Abstract
Aureusimines have been identified as potential virulence factors in Staphylococcus aureus. These pyrazinone secondary metabolites are produced by a nonribosomal peptide synthetase (NRPS) annotated as AusA. We report the overproduction of AusA as a 277 kDa soluble protein with the bimodular architecture A1–T1–C–A2–T2–R. The substrate specificity of each adenylation (A) domain was initially probed using an ATP–pyrophosphate exchange assay with A-domain selective bisubstrate inhibitors to chemically knock-out each companion A-domain. The activity of AusA was then reconstituted in vitro and shown to produce all naturally occurring aureusimines and non-natural pyrazinone products with kcat values ranging from 0.4 to 1.3 min−1. Steady-state kinetic parameters were determined for all substrates and cofactors providing the first comprehensive steady-state characterization of a NRPS employing a product formation assay. The KM values for the amino acids were up to 60-fold lower with the product formation assay versus the ATP–pyrophosphate exchange assay, most commonly used to assess A-domain substrate specificity. The C-terminal reductase (R) domain catalyzes reductive release of the dipeptidyl intermediate leading to formation of an amino aldehyde that cyclizes to a dihydropyrazinone. We show oxidation to the final pyrazinone heterocycle is spontaneous. The activity and specificity of the R-domain was independently investigated using a NADPH consumption assay. AusA is a minimal autonomous two-module NRPS that represents an excellent model system for further kinetic and structural characterization.
The aureusimines are cyclic dipeptide pyrazinone natural products produced by Staphylococcus aureus, a significant human pathogen1 that were independently discovered by the Magarvey and Fischbach research groups.2, 3 Genomic mining of all sequenced S. aureus strains (>50) revealed a conserved gene cluster encoding for two genes (ausA and ausB), whose linkage to aureusimine biosynthesis was confirmed through genetic inactivation by allelic replacement. Aureusimines appear to play a role in virulence as the expression of a wide variety of genes are modulated in a ΔausA mutant including γ-hemolysin, superantigen-like genes, and several respiratory metabolic genes.4 However, the initially reported gene expression profile of the ΔausA mutant2 was largely caused by an inadvertent secondary mutation in saeS, part of a two-component regulator in S. aureus that controls virulence factor expression.5 Although the biological role of the aureusimines remains unknown, they are expected to have an important function given the conservation of their gene cluster among S. aureus isolates coupled with the recent findings that they are overproduced in S. aureus biofilms.6
Aureusimine dipeptides are biosynthesized through a nonribosomal peptide synthetase (NRPS) pathway (Figure 1). NRPSs are multifunctional proteins that synthesize peptide natural products independent of the mRNA ribosomal machinery and employ a modular architecture wherein each module is responsible for the incorporation of one amino acid into the final peptide product.7, 8 The canonical module contains three core domains: an adenylation domain (A-domain) responsible for the selection and activation of an amino acid,9 a thiolation domain (T-domain) onto which the amino acid is covalently tethered as a thioester to the terminal thiol of 4′-phosphopantetheine prosthetic group,10 and a condensation domain (C-domain), which catalyzes peptide bond formation in the N to C direction between the upstream and downstream aminoacyl thioesters. Release of the nascent peptide from the NRPS assembly line is often accompanied by cyclization through the activity of a thioesterase (TE) domain or more rarely by a reductase (R) domain.11 Aureusimines synthesis requires two proteins: AusA encodes for a two module six domain NRPS, whose domain architecture is A1–T1–C–A2–T2–R while AusB encodes for a phosphopantetheinyl transferase responsible for posttranslational modification of apo-AusA through attachment of 4′-phosphopantetheine onto a conserved serine residue of both T-domains. Bioinformatic analysis suggests the first adenylation domain (A1) specifies for valine while the second adenylation domain (A2) is predicted to confer specificity for tyrosine.2 Thus, A1 activates and loads T1 with L-Valine to afford L-Val-S~T1 and A2 activates and loads T2 with L-Tyrosine to afford L-Tyr-S~T2. The condensation domain (C2) then catalyzes peptide bond formation to afford the dipeptide L-Val-L-Tyr~ T2, which is reduced by the R-domain to afford an intermediate amino aldehyde that cyclizes to an imine. Oxidation to the pyrazinone is proposed to occur spontaneously driven by the greater stability associated with aromatization or alternatively catalyzed by an oxidoreductase.3 The isolation of multiple pyrazinone products containing L-Tyr, L-Phe, and L-Leu suggests A2 has relaxed substrate specificity.
Figure 1.
A) biosynthetic pathway of the aureusimines. B) aureusimines and related pyrazinones described in this work.
Unlike most NRPS pathways that require multiple NRPS proteins for assembly of their respective natural product, AusA is predicted to autonomously synthesize the aureusimines. Therefore, AusA represents an excellent model system for structural and kinetic studies as well as to investigate NRPS protein engineering since it is a minimal canonical NRPS. Although, several natural two module NRPSs have been reported including LtxA involved in biosynthesis of the lyngbyatoxin NRPs,12 AnaPS from the acetylaszonalenin indole alkaloid pathway,13 and FtmA that catalyzes diketopiperazine formation of the fumitremorgins14 none have been biochemically characterized.
