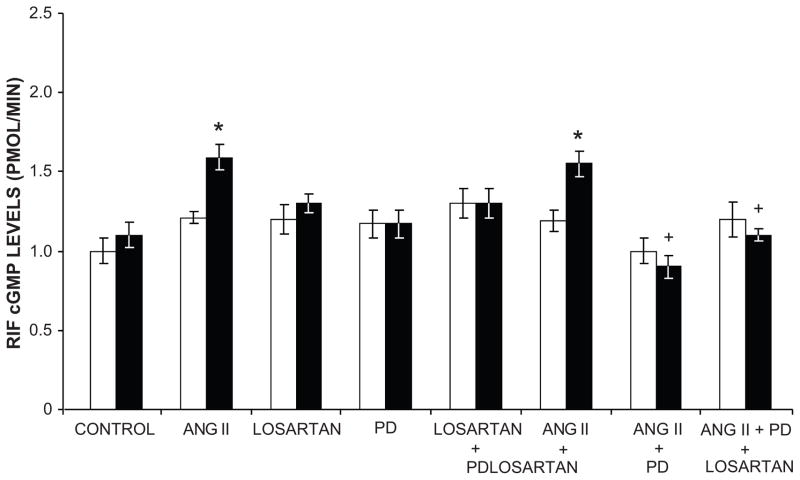
Overwhelming evidence currently exists that AT2Rs mediate vasodilation and oppose the AT1R-mediated vasoconstrictor actions of Ang II (21–35). AT2R-mediated vasodilation has been demonstrated in small resistance arteries of the mesenteric, uterine, adrenal, coronary and peripheral circulations in many animal models and in humans. AT2R-induced vasodilation has also been demonstrated in large capacitance vessels such as the aorta and in the fetus (29–31). AT2R-stimulated vasodilation is mediated by a signaling cascade composed of bradykinin (BK), nitric oxide (NO) and 3′,5′-cyclic guanosine monophosphate (cGMP) (Figures 3 and 4)(21;36–38). AT2Rs increase NO and cGMP production either by increasing BK production with activation of BK B2 receptors or by direct activation of NO production independently of BK (39–41).
Figure 3.
Ang II releases renal bradykinin (BK) by AT2R activation. Renal interstitial fluid BK levels in response to intravenous infusion of Ang II, Losartan, an AT1R antagonist; PD, PD-123319, an AT2R antagonist, and combinations in Sprague-Dawley rats. Control vehicle infusions, white bars; experimental agent infusions, black bars. Data are expressed as mean ± 1 SE. * P<0.0001 from control; + P<0.05, ++P<0.0001 from Ang II alone. Data from Siragy HM et al. Am J Physiol Reg Int Comp Physiol. 1996;271:R1090–R1095 and Siragy HM and Carey RM, Hypertension 1999;33:1237–1242.
Figure 4.
Ang II releases renal cyclic GMP (cGMP) by AT2R activation. Renal interstitial fluid cGMP levels in response to intravenous infusion of Ang II; Losartan, an AT1R antagonist; PD, PD-123319, an AT2R antagonist, and combinations in Sprague-Dawley rats. Control vehicle infusion data, white bars; experimental agent infusion, black bars. Data represent mean ± 1 SE. * P<0.001 from vehicle or time control; + P<0.001 from Ang II alone. Adapted from Siragy *HM and Carey RM. J Clin Invest. 1996;97:1978–1982 and Siragy HM and Carey RM. J Clin Invest. 1997:100:264–269 with permission.
AT2R-mediated vasodilation is most readily demonstrated when AT1Rs are blocked with an AT1R antagonist (22,23,25,26). This is almost certainly because AT1R expression predominates over that of AT2Rs in the vasculature (42,43). AT2R-stimulated vasodilation is also augmented when the RAS is activated, such as during Na+ restriction, Ang II infusion or in renal vascular hypertension (21, 22, 44). Under all three circumstances, AT2Rs are upregulated, enhancing the vasodilator response to Ang II (18,21,44). Another condition which upregulates AT2R expression (by 300%) and unmasks its vasodilator action is increased pressure load from aortic banding (29,30). AT2R blockade with specific antagonist PD-123319 (PD) or BK B2 receptor inhibition with icatibant restores the diminished Ang II contractile responses and abolishes the 9-fold increase in aortic cGMP stimulated by Ang II under these circumstances (29,30). Taken altogether, the results of these studies emphasize the likely importance of counter-regulatory AT2R upregulation and activation in circulatory disorders associated with chronic vasoconstriction via AT1Rs.
