Abstract
The characteristics of early and mild disease in primary progressive aphasia are poorly understood. This report is based on 25 patients with aphasia quotients >85%, 13 of whom were within 2 years of symptom onset. Word-finding and spelling deficits were the most frequent initial signs. Diagnostic imaging was frequently negative and initial consultations seldom reached a correct diagnosis. Functionality was preserved, so that the patients fit current criteria for single-domain mild cognitive impairment. One goal was to determine whether recently published classification guidelines could be implemented at these early and mild disease stages. The quantitative testing of the recommended core and ancillary criteria led to the classification of ∼80% of the sample into agrammatic, logopenic and semantic variants. Biological validity of the resultant classification at these mild impairment stages was demonstrated by clinically concordant cortical atrophy patterns. A two-dimensional template based on orthogonal mapping of word comprehension and grammaticality provided comparable accuracy and led to a flexible road map that can guide the classification process quantitatively or qualitatively. Longitudinal evaluations of initially unclassifiable patients showed that the semantic variant can be preceded by a prodromal stage of focal left anterior temporal atrophy during which prominent anomia exists without word comprehension or object recognition impairments. Patterns of quantitative tests justified the distinction of grammar from speech abnormalities and the desirability of using the ‘agrammatic’ designation exclusively for loss of grammaticality, regardless of fluency or speech status. Two patients with simultaneous impairments of grammatical sentence production and word comprehension displayed focal atrophy of the inferior frontal gyrus and the anterior temporal lobe. These patients represent a fourth variant of ‘mixed’ primary progressive aphasia. Quantitative criteria were least effective in the distinction of the agrammatic from the logopenic variant and left considerable latitude to clinical judgement. The widely followed recommendation to wait for 2 years of relatively isolated and progressive language impairment before making a definitive diagnosis of primary progressive aphasia has promoted diagnostic specificity, but has also diverted attention away from early and mild disease. This study shows that this recommendation is unnecessarily restrictive and that quantitative guidelines can be implemented for the valid root diagnosis and subtyping of mildly impaired patients within 2 years of symptom onset. An emphasis on early diagnosis will promote a better characterization of the disease stages where therapeutic interventions are the most likely to succeed.
Keywords: aphasia, logopenic, semantic, agrammatic, anomic
Introduction
The diagnosis of primary progressive aphasia (PPA) requires the fulfilment of three core criteria. First, the patient should have an aphasic disorder of recent onset as manifested by distortions of word usage or comprehension that cannot be attributed to more elementary motor or perceptual deficits. Second, this language impairment should constitute the most salient neurobehavioural deficit and the chief impediment to the pursuit of customary daily living activities during the initial stages of the illness. Third, the underlying disease should be neurodegenerative and, therefore, progressive (Mesulam, 2001, 2003; Mesulam and Weintraub, 2008).
As the PPA syndrome was being delineated, the heuristic recommendation was made to delay definitive diagnosis until 2 years of a relatively isolated and functionally salient aphasia had elapsed. This ‘2-year rule’ was introduced to exclude patients with the rapidly progressive aphasias of Jacob–Creutzfeldt disease, as well as patients in whom word-finding difficulties could have appeared to lead the clinical picture until further evaluation showed equally prominent amnestic, comportmental or visuospatial deficits characteristic of other dementia phenotypes (Mesulam and Weintraub, 1992; Mesulam, 2001).
The 2-year rule promoted specificity and nosological uniformity by ensuring that only patients with an extended period of predominantly (if not exclusively) aphasic impairments would receive the root diagnosis of PPA. However, this emphasis on established disease also tended to divert attention away from the early and prodromal stages. In fact, a rich literature on the established and advanced phases of PPA has emerged while information on the early stages is rather sparse and anecdotal (Weintraub et al., 1990; LeRhun et al., 2005; Knopman et al., 2009; Sapolsky et al., 2010, 2011; Rogalski et al., 2011).
In contrast to PPA, a great deal of attention has been paid to early and mild impairment stages in typical amnestic dementias of the Alzheimer-type. Such investigations have led to the delineation of preclinical and prodromal disease mechanisms in ways that have promoted diagnostic sensitivity and insights into pathophysiology (Petersen et al., 1999; Jack et al., 2003). Progress along this line of research can be attributed to the high prevalence of Alzheimer’s disease and the importance of age as a major risk factor. These two features enabled large-scale longitudinal studies of high-risk (i.e. elderly) individuals so that prodromal features of Alzheimer’s disease and their neurodiagnostic correlates could be delineated prospectively. This approach is not practical in PPA where prevalence is low and where no major risk factor has yet been established.
A second successful approach for capturing preclinical and very early manifestations of neurodegenerative syndromes has been based on the identification of known carriers of disease-causing mutations prior to the onset of clinical manifestations. This strategy has been fruitful in a number of conditions with autosomal dominant transmission, such as Alzheimer’s disease, Huntington’s disease and frontotemporal degenerations (Geschwind et al., 2001; Ringman et al., 2004; Marshall et al., 2007; Borroni et al., 2008). With the exception of isolated case reports (Cruchaga et al., 2009), this approach is not applicable to PPA since families where genetic mutations lead to a consistent PPA phenotype in affected members are rarely encountered (Mesulam et al., 2007; Beck et al., 2008; Borroni et al., 2008).
This study followed a more indirect approach for identifying early and mild disease stages. It is based on 25 patients, whose aphasia quotient on the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2006) was >85%, 13 of whom were enrolled within 1–2 years since symptom onset. Although there is no test battery that can capture the complexity of a language disorder with a single number, we assumed that an aphasia quotient >85% denoted a mild impairment stage of the disease. This cohort was also used to test whether the recently published classification guidelines for subtyping PPA into agrammatic, semantic and logopenic subtypes (Gorno-Tempini et al., 2011) could be applied in a rigorous and quantitative fashion to patients with mild impairment.
Subjects and methods
Participants
The subjects were selected from a set of 100 patients consecutively referred to the Northwestern University Cognitive Neurology and Alzheimer’s Disease Centre for the evaluation of progressive language impairments between 2007 and 2011. Nineteen of the patients did not receive a PPA diagnosis because the aphasia was accompanied by equally prominent impairments of memory, executive function or comportment, seven did not agree to participate and five were excluded because of left-handedness. The remaining 69 patients fulfilled the criteria for a PPA diagnosis and were recruited into a longitudinal research programme. The aphasia quotients on the WAB-R (Kertesz, 2006) ranged from 35% to 97%. Two of the patients lacked crucial test results. Of the remaining 67 patients, 25 with aphasia quotients >85% yielded the clinical, neuropsychological and imaging data for this study. For the purpose of this longitudinal investigation, the 2-year rule was ignored and a reliable history of an insidiously progressive language impairment was deemed sufficient for inclusion. The 13 patients who did not have symptom duration of >2 years at the time of enrolment received a more definitive PPA diagnosis by the 2-year rule on subsequent clinical contacts in person or by telephone interview.
All participants were Caucasian, native English speakers and right-handed. The study was approved by the Institutional Review Board at Northwestern University and informed consent was obtained from all participants. Thirty-seven normal control subjects were recruited and deemed cognitively unimpaired following evaluation with the Uniform Data Set of the National Alzheimer’s Disease Coordinating Centre (Morris et al., 2006; Weintraub et al., 2009). The initial clinical diagnosis of PPA was made by the same clinician (M.M.) in all cases. Review of records, history from a reliable informant, administration of standardized neuropsychological measures and clinical neuroimaging were used to confirm that the patient fulfilled all three criteria for the root diagnosis of PPA listed in Table 1.
Table 1.
Criteria for the diagnosis of PPA and its subtypes
| A. Criteria for the root diagnosis of PPA |
| Diagnostic criteria for PPA (Mesulam, 2001, 2003) |
| The following three conditions must all be present. |
| 1. A new and progressive language disorder (aphasia) as documented by neuropsychologically determined abnormalities in one or more of the following domains: grammaticality of sentence production, word retrieval in speech, object naming, word and sentence comprehension, spelling, reading, repetition. Isolated impairments of articulation do not qualify. |
| 2. Relative preservation of episodic memory, executive functions, visuospatial skills and comportment as documented by history, medical records and/or neuropsychological testing. |
| 3. Imaging and other pertinent neurodiagnostic test results that rule out causes other than neurodegeneration. |
| B. Criteria for PPA subtypes |
| Non-fluent/agrammatic variant (PPA-G) (Gorno-Tempini et al., 2011) |
| A. One of the following core features must be present. |
| 1. Agrammatism in language production. |
| 2. Effortful, halting speech with inconsistent speech sound errors and distortions (apraxia of speech). |
| B. Two of the following three ancillary features must be present. |
| 1. Impaired comprehension of syntactically complex (non-canonical) sentences. |
| 2. Spared single-word comprehension. |
| 3. Spared object knowledge. |
| Semantic variant (PPA-S) (Gorno-Tempini et al., 2011) |
| A. Both of the following core features must be present. |
| 1. Impaired object naming. |
| 2. Impaired single-word comprehension. |
| B. Three of the following ancillary features must be present. |
| 1. Impaired object knowledge, particularly for low-frequency or low-familiarity items. |
| 2. Surface dyslexia or dysgraphia. |
| 3. Spared repetition. |
| 4. Spared grammaticality and motor aspects of speech. |
| Logopenic variant (PPA-L) (Gorno-Tempini et al., 2011) |
| A. Both of the following core features must be present. |
| 1. Impaired single-word retrieval in spontaneous speech and naming. |
| 2. Impaired repetition of phrases and sentences. |
| B. Three of the following ancillary features must be present. |
| 1. Phonological errors (phonemic paraphasias) in spontaneous speech or naming. |
| 2. Spared single-word comprehension and object knowledge. |
| 3. Spared motor speech. |
| 4. Absence of frank agrammatism. |
| Mixed variant (PPA-M) (Mesulam et al., 2009) |
| A. Both of the following features must be present. |
| 1. Agrammatism in language production. |
| 2. Word comprehension impairments. |
Assessment of aphasia severity
The Western Aphasia Battery-Aphasia Quotient was used as a global measure of aphasia severity. The aphasia quotient is derived from the WAB-R, a comprehensive tool for testing multiple aspects of language function, including speech fluency, communicative content, word and sentence comprehension, repetition, naming, reading and writing. A subset of these tests (speech fluency, content, comprehension, repetition and naming) is used to derive the aphasia quotient, which has a maximum score of 100. The WAB has been validated against other language measures and has good interrater and test–retest reliability (Kertesz, 2006).
