Abstract
Charcot–Marie–Tooth disease type 1B is caused by mutations in myelin protein zero. R98C mice, an authentic model of early onset Charcot–Marie–Tooth disease type 1B, develop neuropathy in part because the misfolded mutant myelin protein zero is retained in the endoplasmic reticulum where it activates the unfolded protein response. Because oral curcumin, a component of the spice turmeric, has been shown to relieve endoplasmic reticulum stress and decrease the activation of the unfolded protein response, we treated R98C mutant mice with daily gastric lavage of curcumin or curcumin derivatives starting at 4 days of age and analysed them for clinical disability, electrophysiological parameters and peripheral nerve morphology. Heterozygous R98C mice treated with curcumin dissolved in sesame oil or phosphatidylcholine curcumin performed as well as wild-type littermates on a rotarod test and had increased numbers of large-diameter axons in their sciatic nerves. Treatment with the latter two compounds also increased compound muscle action potential amplitudes and the innervation of neuromuscular junctions in both heterozygous and homozygous R98C animals, but it did not improve nerve conduction velocity, myelin thickness, G-ratios or myelin period. The expression of c-Jun and suppressed cAMP-inducible POU (SCIP)—transcription factors that inhibit myelination when overexpressed—was also decreased by treatment. Consistent with its role in reducing endoplasmic reticulum stress, treatment with curcumin dissolved in sesame oil or phosphatidylcholine curcumin was associated with decreased X-box binding protein (XBP1) splicing. Taken together, these data demonstrate that treatment with curcumin dissolved in sesame oil or phosphatidylcholine curcumin improves the peripheral neuropathy of R98C mice by alleviating endoplasmic reticulum stress, by reducing the activation of unfolded protein response and by promoting Schwann cell differentiation.
Keywords: Charcot-Marie-Tooth disease 1B, curcumin, myelin protein zero, peripheral neuropathy, unfolded protein response
Introduction
Charcot–Marie–Tooth disease type 1B (CMT1B) is caused by mutations in the Myelin Protein Zero (MPZ) gene and is the second most common form of the autosomal dominant hereditary demyelinating neuropathies, collectively called CMT1 (Nelis et al., 1996; Saporta et al., 2011). More than 120 different disease-causing mutations in MPZ have been identified (http://www.molgen.ua.ac.be/CMTMutations/default.cfm). Most patients with CMT1B present in two phenotypic groups, one with extremely slow nerve conduction velocities and onset of symptoms during the period of motor development; a second with normal or near normal nerve conduction velocities and the onset of symptoms as adults (Shy et al., 2004). Why particular mutations cause early or late onset neuropathy is not understood.
A number of CMT1B mutations cause mutated MPZ to be retained in the endoplasmic reticulum instead of being transported to the cell membrane or myelin sheath. Examples include MpzS51ΔW57 (Grandis et al., 2008), 506delT and 550del3insG (Khajavi et al., 2005), studied in vitro, and Ser63del and R98C mice, studied in vivo (Wrabetz et al., 2006; Pennuto et al., 2008; Saporta et al., 2012). Endoplasmic reticulum stress in both Ser63del and R98C mice contributed to the neuropathy by activating a canonical unfolded protein response (UPR) (Pennuto et al., 2008; Saporta et al., 2012). UPR activation aims to reduce the load of unfolded proteins through upregulation of chaperones, attenuation of protein synthesis and increased protein degradation. The UPR is mediated initially by three molecules located in the endoplasmic reticulum membrane, inositol requiring enzyme (IRE1), activating transcription factor (ATF6) and PKR like ER Kinase (PERK). Three parameters may be used to detect UPR activation (i) XBP1 splicing as an indicator of IRE1 pathway activation; (ii) ATF6 cleavage; and (iii) increase in the levels of the transcription factor C/EBP homology protein (CHOP) and its translocation to the nucleus, as an indicator of PERK pathway. Genetically eliminating Chop improved the neuropathy of Ser63del mice, suggesting that reducing UPR activation caused by endoplasmic reticulum stress is a viable therapeutic strategy for at least some cases of CMT1B (Pennuto et al., 2008).
Sarcoplasmic/endoplasmic reticulum calcium pump (SERCA) inhibitors may reduce endoplasmic reticulum stress, and consequently, UPR activation by inhibiting calcium binding and disrupting calnexin function (Egan et al., 2004). SERCA inhibitors are generally too toxic to be used therapeutically in humans. Curcumin, derived from the curry spice, turmeric, has multiple cellular targets and pleiotropic biological effects (Epstein et al., 2010); in addition, it is safe for human consumption (Corson and Crews, 2007). Curcumin functions as a low-affinity SERCA inhibitor, and it has been shown to relieve endoplasmic reticulum stress and ameliorate the phenotype in several in vitro and in vivo models of UPR activation. Examples include cystic fibrosis models (Egan et al., 2002, 2004), cell culture experiments with MPZ mutants (Khajavi et al., 2005) and TrJ mice; a model for another severe infantile onset neuropathy (CMT1E) caused by a missense mutation in Pmp22 (Khajavi et al., 2007). Taken together, these data demonstrate a proof of principle for using curcumin to treat appropriate animal models and ultimately patients with neuropathies caused by misfolded proteins retained within the endoplasmic reticulum.
R98C MPZ (also called R69C with the leaderless numbering system) causes severe, early onset CMT1B (Bai et al., 2006). We have generated authentic R98C knock-in mice and demonstrated that heterozygous (R98C/+) and homozygous (R98C/R98C) mice develop a neuropathy that resembles the human neuropathy. R98C/R98C mice are more severely affected than R98C/+ mice by clinical, neurophysiological and morphological criteria (Saporta et al., 2012). R98C Mpz is retained in the endoplasmic reticulum in the mice where it also activates a canonical UPR response. These mice serve as a model to test therapies directed at relieving endoplasmic reticulum stress in CMT1B and in related neuropathies (Saporta et al., 2012). We treated R98C mice with orally administered curcumin. We did not obtain significant benefits in mice treated with either curcumin dissolved in alimentum (CA)—the form used to treat TrJ mice (Khajavi et al., 2007)—or with a fluorinated curcumin derivative (CDF) (Padhye et al., 2009). However, a phosphatidylcholine curcumin derivative (PCC) designed to increase bioavailability (Marczylo et al., 2007) and curcumin dissolved in sesame oil (CO) improved the R98C/R98C animals by neurophysiological and morphological criteria and the R98C/+ mice by clinical, neurophysiological and morphological criteria. Furthermore, PCC or CO treatment attenuated the UPR and promoted Schwann cell myelination.
Materials and methods
Animals
All studies carried out on mice were conducted in accordance with experimental protocols approved by the Institutional Animal Care and Use Committee of Wayne State University. R98C knock-in mice were generated using a cre-lox system, kept on a Friend virus-B-Type (mouse) FVB/N background and genotyped as described (Saporta et al., 2012). Mutant mice presented with weakness, tremor, electrophysiological and morphological abnormalities. R98C/R98C mice were more severely affected than R98C/+ animals in all these measurements (Saporta et al., 2012).
Behavioural and electrophysiological parameters were investigated at 6 weeks and 3 months of age. Unless otherwise stated, morphological and molecular studies were carried out at three time points: at post-natal Day 13, 6 weeks and 3 months of age. Ten mice/genotype/time point were used for all experiments unless otherwise stated.
Curcumin treatment
Mice were treated daily with 100 mg curcumin/kg body weight by gastric lavage beginning at post-natal Day 4. They were subsequently evaluated at post-natal Day 13, 6 weeks or 3 months of age. Additional R98C/+ mice were also treated every day beginning at 6 weeks of age and evaluated until 3 months. Some R98C/+ mice whose daily treatment began at post-natal Day 4 had their treatment stopped at 6 weeks but were not analysed until 3 months of age (see ‘Results’ section). Curcumin was purchased from Sigma (catalogue number C7727) and dissolved either in alimentum (CA) or sesame oil (CO). Curcumin derivatives, PCC (gift of Indena Spa) (Marczylo et al., 2007) and CDF (Padhye et al., 2009), were dissolved in sesame oil and dosed equally based on their curcuminoid content.
TaqMan® quantitative polymerase chain reaction analysis
Real-time PCR analysis was performed on sciatic nerve samples that were quickly dissected, from post-natal Day 13 and 3-month-old animals, snap frozen and homogenized. Total RNA was extracted using RNeasy® Micro Kit (50) (Qiagen). Complementary DNA was prepared with SuperScript® III First Strand Synthesis SuperMix (11752-050, Invitrogen), and samples were analysed as triplicates on a MJ Research Opticon (Bio-Rad) detection system using either SYBR® Green or pre-developed TaqMan® assays according to the manufacturer’s guidelines. All samples were normalized to glyceraldehyde 3 phosphate dehydrogenase (GAPDH) as an endogenous control and expressed relative to untreated wild-type sciatic nerve data. The list of primers and probes is included in Supplementary Table 1.
