Abstract
A growing body of evidence demonstrates an association between vascular risk factors and Alzheimer’s disease. This study investigated the frequency and severity of atherosclerotic plaques in the circle of Willis in Alzheimer’s disease and multiple other neurodegenerative diseases. Semi-quantitative data from gross and microscopic neuropathological examinations in 1000 cases were analysed, including 410 with a primary diagnosis of Alzheimer’s disease, 230 with synucleinopathies, 157 with TDP-43 proteinopathies, 144 with tauopathies and 59 with normal ageing. More than 77% of subjects with Alzheimer’s disease had grossly apparent circle of Willis atherosclerosis, a percentage that was significantly higher than normal (47%), or other neurodegenerative diseases (43–67%). Age- and sex-adjusted atherosclerosis ratings were highly correlated with neuritic plaque, paired helical filaments tau neurofibrillary tangle and cerebral amyloid angiopathy ratings in the whole sample and within individual groups. We found no associations between atherosclerosis ratings and α-synuclein or TDP-43 lesion ratings. The association between age-adjusted circle of Willis atherosclerosis and Alzheimer’s disease–type pathology was more robust for female subjects than male subjects. These results provide further confirmation and specificity that vascular disease and Alzheimer’s disease are interrelated and suggest that common aetiologic or reciprocally synergistic pathophysiological mechanisms promote both vascular pathology and plaque and tangle pathology.
Keywords: atherosclerosis, neuritic plaques, neurofibrillary tangles, synuclein, TDP-43
Introduction
Alzheimer’s disease and vascular cognitive impairment are considered the two most common causes of dementia in older adults (Kukull and Ganguli, 2000; Jellinger and Attems, 2010). With existing diagnostic criteria for Alzheimer’s disease (McKhann et al., 1984, 2011), there is an implicit assumption that this is an independent disease from vascular cognitive impairment with different underlying disease processes. Current pathogenesis models of Alzheimer’s disease (Jack et al., 2010) from neuropathological studies and emerging biomarker data emphasize sequential spectra of increasing amyloid-β production and its extracellular oligomerization and aggregation, and hyperphosphorylated tau fibrillization in neurites and neurofibrillary tangles, leading to cell dysfunction and death, synaptic loss and ultimately cognitive and functional deterioration. In the case of vascular cognitive impairment, strategic, large and/or multiple small infarcts are thought to lead to cognitive decline and dementia, although a specific clinical syndrome remains ill-defined and there is still no standard consensus in the clinical or neuropathological definitions for this disease (Wetterling et al., 1996).
A growing body of evidence has begun to link the pathophysiology of Alzheimer’s disease and vascular cognitive impairment. Alzheimer’s disease and atherosclerosis, the major underlying pathology of vascular cognitive impairment, share a number of important genetic and environmental risk factors, including age (Kukull and Ganguli, 2000), apolipoprotein E (APOE) ε4 polymorphism (Farrer et al., 1997), smoking (Price et al., 1991; Hernán et al., 2008), inflammation (Schmidt et al., 2002), obesity (Gustafson et al., 2003; Anstey et al., 2011) and the metabolic syndrome (Razay et al., 2007). Additionally, peripheral artery disease (Lee et al., 2006) and coronary atherosclerosis (Sparks et al., 1990) are more common in patients with Alzheimer’s disease.
We are aware of two recent post-mortem studies that reported greater large vessel atherosclerosis in Alzheimer’s disease compared with non-demented controls (Roher et al., 2003; Beach et al., 2007), and in these studies, cerebrovascular atherosclerosis ratings were associated with increased neuritic plaque and neurofibrillary tangle densities. A third study using data from the US National Alzheimer’s Coordinating Centre also found a strong association between cerebral atherosclerosis and neuritic plaque density, but did not find an association with neurofibrillary tangle density (Honig et al., 2005). All three studies are inconsistent with three other autopsy series reports that found no association between cerebral atherosclerosis and Alzheimer’s disease pathology (Kosunen et al., 1995; Itoh et al., 1999; Dolan et al., 2010). Therefore, greater clarity is needed regarding the association between cerebrovascular atherosclerosis and Alzheimer’s disease. Additionally, whether this phenomenon is specific to Alzheimer’s disease or is a mechanism promoting other neurodegenerative disease processes as well has not been investigated. Here we examined the frequency of circle of Willis atherosclerosis in those with Alzheimer’s disease and multiple neurodegenerative diseases in a large and diverse neurodegenerative disease autopsy series, and the relationship between atherosclerosis ratings and neuritic plaque, paired helical filament-tau neurofibrillary tangle, α-synuclein Lewy bodies and neurites, TDP-43 lesion densities and cerebral amyloid angiopathy prevalence and severity.