The classification of PPA subtypes revolves around the core language domains of grammatical ability, word comprehension, object naming, repetition and motor aspects of speech (Table 1). Ancillary features include syntactic comprehension, object knowledge, phonologic integrity of speech and surface dyslexia. None of the currently existing test batteries capture all of these domains. The WAB was therefore supplemented by more specialized tests as well as a systematic analysis of recorded narrative speech. The recording was obtained by instructing participants to view a wordless picture book of the story of Cinderella and tell the story to the examiner. The narrative was recorded using Praat software (version 5.0, http://www.praat.org) and ‘start’ and ‘end’ times of each narration were automatically recorded, transcribed and coded by experienced personnel in the Aphasia and Neurolinguistics Research Laboratory at Northwestern University (Thompson et al., 1995, 1997). All coded transcripts were entered into the Systematic Analysis of Language Transcripts (SALT; Miller and Chapman, 2000).
Grammaticality of sentence production was assessed on the basis of three factors: (i) qualitative assessment of morphosyntactic errors (of noun morphology, verb morphology, argument structure and word order) in the recorded Cinderella narrative; (ii) scores on Northwestern Anagram Test (NAT; Weintraub et al., 2009); and (iii) scores on the Sentence Production Priming Test (SPPT) of the Northwestern Assessment of Verbs and Sentences (NAVS), an experimental battery in its final stages of standardization (Thompson, 2011). In the NAVS-SPPT, the patient is shown pairs of reversible action pictures and asked to produce sentences of varying complexity according to primes provided by the examiner. In the case of object-extracted ‘Wh’ questions, the examiner would first point to one of the pictures, such as a girl kissing a boy, and prime the patient with a question in the form of ‘who is the girl kissing?’ The patient would then be shown a picture of the reverse action and asked to generate a question with the same object-extracted ‘Wh’ structure. A subset of 15 non-canonical sentences (passive voice, object-extracted ‘Wh’ questions and object relatives) were used to derive a score of grammatical production in the NAVS-SPPT. Performance in this test can potentially be influenced by impairments of working memory or fluency. We therefore also used the NAT (Weintraub et al., 2009). During administration of the NAT, the patient is asked to order single words, each printed on a separate card, to be syntactically consistent with an action depicted in a target picture. This test correlates with other tests of grammatical sentence production but not with tests of naming, single word comprehension or motor speech production. A subset of 15 items from the NAT, representing the same non-canonical sentence types as those we chose for the NAVS-SPPT, were used to derive an additional score of grammaticality. Performance on these two sets of 15 non-canonical sentences was averaged to derive a composite score of grammatical sentence production (NAT and NAVS-SPPT).
The determination of whether the patient had ‘effortful, halting speech with inconsistent speech sound errors and distortions’ as stated in the list of criteria in Table 1 was based on listening to the recorded narrative of the Cinderella story. The presence of word-finding hesitations and phonemic speech errors was also determined by listening to the narrative sample. Transcription and coding of this sample generated a fluency score expressed in words per minute.
The ability to understand syntactic structure was assessed with the Sentence Comprehension Test of the NAVS, based on a subset of 15 non-canonical sentences of the same type as those chosen for the NAVS-SPPT and NAT. In the NAVS-Sentence Comprehension Test, the patient is shown two scenes depicting reversible actions and needs to choose the one corresponding to the stimulus sentence spoken by the examiner.
The Boston Naming Test (BNT) was used to assess the naming of objects (Kaplan et al., 1983). It is a 60-item standardized and challenging test in which items are administered in order of decreasing frequency of occurrence in the language. Word comprehension was tested with a subset of 36 moderately difficult items (157–192) of the Peabody Picture Vocabulary Test (PPVT-IV; Dunn and Dunn, 2006). This set had previously been used to construct a quantitative template for subtyping PPA (Mesulam et al., 2009). Each item requires the patient to match a word representing an object, action or attribute to one of four picture choices. Performance in the PPVT-IV correlates with word–word association tasks but not with tests of fluency (Mesulam et al., 2009). Object knowledge was assessed with the three picture version of the Pyramids and Palm Trees test (Howard and Patterson, 1992) where the patient is asked to decide which of two pictures is conceptually more closely associated with a target object.
We did not find an alternative validated test of repetition that was as comprehensive as the one included in the WAB-R. The WAB-R Repetition Subtest contains 15 items of ascending difficulty ranging from the repetition of single words to word strings, phrases and sentences. The first nine items were too easy and were not failed by any of the subjects. We therefore selected the six most difficult items where perfect performance yields a score of 66. Performance on this subset (REP6) was used to quantitate repetition abilities. Surface dyslexia and dysgraphia were examined with selected examples of exceptional words from the Psycholinguistic Assessment of Language Processing in Aphasia (Kay, 1992). Surface dyslexia was assessed by asking the patient to read the following words: ‘island’, ‘blood’, ‘routine’, ‘ceiling’, ‘mortgage’, ‘debt’, ‘gauge’, ‘tomb’, ‘cough’ and ‘bouquet’. Surface dysgraphia was assessed by asking the patient to write the following words: ‘elephant’, ‘sword’, ‘soldier’, ‘knock’, ‘ghost’, ‘shoe’, ‘queen’, ‘sledge’, ‘watch’ and ‘castle’. A composite score was obtained by averaging performance in the reading and writing of the words.
To control for differently scaled variables, quantitative performance scores were transformed into a percentage of the total possible score. In view of the mild aphasia, we assumed that a performance that fell <90% indicated impairment. In all quantitated domains, this cut-off represented a performance of at least two standard deviations below control values (Table 2). The words per minute counts were not transformed into percentages.
Table 2.
Age, education and language scores
| Subjects (n) | Age | Education | WAB-AQ | NAT and NAVS-SPPT(nc) | PPVT | BNT | REP6 | NAVS-SCTnc | PPT pictures | PALPA (exceptional) | Words per minute |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NC (37) | 62 (6.7) | 16 (2.4) | 99 (1) | 98 (2) | 98 (3) | 97 (3) | 99 (3) | 99 (3) | 98 (2) | 98 (4) | 132 ± 20 |
| PPA (25) | 65 (7.9) | 16 (2.1) | 92 (4) | 88 (19) | 87 (21) | 72 (29) | 86 (12) | 93 (10) | 94 (6) | 84 (17) | 103 ± 33 |
| 2 SD<NC | 97 | 94 | 92 | 91 | 96 | 93 | 94 | 90 | 92 |
Percentage of accuracy (and standard deviation) on the WAB-AQ, NAT and NAVS-SPPT(nc), PPVT, BNT, REP6, NAVS-SCTnc, PPT Pictures and PALPA (exceptional) tests. Thirty-five to 37 normal control (NC) subjects participated in establishing normative values for all tests except for the words per minute, where the control value was derived from 12 subjects.
AQ = aphasia quotient; SCT = Sentence Comprehension Test; NAVS-SCTnc = Sentence Comprehension Test for non-canonical sentences; PALPA (exceptional) = score derived from reading and spelling of exceptional words in the Psycholinguistic Assessment of Language Processing in Aphasia battery; PPT pictures = the picture form of the Pyramids and Palm Trees Test; REP6 = a subset of the six most difficult items in the repetition subtest of the WAB.
The Clinical Dementia Rating Scale (Morris, 1993) and the Mini-Mental State Examination (Folstein et al., 1975) were used to assess global aspects of functionality and cognition, respectively. The Clinical Dementia Rating places the heaviest emphasis on episodic memory dysfunction and its consequences in daily life. The Mini-Mental State Examination is also most focused on memory and orientation. The loss of points on the Mini-Mental State Examination, especially in its memory subtest, can potentially be attributed to the aphasia (Osher et al., 2007). However, a high score is a reliable marker of preserved global cognition.
Magnetic resonance imaging
Structural magnetic resonance imaging (MRI) scans were acquired and reconstructed with the FreeSurfer image analysis suite (version 4.5.0) as previously described (Mesulam et al., 2009; Rogalski et al., 2011). Cortical thickness maps of the PPA group were statistically contrasted against 27 right-handed age- and education-matched healthy volunteers. Differences in cortical thickness between groups were calculated by conducting a general linear model on every vertex along the cortical surface. False Discovery Rate (FDR) was applied at 0.05 to adjust for multiple comparisons and to detect areas of peak cortical thinning (i.e. atrophy) in PPA compared to controls (Genovese et al., 2002).