Western blot analysis
Snap frozen sciatic nerves were pulverized, dissolved in lysis buffer (Radioimmunoprecipitation assay buffer (RIPA) buffer, Sigma R0278 and proteinase inhibitors), kept on ice for at least 30 min and centrifuged at 14 000 g for 10 min at 4°C. The protein content of the supernatant was determined using a bovine serum albumin standard curve. Equal amounts of the protein were loaded on 6–12% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) gels, and electroblotted onto polyvinyl difluoride membranes. Liver samples were taken from mice injected intraperitoneally with 1 μg tunicamycin/g weight and sacrificed 48 h later; served as positive control for UPR activation. Results were normalized for β-actin (1:10 000) and to untreated wild-type samples. All western blots were repeated at least three times, and revealed similar results. The primary antibodies used are listed in Supplementary Table 2. Horseradish peroxidase-conjugated secondary antibodies (1:5000–1:20 000 dilution; Sigma) were detected using enhanced chemiluminescence (ECL) reagents (Bio-Rad) with autoradiography film (Kodak Scientific Imaging Film, Blue XB).
Neuromotor behavioural studies
Grip test
The strength of all four limbs was evaluated using an automated Grip Strength Meter (Columbus Instruments). Within 1 week after training (10 practice trials using a mesh bar), the peak force exerted by each individual mouse was measured 10 times consecutively with 10 s resting periods, averaged and normalized to body weight.
Rotarod
Mice underwent three training trials on an IITC Life Science Roto-Rod (Series 8) with an accelerating ramp speed from 2 to 20 g in 300 s, as has been previously described in detail (Saporta et al., 2012).
Electrophysiology
In addition to electrophysiological techniques published previously (Saporta et al., 2012), we also performed repetitive stimulation (five stimuli) to detect a potential decrement in paw compound muscle action potentials.
Light and electron microscopy, morphometric analysis
Sciatic nerve samples were fixed in 2.5% glutaraldehyde overnight and processed as previously described (Saporta et al., 2009). Tissue blocks were sectioned in 1-μm thickness and stained with methylene blue for light microscopic examination. Semi-thin sections were used to image the whole cross-section of the sciatic nerve (×90 magnification), which was reconstructed from overlapping images. Every fourth image was analysed to quantify the number of axons per field (axonal density). The diameter of at least 200 axons per animal was calculated from the area of the axon in two animals per control group and in three animals per CA-, PCC- and CO-treated groups. Area measurements and manual tagging–used to determine axonal density and the percentage of de- or remyelinated fibres—were carried out using ImagePro Plus software (Media Cybernetics). G-ratio (axonal diameter/fibre diameter) analysis was performed on randomized photos of ultrathin sciatic nerve cross sections imaged with a Zeiss Electron Microscopy (EM900) electron microscope.
Immunohistochemistry on teased nerve fibre preparations and frozen sections
This technique has been described in detail in our previous studies (Bai et al., 2006). In brief, nerves were either fixed in 4% paraformaldehyde for 30 min and teased into individual fibres on glass slides or freshly frozen in O.C.T medium and cut into 10-μm sections. After being dried and washed in PBS, the slides were reacted with primary antibodies (Supplementary Table 2A) and kept at 4°C overnight, followed by 2-h incubation with secondary antibodies (Supplementary Table 2B).
Visualizing the neuromuscular junction
Triangularis sterni, soleus and gastrocnemius muscle samples were dissected bilaterally. The total number of neuromuscular junctions was counted in the soleus muscle, and preterminal axonal segments along with perisynaptic Schwann cells were investigated in the gastrocnemius muscle (Court et al., 2008) (see Supplementary material for details).
The number of fully myelinated junctions was counted, and the length of the demyelinated segment was measured on triangularis sterni samples using ImageJ software on compressed confocal images (×60 magnification). Muscle samples were processed for immunohistochemistry as follows: after being fixed for 2 h in 4% of paraformaldehyde and washed three times in PBS, samples were incubated for 30 min at room temperature in 5 μg/ml tetramethylrhodamine-conjugated α-bungarotoxin (Molecular Probes, Catalogue number T1175) diluted in 1% bovine serum albumin in sterile lactated Ringer’s solution. Samples were blocked in 5% of fish skin gelatin buffer overnight at 4°C, followed by the incubation with primary antibodies [neurofilament, synaptophysin and Myelin Basic Protein (MBP) (Supplementary Table 2)] for 3–4 days. After washing for 5 h in PBS, muscle samples were reacted with secondary antibodies overnight at 4°C (Supplementary Table 2B), washed for 5 h in PBS and whole-mounted.
Cell culture, quantification of XBP1 splicing and confocal microscopy
Cos-7 cells were grown on poly-l-lysine coated coverslips in 24-well plates in Dulbecco’s modified Eagle medium supplemented with 10% foetal bovine serum and 1% penicillin–streptomycin. Coverslips at 80–90% confluence were co-transfected with P0R98C or P0wt, each with the haemagglutinin epitope tag fused to the C-terminus (Tinelli et al., in press) and pXBPd2EGFP3.1 [in which the XBP1/Enhanced green fluorescent protein (EGFP) fusion (Back et al., 2006) was ligated into the pcDNA3.1 plasmid (Invitrogen)] using Fugene 6 according to the supplier’s instructions. Coverslips were either left untreated or were pretreated with 2 or 10 µM curcumin (dissolved in dimethyl sulphoxide, according to the manufacturer’s instructions) 3 h before transfection. Cells were then incubated for 24 h, and curcumin was present for the whole period in treated wells.
Immunocytochemistry was carried out at room temperature. Cells were rinsed twice in PBS and then fixed in 4% paraformaldehyde for 30 min. After being washed twice in PBS, cells were incubated for 2 h with the primary antibody (haemagglutinin 1:50; calnexin 1:300) diluted in a permeabilizing-blocking solution (containing goat serum, 20% Triton™ X-100, 240 mM phosphate buffer, 5 M NaCl and H2O). Following three 10-min washes in PBS and 1 h incubation with secondary antibodies (Alexa® Fluor 633, 1:500; and Alexa® Fluor 555, 1:250), the coverslips were mounted applying Anti-Fade 4_6-diamino-2-phenylindole mounting media that labelled the nuclei.
We determined the percentage of green fluorescent protein (GFP)-positive nuclei (active XBP1 splicing) in transfected cells visualized by standard epifluorescence, and normalized their number to the transfection efficiency (proportion of haemagglutinin-positive cells in each culture dish; Tinelli et al., in press). To demonstrate cell surface expression/endoplasmic reticulum retention of P0R98C or P0wt, transfected Cos-7 cells were imaged at ×189 (×63 times three) magnification with a Leica confocal microscope.
Statistical analysis
Investigators were blinded to the treatment arm, but could not be fully blinded to the genotype when performing behavioural studies because of the characteristic phenotypic presentation of the R98C/R98C mutant. Morphometric, molecular and cell culture studies were carried out in a completely blinded fashion. Data are represented as mean value ± 1 standard error (SE). Normally distributed data (cell culture, several categories of behavioural and morphometric data) were analysed with univariate ANOVA, comparing all the genotypes and treatment groups. Not normally distributed data sets were evaluated by Kruskal–Wallis test followed by Bonferroni correction. Significance levels were marked on figures as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Phosphatidylcholine curcumin and curcumin dissolved in oil ameliorate axonopathic changes in R98C mice
Homozygous (R98C/R98C) and heterozygous (R98C/+) mice were treated with daily gastric lavage of curcumin dissolved in alimentum (CA, 100 mg/kg/day) beginning at post-natal Day 4 and analysed at 6 weeks and 3 months of age to assess peripheral nerve structure and function. CA treatment did not improve balance on a rotating rod (Rotarod), strength on a four-limb grip test, nerve conduction velocities, F-wave latencies or compound muscle action potential amplitudes in either the heterozygous R98C/+ or homozygous R98C/R98C animals. In addition, there was no improvement in morphological features of de/dysmyelination with 3 months of CA treatment, and the reduced number of large calibre axons (diameter > 6 μm) persisted. Supplementary Fig. 1 shows data from 6-week-old mice; all parameters remained unchanged at 3 months of age (data not shown).
To assess whether the lack of effect was of poor bioavalability of curcumin, we investigated whether curcumin was present in nerve tissue following treatment and identified it by mass spectrometry in sciatic nerves at 30 min, and to a lesser extent, 2 h after gavage in animals of various ages (Supplementary Fig. 2). Values were similar to those reported in TrJ mice treated with CA (Khajavi et al., 2007). Alimentum-treated control animals were included in all of the above mentioned studies, and no significant difference was noted compared with untreated or CA-treated animals, confirming that the lack of improvement in CA-treated animals was not due to a potential unexpected negative influence of alimentum (Supplementary Fig. 1).
Curcumin is absorbed poorly from the gastrointestinal tract and remains detectable for only 2 h in plasma, thereby limiting its effectiveness (Marczylo et al., 2007). Therefore, we repeated the above experiments using PCC and CDF, two derivatives with better tissue penetration or retention (Marczylo et al., 2007; Padhye et al., 2009). Because both PCC and CDF were dissolved in sesame oil, we also treated animals with curcumin dissolved in sesame oil (CO). Tissue levels of these compounds 30 min after gavage were similar to those with CA (Supplementary Fig. 2).