The vasodilator and depressor actions of AT2Rs are both acute and chronic and are not accompanied by desensitization, rendering these receptors a potential therapeutic target in hypertension (23,25). Indeed, the BP lowering effects of AT1R blockade may be mediated, at least in part, by AT2R activation as a result of increased renin biosynthesis and release and increased Ang II that can act via unblocked AT2Rs (21,22). An example of this principle was demonstrated in diabetic, hypertensive humans in whom chronic AT1R inhibition upregulated vascular AT2Rs and facilitated a vasodilator response to Ang II in vitro (34). In addition, in spontaneously hypertensive rats during AT1R blockade, pharmacological activation of AT2Rs by Compound 21 (a non-peptide AT2R agonist with >25,000-fold selectivity for AT2Rs over AT1Rs) resulted in decreased BP (35). These observations indicate the potential importance of a non-peptide AT2R agonist combined with an AT1R blocker in the treatment of hypertension.
AT2R-mediated vasodilation and hypotension were confirmed in AT2R-null mice. (45). Although baseline BP was similar between AT2R-null and wild-type (WT) mice, AT2R-null mice demonstrated marked and sustained hypersensitivity to the pressor actions of infused Ang II over the course of 7 days, emphasizing the importance of AT2Rs in counter-regulating Ang II actions via AT1Rs. Ang II pressor hypersensitivity was accompanied by a highly significant reduction in baseline and Ang-II stimulated renal interstitial levels of BK, NO and cGMP in AT2R-null mice.
Intrarenal AT2R Actions and Mechanisms
AT2R-null mice also had marked antinatriuresis (and inhibition of pressure- natriuresis) during the chronic Ang II infusion that was not present in WT mice (45). These results suggested the possibility that intrarenal AT2Rs might increase renal Na+ excretion via BK, NO and cGMP (45).
We subsequently explored and presented definitive evidence that intrarenal AT2R activation mediates natriuresis (46–48). These studies were enabled by the technique of renal interstitial microinfusion of pharmacological agents, which affords direct evaluation of the intrarenal mechanisms governing renal function without systemic hormonal or hemodynamic influences. Selective intrarenal AT1R blockade in rats induced a highly significant natriruesis that was abolished by intrarenal co-administration of AT2R-specific antagonist PD indicating that the natriuretic effect of AT1R blockade is mediated by AT2R activation (46).
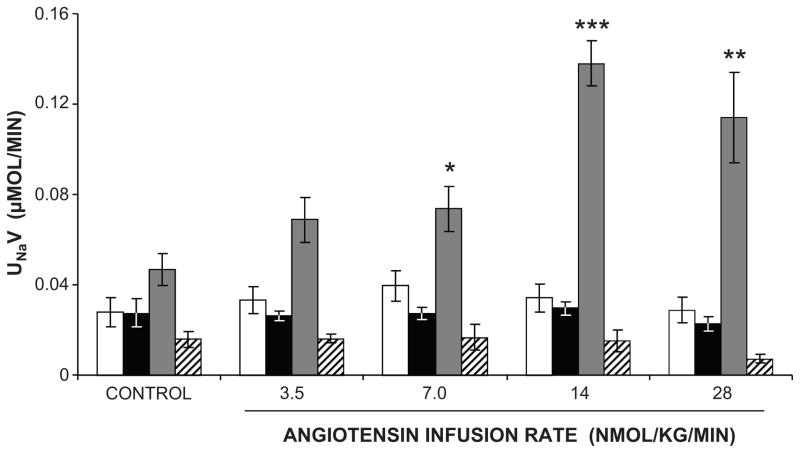
However, we were surprised to find that intrarenal Ang II infusion did not alter Na+ excretion even at high infusion rates. This finding provoked a question as to whether a downstream metabolite of Ang II might be required for renal AT2R activation. Indeed, intrarenal infusion of des-aspartyl1 Ang II (Ang III) into systemically AT1R blocked rats induced a significant natriuretic response, which was abolished with intrarenal co-infusion of PD (46) (Figure 5). Intrarenal Ang III infusion in the absence of systemic AT1R blockade did not change Na+ excretion, similar to AT2R-mediated vascular responses (46). In follow up of this observation, we hypothesized that Ang II needs to be converted to Ang III to interact with AT2Rs within the kidney. Ang II is converted to the heptapeptide Ang III by aminopeptidase A (APA), and Ang III is converted to the hexapeptide Ang IV by aminopeptidase N (APN). In the presence of systemic AT1R blockade, intrarenal infusion of Ang III induced a natriuretic response that was markedly augmented by intrarenal co-administration of APN inhibitor 2-amino-methylsulfonyl-butane-thiol, methane-thiol (PC-18) (47). The PC-18-augmented natriuresis was abolished by intrarenal AT2R inhibition with PD, indicating an AT2R-mediated effect (47). The necessity for conversion of Ang II to Ang III for AT2R-mediated natriuresis was confirmed by demonstrating that intrarenal administration of Ang II is only effective in inducing natriuresis when APN is blocked and that this response is abolished by intrarenal co-administration of APA inhibitor 3-amino-4-thio-butyl-sulfonic acid (EC-33) (48). Taken altogether, these studies demonstrate that Ang III is the preferred agonist for AT2R-mediated natriuresis. In systematic receptor binding studies, Ang III has been found to have about 30-fold selectivity over Ang II for AT2Rs (49). Confirming these results, Ang III has been shown to be the preferred AT2R agonist in other tissues such as the coronary microcirculation and adrenal cortex (50, 51).