Materials and methods
Study population
Demographic, clinical diagnostic and neuropathological data were obtained from the University of Pennsylvania’s Integrated Neurodegenerative Disease Database (Xie et al., 2011) for all autopsy cases from 1985 (when standardized autopsy tissue collection began) to May 2011 that met the following criteria: (i) principal neuropathological diagnosis of a neurodegenerative disease or normal ageing with no history of other complicating neurological (e.g. major strokes, multiple sclerosis) or psychiatric illness; (ii) standardized ratings of circle of Willis atherosclerosis; and (iii) standardized ratings of thioflavin S-labelled neuritic plaques and/or paired helical filament-tau neurofibrillary tangles. Nomenclature for primary neuropathological diagnoses was diverse for non-Alzheimer’s diseases because of the rapid advances in these other areas of neurodegenerative disease research in the past 15 years; thus, these diseases were sorted into their molecular diagnostic category (Table 1). Incidental infarcts were recorded in 5.1% of cases with normal brain ageing, 4.2% of cases with a primary neuropathological diagnosis of Alzheimer’s disease, 7.4% with various synucleinopathies, 0.6% with TDP-43 proteinopathies and 6.25% with tauopathies, but based on size and/or location, these were considered to be non-contributory to clinical dementia status. Although not a requirement for inclusion, many cases had standardized ratings of cerebral amyloid angiopathy, α-synuclein and TDP-43, as well as APOE genotyping. Informed consent for autopsy was obtained in all cases from the patients or legal representative in accordance with Pennsylvania state law and protocols approved by the Institutional Review Board and Hospital of the University of Pennsylvania.
Table 1.
Subject characteristics and stratification by study group and subgroups
| n | Female (%) | Age, years | Brain weight, g | |
|---|---|---|---|---|
| Normal | 59 | 49.2 | 69.6 (15.9) | 1129 (161) |
| AD | 410 | 57.1 | 77.1 (10.5) | 1265 (152) |
| Synucleinopathy | 230 | 24.8 | 74.4 (10.2) | 1315 (140) |
| PD | 117 | 26.5 | 76.3 (10.1) | 1304 (132) |
| PDD | 49 | 10.2 | 77.8 (7.0) | 1336 (145) |
| DLB | 23 | 26.1 | 73.8 (9.6) | 1298 (139) |
| MSA | 41 | 36.6 | 65.1 (8.5) | 1331 (157) |
| Tauopathy | 144 | 42.4 | 72.4 (10.6) | 1170 (178) |
| AGD | 7 | 14.3 | 80.3 (6.0) | 1250 (112) |
| CBD | 33 | 60.6 | 68.2 (8.5) | 1150 (124) |
| FTD P-17 | 5 | 100.0 | 56.2 (14.8) | 952 (103) |
| Pick’s | 15 | 40.0 | 66.4 (12.7) | 1021 (172) |
| PSP | 67 | 31.3 | 75.4 (7.7) | 1221 (182) |
| TPSD | 6 | 66.7 | 83.0 (9.3) | 1104 (209) |
| Unclassifiable tauopathy | 11 | 36.4 | 71.5 (13.9) | 1200 (168) |
| TDP-43 Proteinopathy | 157 | 40.1 | 64.9 (11.7) | 1230 (214) |
| ALS | 85 | 36.5 | 62.2 (11.1) | 1336 (140) |
| FTD | 72 | 44.4 | 68.0 (11.7) | 1105 (219) |
Values given are means (standard deviation) or percentages.
AD = Alzheimer’s disease; AGD = argyophilic grain disease; ALS = amyotrophic lateral sclerosis; CBD = corticobasal degeneration; DLB = dementia with Lewy bodies; FTD = frontotemporal lobar degeneration with TDP-43 or FUS inclusions; FTDP-17 = frontotemporal dementia with parkinsonism linked to chromosome 17; MSA = multiple system atrophy; PD = Parkinson’s disease (without dementia); PDD = Parkinson’s disease with dementia; Pick’s = Pick’s disease; PSP = progressive supranuclear palsy; Synuclein = α-synuclein Lewy bodies and dystrophic neurites; TDP-43 = TDP-43 intracytoplasmic pathological inclusions; TPSD = tangle predominant senile dementia.