R98C/+ mice treated with either PCC or CO from 4 days of age, performed as well as wild-type mice and significantly better (CO P = 0.006) than untreated, CA or CDF-treated R98C/+ animals on the accelerating Rotarod when tested at 6 weeks of age (Fig. 1). While untreated R98C/+ mice improved between 6 weeks and 3 months of age, PCC- or CO-treated animals performed equally at the two time points, resulting in no significant difference at 3 months of age (Supplementary Fig. 3). In contrast, homozygous R98C mice either with or without curcumin treatment could not balance on the Rotarod at all and are, therefore, not shown in Fig. 1. PCC- and CO-treated R98C/+ animals had significantly increased thigh compound muscle action potential amplitudes both at 6 weeks and 3 months of age compared with untreated littermates (PCC P = 0.001, CO P = 0.015), suggesting a persistent amelioration of axonal abnormalities (Fig. 1). Moreover, compound muscle action potential amplitudes of both PCC- and CO-treated R98C/R98C doubled (PCC P = 0.013, CO P = 0.032) compared with untreated homozygous mice (Fig. 1). We obtained corresponding results in paw compound muscle action potential amplitudes (Supplementary Fig. 4). Nerve conduction velocities and F-wave latencies did not change at 6 weeks or 3 months of age in any treatment group (Fig. 1). Oil-treated control animals also demonstrated improved performance on the rotating rod and increased compound muscle action potential amplitudes (Fig. 1 and Supplementary Fig. 4). We cannot exclude the possibility that the oil-rich diet, with its increased sterol content (Saher et al., 2012), synergized with the beneficial effects of curcumin and its derivatives (see ‘Discussion’ section); however, changes seen in the oil-treated control group did not reach significance and treatment with CDF, which was also dissolved in oil, did not have a consistent significant effect on either group of mutant mice (see Supplementary material).
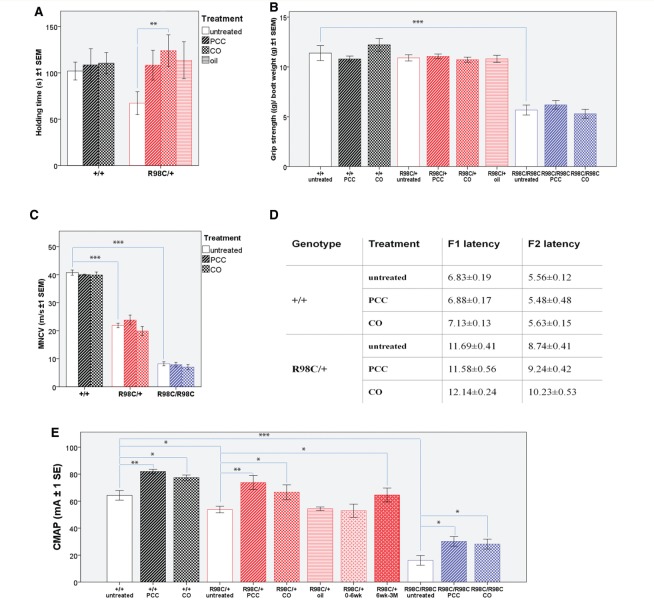
Figure 1.
PCC or CO treatment ameliorates motor function and persistently improves compound muscle action potential amplitudes. (A) Rotating rod analysis of motor function revealed that 6-week-old PCC (P = 0.029) or CO (P = 0.002) treated R98C/+ mice (P = 0.006) remain on the rod for a significantly longer period than untreated R98C/+ littermates. PCC- or oil-treated control animals showed a tendency towards a better performance; however, it did not prove significant (n = 5–10/genotype/treatment group). R98C homozygous animals were unable to remain on the rod even after treatment and are, therefore, not shown on the graph (n = 5–10/genotype/treatment group). (B) We observed no significant difference in muscle strength among untreated or PCC/CO treated +/+ and R98C/+ animals. R98C/R98C animals were significantly (P < 0.001) weaker and did not improve with PCC or CO treatment (n = 10/treatment group/genotype). (C) Three months of PCC or CO treatment did not ameliorate the significantly slowed nerve conduction velocities in either R98C/+ or R98C/R98C mice. (D) Prolonged F-wave latencies in R98C/+ remained unchanged with PCC or CO treatment. F-wave latencies could not be distinguished in untreated or PCC/CO treated R98C/R98C animals because of severe temporal dispersion. F1 latency is the F-wave latency from the distal stimulation point (ankle). F2 latency is the F-wave latency from the more proximal stimulation point (sciatic notch). (E) Of note, compound muscle action potential amplitudes (measured in the thigh) increased significantly in R98C/+ mice when treated with PCC (P = 0.001) or CO (P = 0.015) even if treatment was initiated at 6 weeks of age (P = 0.04). In contrast, oil-treated control animals or heterozygous mice whose treatment was stopped at 6 weeks of age did not show any benefit. PCC/CO treatment increased in wild-type (P = 0.001/P = 0.015) and even doubled R98C/R98C in compound muscle action potential amplitudes (P = 0.013/P = 0.032) (n = 5/treatment group/genotype). CMAP = compound muscle action potential; MNCV = motor nerve conduction velocity. * = P < 0.05; ** = P < 0.01;*** = P < 0.001.
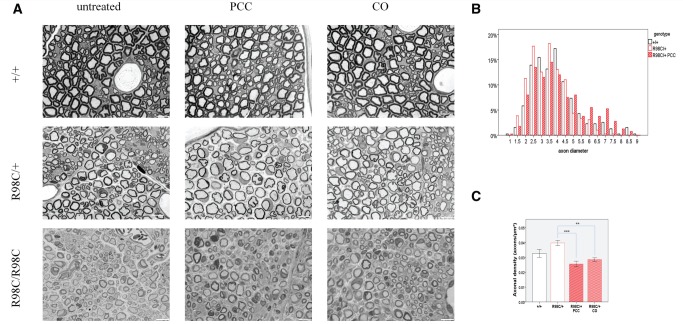
Morphometric analysis of sciatic nerves at the mid-thigh of R98C/+ animals demonstrated a reduced number of the largest diameter axons at both 6 weeks and 3 months of age compared with wild-type (6 week: 7 μm, 3 month: 8.5 μm) (Fig. 2). A similar distribution of fibre sizes was also found in animals treated with CA (data not shown). However, we detected numerous large-diameter axons at 3 months of age in mice treated with PCC or CO, and in fact, there was a return to the normal axonal distribution (P < 0.001 for both CO and PCC when compared with untreated R98C/+; Fig. 2B). In parallel with increased axonal diameter, the axonal density (number of axons/area) decreased significantly in PCC- or CO-treated animals (P < 0.001 for both) (Fig. 2C). The total number of axons did not increase with PCC or CO treatment of R98C/+ mice (R98C/+ untreated, 3529.5 ± 199.5; R98C/+, PCC 3666.5 ± 395.5; R98C/+CO, 3545 ± 20). No differences in myelin thickness (Fig. 2A) or G-ratios were noted following treatment with any curcumin derivative or formulation at any age investigated (data not shown). In addition, X-ray diffraction studies (Agrawal, 2009) (Supplementary Table 3) did not identify any improvement in myelin period or thickness. The percentage of demyelinating (2.6 ± 0.25) or remyelinating (1.58 ± 0.24) fibres in R98C/+ mice remained unchanged in CA/CO/PCC-treated nerves compared with untreated control mice.
Figure 2.
PCC or CO treatment does not affect myelination directly, but exerts its effects on axons. (A) Semi-thin sections illustrate hypomyelination and the absence of large-diameter fibres in R98C/+ animals, and severe dysmyelination in R98C/R98C mice. Several large-diameter fibres can be observed in PCC or CO treated R98C/+ animals. Treatment failed to improve hypomyelination. Bar = 10 μm. (B) By 3 months of age, the distribution of axon diameter returned to normal in PCC or CO treated (not shown) R98C/+ mice (P < 0.001 for both CO and PCC when compared with untreated R98C/+); the proportion of large-diameter fibres was fully corrected (n = 2–3/genotype/treatment group). (C) Increased axonal density in untreated R98C/+ mice was markedly reduced in PCC (P < 0.001) or CO (P = 0.002) treated R98C/+ animals in correlation with the increase in axon size. * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
To determine whether PCC or CO could improve the neuropathy if administered in adult animals, we treated R98C/+ mice with the same set of curcumin derivatives or formulation, starting at 6 weeks of age and analysed them at 3 months. Using this treatment paradigm, curcumin treated mice showed a similar improvement of compound muscle action potential amplitudes (P = 0.04) as those whose treatment began at post-natal Day 4 (Fig. 1C). Alternatively, when treatment was started at post-natal Day 4 and stopped at 6 weeks of age, there were no benefits in any of the groups of mice evaluated at 3 months of age (Fig. 1C). Taken together, these data demonstrate that PCC and CO can improve the clinical performance, increase the compound muscle action potential amplitudes and rescue the deficit of large-diameter fibres in the R98C/+ mouse model of CMT1B. A summary of the effects of PCC or CO treatment is provided in Table 1.
Table 1.
Summary of the effects of PCC or CO treatment
| PCC or CO treatment | R98C/+ | R98C/R98C |
|---|---|---|
| Behavioural tests | ||
| Rotarod | Holding time improved at 6 weeks of age | No change |
| Grip test | No change | No change |
| Electrophysiology | ||
| CMAP amplitude | Increased | Increased |
| NCV | No change | No change |
| Morphology (3 months of age) | ||
| Axon diameter | Increased | No change |
| Myelin thickness (G-ratio) | No change | No change |
| NMJ | Slight, non-significant improvement | Increased percentage of fully myelinated junctions Shorter demyelinated segment |
| Transcription factors (3 month of age) | ||
| SCIP | Reduced (IHC) | Reduced (RT-PCR, IHC) |
| c-Jun | No change | Reduced (IHC) |
| Krox-20 | Reduced (IHC) | Slightly reduced (RT-PCR, IHC) |
| UPR (3 month of age) | ||
| BiP (mRNA) | Slightly reduced | Reduced |
| ATF6 (mRNA) | No change | Reduced |
| CHOP (mRNA) | No change | No change |
| Spliced XBP-1 (mRNA, cell culture) | No change | Reduced |
No change refers to untreated littermates of the same genotype.