Figure 5.
Ang III is the preferred endogenous AT2R agonist mediating natriuresis. Urinary Na+ excretion (UNaV) in anesthetized Sprague-Dawley rats in response to direct renal interstitial infusion of vehicle (white bars), Ang II (black bars), Ang III (gray bars) or Ang III +PD (PD-123319, an AT2R antagonist) (striped bars). Data are expressed as mean ± 1 SE. * P<0.05, ** P<0.01, *** P<0.001 from time control. Adapted from Padia SH et al. Hypertension. 2006.
Recent in vivo studies have demonstrated that Ang III induces natriuresis via AT2R activation in the renal proximal tubule by a NO/cGMP signaling mechanism (52). In vitro studies also have recently shown that AT2Rs reduce AT1R function in the proximal tubule by the common NO/cGMP pathway and also reduce AT1R mRNA via the ubiquitous transcription factor Sp1 (53). Ligand-activated AT2Rs also heterodimerize with AT1Rs reducing their expression via protein-protein action in the plasma membrane (53). Thus, AT2Rs may oppose AT1Rs by several pathways in the kidney.
The recent in vivo investigations cited above have confirmed that Ang III is the preferred endogenous ligand for the activation of renal AT2Rs (52). Unexpectedly, these studies were unable to elicit a natriuretic effect of Ang (1-7), which has counter-regulatory effects offsetting AT1R actions in other tissues (55). No natriuretic response to Ang (1-7) was observed at equimolar doses as Ang III even in the presence of AT1R blockade, ACE inhibition to reduce Ang (1-7) metabolism or APA blockade to augment Ang (1-7) formation from Ang II via ACE-2 (52). However, these studies did demonstrate that intrarenal administration of APN inhibitor PC-18 induces natriuresis even in the absence of systemic AT1R blockade. Furthermore, renal interstitial Ang peptide levels during Ang III administration with and without PC-18 demonstrated a marked augmentation of renal interstitial and tissue Ang III concentrations and AngIII/Ang II ratios during PC-18 administration, consistent with the role of Ang III in the augmented natriuretic effect (52). These studies also demonstrated that systemic administration of the highly selective non-peptide AT2R agonist Compound 21 induces natriruesis that is abolished with intrarenal AT2R antagonist PD in both male and female rats even in the absence of AT1R blockade, suggesting the potential for this compound as a natriuretic/diuretic agent in the treatment of disorders associated with extracellular fluid volume expansion and hypertension.
Recent studies also have suggested that AT2Rs in the thick ascending limb of Henle (TAL) may contribute to the natriuretic response (54,55). Ang II increases NO production in TALs via activation of AT2Rs and NO inhibits the Na+/K+/2Cl- co-transporter and reduces Na+ reabsorption in this nephron segment (55). Whether this response requires Ang II conversion to Ang III awaits further study.
The Intrarenal Dopaminergic System
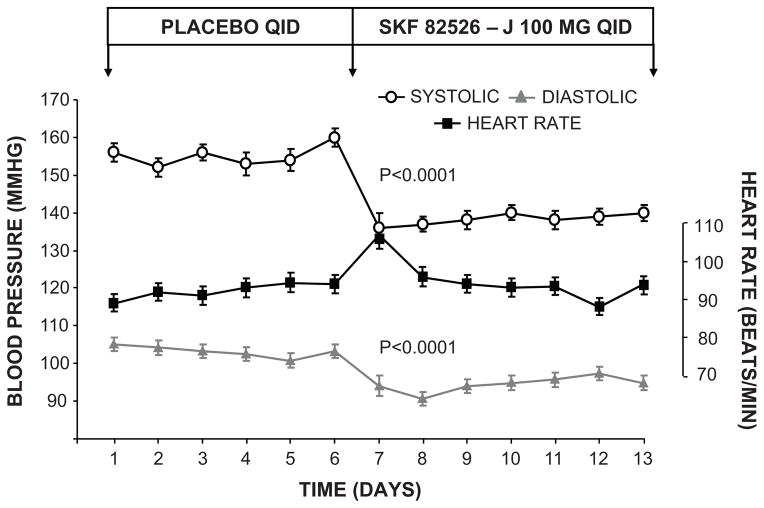
The renal dopaminergic system is a major hormonal system controlling renal Na+ excretion and BP (5). D1-likeR activation inhibits renal Na+ reabsorption through an adenylyl cyclase-cyclic AMP (cAMP) mechanism. In both humans and experimental animals highly selective D1-likeR agonist fenoldopam elicits a substantial natriuretic response that is based almost exclusively on inhibition of renal proximal tubule Na+ reabsorption (5, 56–60). Thus, the renal dopaminergic system is an important counter-regulatory system offsetting the antinatriuretic actions of AT1Rs. Indeed, fenoldopam was demonstrated to be close to ideal as an antihypertensive agent in that it normalized BP without reflex tachycardia and induced natriuresis in patients with primary hypertension (61) (Figure 6). In spite of its low bioavailability, these and other favorable observations led to FDA approval for emergency treatment of hypertension in intensive care settings.
