Abstract
Hydrogen sulfide (H2S) is synthesized intracellularly by the enzymes cystathionine-γ-lyase and cystathionine-β-synthase (CBS), and is proposed to be a gasotransmitter with effects in modulating inflammation and cellular proliferation. We determined a role of H2S in airway smooth muscle (ASM) function. ASM were removed from resection or transplant donor lungs and were placed in culture. Proliferation of ASM was induced by FCS and the proinflammatory cytokine, IL-1β. Proliferation of ASM and IL-8 release were measured by bromodeoxyuridine incorporation and ELISA, respectively. Exposure of ASM to H2S “donors” inhibited this proliferation and IL-8 release. Methemoglobin, a scavenger of endogenous H2S, increased DNA synthesis induced by FCS and IL-1β. In addition, methemoglobin increased IL-8 release induced by FCS, but not by IL-1β, indicating a role for endogenous H2S in these systems. Inhibition of CBS, but not cystathionine-γ-lyase, reversed the inhibitory effect of H2S on proliferation and IL-8 release, indicating that this is dependent on CBS. CBS mRNA and protein expression were inhibited by H2S donors, and were increased by methemoglobin, indicating that CBS is the main enzyme responsible for endogenous H2S production. Finally, we found that exogenous H2S inhibited the phosphorylation of extracellular signal–regulated kinase–1/2 and p38, which could represent a mechanism by which H2S inhibited cellular proliferation and IL-8 release. In summary, H2S production provides a novel mechanism for regulation of ASM proliferation and IL-8 release. Therefore, regulation of H2S may represent a novel approach to controlling ASM proliferation and cytokine release that is found in patients with asthma.
Keywords: hydrogen sulfide, airway smooth muscle, cystathionine-γ-lyase, cystathionine-β-synthase, extracellular signal–regulated kinase–1/2
Hydrogen sulfide (H2S), first discovered in human tissues over 10 years ago, has emerged as an important gaseous mediator in cellular physiology and pathology, being involved in several processes, including chronic inflammation, learning and memory, and regulation of blood pressure (1). H2S is now considered as the third member of a family of gasotransmitters, together with nitric oxide (NO) and carbon monoxide (2). The bulk of endogenous H2S synthesis in mammalian tissues appears to be from the pyridoxal-5′-phosphate–dependent enzymes, cystathionine-γ-lyase (CSE; E.C. 4.4.1.1) and cystathionine-β-synthase (CBS; E.C. 4.2.1.22). CBS is found primarily in nervous tissue, whereas CSE is expressed in vascular and inflammatory cells. A third pathway via 3-mercaptopyruvate sulfurtransferase (E.C. 2.8.1.2) in human vascular endothelial cells has been proposed to generate H2S via enzymatic desulfuration of β-mercaptopyruvate derived from cysteine transamination (3, 4).
The potential role of H2S in airways disease is unknown. We therefore set out to determine its potential role in airway smooth muscle (ASM) cells, which are cells that not only determine the caliber of the airways, but also contribute to airway inflammation and remodeling (5–7). In asthma, there is an increase in ASM mass that could contribute to chronic airflow obstruction, chronic airway inflammation, and airway wall remodeling (8). ASM cells cultured from biopsies of patients with asthma have been shown to be hyperproliferative (9) and to release greater amounts of the chemokine, IL-8 (10). ASM proliferation is increased in response to growth factors, such as FCS, epidermal growth factor, platelet-derived growth factor, and insulin growth factor (11), and also to contractile agonists, such as histamine and leukotriene-D4 (12). In addition, ASM cells can express chemokines, such as regulated upon activation, normal T cell expressed and secreted and eotaxin (6), and, in vitro, have the capacity to release a number of cytokines and chemokines when exposed to other cytokines (13, 14). Due to the fact that there is evidence that H2S can indeed inhibit (and also promote) proliferation of vascular smooth muscle (15–17), we hypothesized that H2S may also mediate ASM proliferation. We examined the effect of both exogenous and intracellular sources of H2S in human ASM on proliferation induced by FCS and IL-1β. We used two extracellular H2S “donors,” the rapidly releasing H2S donor, sodium hydrosulfide (NaSH), and modeled endogenous H2S synthesis with a novel water-soluble, slow H2S-releasing molecule, GYY4137 (18). To examine the role of endogenously synthesized H2S, we used inhibitors of H2S synthesis; namely, DL-propargylglycine (PAG) to inhibit CSE and O-(carboxymethyl)-hydroxylamine hemihydrochloride (CHH) to inhibit CBS. Previously, PAG has been used at a concentration range of between 10 and 50 mg/kg in a rat model (19, 20). Similarly, Wallace and colleagues (21) previously used CHH at 3 mmol/L to inhibit CBS in a rat model of colitis. We observed that H2S could regulate ASM proliferation and the release of IL-8. Because ATP-sensitive potassium channel (K+ATP channel) activation contributes to some of the effects of H2S, such as vasodilatation (22), we determined whether these channels mediate these effects. Finally, we also investigated the role of mitogen-activated protein kinase (MAPK) activation in this process.