Results
The age and education levels of the overall PPA group were not significantly different from that of the 37 control subjects (Table 2). There were 13 males and 12 females in the PPA group (Table 3). Age of onset, as approximated by information derived from the patient, reliable informants and medical records, was in the 50s for 9 patients, in the 60s for 10 patients and in the 70s for 6 patients. All six of the oldest onset patients were female (Patients 6, 7, 18, 19, 22 and 25). Impaired word finding was an initial symptom in all but two of the patients. The next most common initial sign was abnormal spelling, as reported by nine patients. Additional early changes included speech abnormalities (slurring or mispronunciation), word comprehension errors, misuse of words (semantic paraphasias) and difficulty with arithmetic (Table 3). Abnormality of syntax (word order) was reported as an initial symptom in only one patient (Patient 8).
Table 3.
Onset, duration, functionality and clinical imaging
| Case number | Gender/age at onset | Years since onset/WAB-AQ | Initial symptoms as reported by patient/informant |
Functionality in daily living activities in areas other than language | Global function (CDR/MMSE) | Diagnostics prior to entry into this study |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WF | SP | SY | CO | MI | NU | OR | MRI/CT | PET | |||||
| P1-G | M/53 | 5/90 | + | + | + | Unimpaired, out of work for unrelated reasons, active in complex hobby. | 1/24 | Negative | NA | ||||
| P2-G | M/61 | 1–2/93 | + | + | + | Unimpaired, but retired due to the aphasia. | 0/28 | Negative | Left hemisphere | ||||
| P3-G | M/50 | 1–2/93 | + | + | + | + | Unimpaired, working. | 0.5/27 | Left parietal | NA | |||
| P4-G | M/60 | 1–2/95 | + | + | Unimpaired, working. | 1/29 | Negative | NA | |||||
| P5-G | F/55 | 1–2/88 | + | + | Part-time work as health care professional. | 0.5/26 | Negative | Left temporoparietal | |||||
| P6-G | F/79 | 1–2/94 | + | + | + | Unimpaired, holds major board membership. | 0.5/30 | Negative | NA | ||||
| P7-G | F/71 | 4/89 | + | Unimpaired, civic, social activities maintained. | 0.5/30 | Negative | NA | ||||||
| P8-G | F/60 | 1–2/94 | + | + | + | Unimpaired, but retired due to the aphasia. | 0.5/26 | Negative | NA | ||||
| P9-G | M/66 | 1–2/94 | + | + | Unimpaired, working. | 1.5/26 | Non-focal atrophy | NA | |||||
| P10-Sp | M/66 | 3/97 | + | + | + | Unimpaired, but retired due to the aphasia. | 2.5/30 | Negative | Negative | ||||
| P11-S | F/51 | 3/95 | + | + | Unimpaired, but less invested in customary activities. | 2.5/29 | Left hemisphere | NA | |||||
| P12-S | M/59 | 4/88 | + | + | Stopped work, mild disinhibition, drives, independent in daily living. | 4/27 | CT-left anterotemporal | NA | |||||
| P13-S | F/60 | 1–2/85 | + | + | Continues part-time work, pursues complex hobby. | 1.5/24 | Left temporal | NA | |||||
| P14-S | M/64 | 1–2/87 | + | Stopped work for unrelated reasons, travels widely, has complex hobby. | 1/28 | Left anterotemporal | NA | ||||||
| P15-L | M/66 | 3/92 | + | Unimpaired, retired for unrelated reasons, performs complex chores. | 0/30 | Negative | Minimally abnormal | ||||||
| P16-L | M/58 | 5/97 | + | Unimpaired, but retired due to the aphasia, volunteers in high-level activity. | 0.5/27 | Negative | NA | ||||||
| P17-L | M/56 | 1–2/87 | + | + | Had to retire, performs complex chores at home, less confident in driving. | 1/23 | Non-focal atrophy | Left temporoparietal | |||||
| P18-L | F/72 | 1–2/97 | + | Unimpaired, successful in competitive hobby. | 0/28 | Negative | Left hemisphere | ||||||
| P19-L | F/74 | 4/89 | + | + | + | Unimpaired. | 0/24 | Non-focal atrophy | NA | ||||
| P20-L | F/53 | 4/95 | + | Unimpaired, working | 1/30 | Negative | Left hemisphere | ||||||
| P21-M | F/55 | 8/88 | + | + | + | Quit job due to the aphasia, otherwise independent. | 2/26 | CT-negative | NA | ||||
| P22-M | F/76 | 1–2/88 | + | + | Unimpaired. | 0.5/28 | Negative | NA | |||||
| P23 | M/63 | 1–2/90 | + | + | + | Unimpaired, working. | 1/29 | Left hemisphere | Left hemisphere | ||||
| P24 | M/60 | 3/96 | + | Unimpaired, working. | 0/29 | Negative | Negative | ||||||
| P25 | F/75 | 3/92 | + | Unimpaired. | 2/29 | Negative | NA | ||||||
CDR = Clinical Dementia Rating Scale, Sum of Boxes (range 0–18, 18 is most impaired); CO = word comprehension impairment; F = female, M = male; MI = misuse of words (as in semantic paraphasias); MMSE = Mini-Mental State Examination (range 0–30, 30 is most preserved); NA = data not available; NU = impaired handling of numbers (arithmetic); OR = errors in orthography (spelling); SP = speech impairment; SY = abnormal syntax (word order); WAB-AQ = Aphasia quotient of the Western Aphasia Battery; WF = word-finding impairment.
The selectivity of the language impairment was also reflected in the Clinical Dementia Rating Sum of Boxes, which provides a global score of functionality ranging from 0 (unimpaired) to 18 (severely impaired). In the PPA group, median Clinical Dementia Rating Sum of Boxes was 1. The Mini-Mental Status Examination was also high, with a mean of 27.5 ± 2.1, where 30 is the highest possible score. In keeping with these low Clinical Dementia Rating scores, information obtained from patients and informants confirmed preserved functionality. Many patients continued to work, some devoted more time to social and recreational activities, and a few became engaged in new complex hobbies.
Brain CT or MRI obtained at the time that the patient sought initial medical consultation was negative or reported non-focal atrophy in 19 of the 25 cases and was therefore not helpful in reaching a diagnostic formulation in 76% of the sample. PET had been obtained in 8 of the 19 cases where structural scans were uninformative, and revealed the characteristic asymmetric hypometabolism of PPA in 5 cases.
Empirical validation of classification guidelines and the overlapping criteria for agrammatic and logopenic subtypes of primary progressive aphasia
One goal was to determine whether the guidelines listed in Table 1 could be applied strictly and quantitatively to the subtyping of patients at early and mild stages of impairment. Eight patients (Patients 1–8) fulfilled the criteria for the ‘non-fluent/agrammatic’ subtype of Table 1 based on abnormal grammaticality in language production as well as the ancillary conditions of preserved word comprehension and object knowledge (Table 4). Two of the patients (Patients 1 and 2) showed abnormal comprehension of non-canonical sentences, another ancillary criterion for this subtype. Patient 9 was included in this group, despite a Pyramids and Palm Trees Test score that fell under the 90% cut-off, because of the distinct agrammaticality (70% score), preserved word comprehension, low words per minute and failure to fulfil the criteria for another subtype. Although Patient 10 did not fulfil the criteria for agrammaticality, he also fit the ‘non-fluent/agrammatic’ designation according to the criteria in Table 1, because of distinctly effortful and distorted speech on the recorded narrative as well as spared word and object knowledge. In order to promote uniformity in classification, Patients 1–9 were given a suffix of ‘G’ to denote impaired grammaticality and a clinical picture consistent with the PPA-G designation, whereas Patient 10 was given the suffix of ‘Sp’ to indicate that speech, rather than grammatical ability, would have determined his inclusion into the ‘non-fluent/agrammatic’ subgroup of Table 1.
Table 4.
Performance in language tasks
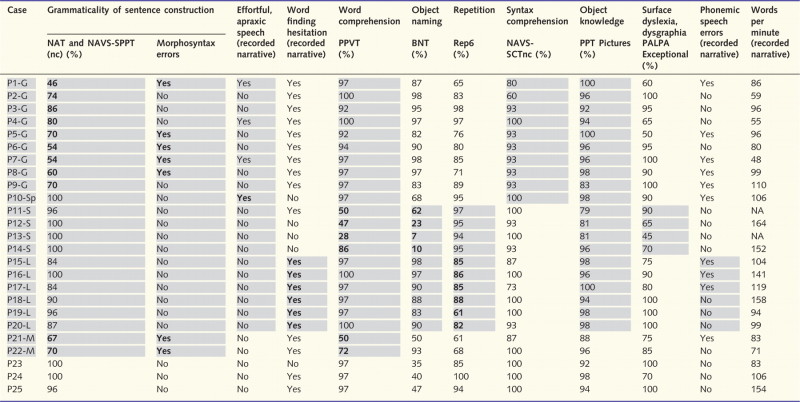 |
Performance with <90% accuracy falls below 2 SDs from normative values and was considered impaired. Shaded cells indicate domains included in the core or ancillary criteria for that subtype. Values in bold indicate presence of the core criteria for that subtype.
BNT = Boston Naming Test; NA = data not available; NAT and NAVS-SPPT(nc) = mean of the scores (for non-canonical sentences) on the Northwestern Anagram Test and on the Sentence Production Priming Test of the Northwestern Assessment of Verbs and Sentences; NAVS-SCTnc = Sentence Comprehension Test for non-canonical sentences in the Northwestern Assessment of Verbs and Sentences; PALPA (Exceptional) = Score derived from reading and spelling of exceptional words in the Psycholinguistic Assessment of Language Processing in Aphasia battery; PPT pictures = the picture form of the Pyramids and Palm Trees Test; REP6 = a subset of the six most difficult items in the repetition subtest of the Western Aphasia Battery.