Herein we describe the expression and functional characterization of AusA, an approximately 280-kDa protein. The substrate specificity of each A-domain is determined with a large panel of natural and non-natural amino acids employing A-domain selective inhibitors to knockout the activity of the other A-domain. The activity of AusA is successfully reconstituted in vitro and shown to produce the naturally occurring aureusimines as well as unnatural pyrazinones. A product formation assay is developed and used to evaluate the steady-state kinetic parameters for all substrates and cofactors. Finally, we demonstrate oxidation of the initially formed imine intermediate to the pyrazinone is spontaneous and also examine the specificity of release by the R-domain. During review of this manuscript, Magarvey reported the expression and in vitro reconstitution of AusA as well as the structural characterization of the R-domain.15
MATERIALS AND METHODS
Materials
The following materials were purchased from the indicated supplier: chemically competent E. coli Mach1 and BL21 STAR (DE3) cells (Invitrogen, Carlsbad, CA, USA), In-Fusion cloning kit (Clonetech, Mountain View, CA, USA), restriction enzymes (New England Biolabs, Ipswich, MA, USA), PrimeSTAR HS DNA polymerase (TAKARA Bio Inc, Otsu, Shiga, Japan), primers for PCR (Integrated DNA Technologies, Coralville, IA, USA), Ni-NTA, and DNA purification/isolation kits (Qiagen Sciences, Germantown, MD, USA), ion exchange and gel filtration columns (GE healthcare, Waukesha WI, USA), NADPH and ATP as well as all biological buffers and components (Sigma-Aldrich, St. Louis, MO, USA), amino acids (Chem-Impex, Wood Dale IL, USA), radioactive [32P]pyrophosphate (PerkinElmer, Waltham MA, USA).
Cloning and Expression of AusA
The gene ausA was amplified by PCR from Staphylococcus aureus ATCC 43300 in three fragments using the primer pairs ausA F1, ausA R1; ausA F2, ausA R2; and ausA F3, ausA R3 (Table 1). The three fragments were ligated into NdeI and HindIII digested pET28b-TEV, an expression vector with the thrombin cleavage site replaced with a TEV cleavage site, using the In- Fusion PCR cloning kit (Clontech) following manufacturer’s instructions. The final construct, pCDD141, expresses AusA with an N-terminal hexahistidine tag (276.6 kDa). Sequencing revealed the expression plasmid to be error free. Similarly the A1–T1 didomain was amplified using primers ausA F1 and ausA 1st AT R (aa 1–999) and the A2–T2–R tridomain (aa 1418–2397) was amplified using ausA 2nd AT F and ausA R3. Both PCR products were cloned into pET28b-TEV as above yielding pCDD142 and pCDD143, respectively.
Table 1.
Oliognucleotide Primers
| Primer name | primer direction | primer sequence | domains |
|---|---|---|---|
| ausA F1 | 5′-forward | ATTTTCAGGGCCATATGAAAGAAGGACTTTTTATG | his-A1-T1-C-A2-T2-R |
| ausA R1 | 3′-reverse | TTCTCCAGGAATACCAACGC | his-A1-T1-C-A2-T2-R |
| ausA F2 | 5′-forward | GCGTTGGTATTCCTGGAGAA | his-A1-T1-C-A2-T2-R |
| ausA R2 | 3′-reverse | TGCGAGACAACTAACATCGC | his-A1-T1-C-A2-T2-R |
| ausA F3 | 5′-forward | GCGATGTTAGTTGTCTCGCA | his-A1-T1-C-A2-T2-R |
| ausA R3 | 3′-reverse | GTGCGGCCGCAAGCTTACTTATTGAATATTGTTTTGATATATTGTG | his-A1-T1-C-A2-T2-R |
| ausA 1st AT R | 3′-reverse | GTGCGGCCGCAAGCTTAAACTATAGTTTCTGGAATCACTTC | his-A1-T1 |
| ausA 2nd AT F | 5′-forward | ATTTTCAGGGCCATATGCCAAACGGCACGGAGGAAC | his-A2-T2-R |
For expression and purification of the apo-AusA pCDD141 was transformed into E. coli BL21(DE3). Overnight cultures (1%) were used to inoculate 1 L of TB media supplemented with 50 μg/mL kanamycin. Cultures were allowed to grow to an A600 of 0.6 at 37 °C and then were induced with 0.4 mM IPTG and allowed to grow 16 h at 18 °C. Cells were pelleted by centrifugation at 5,000 × g for 10 min and resuspended in lysis buffer (50 mM HEPES, 300 mM NaCl, 10 mM imidazole, pH 8.0). Cells were lysed on a Branson Sonifier 250 using five 2 min bursts, power level 8, 30% duty cycle. The lysate was centrifuged at 45,000 × g for 10 min at 4 °C. Cleared lysate was incubated with 0.5 mL Ni-NTA (Qiagen) for 1 h at 4 °C and then loaded onto a gravity column. The Ni-NTA column was washed with 16 mL wash buffer (50 mM HEPES, 300 mM NaCl, 20 mM imidazole, pH 8.0) followed by elution with 3 mL of elution buffer (50 mM HEPES, 300 mM NaCl, 250 mM imidazole, pH 8.0). The eluent was diluted to 4.5 mL with water and then further purified using a MonoQ 5/50 GL strong anion exchange column (GE Life Sciences) running a linear gradient from 0–1 M NaCl in 20 mM Tris pH 8.0 over 40 column volumes at 1.5 mL/min. The fractions containing AusA were pooled and run on a HiPrep 16/60 Sephacryl S-200 gel filtration column (GE Life Sciences) running 10 mM Tris pH 8.0, 200 mM NaCl isocratically. Purified AusA was pooled and glycerol was added to 5% and the protein was stored at −80 °C. The A1–T1 didomain and A2–T2–R tridomain were expressed following an identical protocol to afford 16.7 and 20.0 mg/L, respectively. Holo–AusA was expressed and purified as above except pCDD141 was transformed into E. coli BL21(DE3) containing pSFP a plasmid that constitutively expresses the promiscuous phosphopantetheinyl transferase from B. subtilis. The yield for both apo– and holo–AusA was 0.5 mg/L. All media used was supplemented with 25 μg/mL chloramphenicol to maintain pSFP.