MATERIALS AND METHODS
ASM Cell Isolation and Culture
ASM cells were dissected from main or lobar bronchus removed from resection or transplant donor lungs and were cultured in Dulbecco’s modified Eagles medium supplemented with 4 mM L-glutamine, 20 U/L penicillin, 20 μg/ml streptomycin, and 2.5 μg/ml amphotericin B and 10% FCS. Cells between passages 3 and 6 were used for experiments. Before treatment, cells were incubated for 24 hours in serum-free medium containing phenol-free Dulbecco’s modified Eagles medium supplemented with 1 mM sodium pyruvate, 4 mM L-glutamine, 1:100 nonessential amino acids, 0.1% BSA and antibiotics, as described previously here.
Synthesis of GYY4137 and Exposure of ASM Cells to H2S Donors
GYY4137 was synthesized and characterized as previously described by us (18, 23, 24). Cells were plated in either 96- or 6-well plates, as described previously here, in the presence or absence of 2.5% FCS. Cells were treated with methemoglobin (10 μM) for 1 hour before treatment with H2S donor, NaSH, or GYY4137 (100 μM) with or without IL-1β (1 ng/ml) for a further 48 and 72 hours. Supernatants were removed and IL-8 levels determined by DuoSet ELISA (R&D Systems, Abingdon, UK). Cell proliferation was assessed by measuring the incorporation of bromodeoxyuridine using the Cell Proliferation ELISA bromodeoxyuridine kit (Roche Applied Science, West Sussex, UK) according to the manufacturer’s instructions. In addition, assessment of ASM proliferation was confirmed by cell counting using FACS analysis using a BD FACS Canto II cell sorter (Oxford, UK). Cellular viability was assessed by MTT assay (25). Cellular apoptotic markers were measured by an Apo-ONE Homogeneous Caspase-3/7 Assay (Promega, Southampton, UK). For the inhibitor studies, cells were treated with the indicated concentration of inhibitor for 30 minutes before treatment with NaSH (100 μM) for a further 48 and 72 hours.
Measurement of CBS and CSE mRNA Expression
CBS and CSE mRNA levels were determined by semiquantitative two-step RT-PCR using TaqMan Assay on Demand primer/probe sets obtained from Applied Biosystems (Warrington, UK), as previously described by us (26).
Western Blotting
Proteins were extracted at the indicated times from ASM cells that had been plated in six-well plates, as previously described (27). Samples were separated upon 10% SDS-PAGE gels (Invitrogen, Paisley, UK) and transferred to nitrocellulose (Amersham Ltd., Amersham, UK). Western blotting was performed using a mouse anti-CBS (A-2) antibody, a mouse anti-CSE (30.7) antibody, rabbit anti-p38 MAPK antibody and rabbit anti–phospho–p38 MAPK (Thr180/Tyr182) antibody (all from Santa Cruz Biotechnology, Middlesex, UK) and, rabbit anti–extracellular signal–regulated kinase(ERK)–1/2 (137F5) and rabbit anti–phospho–ERK-1/2 (Thr202/Tyr204; purchased from Cell Signalling Technology, Ely, Cambridgeshire, UK). All primary antibodies were used at a concentration of 1:1,000 or 1:2,000. Labeling of the first antibody was detected using relevant secondary antibodies conjugated to horseradish peroxidase (Dako Ltd., Ely, Cambridgeshire, UK) and detected using ECL reagents (Amersham Ltd.).
Statistical Analysis
Data are shown as means (±SEM) of six or more separate experiments. The effect of the H2S donors NaSH and GYY4137 on the proliferative effect of FCS was analyzed by Wilcoxon paired t test. Concentration-dependent responses were examined using one-way ANOVA (Kruskal-Wallis test), followed by a Dunn’s multiple comparison test. A P value of less than 0.05 was considered significant.