Four patients (Patients 11–14) showed abnormal performance in both object naming and word comprehension tests. The additional sparing of repetition and grammaticality and the abnormal scores in tests of object knowledge or surface dyslexia/dysgraphia fulfilled the criteria for the semantic variant (PPA-S). This is the only group where initial clinical and quantitative scans invariably showed left anterior temporal atrophy and where word comprehension impairments were reported as salient initial symptoms (Table 3).
Six patients (Patients 15–20) fulfilled the core criteria for the logopenic variant (PPA-L) because of abnormalities in repetition and word-finding hesitations, with or without additional object naming impairments. Ancillary criteria included absence of abnormalities in speech, of word/object knowledge and of major impairments of grammaticality. Phonemic paraphasia, another ancillary feature for this variant, was seen in three (Patients 15, 16 and 17) of the six patients in this group. The two core features for PPA-L, word-finding hesitations and poor repetition were also prominent in PPA-G. The possibility that PPA-L and PPA-G have different types of repetition impairment based on sentence or word length was not tested.
The classification guidelines of Table 1 lead to some ambiguity in differentiating PPA-G from PPA-L. For example, a patient with agrammatism, impaired repetition, impaired single-word retrieval and phonemic paraphasias would fulfil the criteria for both variants as long as motor speech, single word comprehension and object knowledge are spared. Thus, Patients 5, 8, 15, 17 and 20 fulfilled criteria for PPA-G as well as PPA-L. We chose to include the former two in the PPA-G group because of the prominent agrammatism and the remaining three in the PPA-L group because we interpreted the exclusionary criterion number 4 for PPA-L in Table 1 (‘absence of “frank” agrammatism’) specifically to designate ‘prominent’ abnormalities of grammaticality, such as those that should lead to overt morphosyntax errors in speech. Conceivably, patients with PPA-G and PPA-L may score poorly in tests of grammaticality for different reasons: PPA-G because of a fundamental syntax processing impairment and PPA-L due to poor verbal working-memory. Longitudinal studies will be needed to show whether the PPA-L patients who also marginally fulfil PPA-G criteria will develop more pronounced grammar impairments in time.
Two patients (Patients 21 and 22) had significant impairments in both grammaticality and word comprehension. This could not be attributed to severity since the WAB aphasia quotient was 88 in both (Tables 3 and 4). These patients can be classified as having a mixed subtype and correspond to the previously proposed PPA-M designation (Mesulam et al., 2009). Three patients (Patients 23–25) were not classifiable by the criteria of Table 1. All three stood out because of pronounced abnormalities in object naming. They came close to fulfilling the criteria for PPA-S except that word comprehension was preserved.
Although fluency, defined as the rate of word production, is no longer a defining variable in the classification of PPA, words per minute measures were computed for 23 of the patients (Table 4). The PPA-G and PPA-M groups had the lowest words per minute scores. However, there was considerable overlap at the level of individual patients. For example, three patients with PPA-L (Patients 15, 19 and 20) and two of the initially unclassifiable patients (Patients 23 and 24) had words per minute scores that fell within the range of those in the PPA-G group. Fluency scores of <60 words per minute were only seen in PPA-G, but such low scores were not necessarily present in all members of this group. The words per minute scores were slightly above normal in the two PPA-S patients for whom the measurements were available, and reflected their tendency for logorrhoea and circumlocution. It remains to be seen whether low fluency in PPA-L predicts future evolution to PPA-G.
Template approach to classification
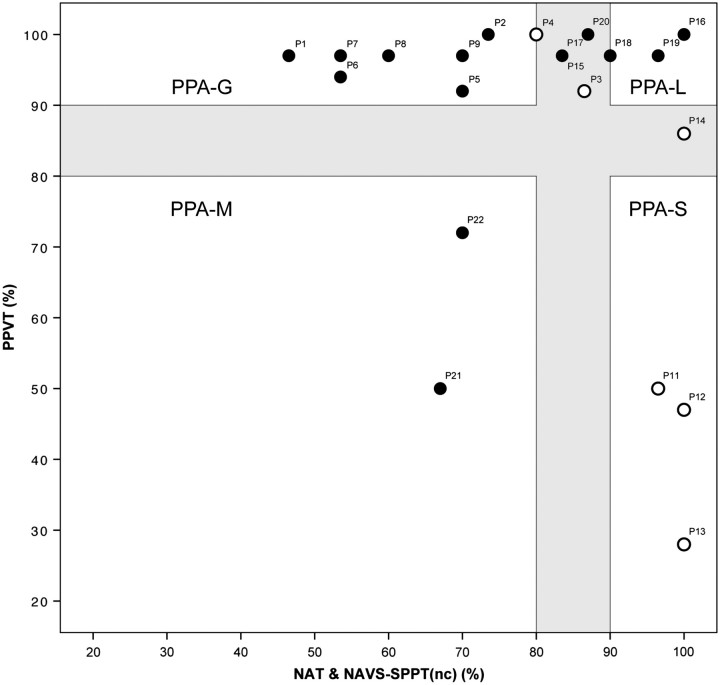
Table 4 lists performance in all 10 domains required by the recent classification criteria for PPA (Gorno-Tempini et al., 2011). We wanted to see whether a more condensed template approach, previously tested on 16 patients (Mesulam et al., 2009), could yield similar results. This template consists of two orthogonal axes, one representing word comprehension, as measured by the PPVT, and the other grammaticality, as measured by the combined scores on the NAT and NAVS-SPPT (Fig. 1). The previous patient sample, which led to the development of this template, had a wide range of aphasia severity and provided subtyping information based on a cut-off level of 60%. For the current sample of less impaired patients, we chose a stringent cut-off at the 90% level of accuracy, and also assumed that each axis would have a ‘grey zone’ of 10 percentage points, so that performance of better than 90% would indicate a definitely preserved domain whereas performance of worse than 80% would indicate definite impairment. In this template, the upper left quadrant beyond the grey zone contains PPA-G, the lower right quadrant beyond the grey zone PPA-S and the lower left quadrant beyond the grey zone PPA-M. The upper right quadrant beyond the grey zone is the most complex. Patients in this quadrant who have repetition impairments are classified as PPA-L, those who do not are unclassifiable by the criteria in Table 1. The grey zone was introduced to indicate that the boundaries between subtypes have a certain amount of blurring and also to allow clinical judgement greater latitude in classifying borderline cases, including those that simultaneously fulfil the PPA-G and PPA-L criteria in Table 1.
Figure 1.
Quantitative template. Filled circles indicate impaired performance (<90% accuracy) in repetition tasks. Open circles indicate preserved repetition. The interval between 80% and 90% accuracy represents a grey zone where boundaries between subtypes are blurred. Numbers correspond to subject numbers in Tables 3 and 4. nc = non-canonical sentences.
The usefulness of the template was tested on the 21 patients with definite PPA-G, PPA-L and PPA-S classifications according to the core and ancillary criteria listed in Table 4. The three unclassifiable patients (Patients 23–25) and the one patient with impairment of speech but not grammar (Patient 10) were excluded. As shown in Fig. 1, this template placed seven of the patients (Patients 1, 2, 5 and 6–9) in the PPA-G quadrant, three in the PPA-S quadrant (Patients 11–13), two in the PPA-M quadrant (Patients 21 and 22) and two in the PPA-L quadrant (Patients 16 and 19). Seven patients fell in the grey zone. Two of these, Patients 3 and 4, were classified as PPA-G because of speech and grammar abnormalities without repetition impairments. Four patients (Patients 15, 17, 18 and 20) were classified as PPA-L because of abnormal repetition and mildly impaired or preserved grammaticality, and Patient 14 was classified as PPA-S because the PPVT score was lower than the 90% cut-off while grammaticality and repetition were preserved. All patients who were classified into the agrammatic, logopenic, semantic and mixed subtypes based on the 10 domains of Table 4 were thus also classified by the relatively simpler rules of this template.
Return of the unclassifiables
The three unclassified subjects were recalled for return assessments after 2-year (Patients 23 and 24) and 1-year (Patient 25) intervals (Fig. 2). According to the template in Fig. 1, Patients 23 and 24 would be classified as PPA-S at the retesting stage because of the drop in PPVT performance to <90% accuracy. However, by the strict criteria of Table 1 and our cut-off at 90% accuracy, Patient 24 would have remained unclassifiable because of the PPT performance of 92% accuracy, indicative of preserved object recognition and the accuracy in the repetition task, which fell just under 90%. One subject, Patient 25, remained unclassifiable by either the template or strict criteria, despite the severe naming impairment that had progressed by the second visit, and can best be characterized as representing an anomic form of PPA. If comprehension or repetition impairments were to arise in the future, the patient could then fulfil the criteria for PPA-S or PPA-L.
Figure 2.