Figure 6.
Oral fenoldopam lowers BP in hypertensive humans. BP responses to oral fenoldopam (SKF-82526-J) in patients with primary hypertension. Data are expressed as mean ± 1 SE. Adapted from Carey RM et al. J Clin Invest. 1984;74:2198–2207 with permission.
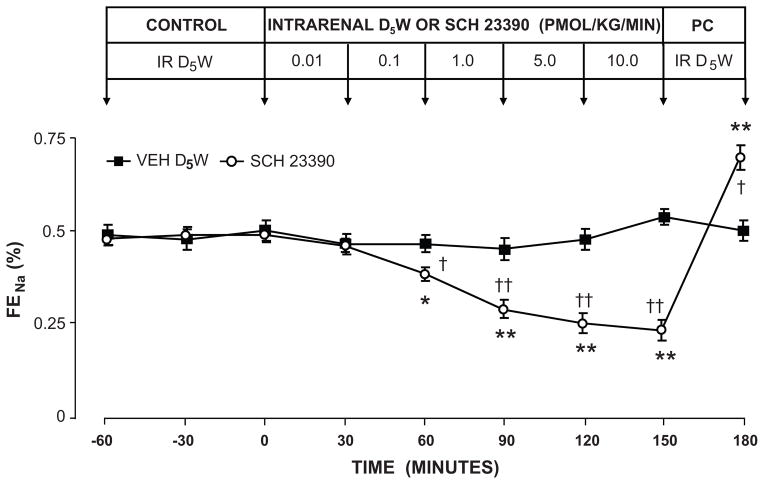
The physiological importance of the renal dopaminergic system in the control of Na+ excretion was demonstrated initially during the 1980s. Studies employing intrarenal arterial administration of highly selective D1-likeR antagonist SCH-23390 revealed that, similar to the intrarenal RAS, DA synthesized within the kidney acts in a local cell-to-cell (paracrine) manner exclusively at the renal proximal tubule to control Na+ excretion (62,63) (Figure 7). These studies demonstrated that approximately 60% of basal Na+ excretion in the Na+-replete state is controlled by intrarenal dopaminergic mechanisms acting at the proximal tubule. These observations were later confirmed using renal interstitial infusion of D1R antisense oligodeoxynucleotides to inhibit receptor expression directly (64). The importance of the renal dopaminergic system in the control of Na+ excretion was further underscored by the demonstration that the natriuretic and diuretic effects of D1-likeRs are dependent on the state of Na+ balance. In Na+ - deplete states, D1-likeR - mediated natriuresis does not occur, whereas in normal or high Na+ states D1-likeRs induce a robust natriuretic response (60). Additional evidence for the physiological importance of renal dopaminergic control of Na+ excretion included the observation that renal DA production is increased during Na+ surfeit but reduced during Na+ depletion (65). Elegant studies in mice with selective proximal tubule knockout of aromatic amino acid decarboxylase, the enzyme generating DA from L-DOPA, inducing intrarenal DA depletion have recently confirmed the importance of intrarenal DA in the control of Na+ excretion and BP (66). Taken altogether, these studies strongly support the physiologic importance of renal DA and D1-likeRs as counter-regulatory systems limiting at least in part the Na+-retaining actions of intrarenal Ang II via AT1Rs.
Figure 7.
Evidence that intrarenal dopamine controls renal Na+ excretion by a paracrine mechanism acting at the level of the renal tubule. Urinary Na+ excretion (UNaV) in uninephrectomized conscious dogs infused intrarenally with D1-LIKE receptor antagonist SCH-23390. Data are expressed as mean ± 1 SE. * P<0.001, ** P<0.0001 from pre-control; † P<0.05, †† P<0.01 from time control. Adapted from Siragy HM et al. Am J Physiol Renal Physiol. 1988;257:F469–F477 with permission.