RESULTS
Effect of H2S on ASM Proliferation and IL-8 Release Induced by FCS and IL-1β
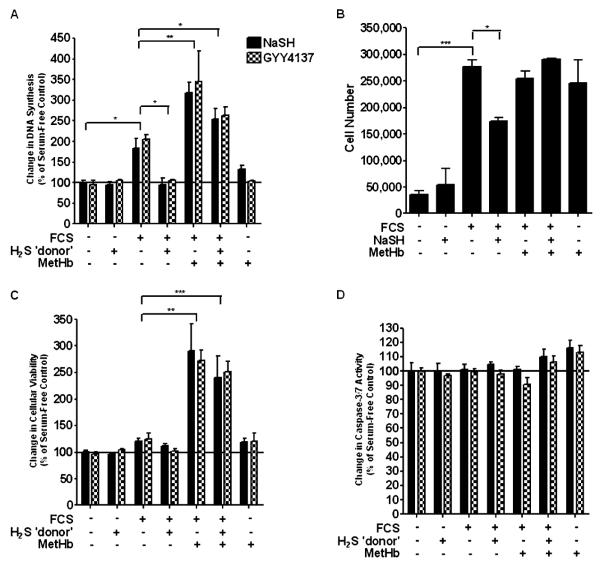
At 48 hours, ASM proliferation increased in the presence of 2.5% FCS (P < 0.05), an effect that was inhibited by both NaSH (100 μM) and GYY4137 (100 μM) (P < 0.05) (Figure 1A). Methemoglobin (10 μM), an extensively used H2S scavenger (28, 29), increased DNA synthesis by roughly 1.5-fold compared with that of FCS alone (P < 0.001). Methemoglobin (10 μM) added 1 hour before either of the H2S donors, NaSH (100 μM) or GYY4137 (100 μM), resulted in DNA synthesis that was approximately 50% greater than FCS alone (P < 0.01), but less than FCS and methemoglobin (Figure 1A). This increase in DNA synthesis was translated into cell number, as confirmed by FACS analysis (Figure 1B). These results were duplicated at 72 hours (data not shown). There was no effect on cell viability or cell apoptosis (Figures 1C and 1D).
Figure 1.
Effect of the hydrogen sulfide (H2S) donors, sodium hydrosulfide (NaSH) and GYY4137, on airway smooth muscle (ASM) proliferation induced by FCS. Both NaSH and GYY4137 inhibited cell proliferation induced by FCS. ASM cells were incubated with methemoglobin (10 μM) for 1 hour; NaSH (100 μM) or GYY4137 (100 μM) was added for another 48 hours. DNA synthesis (A), cell number (B), cell viability (C), and caspase-3/7 activity (D) were subsequently measured by bromodeoxyuridine (BrdU) ELISA, FACS analysis, dimethylthiazol-diphenyltetrazolium bromide (MTT) assay, and Apo-ONE homogeneous caspase-3/7 assay, respectively. Bars represent means (±SEM) of six ASM donors. *P < 0.05; **P < 0.01; ***P < 0.001. MetHb, methemoglobin.
Effect of Inhibiting CSE and CBS on ASM Proliferation Induced by FCS
To ascertain which enzymes are responsible for the endogenous production of H2S, ASM cells were pretreated with an inhibitor of CSE, PAG, or, the inhibitor of CBS, CHH, for 30 minutes before 2.5% FCS with or without NaSH (100 μM) was added for a further 48 and 72 hours. The PAG inhibitor (0.001–1.000 mM) failed to inhibit ASM proliferation induced by 2.5% FCS (Figure 2A). A nonsignificant decrease in ASM proliferation was observed at the highest concentrations of PAG used in the presence of NaSH (100 μM). Similar results were observed in the presence of NaSH (100 μM). CHH (0.03–1.00 mM), and subsequent stimulation with 2.5% FCS plus NaSH (100 μM), caused a significant increase in ASM proliferation induced by 2.5% FCS (P < 0.001 versus cells + 2.5% FCS) (Figure 2B). Upon treatment of the ASM cells with CHH inhibitor (0.1–1.0 μM) and subsequent stimulation with 2.5% FCS plus NaSH (100 μM), a significant increase in ASM proliferation was also observed (P < 0.01 versus cells + 2.5% FCS + 100 μM NaSH). Similar results were obtained at 72 hours (data not shown).
Figure 2.
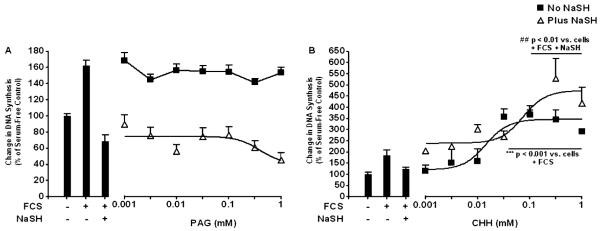
Effect of inhibiting cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) on ASM proliferation induced by FCS. ASM cells were incubated with the indicated concentration of an inhibitor of CSE (DL-propargylglycine [PAG]) (A) or an inhibitor of CBS (O-(carboxymethyl)-hydroxylamine hemihydrochloride [CHH]) (B) for 30 minutes; media with 2.5% FCS was added for a further 48 hours with NaSH (100 μM). DNA synthesis was subsequently measured by BrdU ELISA. Bars represent controls (cells ± FCS ± NaSH), and the curves represent the effect of increasing concentrations of inhibitor with or without NaSH. Data points represent means (±SEM) of six airway smooth muscle cell donors. ***P < 0.001 versus cells plus 2.5% FCS; ##P < 0.01 versus cells plus 2.5% FCS plus NaSH.