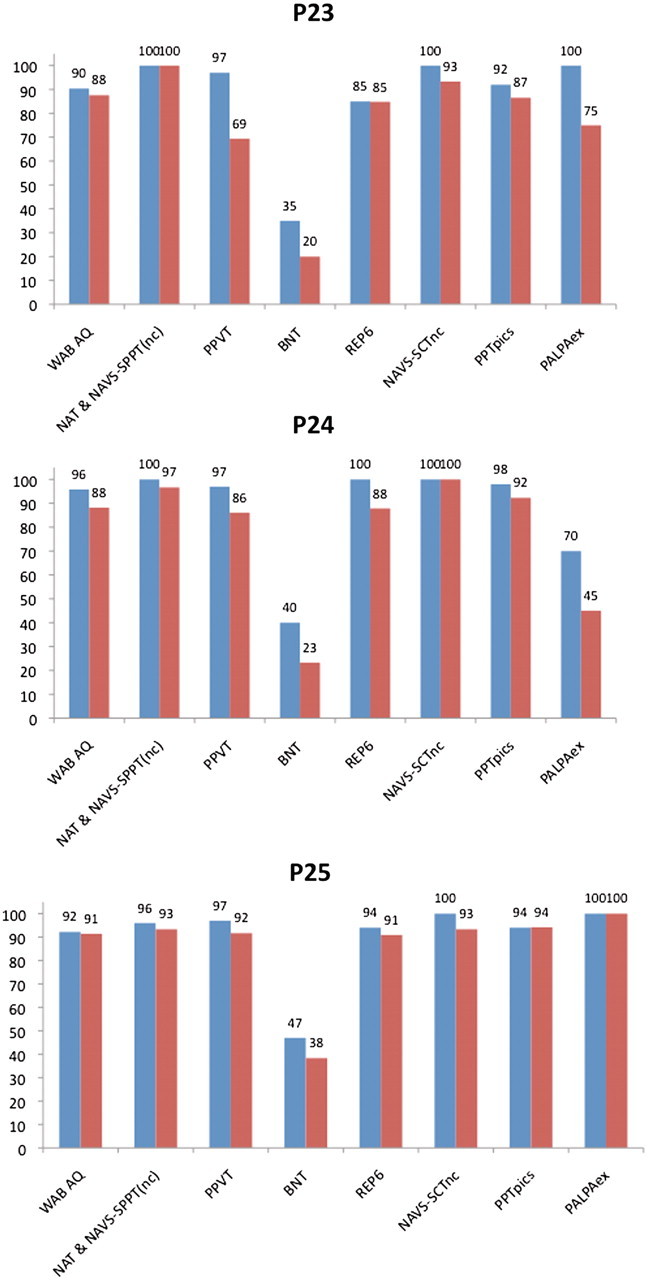
Baseline (blue) and follow-up (red) performance of initially unclassifiable subjects. The vertical axis represents accuracy in percentages. AQ = aphasia quotient; nc = non-canonical sentences; SCT = Sentence Comprehension Test; PALPAex = score derived from reading and spelling of exceptional words in the Psycholinguistic Assessment of Language Processing in Aphasia battery; PPTpics = the picture form of the Pyramids and Palm Trees Test; REP6 = a subset of the six most difficult items in the repetition subtest of the WAB-R.
Anatomy of atrophy
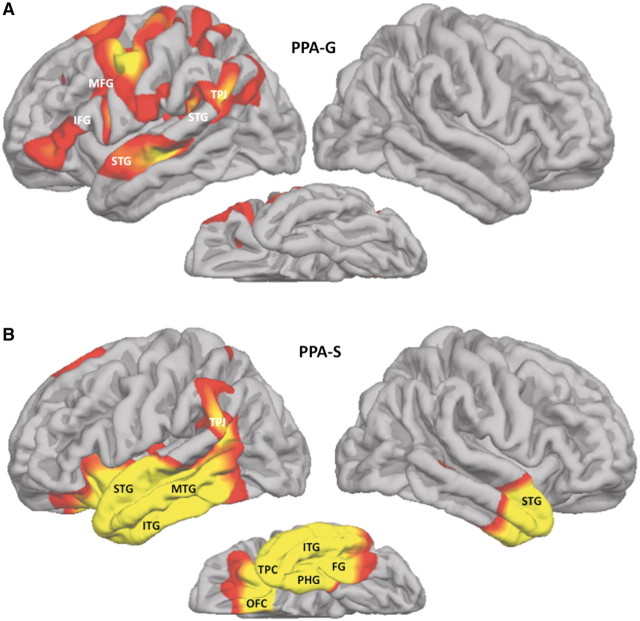
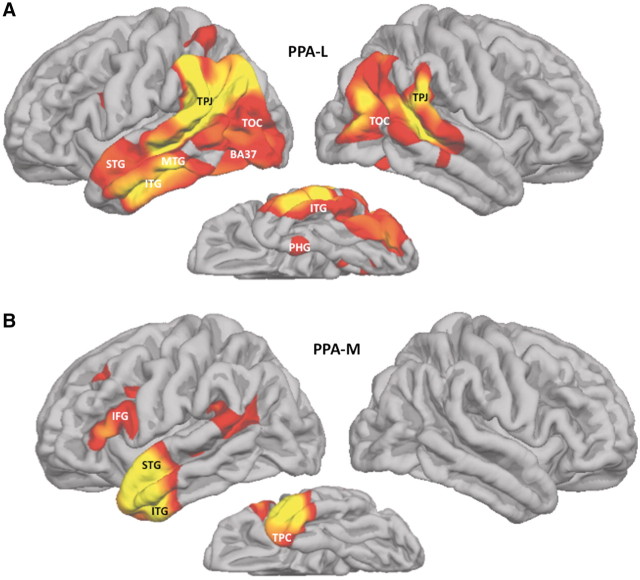
Seven of the nine patients with PPA-G had quantitative MRI (Patients 2 and 4–9). Atrophy in this group was only detected in the left hemisphere and involved the inferior frontal gyrus, the temporoparietal junction, anterior part of the superior temporal gyrus and the posterior part of the middle frontal gyrus, extending into the precentral gyrus (Fig. 3A). Peak atrophy in the four patients with PPA-S was predominantly, though not exclusively, in the left hemisphere and covered nearly the entire temporal lobe with extension into the adjacent temporoparietal junction and posterior orbitofrontal cortex. A much smaller region of the right temporal lobe, mostly at the anterior tip, also showed significant atrophy (Fig. 3B). Peak atrophy in the six patients with PPA-L extended into most of the left temporal lobe with the exception of its medial and temporopolar aspects (Fig. 4A). The temporoparietal junction, temporooccipital cortex and Brodmann area 37 in the inferolateral temporal lobe were also atrophied. The atrophy was mostly in the left hemisphere but there was also prominent atrophy in the temporoparietal junction and temporo-occipital cortex of the right hemisphere. The two patients with PPA-M (Patients 21 and 22) had peak atrophy only in the left hemisphere, involving the inferior frontal gyrus, superior temporal gyrus and the anterior parts of the temporal lobe, including temporopolar cortex (Fig. 4B). These atrophy maps reflect the pooled data for each group. With the exception of the patients with PPA-S, cortical thinning was generally too mild to be detected at the level of individual scans so that a more fine-grained linkage between atrophy patterns and individual performance levels could not be explored.
Figure 3.
Atrophy patterns in PPA-G and PPA-S. Red and yellow areas designate peak atrophy sites at False Discovery Rate = 0.05, full-width at half-maximum = 20 when patient groups were compared to a normal control group of 27 subjects. FG = fusiform gyrus; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; MFG = middle frontal gyrus; MTG = middle temporal gyrus; OFC = orbitofrontal cortex; PHG = parahippocampal gyrus; STG = superior temporal gyrus; TPC = temporopolar cortex; TPJ = temporoparietal junction.
Figure 4.
Atrophy patterns in PPA-L and PPA-M. Red and yellow areas designate peak atrophy sites at False Discovery Rate = 0.05, full-width at half-maximum = 20 when patient groups were compared to a normal control group of 27 subjects. BA37 = Brodmann area 37; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; MTG = middle temporal gyrus; OFC = orbitofrontal cortex; PHG = parahippocampal gyrus; STG = superior temporal gyrus; TPC = temporopolar cortex; TOC = temporooccipital cortex; TPJ = temporoparietal junction.
The three unclassified patients were scanned at baseline and return visits. At baseline, Patient 23 had only a clinical scan. It showed asymmetric atrophy of the left anterior temporal lobe (Fig. 5A). The baseline scan for Patient 24 showed atrophy confined to the anterior tip of the left temporal lobe, extending into the pole (Fig. 5C). Two years later, Patient 23 showed pronounced anterior temporal and lesser inferior frontal gyrus atrophy, both confined to the left hemisphere (Fig. 5B). The 2-year repeat scan of Patient 24 showed a slight increase in the areas of anterior temporal atrophy with no detectable spread to the right hemisphere (Fig. 5D). Patient 25 had no detectable atrophy at baseline or return visit.
Figure 5.