Bold lettering = statistically significant; CMAP = compound muscle action potential; IHC = immunohistochemistry; mRNA = messenger RNA; NMJ = neuromuscular junction; RT-PCR = real-time PCR; NCV = nerve conduction velocity.
Phosphatidylcholine curcumin or curcumin dissolved in sesame oil treatment increased innervation of neuromuscular junctions
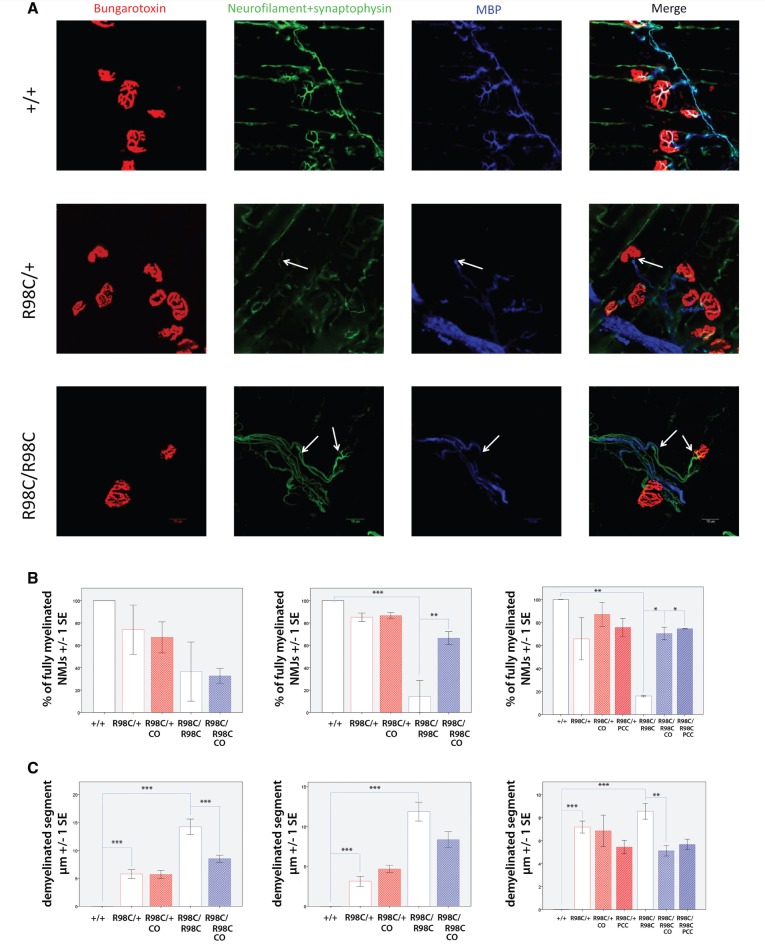
We next investigated whether curcumin derivatives exerted a beneficial effect on the innervation of neuromuscular junctions. We first performed repetitive stimulation and found no decrement in compound muscle action potential amplitudes, suggesting intact synaptic transmission (data not shown). We then investigated the neuromuscular junction morphologically, hypothesizing that improved myelination of the preterminal internode might facilitate reinnervation (Court et al., 2008). In wild-type mice, MBP staining was observed ensheathing preterminal branches labelled with neurofilament + synaptophysin. These MBP-labelled branches made contact with bungarotoxin-labelled acetylcholine receptors in 100% of the cases at all age groups. No evidence of denervation, defined by the absence of neurofilament + synaptophysin staining, was identified.
At post-natal Day 13, we identified a tendency towards a lower overall number of neuromuscular junctions and reduced myelin coverage of preterminal axonal segments in heterozygous and homozygous R98C mice (Supplementary Fig. 5), both of which were slightly ameliorated by PCC/CO treatment. To account for length-dependent effects, we analysed distal (soleus/gastrocnemius) and also proximal (triangularis sterni) muscles and confirmed that changes revealed in the distal muscles were also observed in the triangularis sterni (Fig. 3). Because the findings were similar, we used the triangularis sterni for studies on 6-week- and 3-month-old animals, its flattened structure allowed more precise evaluations of myelin ensheathing the preterminal internodes. By 6 weeks of age, 85% of the preterminal branches were demyelinated (P = 0.004) in untreated R98C/R98C animals; large gaps in MBP staining reached 15 μm in length. Both PCC and CO treatment resulted in a significant improvement; ∼70% of the neuromuscular junctions appeared normal in treated animals (CO P = 0.004), and the length of the demyelinated segment was reduced to ∼8 μm. The beneficial effects of PCC or CO treatment proved to be persistent, as at 3 months of age, 70% of the junctions were fully myelinated (P = 0.017 for CO-treated, P = 0.01 for PCC-treated, demyelinated segment: P = 0.008 for CO-treated; Fig. 3). Changes in preterminal myelination were less pronounced in untreated R98C/+ mice, but they deteriorated with age; 10% of the terminal twigs were demyelinated by 6 weeks of age and 30% by 3 months of age. Treatment improved the deficits, although the changes did not reach significance (Fig. 3).
Figure 3.
PCC or CO treatment improves the innervation of neuromuscular junctions in R98C animals. (A) Magnified (×60) confocal images of neuromuscular junctions of wild-type and R98C animals at 6 weeks of age. Arrows indicate the demyelinated segment. Scale bar = 10 μm. (B and C) Virtually all neuromuscular junctions of untreated and PCC/CO treated wild-type mice were fully myelinated. Untreated wild-type values are included as control in the graphs. Percentage of fully myelinated neuromuscular junctions in the triangularis sterni muscle in R98C mice at post-natal Day 13, 6 weeks and 3 months of age. A tendency towards a lower number of normally myelinated junctions could be seen at post-natal Day 13 in R98C mice. By 6 weeks of age, R98C/R98C animals exhibited a significantly reduced number of normally myelinated junctions, which was rescued by CO treatment (P = 0.004). Benefits of treatment in R98C/R98C animals could be maintained even at 3 months of age (PCC P = 0.013, CO P = 0.017). Virtually all neuromuscular junctions of wild-type mice were fully myelinated, data not shown in the graphs. (C) The length of demyelinated segments proved to be longer in R98C/R98C animals than in R98C/+ mice (post-natal Day 13 P < 0.001, 6 week P = 0.003). CO treatment resulted in shorter demyelinated segments in R98C/R98C animals (post-natal Day 13 P < 0.001, 3 month P = 0.00815). NMJ = neuromuscular junction.
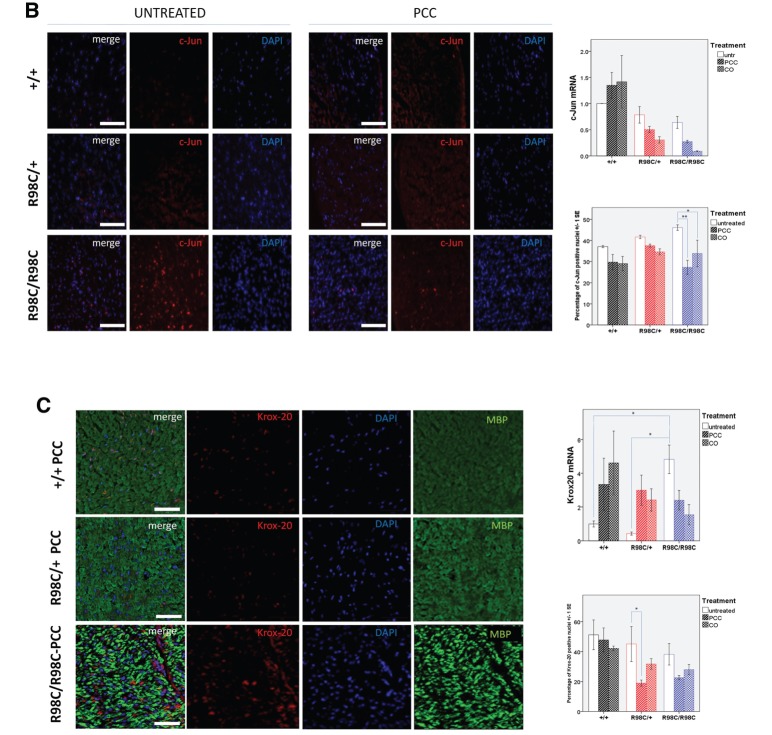
Curcumin derivative and formulation decreased the expression of c-Jun and SCIP in R98C/R98C mice
During development, Schwann cell precursors migrate from the neural crest and pass through a series of stages before becoming myelinating Schwann cells (Jessen and Mirsky, 2005). After establishing a 1:1 relationship with axons, they become ‘promyelinating’ Schwann cells, decrease their expression of the transcription factor c-Jun and upregulate pro-myelinating transcription factors, including SCIP (Oct6, Pou3f1) and Krox20 (Egr2). The coordinate activities of these transcription factors allow promyelinating cells to transition into myelinating Schwann cells (Svaren and Meijer, 2008). Our previous study demonstrated that the R98C mutation alters this progression in mutant nerves, such that near the peak of myelination, at post-natal Day 13, there were increased numbers of c-Jun expressing and decreased numbers of Krox-20 expressing Schwann cells in heterozygous and, to a greater extent, homozygous R98C nerves (Saporta et al., 2012). Particularly, since c-Jun expression has been shown to be upregulated in several human neuropathies (Hutton et al., 2011), we elected to determine whether curcumin therapy altered the expression of these transcription factors. We measured their expression by real-time PCR and immunohistochemistry on frozen sections and teased fibre preparations of sciatic nerve from 3-month-old untreated and PCC- or CO-treated animals, respectively. Because Schwann cell numbers are increased in R98C mutant mice (Saporta et al., 2012), we normalized our results to their number.