Effect of the H2S Donors, NaSH and GYY4137, on CBS and CSE
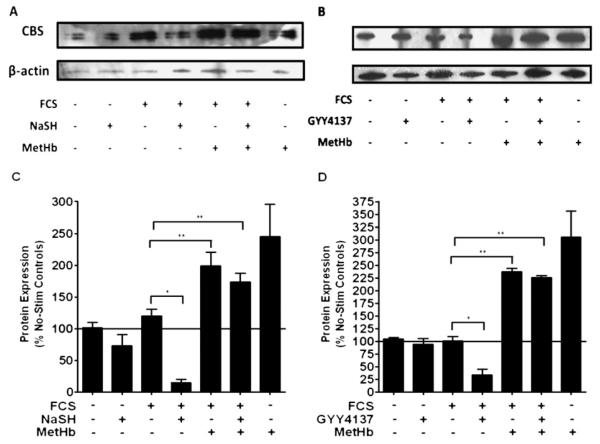
We next examined the effect of exogenous H2S upon CBS expression. NaSH or GYY4137 (100 μM) added to the ASM cells without FCS did not alter mRNA levels at 48 hours (Figure 3A). FCS alone did not increase mRNA levels, but when the ASM cells were subsequently treated with either NaSH or GYY41337 (100 μM), a reduction in mRNA levels was observed (P < 0.05). Methemoglobin (10 μM) induced an increase in CBS mRNA, which was attenuated by the addition of either NaSH or GYY4137 (P < 0.01) (Figure 3A). NaSH or GYY4137 (100 μM) in the absence of FCS did not alter protein expression at 48 hours (Figures 4A and 4B). FCS did not cause an increase in CBS protein, but either of the H2S donors (NaSH or GYY4137 [100 μM]) caused a reduction in CBS protein at 48 hours (P < 0.05). Methemoglobin (10 μM) induced a significant increase in CBS protein expression (P < 0.01), which was then attenuated by either NaSH or GYY4137 (P < 0.01) (Figures 4A and 4B). These results were duplicated at 72 hours (data not shown). Having already shown that inhibition of the CSE enzyme did not induce proliferation (Figure 2A), we examined the effect of exogenous H2S upon CBS expression. The H2S donor compounds, NaSH or GYY4137 (100 μM), had no effect on CSE mRNA or protein expression (Figure 4B), further supporting the notion that endogenous H2S production is solely dependent on CBS. It should be noted that human CBS is a target for sumoylation (a post-translational modification of proteins involving the covalent attachment of small ubiquitin-related modifier to proteins) (30), hence the presence of a protein doublet.
Figure 3.
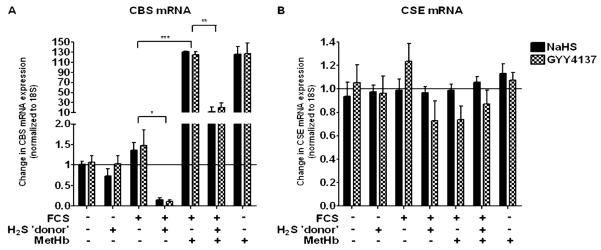
Effect of the H2S donors, NaSH and GYY4137, on CBS and CSE mRNA in human ASM cells. Exogenous H2S inhibited CBS mRNA expression. After the ASM cells were incubated with methemoglobin (10 μM) for 1 hour, NaSH (100 μM) or GYY4137 (100 μM) was added for another 48 hours. Change in CBS (A) or CSE (B) mRNA expression was subsequently measured by TaqMan RT-PCR. Bars represent means (±SEM) of six ASM donors. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 4.
Effect of the H2S donors, NaSH and GYY4137, on CBS protein expression in human ASM cells. Exogenous H2S inhibited CBS protein expression. ASM cells were incubated with methemoglobin (10 μM) for 1 hour; NaSH (A) or GYY4137 (B) (100 μM) was added for another 48 hours. CBS and β-actin were detected by Western blotting. (C and D) Changes in CBS protein expression were quantified by densitometry, normalized against β-actin expression, and then expressed as the percent change versus untreated controls. Bars represent means (±SEM) of six ASM donors. *P < 0.05; **P < 0.01.
Role of K+ATP Channels and Nitric Oxide in the Actions of H2S
Cells were pretreated with the K+ATP channel inhibitor, glibenclamide (0.001–1.000 mM) for 30 minutes before media with 2.5% FCS with or without NaSH (100 μM) was added for a further 48 hours. No increase in ASM proliferation was observed (Figure 5A). We next used Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME), an inhibitor of NO, as there may be interactions between NO and H2S. Pretreatment of the ASM cells with the NO inhibitor (0.001–1.000 mM) had no effect upon ASM proliferation either with or without the NaSH (Figure 5B). These results were duplicated at 72 hours (data not shown).