ATP–PPi Exchange Assay for AusA A-domain Substrate Specificity
All reactions were carried out in duplicate under initial velocity conditions in a final volume of 100 μL. The counts from the bound [32P]–ATP were converted into product concentration using a standard curve of [32P]–pyrophosphate. To determine the substrate specificity of each adenylation domain in AusA, 30 mM of the following L-amino acids (Ala, Arg, Asp, Cys, Gln, Glu, Gly, His, Ile, Leu, Cle, Tle, Lys, Met, Phe, 4-Aph, β-Aph, Pro, Trp, Val) or 10 mM L-Tyr were individually added in a reaction mixture containing 36 nM apo–AusA and 3 mM ATP in ATP–PPi assay buffer (375 mM Bicine pH 8.0, 10 mM MgCl2, 2 mM DTT, 1 mM pyrophosphate, and 0.25 μCi [32P]pyrophosphate). The reaction mixtures were incubated at 37 °C for 20 min and then quenched with 200 μL quenching buffer (350 mM perchloric acid, 100 mM PPi, 1.6 % activated charcoal). Excess [32P]pyrophosphate was removed after centrifugation by washing the charcoal with 500 μL water. The washed pellet was transferred with 200 μL of water to a scintillation vial and 5 mL scintillation fluid (RPI) was added. Radioactive counts were quantitated on a Packard Tri Carb 2900 TR. Substrates that showed activity were rerun in the presence of 100 μM Phe-AMS or Val-AMS.
Determination of the kinetic constants for the A1 domain for L-Val and L-Ile were carried out as above using 18 and 36 nM apo–AusA, respectively. The substrate acids were varied from 41.2 μM to 30 mM using a three-fold dilution series at a fixed concentration of ATP (3 mM). The substrates for the A2 domain were similarly tested using the indicated concentration ranges L-4-Aph (41.2 μM–30 mM), L-Tyr (41.2 μM–10 mM), L-Leu (123.4 μM–30 mM), and L-Phe (41.2 μM–30 mM) at a fixed concentration of ATP (3 mM) using 36 nM apo–AusA for L-Tyr and 72 nM apo–AusA for the remaining substrates. The kinetic parameters for ATP were evaluated at a fixed saturating concentration of L-Phe (30 mM) with 36 nM apo–AusA varying ATP from 13.7 μM to 10 mM. Individual substrate saturation kinetic data were fit by nonlinear regression analysis to either the Michaelis-Menten equation (eq 1) or substrate inhibition model (eq 2) using GraphPad Prism 5.0.
| (eq 1) |
| (eq 2) |
Determination of the Inhibition Constants of Val-AMS and Phe-AMS
Inhibitors against the two AusA adenylation domains were analyzed using the ATP–PPi exchange assay under initial velocity conditions to determine the apparent inhibition constants (appKi). Evaluation of Val-AMS was performed with 10.5 nM apo–AusA, 3 mM L-Val, and 3 mM ATP in ATP–PPi assay buffer and varying Val–AMS from 6.25 to 400 nM using a 2-fold dilution series. Evaluation of Phe-AMS was performed with 21 nM apo–AusA, 1 mM L-Tyr, 3 mM ATP in ATP–PPi assay buffer, and varying Phe-AMS from 3.1 to 200 nM using a 2-fold dilution series. Inhibition studies with the A1-T1 didomain employed 36 nM enzyme, 1 mM L-Val, and 3 mM ATP in ATP–PPi assay buffer. Inhibition studies with the A2-T2-R tridomain employed 360 nM enzyme, 1 mM L-Tyr, and 3 mM ATP in ATP–PPi assay buffer. Fractional initial velocities were fit by nonlinear regression analysis to the Morrison equation (eq 3) using GraphPad Prism 5.0:
| (eq 3) |
where I represents the concentration of inhibitor, is the apparent inhibition constant, E is the active enzyme concentration (determined by active-site titration), vi is the initial rate at each [I], and v0 is the initial rate of the DMSO control.
Reconstitution of AusA Activity and Spontaneous Oxidation to Provide of Aureusimine-B (2)
Reconstitution of AusA activity was initially performed in a 600 μL reaction with 40 nM holo–AusA, 25 mM L-Val, 25 mM L-Phe, 3 mM ATP, and 0.1 mM NADPH in reaction buffer (375 mM bicine pH 8.0, 3 mM MgCl2, 1 mM TCEP). The reaction was incubated at 37 °C for 3 h. The enzyme was removed by passing the mixture through an Amicon Ultra 10,000 MWCO filter. The filtrate was collected and incubated at 100 °C for 0–30 min and then analyzed by HPLC. The separation of 2 was performed on an Agilent 1100 liquid chromatograph system using a Zorbex Eclipse XDB-C8 150 × 3.0 mm, 3.5 μm column. Aureusimine-B (2) eluted at 6.35 min using an isocratic method with 35% acetonitrile and 65% water containing 0.05% formic acid at a flow rate of 0.5 mL/min. The product was detected with a fluorescence detector measuring excitation at 320 nm and emission at 390 nm and quantitated using a standard curve of authentic aureusimine-B (2).