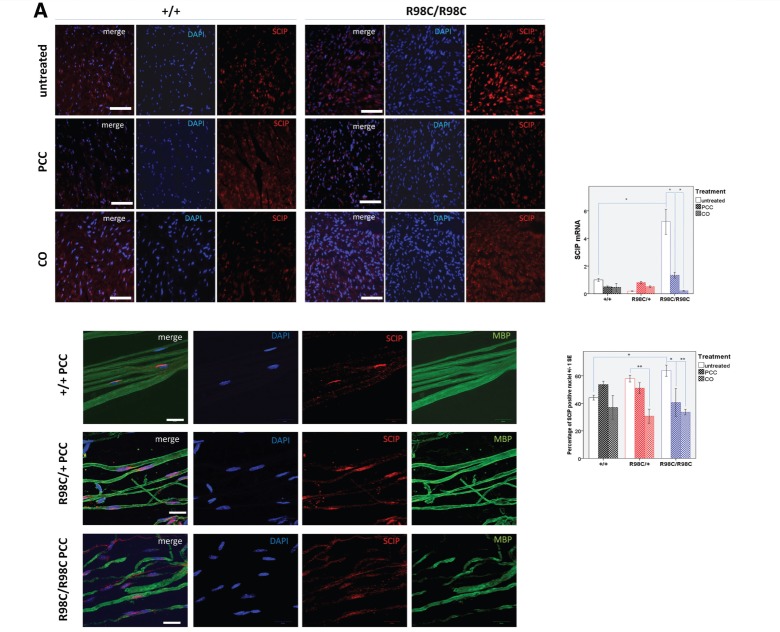
At 3 months of age, PCC (P = 0.002) or CO treatment (P = 0.018) reduced the percentage of c-Jun expressing nuclei in Schwann cells (Fig. 4B). PCC and CO treatment mildly decreased the percentage of Schwann cells expressing Krox-20 in mutant mice. Pronounced changes occurred in SCIP expression. SCIP is normally transiently expressed in rodent myelinating Schwann cells peaking at around the time of birth and barely detectable in myelinating Schwann cells in adulthood (Blanchard et al., 1996; Zorick et al., 1996). Persistent overexpression of SCIP in Schwann cells has been shown to cause Peripheral Nervous System (PNS) hypomyelination (Ryu et al., 2007). The percentage of SCIP-positive nuclei was elevated in untreated R98C/+ nerves and elevated to a greater extent in R98C/R98C nerves (P = 0.029). Both PCC and CO treatment reduced SCIP expression in mutant cells, particularly from R98C/R98C mice (PCC P = 0.013; CO P = 0.003). On teased fibre preparations from PCC-treated mice, in wild-type, occasional SCIP-expressing Schwann cells could be found that also expressed MBP, although most nuclei were SCIP-negative. In R98C/+, a greater number of SCIP-positive Schwann cells were identified associated with MBP-positive internodes. In R98C/R98C teased fibres, not only were the numbers increased but SCIP-expressing Schwann cells were also identified in demyelinated internodes (Fig. 4).
Figure 4.
Curcumin derivatives alter expression of transcription factors in adult R98C mice immunohistochemistry and real-time PCR analyses of SCIP, c-Jun and Krox-20 messenger RNA levels at 3 months of age (n = 2–6/genotype/treatment group). (A) Immunohistochemistry on sciatic nerve cross sections and teased fibre preparations confirmed nuclear SCIP localization. The percentage of SCIP-positive nuclei was elevated in untreated R98C/+ mice, and it was elevated to a greater extent in R98C/R98C mice (P = 0.029). PCC and CO treatment reduced SCIP upregulation in R98C/R98C (PCC P = 0.013; CO P = 0.003) animals. Scale bars : cross section = 50 μm, teased fibre = 20 μm. Markedly elevated SCIP messenger RNA levels in R98C/R98C animals compared with wild-type littermates (P = 0.001) were also significantly reduced in PCC/CO treated R98C/R98C animals (for both PCC and CO P = 0.010). (B) The increased percentage of c-Jun-positive nuclei in the mutant animals was significantly reduced by PCC (P = 0.002) or CO (P = 0.018) treatment in R98C/R98C mice. Scale bar = 50 μm. No significant difference was detected in c-Jun messenger RNA levels between the genotypes and treatment groups. (C) Immunohistochemistry revealed no significant differences between untreated wild-type and mutant animals for Krox-20 expression at 3 months of age; treatment slightly decreased nuclear Krox-20 expression. Scale bar = 50 μm. Krox-20 messenger RNA levels were significantly elevated in R98C/R98C mice (P = 0.014) compared with wild-type animals. PCC- or CO-treated R98C/R98C animals showed a tendency towards reduced Krox-20 messenger RNA expression (CO P = 0.049). * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
Real-time PCR values for these transcription factors were also altered, although not always in parallel with the changes observed with immunohistochemistry. SCIP messenger RNA levels were elevated in mutant nerves, and these values were significantly reduced in the treated animals, consistent with the results from immunohistochemistry (Fig. 4). However, no differences in c-Jun messenger RNA levels were detected between genotypes, and c-Jun messenger RNA levels did not change with treatment. Finally, Krox20 messenger RNA levels were surprisingly increased in R98C/R98C nerves, and these levels were reduced in treated nerves (Fig. 4).
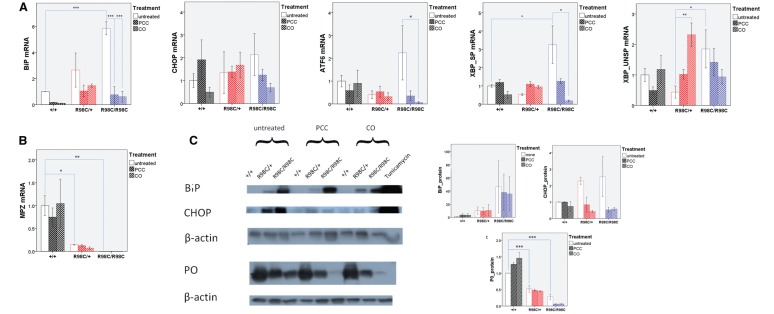
Curcumin treatment altered the unfolded protein response both in vivo and in vitro
We have previously demonstrated that the R98C Mpz mutant protein is retained in the endoplasmic reticulum where it induces a canonical UPR in mice during the peak period of PNS myelination (Saporta et al., 2012). We, therefore, analysed whether curcumin derivative or formulation altered the three parameters that are usually used to detect UPR activation; ATF6 cleavage, XBP1 splicing and the increase in Chop levels along with its translocation to the nucleus.
At post-natal Day 13, neither PCC nor CO treatment attenuated the upregulation of UPR markers; BiP protein levels, as a general marker of UPR activation, remained high at both post-natal Day 13 and 3 months of age (Supplementary Fig. 7). At 3 months of age, there was a prominent increase in messenger RNA levels of mediators involved in all three arms of the UPR in R98C/R98C animals (XBP1 splicing P < 0.001). Treatment significantly reduced XBP1 splicing (PCC and CO P = 0.01). BiP messenger RNA levels, a target of cleaved ATF6 (Yoshida et al., 1998), were also reduced, as were ATF6 messenger RNA levels themselves (PCC P = 0.003, CO P = 0.010). In R98C/R98C untreated animals, western blot analysis demonstrated elevated Chop protein levels, which were reduced by PCC or CO treatment (Fig. 5). However, at 3 months of age, Chop did not translocate to the nuclei even in untreated mice (data not shown). Recent studies suggest that when Chop translocates to the nucleus, it predominantly regulates genes associated with the cell cycle, whereas in the cytoplasm, it appears to affect cell migration genes (Jauhiainen et al., 2012b). Thus, it was difficult to interpret the significance of reducing Chop in terms of UPR activation. Overall, our results indicated that CO and PCC treatment attenuated at least two arms (XBP1 splicing, ATF6) of the UPR at 3 months of age.
Figure 5.