In the mid-1990s, with antibodies directed towards the extracellular domain of D1Rs, receptor protein was localized in the renal proximal tubule and in several other cells and tissues (67–69). Similar to the AT2R, D1R mRNA is expressed only in low copy and was difficult to demonstrate using standard molecular techniques. However, D1R protein cellular distribution was later confirmed using more sensitive in situ amplification of D1R mRNA (70).
D1R/AT2R Interactions
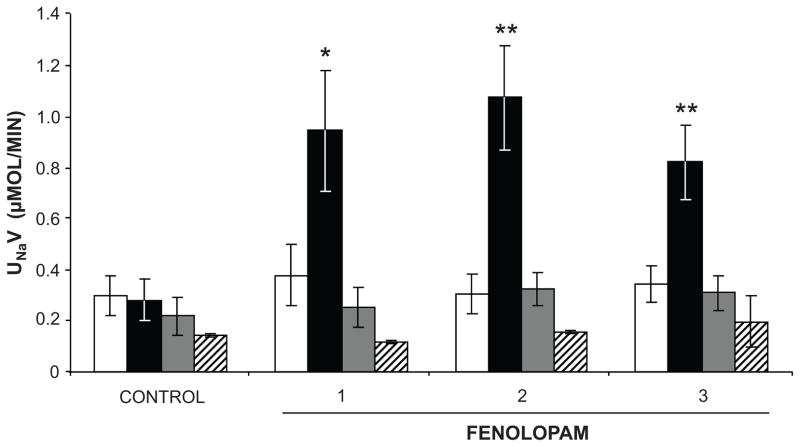
Renal interstitial administration of fenoldopam in Na+-loaded rats elicits a robust natriuretic response that is abolished with intrarenal co-administration of D1-likeR antagonist SCH-23390 (71). However, we were surprised to find that fenoldopam-induced natriuresis is also completely inhibited with intrarenal co-infusion of AT2R antagonist PD (71) (Figure 8). To explore the possible mechanism of AT2R involvement in D1-likeR-induced natriuresis, studies were performed to determine the intracellular trafficking of AT2Rs in RPTCs (71,72). In vivo administration of fenoldopam was associated with translocation of AT2Rs from intracellular sites to the apical plasma membranes of RPTCs. Fenoldopam-induced AT2R translocation to the apical plasma membrane and natriuresis were abolished in the presence of microtubulin inhibitor nocodazole but were unaffected by actin microfilamint inhibitor cytochalasin D, suggesting that microtubules are required for the translocation process (72). Because D1-likeRs signal via an adenylyl cyclase, cAMP and protein kinase A pathway, we explored the role of these signaling processes in D1-likeR – induced AT2R recruitment to the apical plasma membrane and its necessity for the natriuretic response. In vivo experiments demonstrated that intrarenal administration of direct adenylyl cyclase activator forskolin together with 3-isobutyl-1-methyl xanthine (IBMX) to inhibit its metabolism increased renal interstitial fluid levels of cAMP, stimulated AT2R recruitment to the RPTC apical plasma membrane and induced natriruesis that was abolished with AT2R antagonist PD (72). Direct agonist stimulation of D1-likeRs was not necessary for AT2R-mediated natriuresis since forskolin/IBMX-induced AT2R translocation and natriuresis persisted in the presence of D1-likeR blockade with SCH-23390 (72). Therefore, the mechanism by which AT2Rs and D1-likeRs interact during high Na+ conditions to induce natriuresis is D1-likeR-cAMP signaling, which provides the necessary stimulus for AT2R translocation and natriuresis. We also demonstrated that similar to agonist-stimulated D1R recruitment to the plasma membrane, AT2Rs are translocated via microtubules to the apical plasma membrane, where they are required to induce the natriuretic response (72, 73).
Figure 8.
Intrarenal interstitial fenoldopam-induced natriruesis is abolished by intrarenal D1-LIKE receptor antagonist SCH-23390 (SCH) or AT2R antagonist PD-123319 (PD) in anesthetized, uninephrectomized Sprague-Dawley rats. Vehicle infusion, white bars; fenoldopam (1 μg/kg/min) infusion, black bars; fenoldopam + PD infusion, gray bars; fenoldopamn + SCH infusion, striped bars. Data are expressed as mean ± 1 SE. Numbers on the abscissa refer to experimental one-hour periods. * P<0.01, ** P<0.001 from vehicle control. Adapted from Salomone LJ et al. Hypertension. 2007;49:155–161 and Padia SH et al. Hypertension. 2012;59:437–445.
Recently, AT1R-null animals were demonstrated to have increased longevity (74). In current studies, the aging process is beginning to be linked to reduction in AT2R and D1R expression and/or activation in different tissues and at the mitochondrial level (16, 75,76). It is possible that D1R and/or AT2R pharmacological activation may provide a new target for the reversal of certain aspects of the aging process and/or for the extension of lifespan in the future.