Neuropathological assessment
All brain extractions, gross inspections, tissue processing and diagnostic microscopic examinations were conducted by fellows and staff in the University of Pennsylvania’s Centre for Neurodegenerative Disease Research as previously described (Schmidt et al., 1988; Arnold et al., 1995; Neumann et al., 2006). Briefly, after extraction and weighing, visual inspection documented any gross abnormalities of the whole brain, meninges and extracerebral blood vessels. This included semi-quantitative ratings of circle of Willis atherosclerosis. The hindbrain and cerebral hemispheres were separated and coronally sectioned into 1–2-cm slabs for further inspection of any infarctions, tumours or other lesions. Fresh tissues from multiple CNS areas were fixed in 10% neutral buffered formalin or 70% ethanol with 150 mmol of sodium chloride, paraffin embedded and cut into 6-µm sections. Sections were stained with haematoxylin–eosin, thioflavin S and special stains as indicated for establishing diagnosis according to standard criteria (Mirra et al., 1991, 1994; Hyman and Trojanowski, 1997). Immunohistochemistry was conducted with monoclonal antibodies directed at paired helical filament tau (PHF1, 1:1000, a gift of Peter Davies, PhD), α-synuclein (‘303’, 1:4000, generated in the Centre for Neurodegenerative Disease Research) and TDP-43 (1:10 000; Novus Biologicals) and developed as previously described using (i) the avidin–biotin complex detection method (VECTASTAIN ABC kit; Vector Laboratories); or (ii) BioGenex SuperSensitive MultiLink IHC Detection System Kit (BioGenex Laboratories) with 3,3-diaminobenzidine as the chromogen.
DNA was isolated from brain tissue using a commercial kit (Qiagen). APOE genotyping was performed by using a previously described PCR restriction fragment length polymorphism method (Addya et al., 1997). Circle of Willis atherosclerotic plaques were graded from gross inspection of brains using a 4-point scale (0 = none, 1 = rare/mild, 2 = occasional/moderate, 3 = numerous/severe) by experienced neuropathology prosectors without prior knowledge of the clinical diagnosis. Analogous 0–3 ratings were applied in microscopic inspections of the thioflavin S- and immunohistochemically labelled sections with the above antibodies in seven brain regions: entorhinal cortex, hippocampal CA1/subiculum, amygdala, anterior cingulate, mid-frontal cortex, superior/middle temporal gyrus and angular gyrus. For data reduction, the mode of these seven ordinal regional ratings was obtained, providing a whole-brain rating for (i) thioflavin S neuritic plaque load; (ii) paired helical fragments-tau neurofibrillary tangle and dystrophic neurite density; (iii) cerebral amyloid angiopathy (thioflavin S amyloid deposition in blood vessel walls); (iv) α-synuclein Lewy bodies and dystrophic neurites; and (v) TDP-43 intracytoplasmic pathological inclusions. Reliability and accuracy of neuritic plaque and neurofibrillary tangle scoring were confirmed through reassessment of a representative sample of sections from 150 brains using a blinded computer-assisted quantitative method, which found high associations between ordinal ratings and per cent area measures of amyloid plaques (r = 0.83, P < 0.0001) and density counts of neurofibrillary tangles (r = 0.91, P < 0.0001).
Statistical analysis
For all statistical comparisons, circle of Willis atherosclerosis score, plaque, tangle, α-synuclein and TDP-43 densities and cerebral amyloid angiopathy severity were evaluated as ordinal variables. Differences in the characteristics of the study groups were assessed using analysis of variance for continuous variables and χ2 tests for categorical variables. The associations of potential confounders with circle of Willis atherosclerosis scores were examined using ANOVA for continuous variables and χ2 tests for categorical variables. Multinomial logistic regression analysis was used to determine the relationship between circle of Willis atherosclerosis score and disease category membership, with adjustment for age and sex. The relationships between circle of Willis atherosclerosis score and plaque, tangle, α-synuclein and TDP-43 densities and cerebral amyloid angiopathy severity were calculated using ordinal logistic tests, with adjustment for age and sex, two potential confounders. All statistical analyses were conducted using JMP 9 or SAS 9 (both SAS Institute). P-values < 0.05 were considered significant. All statistical tests were two-sided.
Results
A total of 1000 autopsy cases met criteria for inclusion in analyses. There were 410 cases with a primary neuropathological diagnosis of Alzheimer’s disease, 230 with various synucleinopathies, 157 with TDP-43 proteinopathies, 144 with tauopathies and 59 with normal ageing brain. Characteristics of the study groups are displayed in Table 1. Significant differences in age [F(4,995) = 37.2, P < 0.0001] and sex [χ2(4) = 66.5, P < 0.0001] were found among the diagnostic groups. As expected, there were also significant differences for density ratings of the defining pathological lesions of each condition.
For the entire study population, 33.3% of subjects had a circle of Willis atherosclerosis rating of ‘0 = none’, 39.0% had ‘1 = rare/mild’, 15.8% had ‘2 = occasional/moderate’ and 11.9% had ‘3 = numerous/severe’. There was a significant positive association between age and circle of Willis atherosclerosis grade [F(3,996) = 69.2, P < 0.0001]. The mean age of subjects in the none, mild, moderate and severe atherosclerosis groups were 67.2, 74.4, 78.4 and 81.0 years, respectively. Females and males did not differ significantly in atherosclerosis ratings [χ2(3) = 3.3, P = 0.35].