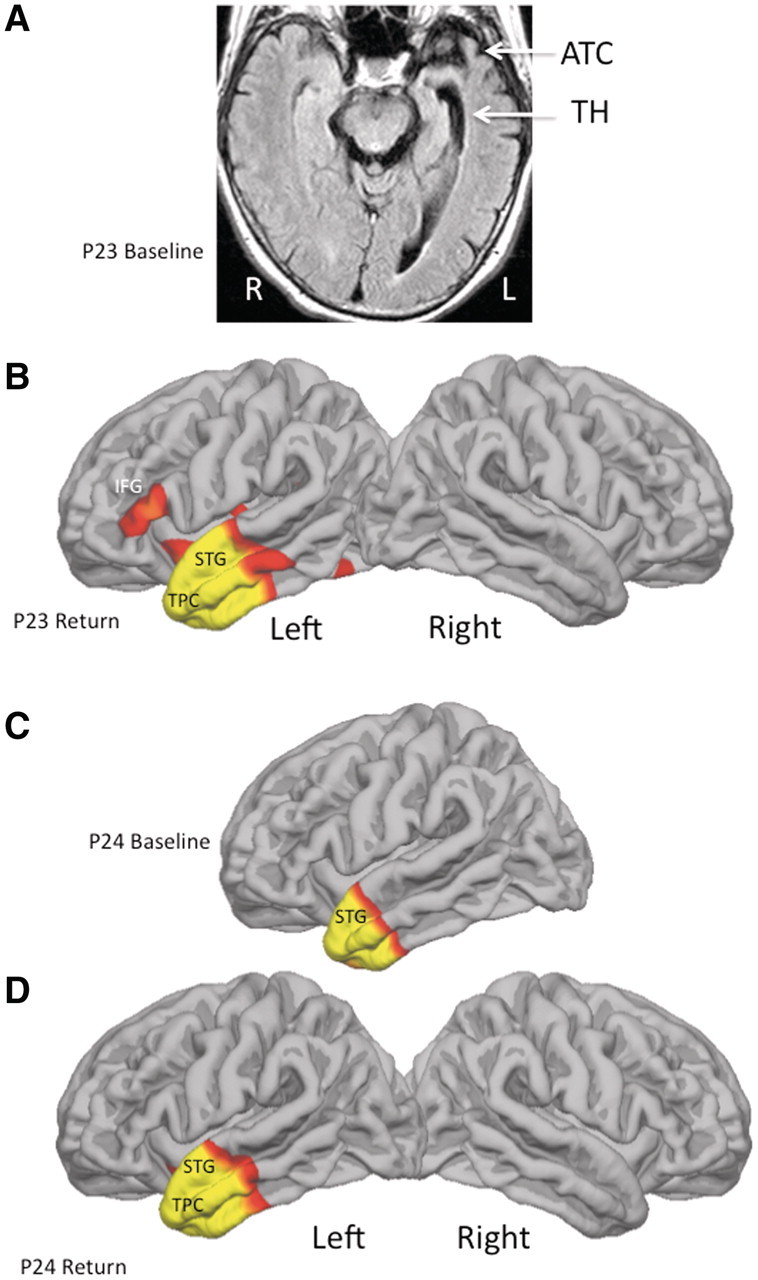
Atrophy at baseline and return visit. Red and yellow areas designate peak atrophy sites at False Discovery Rate = 0.05, full-width at half-maximum = 20 when the patient was compared to a normal control group of 27 subjects. (A) Only a clinical scan was available at baseline for Patient 23 (P23). It shows asymmetrical atrophy of anterior temporal cortex and enlargement of the temporal horn (TH) on the left. (B–D) Quantitative scans of cortical thinning. ATC = anterior temporal cortex; IFG = inferior frontal gyrus; STG = superior temporal gyrus; TPC = temporopolar cortex.
Discussion
This investigation focused on the diagnosis, classification and clinicoanatomical features of early and mild impairment stages in a group of 25 consecutively enrolled patients with PPA. For the purpose of the current investigation, we disregarded the widely adopted recommendation to delay a definitive root diagnosis of PPA until at least 2 years of an isolated aphasia had elapsed (Mesulam and Weintraub, 1992) and included 13 patients with progressive symptoms of lesser duration. The language disorder was relatively mild as reflected by a WAB aphasia quotient >85% in each of the 25 patients. Functionality and global cognitive abilities were largely preserved. While depression and frustration were common, as has been shown in prior work (Medina and Weintraub, 2007), they were not paralysing. According to current nomenclature, each of these 25 patients would fit the diagnosis of single-domain (language) mild cognitive impairment (Petersen et al., 2009). In keeping with the mild severity of the disease, structural and metabolic imaging during the initial medical work-up was frequently uninformative. The clinician must therefore be prepared to consider a diagnosis of PPA in patients who are fully functional, come with the single chief complaint of intermittent word-finding hesitations, have no other detectable impairment in routine assessments of cognition, and whose imaging may be negative. In fact, accurate diagnosis was rarely rendered at the initial medical encounter and the emerging aphasia was occasionally attributed to stress, depression or dysfunctional vocal cords.
The classification of language abnormalities in neurodegenerative disease is at least as challenging as it has been in patients with cerebrovascular accidents. Initial characterizations of the PPA syndrome recognized the heterogeneity of the associated language impairments and their differences from the subtypes identified by classic aphasiology (Mesulam, 1982, 2001; Mesulam and Weintraub, 1992). Some of the aphasic features seen in PPA were incorporated into diagnostic criteria that were being compiled for the clinical classification of frontotemporal lobar degenerations (Neary et al., 1998). However, the resultant syndromes did not fully capture the clinical spectrum of PPA and left gaps that had to be filled-in by new sets of guidelines (Gorno-Tempini et al., 2004). The resultant Gorno-Tempini et al. (2011) classification of PPA has introduced a more comprehensive platform for research and clinical practice (Gorno-Tempini et al., 2004, 2011). Algorithms and templates have been published to implement this classification but numerous details remain to be worked out before the guidelines can be transformed from principles into specific procedures (Mesulam et al., 2009; Leyton et al., 2011). First, the 2011 guidelines do not specify the individual tests to be administered or cut-off scores to be used. Secondly, they do not specify whether the resultant subtyping is detectable early in the disease or whether it can still be discerned beyond a certain stage of severity. Moreover, investigations in this area have illustrated the practicability of the guidelines by showing differences at the ‘group’ rather than ‘individual patient’ level (Gorno-Tempini et al., 2004; Leyton et al., 2011). This is a potentially important distinction since a classification method may succeed in reaching a certain inclusionary or exclusionary criterion at the group level without necessarily ensuring that this is also the case at the level of each member of the group. One goal for the current investigation was to address some of these procedural questions by determining whether a rigorous application of the published criteria could be achieved through the use of specific tests and explicit cut-off scores in successively enrolled individual patients at the mild and early stages of PPA.
Mild impairment stage of the agrammatic variant of primary progressive aphasia
The PPA-G subgroup had the highest proportion of patients seen within 2 years of symptom onset, probably because the relatively frequent speech distortions, such as mispronunciations of multi-syllable words, led to early detection and referral. The shared core feature was the impaired grammaticality of sentence construction as revealed by the combined NAT and NAVS-SPPT scores. Only patients with PPA-G and PPA-M had <80% accuracy in this domain. This feature and the preservation of single word comprehension allowed the 2D template of Fig. 1 to correctly classify these patients, even without taking the other ancillary criteria into consideration. Although not listed as an ancillary feature for PPA-G, the next most frequent abnormality was in phrase and sentence repetition. Impaired comprehension of syntactically complex sentences, an ancillary criterion in the comprehensive classification system of Table 1, was seen in only two of the patients and was also detected in the PPA-L group. Even at these early stages of the disease, cortical maps showed significant atrophy sites in the inferior frontal gyrus, temporoparietal junction, superior temporal gyrus, posterior middle frontal gyrus and the precentral gyrus (Fig. 3A). A previous study on a group of patients with PPA with a much broader range of severity had demonstrated correlations of atrophy with grammatical competence in the inferior frontal gyrus, with fluency in the middle frontal gyrus and with repetition in the posterior superior temporal gyrus (Amici et al., 2007; Rogalski et al., 2011). The presence of atrophy in these three regions, and the impairments of grammaticality, repetition and fluency in the patients, suggests that similar clinicoanatomical correlations are likely to exist at the early stages of PPA-G as well. Atrophy was confined to the left hemisphere. The degree of asymmetry was greater than what had been shown in a group of patients with PPA-G with more advanced disease (Mesulam et al., 2009).
The assessment of grammaticality and fluency
In clinical practice, grammaticality is usually determined qualitatively by detecting morphosyntactic errors in spoken or written sentences. Five of the patients with PPA-G displayed this feature but three did not, despite their abnormal NAT and NAVS-SPPT score, indicating that the quantitative tests may provide greater sensitivity for detecting impairments of grammaticality, especially in mildly impaired patients. Fluency in neurodegenerative aphasia is not necessarily correlated with grammaticality and these features of language may have different, albeit partially overlapping, neuroanatomical substrates (Weintraub et al., 2009; Rogalski et al., 2011; Thompson et al., 2012). The complex relationship between grammaticality and fluency is illustrated in Table 4. In general, the patients with PPA-G had the lowest words per minute scores. However, there was also overlap between the range of scores seen in PPA-G and those noted in members of all other groups except for PPA-S.
Although the newer classification system of Table 1 no longer lists fluency as a criterion, it uses the ‘non-fluent/agrammatic’ designation for one of the variants. The new classification system also allows patients with effortful or apraxic speech to receive this designation even if they have no significant impairment of grammaticality, as illustrated by Patient 10 (Table 4). Our approach has been to sharpen the scope of this variant by emphasizing impaired grammaticality as the single core feature and shortening the designation to ‘agrammatic variant’. Aphasic patients with speech apraxia but preserved grammatical ability may need to be placed in a separate category, as was done in the case of Patient 10, in order to maintain uniformity of the resultant symptom clusters. In fact, the quantitative brain scan of Patient 10 showed atrophy confined to the left anterior temporal lobe, more in keeping with his anomia (68% accuracy in the BNT) than with the pattern characteristic of the ‘non-fluent/agrammatic’ PPA variant.