Figure 5.
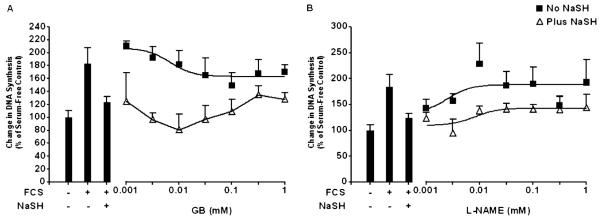
Effect of inhibiting K+ATP channels and nitric oxide (NO) synthesis on ASM proliferation induced by FCS. After the ASM cells were incubated with the indicated concentration of glybenclamide (GB) (A) or Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) (B) for 30 minutes, media with 2.5% FCS were added for a further 48 hours with or without NaSH (100 μM). DNA synthesis was subsequently measured by BrdU ELISA. Bars represent controls (cells ± FCS ± NaSH), and the curves represent the effect of increasing concentrations of inhibitor with or without NaSH. Data points represent means (±SEM) of six ASMC donors.
Effect of NaSH on Activation of ERK-1/2 and p38 MAPK
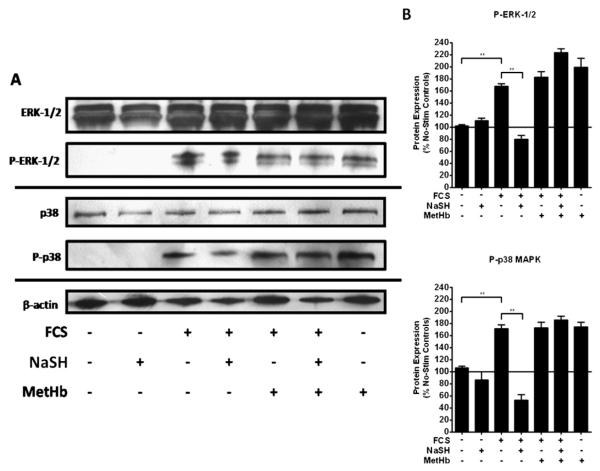
We next examined the role of NaSH upon both ERK-1/2 and p38 phosphorylation in the human ASM cells. NaSH (100 μM) alone did not induce phosphorylation of either ERK-1/2 or p38 in human ASM cells (Figures 6A and 6B). However, ASM cells grown in the presence of 2.5% FCS showed a significant increase in phosphorylation of both ERK-1/2 and p38 (P < 0.01), which was subsequently reduced by NaSH (P < 0.01) (Figures 6A and 6B). Pretreatment of the ASM cells with methemoglobin (10 μM) inhibited this reduction in phosphorylation. These results were duplicated at 72 hours (data not shown).
Figure 6.
Effect of the H2S donor, NaSH, on FCS-induced activation of extracellular signal–regulated kinase (ERK)–1/2 and p38 mitogen-activated protein kinase (MAPK) in human ASM cells. NaSH inhibited FCS-induced ERK-1/2 and p38 phosphorylation. ASM cells were incubated with methemoglobin (10 μM) for 1 hour; NaSH (100 μM) was added for another 48 hours. Total and phospho–ERK-1/2, total and phospho-p38 and β-actin were detected by Western blotting (A). (B) Changes in phospho-MAPK expression were quantitated by densitometry, normalized against β-actin expression, and then expressed as the percent change versus nonphosphorylated controls. Bars represent means (±SEM) of six ASM donors. **P < 0.01. P-ERK, phosphorylated-ERK; P-p38, phosphorylated-p38; Stim, stimulated.
Finally, we examined the role of the MAPKs, ERK-1/2 and p38, on FCS-induced proliferation and IL-8 release in human ASM cells. The ERK-1/2 inhibitor, PD98059 (5 μM), inhibited FCS-induced proliferation (P < 0.05) and IL-8 release (P < 0.01) (Figures 7A and 7C). With the p38 MAPK inhibitor, SB202190 (5 μM), a similar, but nonsignificant, trend was observed. When both the ERK-1/2 and p38 inhibitors were added together, cellular proliferation and IL-8 were decreased to a greater degree (P < 0.01 and P < 0.001, respectively). Furthermore, when the ASM cells were treated with NaSH (100 μM), after addition of both inhibitors, a further decrease in IL-8 release was observed (P < 0.001) (Figure 7C). These results were duplicated at 72 hours (data not shown).
Figure 7.