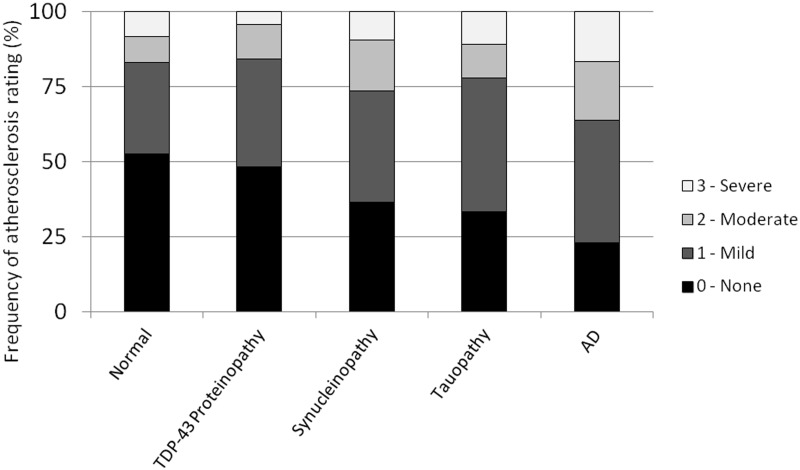
There were significant differences in the frequencies of occurrence and severity of circle of Willis atherosclerosis ratings among the diagnostic groups, and these differences remained significant after adjustment for age and sex [χ2(6) = 15.4, P = 0.004]. Atherosclerosis ratings stratified by group are displayed in Fig. 1. The highest frequency and severity of circle of Willis atherosclerosis were in the Alzheimer’s disease group. More than 77% of Alzheimer’s disease subjects had circle of Willis atherosclerosis, a rate that was higher than normal (47%), and other neurodegenerative diseases (52–67%). Approximately 36% of subjects with Alzheimer’s disease had moderate or severe grades of atherosclerosis compared with 17% of normal subjects and 16–26% of subjects with other neurodegenerative diseases. In individual between-group comparisons, adjusted for age and sex, the Alzheimer’s disease group had significantly greater circle of Willis atherosclerosis than the normal group [χ2(3) = 9.8, P = 0.0017], TDP-43 proteinopathy group [χ2(3) = 4.1, P = 0.043] and synucleinopathy group [χ2(3) = 5.7, P = 0.017]. Although atherosclerosis ratings were higher in the Alzheimer’s disease group than the tauopathy group, this difference did not reach statistical significance [χ2(3) = 2.3, P = 0.13].
Figure 1.
Circle of Willis atherosclerosis rating by disease category. Disease categories ordered by frequency of atherosclerosis rating of ‘0 = None’ from greatest to least. AD = Alzheimer’s disease.
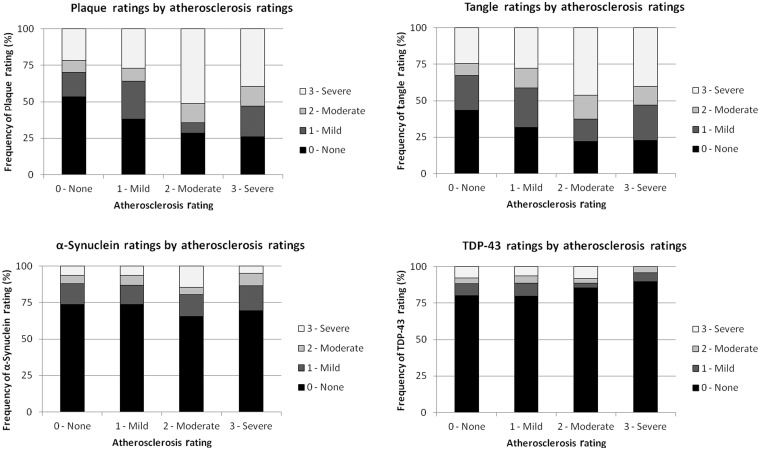
The relationships between circle of Willis atherosclerosis rating and the specific pathological lesions were assessed across the entire study population and within each diagnostic category for its defining lesion. For all subjects, age- and sex-adjusted circle of Willis atherosclerosis ratings were positively associated with plaque [χ2(5) = 22.0, P < 0.0001] and tangle [χ2(5) = 24.5, P < 0.0001] densities as well as cerebral amyloid angiopathy severity [χ2(5) = 9.2, P = 0.027] (Fig. 2). No such associations were found between atherosclerosis ratings and α-synuclein [χ2(5) = 3.3, P = 0.35] or TDP-43 lesion densities [χ2(5) = 1.6, P = 0.67]. In those with Alzheimer’s disease, atherosclerosis severity was significantly associated with densities of plaques [χ2(5) = 38.6, P < 0.0001] and tangles [χ2(5) = 23.4, P < 0.0001] and cerebral amyloid angiopathy severity [χ2(5) = 12.8, P = 0.0052]. By comparison, in those with synucleinopathies, atherosclerosis severity was unrelated to synuclein ratings [χ2(5) = 7.6, P = 0.059]; in those with TDP-43 proteinopathies, atherosclerosis was unrelated to TDP-43 ratings [χ2(5) = 5.5, P = 0.14], and in those with tauopathies, atherosclerosis was unrelated to tau pathology ratings [χ2(5) = 0.55, P = 0.91].