Prodromal and mild impairment stage of the semantic variant of primary progressive aphasia
The longitudinal course of the two initially unclassifiable patients, Patients 23 and 24, shows that PPA-S has a prodromal stage during which severe naming impairments emerge in isolation and in the absence of detectable abnormality of single word comprehension or object knowledge. During this prodromal period, peak cortical atrophy can be confined to the anterior tip of the temporal lobe, exclusively within the left hemisphere (Fig. 5A and C). As the word comprehension and lesser object knowledge impairments in PPA-S become established (e.g. in Patients 11–14), the atrophy expands posteriorly into the left temporal lobe, anteriorly into the adjacent orbitofrontal cortex and contralaterally into the anterior tip of the right temporal lobe (Fig. 3B). The emergence of lesser but significant object knowledge impairments at this stage may reflect the spread of the temporopolar atrophy to the homologous parts of the right hemisphere, while the extension into orbitofrontal cortex may explain the high incidence of comportmental abnormalities in PPA-S as the disease progresses. PPA-S was the only group where initial diagnostic brain scans frequently revealed asymmetric atrophy of the left anterior temporal cortex in individual patients (Table 3) and where the initial symptoms could be reported as ‘forgetting the meaning of words’ rather than word finding hesitations. In all patients with PPA-S, performance on tests of word comprehension (PPVT) was distinctly inferior to performance on tests of object knowledge (Pyramids and Palm Trees Test), further illustrating the fact that aphasia rather than a more general impairment of object knowledge is the core feature of this variant (Mesulam et al., 2009). Although anomia, as measured by BNT scores, was seen in all subtypes, BNT accuracy <50% was confined to PPA-S and its prodromal stages (Table 4). Surface dyslexia or dysgraphia, listed as an ancillary feature, was seen in three of the four patients with PPA-S but was also present in other PPA subtypes (Table 4).
Investigations on patients with semantic dementia (a syndrome that partially overlaps with PPA-S) had led to the suggestion that the temporopolar regions contain ‘amodal’ object representations (Adlam et al., 2006). If so, atrophy in the temporopolar areas should trigger comparable if not simultaneous impairments of naming an object, recognizing its name and understanding its nature. This was not observed in Patients 23 and 24, both of whom had left anterior and polar temporal atrophy. The severe impairment of object naming in these two patients at a time when object knowledge and word comprehension were both relatively preserved, is therefore inconsistent with the postulated presence of amodal object representations in the anterior temporal lobe, at least in the left hemisphere. The severe anomia in Patients 23 and 24, at a time when the atrophy was confined to the left anterior temporal lobe, adds to the growing evidence that this region mediates critical language functions related to lexical representations and their linkage to object representations (Gitelman et al., 2005; DeLeon et al., 2007; Schwartz et al., 2009; Lambon Ralph et al., 2010).
Mild impairment stage of the logopenic variant of primary progressive aphasia
The most conspicuous clinical feature of PPA-L is an intermittent word-finding (or retrieval) impairment. There is currently no method for the meaningful quantitation of word-finding hesitations in PPA-L. The average words per minute score may be uninformative since patients may produce voluminous circumlocutions in response to some word finding obstacles and silent gaps in response to others. The BNT is not adequate either since some patients who have word-finding hesitations during the verbalization of ideas may have little difficulty naming externally presented objects. Impaired retrieval in speech is currently assessed qualitatively by evaluating narrative output. It is a core feature of PPA-L but also commonly seen in the other subtypes (Table 4). The second core feature of PPA-L is impaired repetition, reflecting dysfunction of the phonological loop within the language network (Gorno-Tempini et al., 2008). This feature is also non-specific since it is frequently present in PPA-G, although the mechanisms for repetition impairments in these two variants may be different. Phonemic paraphasias, listed as an ancillary feature, are also neither specific to nor particularly frequent in PPA-L, at least at the mild disease stages (Table 4).
Although the two core features may not be specific for PPA-L, they fit the distribution of the atrophy shown in Fig. 4A. The atrophy of temporoparietal junction and adjacent parts of the superior temporal gyrus in this group may account for the impairment of phonological loop function while the atrophy of lateral temporal cortex and BA37 may account for the word retrieval impairments (Amici et al., 2007; DeLeon et al., 2007; Gorno-Tempini et al., 2008; Rogalski et al., 2011). The absence of atrophy in the inferior frontal gyrus is in keeping with the preservation of grammaticality. In contrast to PPA-G and PPA-S, where the underlying pathology usually belongs to the family of frontotemporal lobar degenerations, the majority of autopsies in PPA-L have shown the pathology of Alzheimer’s disease, a pathology that typically causes the greatest atrophy in the medial temporal lobe of both hemispheres (Knibb et al., 2006; Mesulam et al., 2008; Rohrer et al., 2012). It is therefore interesting to note that there was only very minimal medial temporal atrophy in PPA-L at this early disease stage. If Alzheimer pathology is responsible for neuronal destruction in the majority of the PPA-L patients in this study, it must represent a highly atypical form of the disease that preferentially attacks language rather than memory networks of the brain.
The mixed and anomic variants of primary progressive aphasia phenotypes
The classification proposed by Gorno-Tempini et al. (2011) does not include a ‘mixed’ subtype. Such a subtype, PPA-M, characterized by the combined presence of impaired grammaticality and word comprehension was previously described, but the possibility that it merely designated end-stage disease could not be ruled out (Mesulam et al., 2009). In the current cohort, mildly impaired Patients 21 and 22, both with WAB aphasia quotients of 88%, show that the mixed phenotype can emerge as a distinct clinical form in mild or early disease. In keeping with this phenotype, peak atrophy sites in these two patients were confined to the anterior tip of the left temporal lobe and the left inferior frontal gyrus, the former accounting for the comprehension impairment and the latter for the agrammaticality (Fig. 4B). The three patients who could not be classified at the initial encounter, Patients 23–25, displayed a relatively isolated but severe anomia. Two of these progressed to fulfil the PPA-S criteria. Conceivably, the third may also do so in time. However, the possibility exists that some patients will maintain such a profile, representing an anomic variant of PPA (PPA-A).
Conclusions and comments on classification guidelines
In clinical practice as in the research laboratory, the classification of PPA occurs one patient at a time. It is therefore necessary to have explicit and quantitative guidelines that can be validated at the individual patient level. Since understanding disease evolution and exploring therapeutic options both place a premium on early diagnosis, such classification methods should also be practicable at the stages of mild impairment. This study shows that the root diagnosis of PPA can be made in patients with symptom duration of <2 years. It also shows that a strict application of the Gorno-Tempini et al. (2011) core and ancillary guidelines, through the uniform administration of standardized tests and explicit cut-off scores, led to the classification of ∼80% of patients at the mild and early disease stages. The inclusion of a ‘mixed’ phenotype (PPA-M) into the list of variants raises the success rate to nearly 90%. Some patients fit criteria for both PPA-G and PPA-L and required clinical judgement for disambiguation. This overlap in the criteria for PPA-G and PPA-L may well become less prominent in patients with more advanced disease. Furthermore, the necessarily qualitative assessment of speech characteristics (e.g. paraphasias, word-finding and apraxia) introduced additional components of clinical judgement into the process of subtyping. Nonetheless, the resultant classification displayed considerable biological validity as demonstrated by the phenotypically concordant patterns of cortical atrophy.
The choice of tests was necessarily arbitrary. Different tests that assess analogous domains will obviously need to be chosen for patients who speak languages other than English. In patients with more advanced disease or lower education levels, cut-offs will also need to be lower (Mesulam et al., 2009). The core variables of word comprehension and grammatical competence frequently showed pronounced quantitative deviation from control values when abnormal, and led to relatively unambiguous identification of PPA-S, PPA-G and PPA-M even at the early disease stages, as shown by the template of Fig. 1. The one core feature that did not show such profound abnormality was repetition in PPA-L. Either repetition impairment becomes prominent later in the disease in PPA-L or the test we chose was not sufficiently sensitive. The current investigation also suggests that mildly impaired patients with intact grammar and comprehension but severe anomia should be suspected of being at a prodromal stage of PPA-S, that some of these patients may remain at the anomic stage and reflect an anomic form of PPA, that the ‘non-fluent/agrammatic’ variant should be divided into an agrammatic subgroup (with or without abnormal speech) and a subgroup with disturbance of speech but not grammar, and that the ancillary criteria of surface dyslexia, phonemic paraphasia and syntactic comprehension are not particularly useful in the subtyping process. The resultant classification strategy can be outlined in the form of a road map, modelled after the algorithm of Leyton et al. (2011), which can be navigated quantitatively or qualitatively (Fig. 6).
Figure 6.