Kinetic Characterization of AusA Using the Product Formation Assay
Reactions were carried out under initial velocity conditions. Reactions (100 μL final volume) were assayed using 40 nM holo–AusA in 375 mM Bicine pH 8.0, 10 mM MgCl2, and 1 mM TCEP. Kinetic parameters for the varied substrate used a 2-fold dilution series at the indicated concentration range with fixed concentrations of the remaining substrates at the indicated concentration. Experiments with ATP (13.7 μM–10 mM) contained 100 μM NADPH, 5 mM L-Val, and either 10 mM L-4-Aph, 10 mM L-Tyr, 30 mM L-Leu, or 10 mM L-Phe. Experiments with -4-Aph (13.7 μM–30 mM), L-Tyr (4.6 μM–10 mM), L-Leu (13.7 μM–30 mM), and L-Phe (4.6 μM–10 mM) contained 3 mM ATP, 100 μM NADPH, and 5 mM L-Val. Experiments with L-Val (2.3 μM–5 mM) contained 3 mM ATP, 100 μM NADPH and 1 mM L-Tyr. Experiments with NADPH (1.4 μM–1 mM) contained 3 mM ATP, 5 mM L-Val, and either 30 mM L-4-Aph, 10 mM L-Tyr, 30 mM L-Leu, or 10 mM L-Phe. Reactions were incubated at 37 °C for 2.5 h and then inactivated at 100 °C for 20 min. For experiments with ATP, amino acids (except L-Val), and NADPH, the quenched reactions containing the same concentration of the varied substrate were pooled before analysis by LC-MS to allow for the simultaneous quantification of each of the four measured products. To accurately determine the concentration of holo–AusA, the enzyme was titrated with Phe-AMS (3.1–400 nM) using 200 nM holo–AusA, 3 mM L-Val, 0.2 mM L-Tyr, 0.2 mM ATP, and 0.1 mM NADPH.
LC-MS Analysis of AusA Reaction Products
LC-MS/MS was performed on a Shimadzu HPLC coupled with an AB Sciex QTRAP 5500 mass spectrometer. Samples were analyzed with an electrospray source run in the positive mode. Source and gas parameters were set to the following: Curtain gas: 40; CAD gas: Medium; Ion Spray Voltage: 3000; Temperature: 600; Gas 1: 45; Gas 2: 40. Pyrazinones, tyrosinol, and tyrosinal oxime were analyzed using Multiple Reaction Monitoring (MRM) using the parameters in Table 2 below.
Table 2.
MS Parameters for Detection of Pyrazinone Products
| Pyrazinones | Precursor Mass, Da | Product Mass, Da | Dwell Time, ms | DPa | EPb | CEc | CXPd |
|---|---|---|---|---|---|---|---|
| 1 | 245.2 | 136.1 | 100 | 100 | 10 | 39 | 17 |
| 2 | 229.1 | 100 | 100 | 100 | 10 | 27 | 16 |
| 3 | 195.1 | 137.1 | 100 | 100 | 10 | 37 | 17 |
| 4 | 244.1 | 151.2 | 100 | 100 | 10 | 27 | 20 |
| Tyrosinol (S8) | 168.1 | 107 | 100 | 60 | 10 | 22 | 14 |
| Tyrosinal oxime (S10) | 361 | 181.1 | 100 | 130 | 10 | 47 | 23 |
DP: Declustering Potential;
EP: Entrance Potential;
CE: Collision Energy; CXP: collision Cell Exit Potential
The samples were separated using a 50 × 2.0 mm Varian Polaris C-18 column (5 μm) with a mobile phase of 0.1% aqueous formic acid (mobile phase A) and acetonitrile containing 0.1% formic acid (mobile phase B) with a flow rate of 0.5 mL/min. Products were eluted after running isocratically with mobile phase A for 0.2 min followed by a linear gradient of 0–95% mobile phase B for 1.5 min. Subsequently, the column was washed with mobile phase B (95%) for 1.2 min and reequilibrated with mobile phase A (100%) for 4 min. Pyrazinone products 1–4 eluted at 1.6, 2.3, 1.8, and 2.1 min respectively. Tyrosinol S8 and tyrosinal oxime S10 eluted at 0.76 and 1.59 min, respectively (see Supplemenatry Information for structures).
For the identification of tyrosinal, the aldehyde was converted to a pentafluorobenzyl oxime following the protocol of Li and co-workers.16 Briefly reactions were extracted 3 times with ethyl acetate. The extract was then dried to completion and resupended in 100 μL methanol. Pentafluorobenxyl hydroxylamine (Sigma) was added to the extract (5 μL of a 10 mg/mL solution) and allowed to react at room temperature for 3 h. The solution was diluted with 3 volumes of 0.1 % formic acid and analyzed as above using LC-MS.
Each LC-MS run was exported into the MultiQuant (AB SCIEX) software package, which calculated the concentration of each peak using a standard curve of the four synthesized standards (2.7 nM–2 μM) that was run with each experiment. The concentration of product was transformed into vi values and the saturation curves generated were fit to either equation 1 or 2.
NADPH consumption assay
The reductase domain activity was independently investigated by monitoring the consumption of NADPH by following the change in absorbance at 360 nm on a Molecular Devices M5e multimode plate reader. Assays were set up in duplicate and contained 80 nM holo–AusA in reaction buffer (375 mM bicine pH 8.0, 3 mM MgCl2, 3 mM ATP, 1 mM TCEP) containing 100 μM NADPH and either 1 mM L-Tyr, 1 mM L-Val, or both amino acids. Reactions were monitored continuously at ambient temperature for 45 min. The extinction coefficient of NADPH (ε340 = 6220 M−1 cm−1) was used to convert slopes into activity. The reaction solutions were then transferred into individual tubes and the reaction products were analyzed by LC-MS as described above.