PCC or CO treatment partially attenuated the UPR at 3 months of age, but did not change MPZ protein levels. (A) Real-time PCR analyses of UPR activation at 3 months of age (n = 3–6). BiP, the general marker of UPR activation, was highly upregulated (P < 0.001) in untreated R98C/R98C animals compared with wild-type littermates. PCC/CO treatment reduced significantly (for both P < 0.001) the elevation of BiP in R98C/R98C mice. Note the activation of all three arms of the UPR [spliced XBP1 (XBP SP) P < 0.001]. The upregulation of two arms was significantly reduced at the messenger RNA level by PCC/CO treatment (spliced XBP1 both for PCC and CO P = 0.010; ATF6 PCC P = 0.003, CO P = 0.010) (n = 3–6). (B) Real-time PCR confirmed reduced MPZ levels in untreated (R98C/+ P = 0.023; R98C/R98C P = 0.01) and treated knock-in mice compared with wild-type animals (n = 3). (C) Western blot analyses indicated that BiP was highly upregulated in untreated (both R98C/+ and R98C/R98C P < 0.001) and treated mutants. Elevated CHOP protein levels were slightly but significantly reduced in treated animals (R98C/+ CO P = 0.019; R98C/R98C PCC P = 0.012, CO P = 0.014), but it was considered functionally uncertain because CHOP translocation to the nucleus is no longer observed at this age (data not shown). Positive control: 10-fold diluted liver samples from tunicamycin treated animals. P0 protein levels were reduced similarly in untreated and PCC/CO treated R98C/+ and R98C/R98C animals when compared with wild-type littermates (P < 0.001 both for R98C/+ and R98C/R98C). UNSP = unspliced. * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
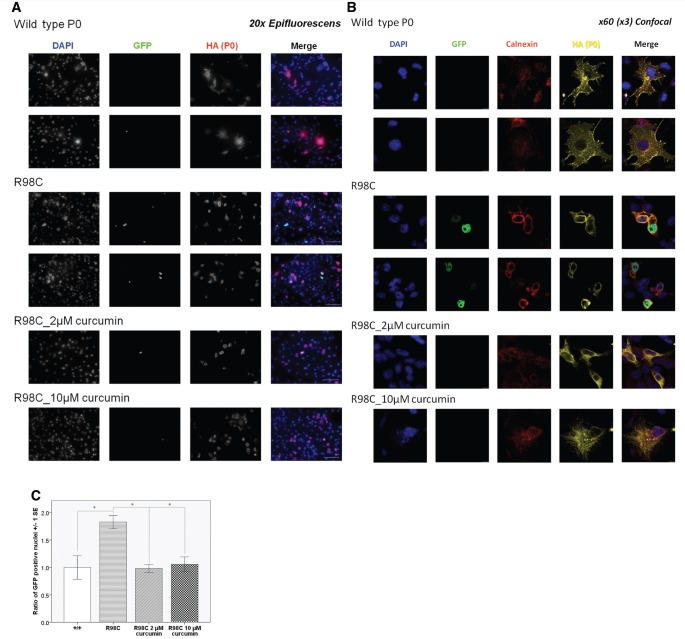
To address UPR activation in an independent assay, we performed in vitro studies, assessing XBP1 splicing. Cos-7 cells were co-transfected with wild-type or R98C MPZ and a plasmid containing XBP1 fused out of frame with EGFP. EGFP is pulled into frame by XBP1 splicing, and results in nuclear green fluorescence on UPR activation (Saporta et al., 2012). Transfection with wild-type MPZ revealed minimal XBP1 splicing (4–10% of cells); presumably this was secondary to protein overexpression in the small percentage of cells. Nevertheless, transfection with the R98C mutant elicited significantly increased (P = 0.05) XBP1 splicing when quantified as the ratio of MPZ transfected cells with GFP-positive nuclei and normalized for transfection efficiency. We treated the cells with two different curcumin concentrations, 10 μM as suggested in the literature in cases of other MPZ mutants (Khajavi et al., 2005) and 2 μM, which was the concentration we measured in post-natal Day 13 sciatic nerves by mass spectrometry. We found that both curcumin concentrations reduced XBP1 splicing equally, as illustrated by the pronounced decrease (70%) in the number of GFP-positive nuclei (P = 0.05 for both concentrations) (Fig. 6). Therefore, curcumin treatment did attenuate the activation of the XBP1 arm of the UPR in stressed transfected cells. Nevertheless, as shown by confocal images, mutant Mpz remained in the endoplasmic reticulum after 2 μM curcumin treatment and was only somewhat transported to the cell surface after 10 μM curcumin, suggesting that cell surface expression and UPR activation may be regulated distinctly (Fig. 6). The decreased MPZ messenger RNA (R98C/+ P = 0.017; R98C/R98C P = 0.004) and protein levels (R98C/R98C P < 0.001) that were found in untreated knock-in animals did not improve with PCC or CO treatment (Fig. 5), which is consistent with the morphological, neurophysiological and cell culture findings.
Figure 6.
Curcumin treatment attenuated XBP1 splicing elicited by altered trafficking of the R98C mutant in Cos-7 cells. (A) Green fluorescence in the nucleus indicates the activation of the IRE1 pathway of the unfolded protein response in a cell culture reporter system where alternative splicing of XBP1 pulls GFP into frame. Representative epifluorescent images demonstrate that XBP1 splicing occurred more frequently in Cos-7 cells transfected with R98C than in those transfected with wild-type P0 and that curcumin treatment reduced the number of GFP-positive nuclei. Scale bar = 100 μm. (B) Confocal images illustrate that although wild-type P0 reached the cell surface, the R98C mutant was retained in the endoplasmic reticulum as well as elicited XBP1 splicing. At 2 μM curcumin treatment altered trafficking of P0R98C protein remained, but the number of GFP-positive nuclei decreased; whereas at 10€ μM curcumin treatment, the number of GFP-positive nuclei decreased but some mutant protein appeared to reach the cell surface, as every cell process and protrusion is labelled with haemagglutinin staining (see arrows). Scale bar = 10 μm. (C) Quantification of XBP1 splicing in vitro. The percentage of GFP-positive nuclei was significantly higher (P = 0.05) in Cos-7 cells transfected with the R98C mutant as in those transfected with the wild-type construct. In the end, 2 and 10 μM curcumin treatment reduced XBP1 splicing to the same extent (for both P = 0.05).
Discussion
We have shown that phosphatidyl choline curcumin and curcumin dissolved in sesame oil, but not curcumin dissolved in alimentum or fluorinated carbon curcumin, improved the phenotype of R98C animals. PCC- and CO-treated R98C/+ mice performed better on the Rotarod, had improved compound muscle action potential amplitudes and exhibited increased numbers of large-diameter axons compared with untreated mice or mice treated with CA or CDF curcumin. R98C/R98C mice showed increased compound muscle action potential amplitudes, although no clinical improvements or increased large diameter axons were identified. We did detect an increased number of normally myelinated internodes adjacent to neuromuscular junction and shorter demyelinated segments in R98C/R98C mice. We identified no changes in myelin thickness, nerve conduction velocity, F-wave latencies or X-ray diffraction patterns of myelin, although MPZ protein levels appeared increased in PCC- and CO-treated animals. Our findings suggest that the clinical improvement was due to the attenuation of a toxic gain of function mechanism and consequent indirect positive effects on axonopathic changes, as discussed later in the text.
Neuromuscular junction and axonal calibre
We hypothesize, for reasons discussed later, that the increased percentages of fully myelinated preterminal internodes and decreased length of demyelinated segments approaching the neuromuscular junction of treated animals contribute to the clinical improvement in the mice and perhaps also to the increased numbers of large-diameter axons in the treated nerves. Demyelinated axons have been shown to impair the regeneration of nerve terminals, resulting in dysfunctional presynaptic neuromuscular junction following retraction (Yin et al., 2004). At post-natal Day 13 when the neuromuscular junctions are still in a highly plastic phase (Brill et al., 2011), the deficits are not yet pronounced, but they become gradually aggravated with ageing. Normally functioning neuromuscular junction are necessary to provide retrograde transport from muscle to the neuronal cell body of growth factors, such as nerve growth factor (NGF), brain derived neurotrophic factor or ciliary neurotrophic factor (CNTF) (Butowt and von Bartheld, 2009). Decreased numbers of normal functioning neuromuscular junctions could thus compromise neuronal health resulting in reduced numbers of large calibre axons such as we found in R98C mice. Abnormalities at the neuromuscular junction have also been shown to cause asynchronous failure of action potential transmission at high-stimulation frequencies in periaxin-null mice, a model of the demyelinating autosomal recessive neuropathy CMT4F (Court et al., 2008). Increasing the number of fully functioning myelinated neuromuscular junctions with PCC or CO may induce more effective synapse reformation and regeneration.
An alternative explanation is that the alterations we observed at the neuromuscular junction result from PCC or CO, inducing the regrowth of the largest diameter axons through some other process, possibly through improved myelination around the larger axons. However, we observed no evidence of increased compact myelin around the large axons or around any other calibre axon we evaluated, and no differences were observed in G-ratios following treatment that would support increased myelination. Moreover, nerve conduction velocity and F-wave latencies are calculated based on saltatory conduction along the large-diameter axons (Kimura, 2005), and these values did not improve with treatment. Therefore, we have no data to support PCC or CO improving compact myelin along the larger diameter axons.
Unfolded protein response: IRE-1, PERK and ATF6
Abnormalities in the UPR contributed not only to the pathogenesis of the neuropathy in R98C mutant mice but also responded to treatment by PCC and CO. For example, by 3 months of age, XBP1-splicing and BiP, as well as ATF6, messenger RNA levels were significantly attenuated in treated R98C/R98C animals compared with untreated mice. We also detected attenuation in XBP1 splicing in an in vitro assay between curcumin-treated and untreated R98C transfected cells. Taken together, these data suggested that reduced activation of the IRE1 and ATF6 arms of the UPR might have contributed to the improvement of treated mice. In contrast, BiP protein levels remained elevated with PCC and CO treatment, and we also could not identify differences in the levels of Chop translocation into the nucleus between treated and untreated mice. Although the observed reduction of Chop levels in the cytoplasm may also affect Schwann cell behaviour in ways that are independent of DNA binding (Jauhiainen et al., 2012a), we did not detect changes in the traditional PERK pathway of the UPR following treatment. These results are consistent with our previous findings that genetically eliminating the Chop arm of the UPR, by crossing R98C mice with Chop-null mice, did not improve the neuropathy of R98C/+ or R98C/R98C mice (Saporta et al., 2012). Taken together, these results suggest that signalling through IRE1 or ATF6, of the UPR, contribute to the pathogenesis of the neuropathy caused by R98C MPZ.