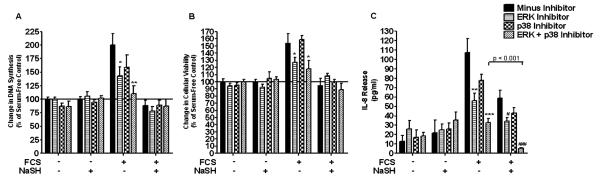
Effect of mitogen-activated protein kinase kinase 1/2 (MEK-1/2) and p38 MAPK inhibition upon FCS-induced proliferation and IL-8 release in human ASM cells. Inhibition of FCS-induced ERK-1/2 and p38 phosphorylation results in a decrease in proliferation and IL-8 release (A and C). ASM cells were incubated with an MEK-1/2 (5 μM) inhibitor, a p38 (5 μM) inhibitor, or both for 30 minutes. The ASM cells were then further incubated with NaSH (100 μM) for another 48 hours. DNA synthesis was subsequently measured by BrdU ELISA (A and B). Bars represent means (±SEM) of six ASMC donors. *P < 0.05 versus cells plus 2.5% FCS; **P < 0.01 versus cells plus 2.5% FCS; ***P < 0.001 versus cells plus 2.5% FCS; #P < 0.05 versus cells plus 2.5% FCS plus NaSH; ###P < 0.001 versus cells plus 2.5% FCS plus NaSH.
DISCUSSION
Both endogenous and exogenous H2S inhibited human ASM cell proliferation and IL-8 release induced by FCS. Furthermore, we have shown that endogenous H2S is produced solely by the enzyme, CBS, and not by CSE. This inhibitory action of H2S was not mediated through the activation of ATP-sensitive potassium channels or controlled by any “cross-talk” with NO. We found that H2S inhibited the phosphorylation of the MAPKs, ERK-1/2 and p38, which could be a mechanism by which H2S inhibited cellular proliferation and IL-8 release (7, 31, 32).
ASM proliferation is increased in response to FCS (11) and the inflammatory mediator, IL-1β (33). Previous studies have examined the role of H2S upon cell proliferation, and have concluded that this gas can induce proliferation (34) or, conversely, inhibit it (15, 28), depending upon the cell type examined. Our data are consistent with previous studies showing the inhibitory effect of H2S in other smooth muscle cells, such as vascular smooth muscle cells (17). In ASM cells, H2S did not promote apoptosis as it does in aortic vascular smooth muscle cells (35). We used both the fast-release H2S donor, NaSH, and the slow-release donor, GYY4137, and found that both donor compounds caused similar inhibitory effects. Whiteman and colleagues (23) have recently shown that NaSH had an inhibitory action upon cytokine/chemokine release that peaked at 200 μM, whereas GYY4137 was an effective inhibitor of the inflammatory mediators, IL-1β, IL-6, and TNF-α, from LPS-stimulated macrophages, perhaps at least in part explained by the contrasting rates of H2S delivery by these two different compounds. We very recently showed that H2S release from NaSH is instantaneous, and generates a bolus of concentrated H2S, whereas GYY4137 releases H2S in a slow and sustained manner as per CSE and CBS. It is unlikely that cells or tissues will be exposed to H2S as a concentrated bolus, but it is important to compare and contrast all aspects of H2S physiology/pathology. The bolus approach may be important in the lung after inhalation of H2S (23). Our data show that both fast and slow release of H2S inhibited cellular proliferation and IL-8 release, indicating that the rate of release does not determine the inhibitory effect of H2S in ASM.
Several inhibitors of endogenous H2S are available. For example, PAG is an irreversible inhibitor of CSE, which has previously been shown to be effective in a rat model of colitis at concentrations of 10–50 mg/kg (19, 20). Similarly, Wallace and colleagues (21) have previously used CHH at 3 mmol/L to inhibit CBS in a rat model of colitis. Using these inhibitors, we found that endogenous H2S production is likely to be through the enzyme, CBS, and not CSE. We also found that, although both enzymes were expressed in ASM cells, CBS, and not CSE, mRNA and, to a lesser degree, CBS protein, is increased in expression by FCS, suggesting that this growth factor may induce the cell to produce more H2S. Only CBS was modulated by the addition of methemoglobin in the presence of FCS and the H2S donors. Exogenous donors of H2S inhibited CBS, but not CSE, expression, perhaps as a negative-feedback inhibitory mechanism. Currently, CBS appears to be involved in the generation of endogenous H2S in neural pathways and in the brain (36–39). On the other hand, CSE appears to be the enzyme predominantly involved in endogenous H2S production in rodent smooth muscle and the lung (3, 17, 35, 40, 41). The work presented here is the first of its kind in human ASM cells. Interestingly, Chasse and colleagues (42) have previously demonstrated that the CBS gene isolated from human tissue differs from that of rat, which could explain the disparate finding between species. In addition, a very recent study has demonstrated a possible correlation between two polymorphisms of CBS and lung cancer, the epitome of cell proliferation (43). Therefore, species differences should be taken into consideration when investigating the production of endogenous H2S.