RESULTS
Cloning and Expression of AusA Constructs
AusA was cloned directly from 3 PCR fragments into an expression vector in a single step using Clonetech’s In-Fusion system and confirmed by sequencing the whole gene for PCR errors. This strategy greatly increased the efficiency of cloning such a large construct (7.2 kb) and avoided the necessity of utilizing compatible restriction enzymes, which can be quite challenging for large inserts. AusA was expressed as the full-length apo–protein containing an N-terminal 6×His-tag in E. coli at 18 °C inducing with 0.4 mM IPTG. Initial expression profiling showed that a significant amount of proteolysis occurs near the N-terminus during induction. Consequently, we found the N-terminus was a superior location for the 6×His-tag as the smaller 6×His-tagged fragments were easily separated by gel filtration. We also attempted to purify a doubly-tagged protein with an N-terminal 6×His-tag and C-terminal Strep-tag, but the yield was not improved (data not shown). The 277 kDa AusA protein was purified by sequential nickel-affinity chromatography, ion-exchange, and gel-filtration to afford approximately 0.5 mg/L of culture in greater than 90% purity. The A1-T1 didomain and A2-T2-R tridomain (attempts to prepare a A2-T2 didomain were unsuccessful, data not shown) were also cloned and expressed using similar methods.
Adenylation Domain Specificity
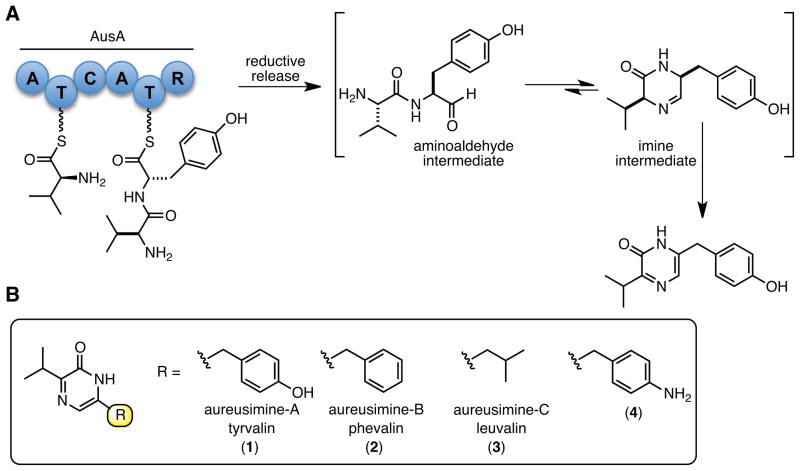
The two adenylation domains in full-length AusA were initially probed using the classic ATP–[32P]pyrophosphate (PPi) exchange assay. Twenty-one natural and non-natural L-amino acids were screened for activity against AusA (Figure 3A). In addition to known substrates L-Val, L-Leu, L-Phe, and L-Tyr, we also identified seven other amino acids whose activity was above background including 4-aminophenylalanine (L-4Aph), L-Ile, cycloleucine (L-Cle), and tert-butylleucine (L-Tle).
Figure 3.
Substrate specificity of AusA using pyrophosphate exchange assay. Each reaction contained 36 nM apo–AusA, 375 mM Bicine pH 8.0, 10 mM MgCl2, 3 mM ATP, 2 mM DTT, 1 mM pyrophosphate, 0.25 μCi [32P]pyrophosphate, and 30 mM of the selected amino acid except for tyrosine, which was held at 10 mM. Nonstandard amino acid abbreviations: L-cycloleucine (Cle), L-tert-leucine (Tle), L-4-aminophenylalanine (4Aph), α-methylphenylalanine (Mpa), β-aminophenylalanine (β-Phe) A) no inhibitor. B) minus (−) or plus (+) 100 μM Phe-AMS. C) minus (−) or plus (+) 100 μM Val-AMS.