R98C Mpz prevents or delays the transition of Schwann cells from a promyelinating to a myelinating state (Saporta et al., 2012). Thus, it can be considered as a dysmyelinating or congenital hypomyelinating neuropathy. Ser63del MPZ causes a clinically milder neuropathy, in which patients’ early milestones, such as walking independently, are normal (Miller et al., 2012). Ser63del Mpz mice develop a demyelinating rather than a dysmyelinating neuropathy, and these mice improve by clinical, physiological and morphological criteria when crossed with Chop-null animals (Pennuto et al., 2008). These results suggest that activation of PERK is important in deymelination, whereas IRE1 and/or ATF6 contribute more strongly to dysmyelination caused by MPZ mutations. In this regard, it will be important to determine whether Ser63del mice respond as well to PCC or CO as do R98C mice. Our previous study also suggested that UPR activation might contribute to the upregulation of c-Jun, through the IRE pathway. c-Jun, as a negative regulator, inhibits PNS myelination (Parkinson et al., 2008). The decrease in c-Jun expression that accompanied attenuation of XBP1 splicing is consistent with this role. Because c-Jun upregulation has been identified in other neuropathies (Hutton et al., 2011), targeting IRE signalling may prove to be a novel therapeutic strategy for multiple demyelinating neuropathies.
Potential effects independent of the unfolded protein response
Curcumin may also be affecting R98C mice through cellular pathways that are independent of UPR activation. Curcumin, in fact, has been shown to affect cells through multiple other intracellular signalling mechanisms, including anti-inflammatory effects altering cytochrome c oxidase (COX)-2 expression, altering cytokine release from white blood cells and inhibition of NF-κB activity (Agrawal and Mishra, 2009). NF-κB is of particular interest, as it is a transcription factor that is necessary for Schwann cell myelination. Neuregulin-1 signalling from the axon induces protein kinase A phosphorylation of the p65 subunit of NF-κB that promotes Schwann cell differentiation into a myelinating phenotype (Yoon et al., 2008; Limpert and Carter, 2010). Specifically, NF-κB is required for activation of the transcription factor SCIP in Schwann cells (Nickols et al., 2003), which then proceeds to activate Krox-20 as part of a ‘feed forward’ loop reviewed in Svaren and Meijer, 2008). Much of this signalling occurs during the period of peak myelination, the same time the R98C neuropathy develops. Therefore, it is intriguing to hypothesize that curcumin induced alteration of the NF-κB pathway might also play a role in the improvement shown by PCC- and CO-treated animals. Consistent with this possibility is the observed reduction in elevated SCIP messenger RNA levels and nuclear expression by immunohistochemistry after PCC and CO treatment at an adult age, which would be predicted from inhibition of NF-κB in myelinating Schwann cells (Limpert and Carter, 2010). Induced overexpression of SCIP has been shown to cause PNS hypomyelination without altering Krox-20 expression (Ryu et al., 2007), which is precisely what we observed in our untreated animals. Thus, it is attractive to speculate that PCC or CO treatment caused improvement by reducing SCIP expression that then permitted at least Schwann cells ensheathing nerve terminals to proceed towards myelination.
Sesame oil, curcumin dissolved in sesame oil and phosphatidylcholine curcumin
We were surprised that R98C mice treated with sesame oil showed a trend towards increased compound muscle action potential amplitudes and improved Rotarod performance (Fig. 1 and Supplementary Fig. 4). The recent study in which cholesterol treatment improved transgenic mice with the leukodystrophy, Pelizaeus–Merzbacher disease may provide an explanation for these results (Saher et al., 2012). The most common form of Pelizaeus–Merzbacher disease is caused by a duplication of the major CNS myelin protein proteolipid protein-1 (Inoue et al., 1996). Dietary supplementation with cholesterol improved Plp1 overexpressing mice clinically and increased their myelin content, at least in part by facilitating the incorporation of PLP-1 into CNS myelin membranes through lipid rafts (Saher et al., 2012). Moreover, a previous study by the same group showed that cholesterol may act similarly on Mpz (Saher et al., 2009). Sesame oil contains negligible cholesterol, but it does contain multiple plant sterols that function through similar pathways in humans, and that presumably contribute to cholesterol-lowering properties of sesame and other plant oils (Abidi, 2001). Thus, we hypothesize that sesame oil may act like cholesterol to promote trafficking of MpzR98C into myelin, and contribute to the improvement of R98C mice, a hypothesis that is supported by the fact that CO treatment was as effective as PCC in treating the animals. The main advantage of PCC over CA may be simply that it is dissolved in sesame oil. As curcumin is poorly absorbed from the gastrointestinal tract by itself (Marczylo et al., 2007), we suggest that sesame oil should be considered as a component of the medication that improved R98C mice rather than as a simple diluent for the curcumin.
R98C Mpz compared with TrJ and Ser63del Mpz mice
We were surprised by the lack of effect in R98C/+ mice with CA treatment because this therapy had proved effective with the TrJ mouse model of CMT1E (Khajavi et al., 2007). TrJ mice also have endoplasmic reticulum retention of their mutant protein, although in this case, the protein is Pmp22. MPZ comprises >50% of PNS myelin proteins, whereas Peripheral Myelin Protein (PMP22) constitutes ∼5% (Trapp et al., 2003). Thus, TrJ Schwann cells may have fewer misfolded proteins to process than R98C Mpz animals and require less curcumin to cause an improvement. Benefits from PCC or CO in R98C mice presumably result from increased gastrointestinal absorption of curcumin and prolonged plasma levels with these preparations (Marczylo et al., 2007) that would aid Schwann cells in processing the additional protein load. Curcumin itself, as noted above, is poorly absorbed after oral intake and largely excreted by the gut. It has been estimated that a patient would have to ingest oral doses of 10–12 g of curcumin daily to achieve therapeutic plasma levels, a particular problem because of a bad aftertaste with such high doses (Singh, 2007). The fact that we also observed similar benefits with CO, and to a lesser extent sesame oil, was consistent with this explanation, since curcumin is more soluble in sesame oil than in alimentum. We observed that curcumin levels in sciatic nerve were similar in CA, PCC and CO treated animals 30 min after gavage. However, PCC is known to remain in plasma for at least 6 h, unformulated curcumin disappears within 2 h of oral administration (Marczylo et al., 2007).
An alternative explanation is that pathogenic mechanisms caused by UPR activation may differ between R98C Mpz and TrJ mice. The R98C mutation causes a particularly severe neuropathy due to of congenital hypomyelination, a form of dysmyelination resulting in severe disability of patients (Warner et al., 1996; Bai et al., 2006). Ser63del MPZ causes a milder clinical form of CMT1B in which patients attain early milestones normally and usually remain ambulatory (Miller et al., 2012). Ser63del Mpz also activates the UPR but predominantly have demyelination rather than dysmyelination (Pennuto et al., 2008). Eliminating the Chop arm of the UPR has minimal effects on R98C mice (Saporta et al., 2012), but rescues Ser63del Mpz mice (Pennuto et al., 2008). Dysmyelination and demyelination may respond differently to SERCA inhibition, and it may be that TrJ mice also suffer predominantly from demyelination.
Toxic gain of function versus haploinsufficiency
It is unlikely that PCC or CO improves the neuropathy by releasing mutant Mpz from the endoplasmic reticulum, such that it can be incorporated into myelin. Not only were there no changes in myelin thickness or G-ratios in treated compared with non-treated mice, but also, although curcumin did alter XBP-splicing in vitro, it did not induce R98C MPZ to travel to the cell surface. We speculate that the additional cysteine introduced at amino acid 98 would so disrupt the secondary and tertiary structure of the Mpz extracellular domain that tetramers of R98C would be unable to form in cis or interact in trans (Shapiro et al., 1996). Rather, we hypothesize that relief or partial relief from the ‘toxic gain of function’ caused by R98C Mpz permits Schwann cells to more easily enter or maintain their myelinating phenotype. The benefits from this relief would not be limited to an increase in wild-type Mpz expression in treated heterozygous R98C/+ mice. In other words, the toxic gain of function would not be limited to a dominant-negative effect on the wild-type Mpz allele. We have shown that the R98C mutation causes many Schwann cells to express transcription factors, c-Jun and SCIP, that prevent them from entering into a myelinating state. Schwann cells in this ‘pre-myelinating’ state would not express any myelin proteins, consistent with our previously published data that show reduced levels of all myelin proteins, particularly in homozygous mice (Saporta et al., 2012). By decreasing the number of Schwann cells that express c-Jun and or SCIP, curcumin treatment would allow more Schwann cells to enter and remain in a myelinating state even if they make no normal Mpz, as is the case with R98C/R98C mice. We hypothesize that Schwann cells that remain in a myelinating phenotype without making normal Mpz are better for nerves than Schwann cells that express no myelin proteins. It is for this reason that it is important to minimize endoplasmic reticulum stress, UPR activation and other toxic gain of function mechanisms caused by R98C and related mutations. Improvement to haploinsufficiency in these heterozygous disorders should dramatically improve the quality of life in affected patients. Patients with haploinsufficiency of MPZ are often asymptomatic well into their adult years (Shy et al., 2004), and Mpz ± mice are much milder than R98C/+ mice by clinical, physiological and morphological criteria (Saporta et al., 2012). Determining which MPZ mutations respond to curcumin derivatives by improvement towards haploinsufficiency should identify candidate patients for clinical trials with these medications.