To date, little is known regarding the effect of methemoglobin both in vivo and in vitro. We have shown that, on ASM cells, methemoglobin not only induces proliferation, but also causes IL-8 release. Interestingly, a dose- (1–50 μM)- and time (2–16 h) -dependent increase in IL-6 and IL-8 release from venular endothelial cells has been demonstrated (44), as well as an increase in cellular proliferation by as much as 200% in a human hepatocellular carcinoma cell line (45), and up to 600% in epithelial cells at concentrations comparable to ours (46).
We investigated the downstream effectors of H2S that could inhibit both cellular proliferation and IL-8 release. Several actions of H2S, including vasodilatation, inhibition of leukocyte adherence, and visceral analgesia, have been shown to be mediated via activation of K+ATP channels (47–49). However, glibenclamide, an inhibitor of ATP-sensitive potassium channels, had no effect on the inhibitory effects of H2S in our studies. To examine the possibility of cross-talk between H2S and NO, a nonselective NO synthesis inhibitor (which is an analog of arginine) that inhibits NO production, l-NAME, was used (41, 50, 51). l-NAME had no effect upon the inhibitory effects of H2S.
High concentrations of H2S have been shown to increase phosphorylation of the MAPKs, ERK-1/2 and p38 (52, 53), whereas Hu and colleagues (54) showed that p38 phosphorylation was prevented in an in vitro model of Parkinson’s disease. Hence, we examined the degree of phosphorylation of these kinases in ASM cells. We noted that FCS induced both ERK-1/2 and p38 phosphorylation, which was reduced by NaSH at the relatively low concentration of 100 μM. Induction and cessation of the phosphorylation of ERK-1/2 and p38 appear to be mediated in a concentration-dependent manner by H2S. Inhibiting these kinases not only stopped the ASM proliferation, but when they were used before treatment with NaSH, a further decrease in IL-8 release was observed, further supporting the possibility that the mechanism of H2S, at least in part, is via the inhibition of these kinases.
In conclusion, we have shown, for the first time, that H2S inhibits both human ASM proliferation and IL-8 release induced by FCS and IL-1β. It is likely that exogenous H2S targets the production of endogenous H2S by inhibiting the transcription and subsequent translation of the CBS enzyme, and proliferation of the cell is controlled by H2S through a negative-feedback pathway. H2S inhibits the activity of the MAPKs, ERK-1/2 and p38, which may result in decreased human ASM proliferation and IL-8 release. H2S may provide a novel therapeutic avenue in the stabilization of ASM proliferation, which is increased in asthma.
Supplementary Material
CLINICAL RELEVANCE.
Hydrogen sulfide (H2S) production provides a novel mechanism for regulation of airway smooth muscle (ASM) proliferation and IL-8 release. Therefore, regulation of H2S may represent a novel approach to controlling ASM proliferation and cytokine release that is found in patients with asthma.
Acknowledgments
This work was supported by Asthma UK and Wellcome Trust grants (K.F.C.).
I.A. serves on the advisory board of GlaxoSmithKline and Chiesi, and has received sponsored grants from Glaxo-SmithKline, AstraZeneca, Pfizer, and Novartis, and has also received lecture fees from GlaxoSmithKline, AstraZeneca, Nycomed, Daiichi-Sankyo, Pfizer, and Novartis.
Footnotes
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author Disclosure: K.F.C. serves on the advisory board of GlaxoSmith Kline, Gilead, and Boehringer Ingelheim and received lecture fees from Glaxo-SmithKline, Novartis, and AstraZeneca.