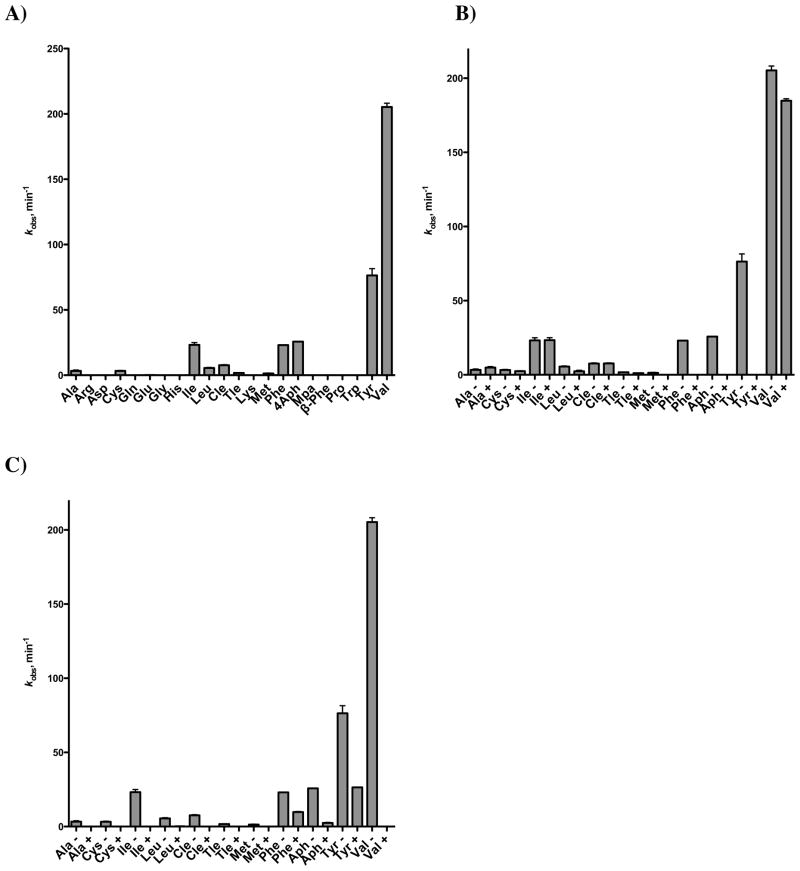
In order to distinguish the substrate specificity of each A-domain we used small molecule bisubstrate inhibitors initially described for the functionally related aminoacyl tRNA synthetases17 to “chemically knockout” each A-domain and then tested the activity of the other A-domain. We synthesized 5′-O-[N-(valinyl)sulfamoyl]adenosine (Val-AMS) and 5′-O-[N-(phenylalanyl)sulfamoyl]adenosine (Phe-AMS) (Figure 2B) as inhibitors of A1 and A2, respectively. These inhibitors mimic the intermediate acyl-adenylate formed in the A-domain adenylation half reaction by replacement of the labile acylphosphate moiety with a chemically stabile acylsulfamate isostere and derive their binding affinity through simultaneous interaction with the amino acid and ATP binding pockets.17 We evaluated the apparent inhibition constants of Val-AMS and Phe-AMS using the ATP–PPi exchange assay by measuring the initial rates (v0) at various inhibitor concentrations [I] at fixed substrate concentrations.18 Due to the tight-binding nature of both inhibitors, the resulting concentration–response plots were fit to the Morrison equation (equation 3, Materials and Methods) to provide apparent Ki values of 44 nM and 0.8 nM for Val-AMS and Phe-AMS, respectively (Figure 2A and Table 3).19 To confirm the inhibitor selectivity, we also measured the apparent Ki’s of each inhibitor against the individual apo–A–T–(R) di- and tri-domain constructs (Table 3). Val-AMS exhibits potent inhibition of A1–T1, but unexpectedly shows modest inhibition of A2–T2–R with an appKi of 10 μM providing a selectivity factor of 158 (i.e. ). Conversely, Phe-AMS is a potent inhibitor of A2–T2 with an appKi less than 4 nM and displays little inhibition of A1–T1 even up to 100 μM, providing a selectivity factor of >104. These results confirm the high specificity of these inhibitors for their cognate A-domains. The residues predicted to form the valine binding pocket in the A1 domain are highly conserved with the active of PA1221, an A-T didomain NRPS protein that was recently crystallized in the presence of a valine-adenosine vinylsulfonamide inhibitor.20 A sequence comparison shows that only one residue, which is ~7 Å from the valine side chain, is changed between the two sequences (Supporting Information, Figure S1).
Figure 2.
A) Concentration–response of fractional initial velocity of [32P]–ATP formation catalyzed by AusA as a function of Phe-AMS concentration (solid line) and Val-AMS (dashed line). The curve represents the best nonlinear fit of the data to the Morrison equation. The data points represent the mean with standard error of duplicate experiments. B) Structures of Val-AMS and Phe-AMS.
Table 3.
Inhibition Constants of Val-AMS and Phe-AMS with AusA and individual A–T constructs
| protein | appKi (nM)
|
|
|---|---|---|
| Val-AMS | Phe-AMS | |
| AusA (A1–T1–C–A2–T2–R) | 44 ± 7 | 0.8 ± 0.5 |
| A1–T1 | 63 ± 5 | > 105 |
| A2–T2–R | (10 ± 1) × 103 | < 4a |
Due to lower activity of the A2–T2–R tridomain, larger amounts of protein were required resulting in protein titration and thus we are only able to calculate a lower limit for the appKi
Each of the 11 amino acids that showed activity in the initial ATP–PPi exchange assay with AusA was rerun in the presence of saturating amounts of one of the inhibitors (Figure 3B and 3C). Incubation with Phe-AMS, which inhibits A2, eliminates the enzymatic activity with L-Phe, L-4Aph, L-Tyr, and L-Met. In the presence of Phe-AMS, AusA is capable of activating L-Val, L-Ile, L-Cle, L-Leu, L-Ala, L-Cys, and L-Tle; though the exchange rate of the second best substrate L-Ile is almost 10-fold lower than with L-Val. To assess the substrate specificity of A2, we incubated AusA with Val-AMS to inhibit A1 and again measured the amino acid-dependent ATP–PPi exchange rates. The activity of L-Val, L-Ile, L-Cle, L-Ala, L-Cys, L-Tle, and L-Met, are eliminated. In the presence of Val-AMS, AusA is only capable of activating L-Tyr, L-Phe, L-4Aph, and L-Leu; although in all cases the activity was reduced more than 2-fold relative to the uninhibited control due to off-target inhibition of A2 by Val-AMS.