Figure 2.
Circle of Willis atherosclerosis ratings versus plaque, tangle, α-synuclein and TDP-43 lesion density ratings across the entire study population.
APOE is a constituent of high-density lipoproteins that is related to atherosclerosis (Elosua et al., 2004), and the APOE ε4 genotype is highly associated with Alzheimer’s disease and its pathology (Bennett et al., 2005). The association and effects of APOE genotype with circle of Willis atherosclerosis and plaque and tangle ratings were assessed among all subjects with APOE genotype data (n = 559) and within the Alzheimer’s disease subgroup alone (n = 243). Adjusted for age and sex, atherosclerosis ratings were not significantly greater among APOE ε4 carriers than non-carriers [χ2(3) = 3.20, P = 0.073], whereas, as expected, Alzheimer’s disease lesion density ratings were significantly higher among ε4 carriers for plaques [χ2(3) = 82.8, P < 0.0001], tangles [χ2(3) = 29.5, P < 0.0001] and cerebral amyloid angiopathy severity [χ2(3) = 48.8, P < 0.0001]. When APOE ε4 status was entered into an ordinal logistic model (along with age and sex) testing the associations of atherosclerosis with Alzheimer’s disease lesion ratings, these associations remained significant for tangles [χ2(6) = 26.8, P < 0.0001], but not for plaques [χ2(6) = 7.0, P = 0.073] or cerebral amyloid angiopathy severity [χ2(6) = 4.5, P = 0.22]. Findings were similar within the Alzheimer’s disease group only with APOE genotype data (data not shown). Finally, we tested for an effect of interaction of APOE ”4 genotype*atherosclerosis for plaque and tangle density and cerebral amyloid angiopathy severity ratings and found none in either the whole group or the Alzheimer’s disease subgroup (data not shown).
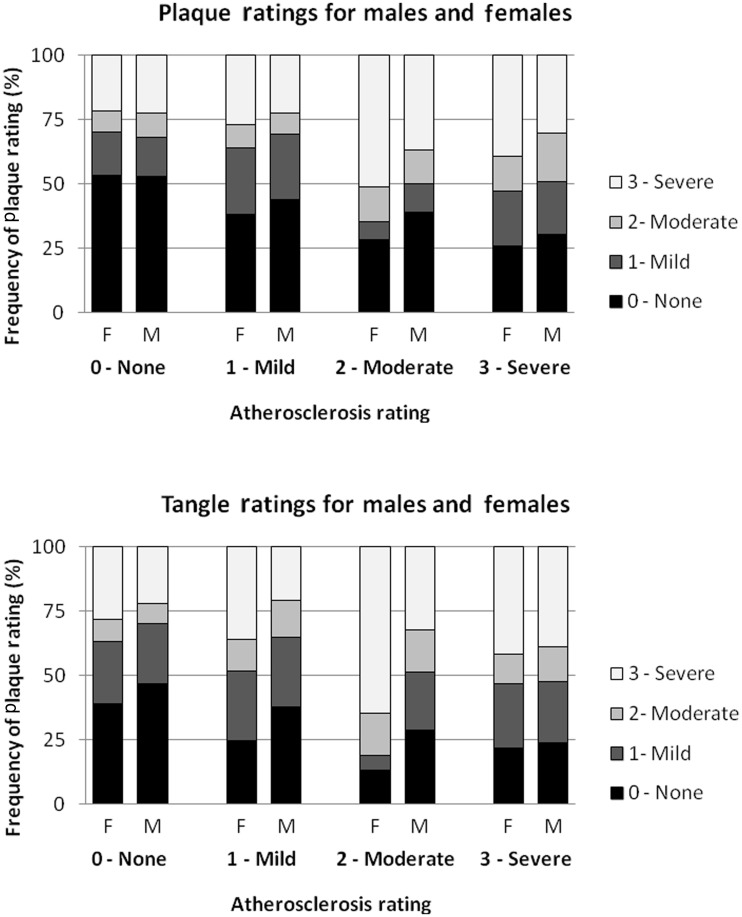
There were notable sex differences in plaque and tangle densities and the association of these lesions with age-adjusted circle of Willis atherosclerosis. Adjusted for age, female subjects (n = 444) had significantly greater average plaque [χ2(2) = 188.1, P < 0.0001] and tangle [χ2(2) = 18.7, P < 0.0001] lesion densities than male subjects. For females, age-adjusted circle of Willis atherosclerosis was strongly associated with both plaque [χ2(4) = 41.8, P < 0.0001] and tangle densities [χ2(4) = 30.7, P < 0.0001], whereas for males, there were no such associations with plaque density [χ2(4) = 2.0, P = 0.56] or tangle density [χ2(4) = 5.4, P = 0.14] (Fig. 3). Within the Alzheimer’s disease subgroup, however, there were no significant differences in average age-adjusted plaque [χ2(2) = 0.067, P = 0.80] and tangle [χ2(2) = 0.42, P = 0.52] densities between female (n = 234) and male (n = 176) subjects. Age-adjusted circle of Willis atherosclerosis ratings in female subjects within the Alzheimer’s disease subgroup was strongly associated with both plaque [χ2(4) = 29.1, P < 0.0001] and tangle density [χ2(4) = 22.0, P < 0.0001], whereas for male subjects within this subgroup, there was an association with plaque [χ2(4) = 12.9, P = 0.0048] but not tangle density [χ2(4) = 5.8, P = 0.12].