Conclusions and Perspectives
In conclusion, the intrarenal renin-angiotensin and dopaminergic systems play a major critical role in cardiovascular and renal function, the subject of this brief review. AT2Rs and D1Rs cooperatively oppose the vasoconstrictor and antinatriuretic functions mediated by Ang II at AT1Rs. Reduced AT2R expression and/or activity may contribute to the initiation and/or acceleration of disease processes including hypertension, edema-forming states and inflammation/fibrosis leading to cardiovascular and renal damage. Conversely, pharmacological activation of AT2Rs and/or D1Rs may provide therapeutic advantages or even preventive strategies in the presence or absence of AT1R blockade. Increased understanding of angiotensin and dopamine receptor functions and interactions currently provides hope for improved treatment and prevention of hypertension and other Na+/fluid retaining states and for the extension of healthier lives in the future.
Acknowledgments
SOURCES OF FUNDING: None
Footnotes
DISCLOSURES: None
References
- 1.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal disease. Endocr Rev. 2003;24:261–267. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 2.deGasparo M, Catt KJ, Inagami T, Wright JW, Unger T International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;53:415–472. [PubMed] [Google Scholar]
- 3.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: the AT2 receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 4.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey RM. Theodore Cooper Lecture. Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZQ, Siragy HM, Felder RA, Carey RM. Intrarenal dopamine production and distribution in the rat. Physiological control of sodium excretion. Hypertension. 1997;29:228–234. doi: 10.1161/01.hyp.29.1.228. [DOI] [PubMed] [Google Scholar]
- 7.Kimbrough HM, Vaughan ED, Jr, Carey RM, Ayers CR. Effect of intrarenal angiotensin II blockade on renal function in conscious dogs. Circ Res. 1977;40:174–178. doi: 10.1161/01.res.40.2.174. [DOI] [PubMed] [Google Scholar]
- 8.Levens NR, Freedlender AE, Peach MJ, Carey RM. Control of renal function by intrarenal angiotensin II. Endocrinol. 1983;112:43–49. doi: 10.1210/endo-112-1-43. [DOI] [PubMed] [Google Scholar]
- 9.Levens NR, Peach MJ, Carey RM. Role of the intrarenal rennin-angiotensin system in the control of renal function. Circ Res. 1981;48:157–167. doi: 10.1161/01.res.48.2.157. [DOI] [PubMed] [Google Scholar]
- 10.Siragy HM, Howell NL, Peach MJ, Carey RM. Renal interstitial fluid angiotensin. Modulation by anesthesia, epinephrine, sodium depletion and renin inhibition. Hypertension. 1995;25:1021–1024. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 11.Hunt MK, Ramos SP, Geary KM, Norling LL, Peach MJ, Gomez RA, Carey RM. Colocalization and release of angiotensin and rennin in renal cortical cells. Am J Physiol. 1992;263:F363–F373. doi: 10.1152/ajprenal.1992.263.3.F363. [DOI] [PubMed] [Google Scholar]
- 12.Siragy HM, Howell NL, Peach MJ, Carey RM. Combined intrarenal blockade of the renin-angiotensin system in the conscious dog. Am J Physiol. 1990;258:F522–F529. doi: 10.1152/ajprenal.1990.258.3.F522. [DOI] [PubMed] [Google Scholar]
- 13.Davisson RL, Ding Y, Stec DE, Caterall JP, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;15:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwathmey TM, Alzayadneh EM, Pendergast KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Reg Integr Comp Physiol. 2012;302:R518–530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abadir PM, Foster DB, Crowe M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burk TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuo JAA, Alcom D, Aldred GP, MacGregor DP, Mendelsohn FA. The distribution of angiotensin II receptors. Hypertension. 1995;35:155–163. [Google Scholar]
- 18.Ozono R, Wang Z-Q, Moore AF, Inagami T Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1248. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 19.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z-Q, Moore AR, Ozono R, Siragy HM, Carey RM. Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension. 1998;32:78–83. doi: 10.1161/01.hyp.32.1.78. [DOI] [PubMed] [Google Scholar]
- 21.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 22.Siragy HM, deGaspero M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- 23.Carey RM, Howell NL, Jin X-H, Siragy HM. Angiotensin type 2 receptor- mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38:1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- 24.Katada J, Majima M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br J Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widdop RE, Matrougui K, Levy BI, Henrion D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- 26.Hannan RE, Davis EA, Widdop RE. Functional role of the angiotensin II AT2 receptor in modulation of AT1 receptor-mediated contraction in rat uterine artery: involvement of bradykinin and nitric oxide. Br J Pharmacol. 2003;140:987–995. doi: 10.1038/sj.bjp.0705484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AH. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 28.Bergaya S, Hilgers RH, Meneton P, Dong Y, Bloch-Faure M, Inagami T, Alhenc-Galas F, Levy BI, Boulanger CM. Flow-dependent dilation mediated by endogenous kinins requires angiotensin AT2 receptors. Circ Res. 2004;94:1623–1629. doi: 10.1161/01.RES.0000131497.73744.1a. [DOI] [PubMed] [Google Scholar]