Having established the substrate specificities of each A-domain were mutually exclusive except for L-Leu, we proceeded to examine the apparent steady-state kinetic parameters of the most active substrates using the ATP–PPi exchange assay with AusA (Table 4). The kinetic parameters for L-Val, L-Ile, L-Leu, L-Tyr, L-Phe, and L-4Aph were determined at subsaturating concentrations of ATP due to substrate inhibition by ATP (Table 4, entry 7) and thus are reported as apparent values. The specificity constant (kcat/KM ) for L-Val with A1 is 2319 M−1 s−1, which is 49-fold greater than L-Ile, the next best substrate, confirming the strict substrate specificity of A1. Indeed the three naturally occurring aureusimines all contain valine as the first amino acid. The kcat/KM values for L-Tyr, L-Phe, and L-4Aph with A2 are 2141, 320, and 49 M−1 s−1, respectively. The attenuation in kcat/KM for L-4Aph relative to L-Tyr is primarily due to a 31-fold increase in the KM value for this non-natural substrate with the kcat displaying a modest 30% decrease relative to the value for L-Tyr. The specificity constants for A1 and A2 with their optimal substrates are nearly identical, indicating a balanced activity for these two A-domains in AusA. Both A1 and A2 were shown to support PPi exchange with L-Leu, thus the kinetic parameters represent aggregate values. The kcat/KM for L-Leu is only 10 M−1 s−1, a value more than 200-fold less than the best substrates (L-Val and L-Phe) with their cognate A-domains.
Table 4.
ATP–PPi Exchange Kinetic Parameters of AusA
| entry | A-domain | varied substrate | non-varied substrate | kcat, min−1 | KM, mM | kcat/KM, M−1 s−1 | Ki, mM |
|---|---|---|---|---|---|---|---|
| 1 | A1 | L-Val | ATP | 461 ± 3 | 3.3 ± 0.1 | 2328 ± 72 | NA |
| 2 | A1 | L-Ile | ATP | 33.9 ± 1.1 | 11.9 ± 0.9 | 47 ± 4 | NA |
| 3 | A1/A2 | L-Leu | ATP | 14.9 ± 1.0 | 24 ± 3 | 10.3 ± 1.5 | NA |
| 4 | A2 | L-Tyr | ATP | 129 ± 1 | 1.0 ± 0 | 2150 ± 17 | NA |
| 5 | A2 | L-Phe | ATP | 40.5 ± 0.6 | 2.1 ± 0.1 | 321 ± 16 | NA |
| 6 | A2 | L-4Aph | ATP | 92 ± 2 | 31 ± 1 | 49 ± 2 | NA |
| 7 | A2 | ATP | L-Phe | 63 ± 7 | 3.0 ± 0.5 | 350 ± 70 | 4.1 ± 0.7 |
Reconstitution of Aureusimine Synthetase Activity
AusA was initially expressed as the apo–enzyme. In order to obtain a functional enzyme capable of producing aureusimine, AusA must be converted to the active holo-enzyme by phosphopantetheinylation of the two thiolation domains. Ser947 and Ser1976 are predicted to be the sites of phosphopantetheinylation in T1 and T2, respectively. Although the aureusimine biosynthetic gene cluster encodes for a dedicated phosphopantetheinyl transferase (PPTase), we elected to use Sfp, a promiscuous PPTase from B. subtilis that has been utilized extensively for the heterologous priming of NRPSs.21 AusA was co-expressed with a plasmid that constitutively expresses Sfp to provide holo-AusA in comparable yields as the apo–enzyme.
Incubation of holo–AusA (40 nM) with L-Val (25 mM), L-Phe (25 mM), NADPH (0.1 mM), and ATP (3 mM) at pH 8.0 at 37 °C led to the time-dependent formation of aureusimine-B (2). The reaction product co-migrated on HPLC with an authentic standard that we synthesized (Supporting Information) and possessed an identical fragmentation pattern in tandem MS/MS (229.1→100.0). Exclusion of any of the four substrates resulted in complete loss of aureusimine synthesis activity. These results demonstrate recombinant AusA is capable of synthesizing aureusimine-B (2) in vitro.
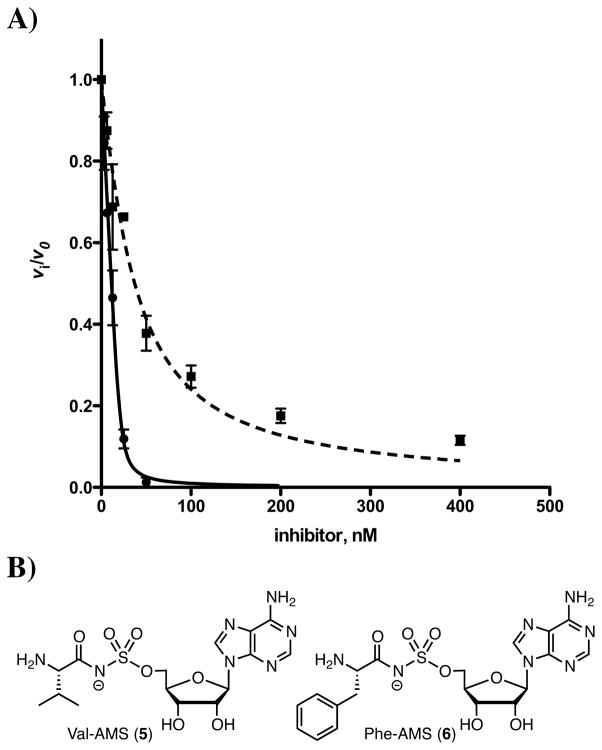
Since oxidation of the initial imine product to aureusimine-B (2) (Figure 1) is likely not catalyzed by AusA, we were concerned oxidative conversion to aureusimine-B (2) may be incomplete. As a result, we separated the reaction products from AusA using a centrifugal filter and heated the filtrate at 100 °C to promote oxidation. We conducted a time course study and found that heating the reaction filtrate for 20 minutes at 100 °C provided full conversion to aureusimine-B (2) (Figure 4). Subsequently, detection and quantitation of the pyrazinones was determined by heating reaction filtrates for 20 minutes at 100 °C to ensure full conversion to the respective pyrazinone products. Zimmerman and co-workers propose oxidation of dihydro-aureusimine (i.e. the imine intermediate in Figure 1) is either spontaneous or catalyzed by the reductase domain of AusA operating in reverse as an oxidoreducatase.3 Our results suggest oxidation to aureusimine-B (2) is spontaneous driven by the aromatic stabilization energy of the pyrazinone.