Figure 3.
Plaque and tangle ratings by circle of Willis atherosclerosis ratings, stratified by sex, across the entire study population. F = female; M = male.
Discussion
Both Alzheimer’s disease and vascular disease are common age-related disease processes and so it is not surprising to find comorbidity. However, we find that cerebrovascular atherosclerosis is significantly more frequent and severe in those with Alzheimer’s disease compared with normal ageing and those with other neurodegenerative diseases. Our results confirm the reports by Roher et al. (2003) and Beach et al. (2007) of an association between circle of Willis atherosclerosis and Alzheimer’s disease, and we further find that this association is relatively specific to Alzheimer’s disease and not other major neurodegenerative diseases.
Frank cerebrovascular infarcts (multiple or strategic) and/or confluent ischaemic damage to deep white matter are the principal criteria by which vascular cognitive impairment is diagnosed. Large-scale clinicopathological studies of cognition in ageing have indicated that macroscopic infarcts and Alzheimer’s disease pathology both contribute to risk of dementia and severity of cognitive impairment, but do so independently (Schneider et al., 2004, 2007a, b). Therefore, one possible interpretation of our data showing an association between circle of Willis atherosclerosis and Alzheimer’s disease is that our Alzheimer’s disease study group was contaminated with subjects with vascular cognitive impairment who were inappropriately categorized as having Alzheimer’s disease. However, all disease category assignments were made on the basis of primary neuropathological rather than clinical diagnosis, and therefore case inclusion of dementia caused by macroscopic infarcts into the Alzheimer’s disease study group would not be expected. Additionally, the rate of macroscopic infarcts in the Alzheimer’s disease group was not dissimilar from the rate of infarcts in the normal group, and was also within the range observed for the other neurodegenerative diseases in our sample. In further support of a common neurobiological substrate for atherosclerosis and Alzheimer’s disease, our linear regression models found highly correlated circle of Willis atherosclerosis ratings specifically with neuritic plaque and paired helical filament-tau neurofibrillary lesion densities and cerebral amyloid angiopathy severity, even after adjusting for age and sex. This relationship was present in the subset of subjects with a primary neuropathological diagnosis of Alzheimer’s disease as well as the total study population. Therefore, cerebrovascular atherosclerosis appears to be intimately related with the defining pathological lesions of Alzheimer’s disease, independent of neuropathological diagnosis. We found no such associations between atherosclerosis ratings and α-synuclein or TDP-43 lesion densities. This further suggests that the association between circle of Willis atherosclerosis and Alzheimer’s disease is relatively specific and is not shared by other major neurodegenerative disease processes.
Our results contrast with three earlier studies in the USA, Finland and Japan that found no association between cerebral atherosclerosis and Alzheimer’s disease pathology (Kosunen et al., 1995; Itoh et al., 1999; Dolan et al., 2010). With 200 or fewer subjects in any of these series, it is possible that these studies were underpowered to show such an association. Alternatively, differences in the study populations or methods of neuropathological examination may also explain the differences in the study results. One important difference between our study population and that of Dolan et al. (2010) was the relatively small percentage of mixed Alzheimer’s disease and vascular pathology in our sample. Coexisting vascular pathology has been described to occur in 24–28% of cases with Alzheimer’s disease in other series (Langa et al., 2004), whereas we excluded subjects with major strokes, and incidental infarcts were observed in only 4.7% of subjects in our series. The relatively low rate of infarcts in our series may have resulted from the recruitment of subjects through our neurodegenerative disease programme, which systematically biased recruitment in favour of subjects without a clinical history of stroke.