Funding
The Muscular Dystrophy Association (to M.E.S.), NINDS R01 NS41319A (to M.E.S.), R01 NS055256 (to L.W.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Paola Saveri for excellent technical assistance and Elisa Tinelli for suggestions on neuromuscular junction analysis.
Glossary
Abbreviations
- CA
curcumin dissolved in alimentum
- CDF
fluorinated curcumin derivative
- CMT1B
Charcot–Marie–Tooth disease type 1B
- CO
curcumin dissolved in sesame oil
- GFP
green fluorescent protein
- PCC
phosphatidylcholine curcumin
- SERCA
sarcoplasmic/endoplasmic reticulum calcium pump
- UPR
unfolded protein response
References
- Abidi AL. Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A. 2001;935:173–201. doi: 10.1016/s0021-9673(01)00946-3. [DOI] [PubMed] [Google Scholar]
- Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2009;30:818–60. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- Agrawal D, Hawk R, Avila RL, Inouye H, Kirschner DA. Internodal myelination during development quantitated using X-ray diffraction. J Struct Biol. 2009;168:521–6. doi: 10.1016/j.jsb.2009.06.019. [DOI] [PubMed] [Google Scholar]
- Back SH, Lee K, Vink E, Kaufman RJ. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J Biol Chem. 2006;281:18691–706. doi: 10.1074/jbc.M602030200. [DOI] [PubMed] [Google Scholar]
- Bai Y, Ianokova E, Pu Q, Ghandour K, Levinson R, Martin JJ, et al. Effect of an R69C mutation in the myelin protein zero gene on myelination and ion channel subtypes. Arch Neurol. 2006;63:1787–94. doi: 10.1001/archneur.63.12.1787. [DOI] [PubMed] [Google Scholar]
- Blanchard AD, Sinanan A, Parmantier E, Zwart R, Broos L, Meijer D, et al. Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20, and Pax-3. J Neurosci Res. 1996;46:630–40. doi: 10.1002/(SICI)1097-4547(19961201)46:5<630::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Brill MS, Lichtman JW, Thompson W, Zuo Y, Misgeld T. Spatial constraints dictate glial territories at murine neuromuscular junctions. J Cell Biol. 2011;195:293–305. doi: 10.1083/jcb.201108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, von Bartheld CS. Fates of neurotrophins after retrograde axonal transport: phosphorylation of p75NTR is a sorting signal for delayed degradation. J Neurosci. 2009;29:10715–29. doi: 10.1523/JNEUROSCI.2512-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–74. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Brophy PJ, Ribchester RR. Remodeling of motor nerve terminals in demyelinating axons of periaxin-null mice. Glia. 2008;56:471–9. doi: 10.1002/glia.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan ME, Glockner-Pagel J, Ambrose C, Cahill PA, Pappoe L, Balamuth N, et al. Calcium-pump inhibitors induce functional surface expression of Delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat Med. 2002;8:485–92. doi: 10.1038/nm0502-485. [DOI] [PubMed] [Google Scholar]
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–2. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–57. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- Grandis M, Vigo T, Passalacqua M, Jain M, Scazzola S, La Padula V, et al. Different cellular and molecular mechanisms for early and late-onset myelin protein zero mutations. Hum Mol Genet. 2008;17:1877–89. doi: 10.1093/hmg/ddn083. [DOI] [PubMed] [Google Scholar]
- Hutton EJ, Carty L, Laura M, Houlden H, Lunn MP, Brandner S, et al. c-Jun expression in human neuropathies: a pilot study. Journal of the peripheral nervous system. J Peripher Nerv Syst. 2011;16:295–303. doi: 10.1111/j.1529-8027.2011.00360.x. [DOI] [PubMed] [Google Scholar]
- Inoue K, Osaka H, Sugiyama N, Kawanishi C, Onishi H, Nezu A, et al. A duplicated PLP gene causing Pelizaeus-Merzbacher disease detected by comparative multiplex PCR. Am J Hum Genet. 1996;59:32–9. [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen A, Thomsen C, Strombom L, Grundevik P, Andersson C, Danielsson A, et al. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153. PloS One. 2012;7:e33208. doi: 10.1371/journal.pone.0033208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Khajavi M, Inoue K, Wiszniewski W, Ohyama T, Snipes GJ, Lupski JR. Curcumin treatment abrogates endoplasmic reticulum retention and aggregation-induced apoptosis associated with neuropathy-causing myelin protein zero-truncating mutants. Am J Hum Genet. 2005;77:841–50. doi: 10.1086/497541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajavi M, Shiga K, Wiszniewski W, He F, Shaw CA, Yan J, et al. Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy. Am J Hum Genet. 2007;81:438–53. doi: 10.1086/519926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. Nerve conduction and electromyography. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy. Philadelphia, PA: Elsevier/Saunders; 2005. pp. 899–970. [Google Scholar]
- Limpert AS, Carter BD. Axonal neuregulin 1 type III activates NF-kappaB in Schwann cells during myelin formation. J Biol Chem. 2010;285:16614–622. doi: 10.1074/jbc.M109.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–7. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Miller LJ, Patzko A, Lewis RA, Shy ME. Phenotypic presentation of the Ser63Del MPZ mutation. Journal of the peripheral nervous system. J Peripher Nerv Syst. 2012;17:197–200. doi: 10.1111/j.1529-8027.2012.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelis E, Van Broeckhoven C, De Jonghe P, Lofgren A, Vandenberghe A, Latour P, et al. Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur J Hum Genet. 1996;4:25–33. doi: 10.1159/000472166. [DOI] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–7. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438–45. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–37. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D'Antonio M, Ron D, et al. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in charcot-marie-tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu EJ, Wang JY, Le N, Baloh RH, Gustin JA, Schmidt RE, et al. Misexpression of Pou3f1 results in peripheral nerve hypomyelination and axonal loss. J Neurosci. 2007;27:11552–9. doi: 10.1523/JNEUROSCI.5497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Quintes S, Mobius W, Wehr MC, Kramer-Albers EM, Brugger B, et al. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci. 2009;29:6094–104. doi: 10.1523/JNEUROSCI.0686-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Rudolphi F, Corthals K, Ruhwedel T, Schmidt KF, Lowel S, et al. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat Med. 2012;18:1130–5. doi: 10.1038/nm.2833. [DOI] [PubMed] [Google Scholar]
- Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot marie tooth (CMT) subtypes and genetic testing strategies. Ann Neurol. 2011;69:22–33. doi: 10.1002/ana.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporta MA, Katona I, Lewis RA, Masse S, Shy ME, Li J. Shortened internodal length of dermal myelinated nerve fibres in Charcot-Marie-Tooth disease type 1A. Brain. 2009;132:3263–73. doi: 10.1093/brain/awp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporta MA, Shy BR, Patzko A, Bai Y, Pennuto M, Ferri C, et al. MpzR98C arrests Schwann cell development in a mouse model of early-onset Charcot-Marie-Tooth disease type 1B. Brain. 2012;135:2032–47. doi: 10.1093/brain/aws140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Doyle JP, Hensley P, Colman DR, Hendrickson WA. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron. 1996;17:435–49. doi: 10.1016/s0896-6273(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Shy ME, Jani A, Krajewski KM, Lewis RA, Li J, Shy RR, et al. Phenotypic clustering in MPZ mutations. Brain. 2004;127:371–84. doi: 10.1093/brain/awh048. [DOI] [PubMed] [Google Scholar]
- Singh S. Indian pharma enters the global arena. Cell. 2007;128:811–14. doi: 10.1016/j.cell.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–51. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Pfeiffer SE, Anitei A, Kidd GJ. Cell biology and myelin assembly. In: Lazzarini RA, editor. Myelin biology and disorders. San Diego/London: Elsevier Academic Press; 2003. pp. 29–56. [Google Scholar]
- Warner LE, Hilz MJ, Appel SH, Killian JM, Kolodry EH, Karpati G, et al. Clinical phenotypes of different MPZ (P0) mutations may include Charcot-Marie-Tooth type 1B, Dejerine-Sottas, and congenital hypomyelination. Neuron. 1996;17:451–60. doi: 10.1016/s0896-6273(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Wrabetz L, D'Antonio M, Pennuto M, Dati G, Tinelli E, Fratta P, et al. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J Neurosci. 2006;26:2358–68. doi: 10.1523/JNEUROSCI.3819-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Kidd GJ, Pioro EP, McDonough J, Dutta R, Feltri ML, et al. Dysmyelinated lower motor neurons retract and regenerate dysfunctional synaptic terminals. J Neurosci. 2004;24:3890–8. doi: 10.1523/JNEUROSCI.4617-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Korade Z, Carter BD. Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-kappaB promotes Schwann cell differentiation into a myelinating phenotype. J Neurosci. 2008;28:3738–46. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–9. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol Cell Neurosci. 1996;8:129–45. doi: 10.1006/mcne.1996.0052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.