- 29.Hiyoshi H, Yayama K, Takano M, Okamoto H. Stimulation of cyclic GMP production via AT2 and B2 receptors in the pressure-overloaded aorta after banding. Hypertension. 2004;43:1258–1263. doi: 10.1161/01.HYP.0000128022.24598.4f. [DOI] [PubMed] [Google Scholar]
- 30.Yayama K, Horii M, Hiyoshi H, Takano M, Okamoto H, Kagota S, Kunitomo M. Up-regulation of angiotensin II type 2 receptor in rat thoracic aorta by pressure overload. J Pharmacol Exp Ther. 2004;308:736–743. doi: 10.1124/jpet.103.058420. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier KM, Zhang DX, Edwards EM, Holmes B, Campbell WB. Angiotensin II dilates bovine adrenal cortical arterioles: role of endothelial nitric oxide. Endocrinology. 2005;146:3319–3324. doi: 10.1210/en.2005-0129. [DOI] [PubMed] [Google Scholar]
- 32.Perlegas D, Xie H, Sinha S, Somlyo AV, Owens GK. Ang II type 2 receptor regulates smooth muscle growth and force generation in late fetal mouse development. Am J Physiol Heart Circ Physiol. 2005;288:H96–H102. doi: 10.1152/ajpheart.00620.2004. [DOI] [PubMed] [Google Scholar]
- 33.Savoia C, Ebrahimian T, He Y, Gratton JP, Schiffrin EL, Touyz RM. Angiotensin II/AT2 receptor-induced vasodilation in stroke-prone spontaneously hypertensive rats involves nitric oxide and cyclic GMP- dependent protein kinase. J Hypertens. 2006;24:2417–2422. doi: 10.1097/01.hjh.0000251902.85675.7e. [DOI] [PubMed] [Google Scholar]
- 34.Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor In resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49:341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- 35.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;59:709–716. doi: 10.1111/j.1476-5381.2009.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′,5′-monophosphate and AT1 receptor- mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol Reg Int Comp Physiol. 1996;27:R1090–R1095. doi: 10.1152/ajpregu.1996.271.4.R1090. [DOI] [PubMed] [Google Scholar]
- 38.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siragy HM, Jaffa AA, Margolius HS. Bradykinin B2 receptor modulates renal prostaglandin E2 and nitric oxide. Hypertension. 1997;29:757–762. doi: 10.1161/01.hyp.29.3.757. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasewa S, Takai S, Miyazaki M, Nozawa Y, Ozono R, Nakagawa K, Miwa T, Kawada N, Mori Y, Shibasaki Y, Tanaka Y, Fujiyama S, Koyama Y, Fujiyama A, Takahashi H, Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2 receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 42.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology and interactions with ceremide. Am J Physiol Heart Circ Physiol. 2001;281:H2337–H2365. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- 43.Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol. 2003;140:809–824. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet F, Cooper ME, Carey RM, Casley D, Cao Z. Vascular expression of angiotensin type 2 receptor in the adult rat: influence of angiotnesin II infusion. J Hypertens. 2001;19:1075–1081. doi: 10.1097/00004872-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 47.Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski M-C, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625–630. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- 48.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski M-C, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 49.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 50.van Esch JH, Oosterveer CR, Batenburg WW, van Veghel R, Jan Danser AH. Effects of angiotensin II and its metabolites in the rat coronary vascular bed: Is angiotensin III the preferred ligand of the angiotensin AT2 receptor? Eur J Pharmacol. 2008;588:286–293. doi: 10.1016/j.ejphar.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 51.Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from the adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011;152:1582–1588. doi: 10.1210/en.2010-1070. [DOI] [PubMed] [Google Scholar]