There were significant sex differences in the association between circle of Willis atherosclerosis and Alzheimer’s disease pathology in our sample. While male and female subjects did not differ significantly with regard to frequency of atherosclerosis ratings, we observed that the association between age-adjusted circle of Willis atherosclerosis and Alzheimer’s disease lesion densities was stronger for female subjects than male subjects. A similar observation was made by Roher et al. (2003), and future research is needed to elucidate the basis of this sex difference. Although atherosclerosis was positively associated with Alzheimer’s disease pathology across the entire sample and within the Alzheimer’s disease group, this association was non-linear and the greatest mean tau and plaque density was in the moderate atherosclerosis group. One possible explanation of this trend, as well as the observed sex differences in the relationship between atherosclerosis and Alzheimer’s disease pathology, may relate to differences in prior mortality from cardiovascular disease. Because cardiovascular mortality is markedly higher in males versus females as well as individuals with severe atherosclerosis versus moderate atherosclerosis (Lerner and Kannel, 1986), fewer males or subjects with longstanding severe atherosclerosis may have lived long enough to develop Alzheimer’s disease pathology.
It is not clear from our results whether circle of Willis atherosclerosis promotes Alzheimer’s disease, whether Alzheimer’s disease promotes atherosclerosis or whether they are instead driven by some common pathophysiological mechanism(s). Both pathologies develop chronically, and age is an accompanying factor in both disease processes (Flora et al., 1968; Kukull and Ganguli, 2000). One mechanism by which cerebral atherosclerosis could directly contribute to Alzheimer’s disease pathology is through its effects on cerebral blood flow. A link between cerebral blood flow and Alzheimer’s disease pathology is supported by data in animal models showing that chronic cerebral hypoperfusion leads to learning impairment, neuronal loss and increased amyloid-β levels (Koike et al., 2010; Yamada et al., 2011). Alternatively, the finding that amyloid-β is present in advanced human atherosclerotic lesions suggests that Alzheimer’s disease pathology may directly promote atherosclerosis, potentially by altering the metabolism of oxidized lipoproteins (Moore et al., 2002; Kunjathoor et al., 2004).
If circle of Willis atherosclerosis and Alzheimer’s disease pathology are instead (or also) linked by one or more common pathophysiological mechanisms, hyperlipidaemia is one candidate for mediating this link. Non–high-density lipoprotein cholesterol is a strong risk factor for cerebral atherosclerosis (Reed et al., 1989), and a cholesterol-rich diet accentuates Alzheimer’s disease pathology in rabbit and mouse models of Alzheimer’s disease (Sparks et al., 1994; Refolo et al., 2000). Inflammation, abdominal obesity and insulin resistance may also contribute to both circle of Willis atherosclerosis and Alzheimer’s disease pathology (Schmidt et al., 2002; Gustafson et al., 2003; Razay et al., 2007). Additional research is needed to further evaluate the contribution of these risk factors to cerebral atherosclerosis and Alzheimer’s disease.
Importantly, the link between circle of Willis atherosclerosis and Alzheimer’s disease pathology is not fully explained by the APOE ε4 genotype in our sample. Although this polymorphism is a major risk factor for Alzheimer’s disease (Farrer et al., 1997), we found no association between APOE ε4 and circle of Willis atherosclerosis, which is consistent with previous studies (Kosunen et al., 1995; Traykov et al., 2002; Honig et al., 2005). Still, the lack of an association between APOE ε4 and circle of Willis atherosclerosis is surprising given that the APOE ε4 allele is a risk factor for atherosclerosis in multiple anatomical locations (Elosua et al., 2004).
Strengths of this investigation included a large sample size and the inclusion of multiple neurodegenerative disease syndromes to test disease specificity, as well as the use of multiple molecular pathological markers of Alzheimer’s disease and other neurodegenerative diseases. Although circle of Willis atherosclerosis ratings were adjusted for age and sex, one limitation of this study is that we did not assess for other possible covariates, including hypertension, hyperlipidaemia, diabetes and medication use such as statins or anti-hypertensives. Another limitation of our study is that circle of Willis atherosclerosis and pathological lesions were assessed by several investigators over several decades of brain collection. However, one co-author (J.Q.T.) reviewed the microscopic findings of every case in the sample from its inception, and any sampling error would most likely bias our results towards the null hypothesis.
Despite the recognition for >100 years that cerebrovascular pathology contributes to age-related dementia (Blass et al., 1991), there has been difficulty defining vascular cognitive impairment and its relationship to Alzheimer’s disease. Our results and similar results from other independent groups suggest that vascular cognitive impairment and Alzheimer’s disease are intimately related. Therefore, we suggest that a greater emphasis should be placed on understanding the common pathophysiological mechanisms underlying these syndromes rather than attempting to distinguish between them. From a therapeutic perspective, the convergence of vascular cognitive impairment and Alzheimer’s disease demonstrated in this study suggests that strategies proven to delay the progression of vascular pathology may be useful for preventing or treating Alzheimer’s disease.