None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 4.Whiteman M, Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung KF. Should treatments for asthma be aimed at the airway smooth muscle? Expert Rev Respir Med. 2007;1:209–217. doi: 10.1586/17476348.1.2.209. [DOI] [PubMed] [Google Scholar]
- 6.Oltmanns U, Chung KF, Walters M, John M, Mitchell JA. Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Respir Res. 2005;6:74. doi: 10.1186/1465-9921-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L245–L253. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 10.Fong CY, Pang L, Holland E, Knox AJ. TGF-beta1 stimulates IL-8 release, COX-2 expression, and PGE(2) release in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L201–L207. doi: 10.1152/ajplung.2000.279.1.L201. [DOI] [PubMed] [Google Scholar]
- 11.Shiels IA, Bowler SD, Taylor SM. Airway smooth muscle proliferation in asthma: the potential of vascular leakage to contribute to pathogenesis. Med Hypotheses. 1995;45:37–40. doi: 10.1016/0306-9877(95)90198-1. [DOI] [PubMed] [Google Scholar]
- 12.Ammit AJ, Panettieri RA., Jr. Invited review: the circle of life: cell cycle regulation in airway smooth muscle. J Appl Physiol. 2001;91:1431–1437. doi: 10.1152/jappl.2001.91.3.1431. [DOI] [PubMed] [Google Scholar]
- 13.Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DW, Torphy TJ, Penn RB, Panettieri RA., Jr. Tumor necrosis factor–alpha–induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells: modulation by cyclic adenosine monophosphate. Am J Respir Cell Mol Biol. 2000;23:794–802. doi: 10.1165/ajrcmb.23.6.4184. [DOI] [PubMed] [Google Scholar]
- 14.Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6–type cytokines. Am J Physiol. 1997;273:L648–L655. doi: 10.1152/ajplung.1997.273.3.L648. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, Tang C. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels. 2004;19:75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- 16.Baskar R, Sparatore A, Del SP, Moore PK. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur J Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Wu L, Bryan S, Khaper N, Mani S, Wang R. Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc Res. 2010;86:487–495. doi: 10.1093/cvr/cvp420. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 19.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di SM, Morelli A, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 20.Mok YY, Atan MS, Yoke PC, Zhong JW, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 23.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide–releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA. Lung delivery studies using siRNA conjugated to TAT(48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug Chem. 2007;18:1450–1459. doi: 10.1021/bc070077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1{beta}-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180:5689–5698. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, Cao K, Wu L, Wang R. Cystathionine gamma-lyase over-expression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J Biol Chem. 2004;279:49199–49205. doi: 10.1074/jbc.M408997200. [DOI] [PubMed] [Google Scholar]
- 29.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 30.Kabil O, Zhou Y, Banerjee R. Human cystathionine beta-synthase is a target for sumoylation. Biochemistry. 2006;45:13528–13536. doi: 10.1021/bi0615644. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Liang Q, Balzar S, Wenzel S, Gorska M, Alam R. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J Allergy Clin Immunol. 2008;121:893–902. doi: 10.1016/j.jaci.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Nath P, Leung SY, Williams A, Noble A, Chakravarty SD, Luedtke GR, Medicherla S, Higgins LS, Protter A, Chung KF. Importance of p38 mitogen-activated protein kinase pathway in allergic airway remodelling and bronchial hyperresponsiveness. Eur J Pharmacol. 2006;544:160–167. doi: 10.1016/j.ejphar.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor–beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol. 2006;34:247–254. doi: 10.1165/rcmb.2005-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christl SU, Eisner HD, Dusel G, Kasper H, Scheppach W. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa: a potential role for these agents in the pathogenesis of ulcerative colitis. Dig Dis Sci. 1996;41:2477–2481. doi: 10.1007/BF02100146. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Wu L, Wang R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006;20:553–555. doi: 10.1096/fj.05-4712fje. [DOI] [PubMed] [Google Scholar]
- 36.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Jhee KH, Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 38.Eto K, Kimura H. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. J Biol Chem. 2002;277:42680–42685. doi: 10.1074/jbc.M205835200. [DOI] [PubMed] [Google Scholar]
- 39.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 40.Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–123. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 42.Chasse JF, Paly E, Paris D, Paul V, Sinet PM, Kamoun P, London J. Genomic organization of the human cystathionine beta-synthase gene: evidence for various cDNAs. Biochem Biophys Res Commun. 1995;211:826–832. doi: 10.1006/bbrc.1995.1886. [DOI] [PubMed] [Google Scholar]
- 43.Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, Basu A, Porter KE, Hubbard A, Bates MN, et al. Individual differences in arsenic metabolism and lung cancer in a case–control study in Cordoba, Argentina. Toxicol Appl Pharmacol. 2010;247:138–145. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Spolarics Z. Methemoglobin is a potent activator of endothelial cells by stimulating IL-6 and IL-8 production and E-selectin membrane expression. Am J Physiol Cell Physiol. 2003;285:C1036–C1046. doi: 10.1152/ajpcell.00164.2003. [DOI] [PubMed] [Google Scholar]
- 45.Wen WN. Methemoglobin contributes to the growth of human tumor cells. Life Sci. 2002;70:907–916. doi: 10.1016/s0024-3205(01)01465-5. [DOI] [PubMed] [Google Scholar]
- 46.Wen W-N. Methaemoglobin enhances the proliferation of transformed human epithelial cells: a possible outcome of neovascularisation and haemorrhage in tumours? Eur J Cancer. 2001;37:1921–1929. doi: 10.1016/s0959-8049(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 47.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 48.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 49.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide–induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180–186. doi: 10.1016/j.ejphar.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 52.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stuhlmeier KM, Broll J, Iliev B. NF-kappaB independent activation of a series of proinflammatory genes by hydrogen sulfide. Exp Biol Med (Maywood) 2009;234:1327–1338. doi: 10.3181/0904-RM-137. [DOI] [PubMed] [Google Scholar]
- 54.Hu LF, Lu M, Wu ZY, Wong PT, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.