- 52.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal angiotensin AT2 receptors. Hypertension. 2012;60:387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang J, Chen C, Ren H, Han Y, He D, Zhou L, Hopfer U, Jose PA, Aeng C. Angiotensin II AT2 receptor decreases AT1 receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J Hypertens. 2012;30:1176–1184. doi: 10.1097/HJH.0b013e3283532099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrera M, Garvin JL. Angiotensin II stimulates thick ascending limb NO production via AT(2) receptors and Akt1-dependent nitric oxide synthase 3 (NOS3) activation. J Biol Chem. 2010;285:14932–14940. doi: 10.1074/jbc.M110.109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong NJ, Garvin JL. Angiotensin II type 2 receptor-mediated inhibition of NaCl absorption is blunted in thick ascending limbs from Dahl salt- sensitive rats. Hypertension. 2012;60:765–769. doi: 10.1161/HYPERTENSIONAHA.112.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes JM, Beck TR, Rose CE, Jr, Carey RM. Selective dopamine-1 receptor stimulation produces natriuresis by a direct tubular action. J Hypertens Suppl. 1986;4:S106–S108. [PubMed] [Google Scholar]
- 57.Carey RM, Hughes JM. Selective renal dopamine-1 receptor stimulation in man. Clin Exp Hypertens. 1987;9:1009–1020. doi: 10.3109/10641968709161462. [DOI] [PubMed] [Google Scholar]
- 58.Hughes JM, Ragsdale NV, Felder RA, Chevalier RL, Keng B, Carey RM. Diuresis and natriuresis during continuous dopamine-1 receptor stimulation. Hypertension. 1988;11:169–174. doi: 10.1161/01.hyp.11.2_pt_2.i69. [DOI] [PubMed] [Google Scholar]
- 59.Hughes JM, Beck TR, Rose CE, Jr, Carey The effect of selective dopamine-1 receptor stimulation on renal and adrenal function. J Clin Endocrinol Metab. 1988;66:518–525. doi: 10.1210/jcem-66-3-518. [DOI] [PubMed] [Google Scholar]
- 60.Ragsdale NV, Lynd M, Chevalier RL, Felder RA, Carey RM. Selective peripheral dopamine-1 receptor stimulation: differential responses to sodium loading and depletion in humans. Hypertension. 1990;15:914–921. doi: 10.1161/01.hyp.15.6.914. [DOI] [PubMed] [Google Scholar]
- 61.Carey RM, Stote RM, Dubb JW, Townsend LH, Rose CE, Jr, Kaiser DL. Selective peripheral dopamine-1 receptor stimulation with fenoldopam in human essential hypertension. J Clin Invest. 1984;74:2198–2207. doi: 10.1172/JCI111646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Intrarenal DA acts at the dopamine-1 receptor to control renal function. J Hypertens Suppl. 1988;6:S479–S481. doi: 10.1097/00004872-198812040-00151. [DOI] [PubMed] [Google Scholar]
- 63.Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol Renal Physiol. 1989;257:F469–F477. doi: 10.1152/ajprenal.1989.257.3.F469. [DOI] [PubMed] [Google Scholar]
- 64.Wang ZQ, Felder RA, Carey RM. Selective inhibition of the renal dopamine subtype D1A receptor induces antinatriuresis in conscious rats. Hypertension. 1999;33:504–510. doi: 10.1161/01.hyp.33.1.504. [DOI] [PubMed] [Google Scholar]
- 65.Carey RM, Van Loon GR, Baines AD, Ortt EM. Decreased plasma and urinary dopamine during dietary sodium depletion in man. J Clin Endocrinol Metab. 1981;52:903–909. doi: 10.1210/jcem-52-5-903. [DOI] [PubMed] [Google Scholar]
- 66.Zhang M-Z, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011:2845–2854. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Connell DP, Botkin SJ, Ramos SI, Sibley DR, Ariano MA, Felder RA, Carey RM. Localization of the dopamine D1A receptor protein in rat kidneys. Am J Physiol Renal Physiol. 1995:F1185–F1197. doi: 10.1152/ajprenal.1995.268.6.F1185. [DOI] [PubMed] [Google Scholar]
- 68.Ozono R, O’Connell DP, Vaughan C, Botkin SJ, Walk SF, Felder RA, Carey RM. Expression of the subtype 1A dopamine receptor in the rat heart. Hypertension. 1996;27:693–703. doi: 10.1161/01.hyp.27.3.693. [DOI] [PubMed] [Google Scholar]
- 69.Ozono R, O’Connell DP, Wang ZQ, Moore AF, Sanada H, Felder RA, Carey RM. Localization of the dopamine 1 receptor protein in the human heart and kidney. Hypertension. 1997;30:725–729. doi: 10.1161/01.hyp.30.3.725. [DOI] [PubMed] [Google Scholar]
- 70.O’Connell DP, Aherne AM, Lane E, Felder RA, Carey RM. Detection of dopamine receptor D1A subtype-specific mRNA in rat kidney by in situ amplification. Am J Physiol Renal Physiol. 1998;274:F232–F241. doi: 10.1152/ajprenal.1998.274.1.F232. [DOI] [PubMed] [Google Scholar]
- 71.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 72.Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D(1) and angiotensin type-2 receptor interaction in natriuresis. Hypertension. 2012;59:437–445. doi: 10.1161/HYPERTENSIONAHA.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine- induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci USA. 1998;95:5573–5578. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bengini A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morgi M, Coffman TM, Remuzzi G. Disruption of the angiotensin type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–2854. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012 doi: 10.1016/j.peptides.2012.09.016. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]