Glossary
Abbreviation
- APOE
apolipoprotein E
References
- Addya K, Wang Y, Leonard D. Optimization of apolipoprotein E genotyping. Mol Diagn. 1997;2:271–6. doi: 10.1054/MODI00200271. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Gur RE, Shapiro RM, Fisher KR, Moberg PJ, Gibney MR, et al. Prospective clinicopathologic studies of schizophrenia: accrual and assessment of patients. Am J Psychiatry. 1995;152:731–7. doi: 10.1176/ajp.152.5.731. [DOI] [PubMed] [Google Scholar]
- Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, et al. Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005;76:1194–9. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass JP, Hoyer S, Nitsch R. A translation of Otto Binswanger’s article, “The delineation of the generalized progressive paralyses”. 1894. Arch Neurol. 1991;48:961–72. doi: 10.1001/archneur.1991.00530210089029. [DOI] [PubMed] [Google Scholar]
- Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68:231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosua R, Ordovas JM, Cupples LA, Fox CS, Polak JF, Wolf PA, et al. Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res. 2004;45:1868–75. doi: 10.1194/jlr.M400114-JLR200. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- Flora GC, Baker AB, Klassen A. Age and cerebral atherosclerosis. J Neurol Sci. 1968;6:357–72. doi: 10.1016/0022-510x(68)90102-0. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology (Cambridge, Mass.) 2008;19:448–50. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- Honig LS, Kukull W, Mayeux R. Atherosclerosis and Alzheimer’s disease: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Yamada M, Sodeyama N, Suematsu N, Matsushita M, Otomo E, et al. Atherosclerosis is not implicated in association of APOE epsilon4 with Alzheimer’s disease. Neurology. 1999;53:236–7. doi: 10.1212/wnl.53.1.236. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–33. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta} Am J Pathol. 2010;177:300–10. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosunen O, Talasniemi S, Lehtovirta M, Heinonen O, Helisalmi S, Mannermaa A, et al. Relation of coronary atherosclerosis and apolipoprotein E genotypes in Alzheimer patients. Stroke. 1995;26:743–8. doi: 10.1161/01.str.26.5.743. [DOI] [PubMed] [Google Scholar]
- Kukull WA, Ganguli M. Epidemiology of dementia: concepts and overview. Neurol Clin. 2000;18:923–50. doi: 10.1016/s0733-8619(05)70233-4. [DOI] [PubMed] [Google Scholar]
- Kunjathoor VV, Tseng AA, Medeiros LA, Khan T, Moore KJ. beta-Amyloid promotes accumulation of lipid peroxides by inhibiting CD36-mediated clearance of oxidized lipoproteins. J Neuroinflammation. 2004;1:23. doi: 10.1186/1742-2094-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901–8. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- Lee AY, Jeong SH, Choi BH, Sohn EH, Chui H. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Arch Gerontol Geriatr. 2006;42:157–66. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–90. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimeŕs disease: report of the NINCDS-ADRDA Work group under the auspices of Department of Health and Human Services Task Force on Alzheimeŕs diesease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Gearing M, McKeel DW, Crain BJ, Hughes JP, van Belle G, et al. Interlaboratory comparison of neuropathology assessments in Alzheimer’s disease: a study of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) J Neuropathol Exp Neurol. 1994;53:303–15. doi: 10.1097/00005072-199405000-00012. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, et al. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem. 2002;277:47373–9. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science (New York, N.Y.) 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64:93–6. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- Reed DM, Strong JP, Resch J, Hayashi T. Serum lipids and lipoproteins as predictors of atherosclerosis. An autopsy study. Arteriosclerosis. 1989;9:560–4. doi: 10.1161/01.atv.9.4.560. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–31. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–62. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Gur RE, Gur RC, Trojanowski JQ. Intraneuronal and extracellular neurofibrillary tangles exhibit mutually exclusive cytoskeletal antigens. Ann Neurol. 1988;23:184–9. doi: 10.1002/ana.410230212. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007a;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007b;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hunsaker JC, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11:601–7. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Hunsaker JC, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- Traykov L, Rigaud AS, Baudic S, Smagghe A, Boller F, Forette F. Apolipoprotein E epsilon 4 allele frequency in demented and cognitively impaired patients with and without cerebrovascular disease. J Neurol Sci. 2002;203–204:177–81. doi: 10.1016/s0022-510x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN) Stroke. 1996;27:30–6. doi: 10.1161/01.str.27.1.30. [DOI] [PubMed] [Google Scholar]
- Xie SX, Baek Y, Grossman M, Arnold SE, Karlawish J, Siderowf A, et al. Building an integrated neurodegenerative disease database at an academic health center. Alzheimers Dement. 2011;7:e84–93. doi: 10.1016/j.jalz.2010.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ihara M, Okamoto Y, Maki T, Washida K, Kitamura A, et al. The influence of chronic cerebral hypoperfusion on cognitive function and amyloid β metabolism in APP overexpressing mice. PloS One. 2011;6:e16567. doi: 10.1371/journal.pone.0016567. [DOI] [PMC free article] [PubMed] [Google Scholar]