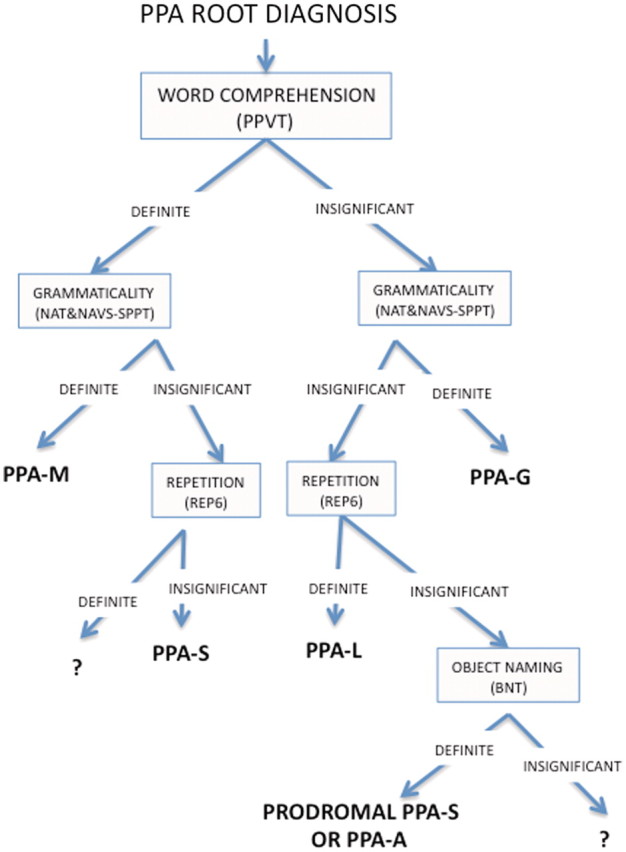
Road map for subtyping PPA. The road map can be used quantitatively, in which case ‘definite’ impairment can be defined through a z-score or deviation from normative values by a certain number of standard deviations. It can also be used qualitatively, in which case, ‘definite’ and ‘insignificant’ can be assessed on the basis of their prominence in the clinical assessment or impact on daily activities. The choice of tests can vary based on language, education and severity level. The branch point that depends on the integrity of repetition is particularly challenging in mild disease stages since so few of the patients, other than those in the PPA-G group, showed major impairments in this domain. NAT and SPPT(nc) = mean of the scores (for non-canonical sentences) on the NAT and on the Sentence Production Priming Test of the Northwestern Assessment of Verbs and Sentences; REP6 = a subset of the six most difficult items in the repetition subtest of the WAB; ? = unclassifiable.
The defining biological feature of PPA is a distinctly asymmetric atrophy revolving around the left hemisphere language network of the brain. The asymmetry is particularly striking at the early and mild disease stages covered in this report. In some patients, such as those with PPA-S, the atrophy seems to emanate from a temporopolar focus, and in others, such as those with PPA-G and PPA-L, from a perisylvian inferofrontal-temporoparietal axis. However, the distinctions are relative rather than absolute. For example, PPA-S patients may also have less but significant temporoparietal junction and inferior frontal gyrus atrophy (Figs 3B and 5B) while patients with PPA-M may show that atrophy can arise simultaneously from both anterotemporal and perisylvian foci (Fig. 4B). Even as the disease reaches its advanced stages, the atrophy spreads preferentially into components of the language network and maintains its asymmetry (Rogalski et al., 2011). Further research on the early stages of PPA is likely to generate pivotal insights into the molecular markers that make the language network selectively vulnerable to neurodegeneration, and at the same time provide crucial information on the natural course of progression against which the efficacy of therapeutic interventions can be assessed.
Funding
National Institute on Deafness and Communication Disorders (DC008552); National Institute on Ageing [AG13854 (Alzheimer Disease Centre)]; National Centre for Research Resources (5KL2RR025740).
Acknowledgements
Imaging was performed at the Northwestern University Department of Radiology Centre for Advanced MRI (CAMRI).
Glossary
Abbreviations
- BNT
Boston Naming Test
- NAT
Northwestern Anagram Test
- NAVS
Northwestern Assessment of Verbs and Sentences
- PPA
primary progressive aphasia
- PPA-G
agrammatic variant of PPA
- PPA-L
logopenic variant of PPA
- PPA-M
mixed PPA variant
- PPA-S
semantic variant of PPA
- PPVT
Peabody Picture Vocabulary Test
- SPPT
Sentence Production Priming Test
- WAB-R
Western Aphasia Battery-Revised
References
- Adlam A-L, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–80. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Amici S, Ogar J, Brambati SM, Miller B, Neuhaus J, Dronkers NF, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol. 2007;20:203–11. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Beck JA, Rohrer JD, Campbell T, Isaacs I, Morrison KE, Goodall EF, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131:706–20. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni B, Alberici A, Premi E, Archetti S, Garibotto V, Agosti C, et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin carriers. Rejuvenation Res. 2008;11:585–95. doi: 10.1089/rej.2007.0623. [DOI] [PubMed] [Google Scholar]
- Cruchaga C, Fernández-Seara MA, Seijo-Martínez M, Samaranch L, Lorenzo E, Hinrichs A, et al. Cortical atrophy and language network reorganization associated with a novel Progranulin mutation. Cereb Cortex. 2009;19:1751–60. doi: 10.1093/cercor/bhn202. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–22. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dunn LA, Dunn LM. Peabody Picture Vocabulary Test. 4th edn. San Antonio, Texas: Pearson; 2006. [Google Scholar]
- Folstein M, Folstein S, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional imaging using the false discovery rate. NeuroImage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Robidoux J, Alarcón M, Miller BL, Wilhelmsen KC, Cummings JL, et al. Dementia and neurodevelopmental predisposition: cognitive dysfunction in presymptomatic subjects precedes dementia by decades in frontotemporal dementia. Ann Neurol. 2001;50:741–6. doi: 10.1002/ana.10024. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam M-M. Language network specializations: an analysis with parallel task design and functional magnetic resonance imaging. NeuroImage. 2005;26:975–85. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis A, Weintraub S, Kertesz A, Mendez MF, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees: a test of symantic sccess from pictures and words. Bury St. Edmonds, Suffolk, UK: Thames Valley Test Company; 1992. [Google Scholar]
- Jack CR, Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–60. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia. Lea & Febiger; 1983. [Google Scholar]
- Kay JLR. Psycholinguistic assesment of language processing in aphasia. Hove: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Kertesz A. Western Aphasia Battery-Revised (WAB-R) Austin, Texas: Pro-Ed; 2006. [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Knopman D, Jack CR, Jr, Kramer JH, Boeve B, Caselli R, Graff-Radford N, et al. Brain and ventricular volumetric changes in frontotemporal lobar degeneration over 1 year. Neurology. 2009;72:1843–9. doi: 10.1212/WNL.0b013e3181a71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133:3243–55. doi: 10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- LeRhun E, Richard F, Pasquier F. Natural history of primary progressive aphasia. Neurology. 2005;65:887–91. doi: 10.1212/01.wnl.0000175982.57472.84. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Villemange VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the international consensus criteria and validation using β-amyloid imaging. Brain. 2011;134:3030–43. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- Marshall JC, White K, Weaver M, Etherill FL, Hui S, Stout JC, et al. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007;64:116–21. doi: 10.1001/archneur.64.1.116. [DOI] [PubMed] [Google Scholar]
- Medina J, Weintraub S. Depression in primary progressive aphasia. J Geriatr Psychiatry Neurol. 2007;20:153–60. doi: 10.1177/0891988707303603. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–51. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL, et al. Progranulin mutations in primary progressive aphasia. Arch Neurol. 2007;64:43–7. doi: 10.1001/archneur.64.1.43. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. [PubMed] [Google Scholar]
- Mesulam M-M. Primary progressive aphasia: a language-based dementia. New Eng J Med. 2003;348:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Weintraub S. Spectrum of primary progressive aphasia. In: Rossor MN, editor. Unusual dementias. London: Baillière Tindall; 1992. pp. 583–609. [PubMed] [Google Scholar]
- Mesulam M-M, Weintraub S. Primary progressive aphasia and kindred disorders. In: Duyckaerts C, Litvan I, editors. Handbook of clinical neurology. New York: Elsevier; 2008. pp. 573–87. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic subtype of primary progressive aphasia. Brain. 2009;132:2553–65. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Chapman R. Systematic analysis of language transcripts (SALT) Research Version 6.1] edn. Madison, WI: University of Wisconsin, Language Analysis Lab; 2000. . p. Computer software, SALT for Windows. [Google Scholar]
- Morris JC. The clinical dementia rating (Clinical Dementia Rating): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Osher J, Wicklund A, Rademaker A, Johnson N, Weintraub S. The Mini-Mental State Examination in behavioural variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimer’s Dis Other Demen. 2007;22:468–73. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R, Roberts RO, Knopman D, Boeve B, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–55. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Diaz-Olavarietta C, Rodriguez Y, Chavez M, Paz F, Murell J, et al. Female preclinical presenilin-1 mutation carriers unaware of their genetic status have higher levels of depression than their non-mutation carrying kin. J Neurol Neurosurg Psychiatr. 2004;75:500–2. doi: 10.1136/jnnp.2002.005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam M-M. Progression of language impairments and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–10. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson C, Weintraub S, et al. Anatomy in language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–50. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Rossor MN, Warren J. Alzheimer pathology in primary progressive aphasia. Neurobiol Aging. 2012;33:744–52. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam M-M, et al. Cortical neuoanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–66. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky D, Domoto-Reilly K, Negreira A, Brickhouse M, McGinnis S, Dickerson BC. Monitoring progression of primary progressive aphasia: current approaches and future directions. Neurodegener Dis Manag. 2011;1:43–55. [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–27. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK. Northwestern Assessment of Verbs and Sentences (NAVS). Unpublished developmental version. Evanston, IL: Aphasia and Neurolinguistics Laboratory, Northwestern University School of Communication; 2011. [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- Thompson CK, Cho S, Hsu C-J, Wieneke C, Rademaker A, Weitner BB, et al. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26:20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Shapiro LP, Tait ME, Jacobs B, Schneider SL, Ballard K. A system for the linguistic analysis of agrammatic language production. Brain Lang. 1995;51:124–9. [Google Scholar]
- Weintraub S, Mesulam M-M, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The Northwestern Anagram Test: measuring sentence production in primary progressive aphasia. Am J Alzheimer’s Dis Other Demen. 2009;24:408–16. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–35. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon DP, Mercaldo N, Ferris S, Graff-Radford N, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychological test battery. Alzheimer’s Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]