Figure 4.
Thermal conversion to aureusimine-B (2).
Kinetics of Pyrazinone Formation by AusA
We evaluated the apparent kinetic parameters for pyrazinone formation for each substrate (L-Tyr, L-Val, ATP, and NADPH) at fixed saturating concentrations of the other substrates and also evaluated several additional A2 substrates including L-Phe, L-4Aph, and L-Leu. Finally, we examined how the kinetic parameters for ATP and NADPH change with different non-varied A2 substrates. We synthesized authentic standards of all pyrazinone products 1–4 (Supporting Information). Active-site titration of AusA with Phe-AMS showed it to be ~80% active and the corrected concentration was used for determination of kcat values (Figure S2, Supporting Information).
The initial rates, v0, at a given [S] were determined by single time point stopped-time incubation at 150 minutes. The reaction velocity remained linear for up to 4 hours (the longest time measured). All reactions were run in duplicate employing a fresh enzyme preparation and the pyrazinone products were quantified by LC-MS/MS (Materials and Methods) employing a standard curve generated from our synthetic standards. Apparent steady-state kinetic parameters were determined by fitting the v0 vs. [S] plots to the Michaelis–Menten equation (equation 1, Materials and Methods) or substrate inhibition model (equation 2, Materials and Methods). Representative LC-MS data and the corresponding saturation curve for determination of the steady-state kinetic parameters for L-Val are shown in Figure 5. Initial velocity experiments provided KM values of 132, 50, 104, and 22 μM for L-Tyr, L-Val, ATP, and NADPH, respectively with an average kcat equal to 0.38 ± 0.01 min−1 for L-Val, L-Tyr, and ATP (Table 5, entries, 1, 5, 6, and 10). The kcat of 0.71 min−1 using NADPH as the varied substrate is a result of fitting the saturation curve to the substrate inhibition model, which showed NADPH exhibits substrate inhibition with a Ki of 2.2 mM. Notably, at 0.1 mM NADPH (the concentration employed when NADPH was the non-varied substrate), the kobs is 0.39 min−1, consistent with the other values.
Figure 5.
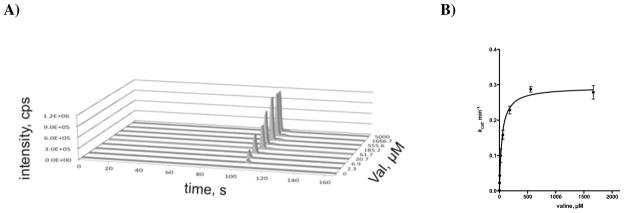
A) LC–MS data for production of aureusimine-A (1) at varying concentrations of L-Val. B) Normalized plot of the data shown in panel A used to determine the steady-state kinetic parameters of AusA with L-Val. Each reaction contained 40 nM holo–AusA, 375 mM Bicine pH 8.0, 10 mM MgCl2, 3 mM ATP, 0.1 mM NADPH, 1 mM TCEP, 1 mM L-Tyr and 2.3 μM–5 mM L-Val.
Table 5.
Kinetic Parameters of Pyrazinone Formation
| Entry | Pyrazinone Product | Varied Substrate | Non-varied Substrates | kcat, min−1 | KM, M | kcat/KM, M−1 s−1 | Ki, mM | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | L-Tyr | L-Val | ATP | NADPH | 0.36 ± 0.01 | (1.32 ± 0.19) × 10−4 | 45.3 ± 6.48 | NA |
| 2 | 2 | L-Phe | L-Val | ATP | NADPH | 0.99 ± 0.04 | (1.03 ± 0.14) × 10−3 | 16.0 ± 2.26 | NA |
| 3 | 4 | L-4Aph | L-Val | ATP | NADPH | 1.08 ± 0.04 | (6.67 ± 0.66) × 10−3 | 2.70 ± 0.28 | NA |
| 4 | 3 | L-Leu | L-Val | ATP | NADPH | 1.32 ± 0.46 | (1.49) ± 0.60) × 10−1 | 0.15 ± 0.08 | NA |
| 5 | 1 | L-Val | L-Tyr | ATP | NADPH | 0.39 ± 0.02 | (5.03 ± 0.70) × 10−5 | 130 ± 19.0 | NA |
| 6 | 1 | ATP | L-Val | L-Tyr | NADPH | 0.39 ± 0.02 | (1.04 ± 0.15) × 10−4 | 62.5 ± 9.32 | NA |
| 7 | 2 | ATP | L-Val | L-Phe | NADPH | 1.22 ± 0.15 | (4.55 ± 1.42) × 10−4 | 44.8 ± 15.0 | NA |
| 8 | 4 | ATP | L-Val | L-4Aph | NADPH | 0.90 ± 0.09 | (1.12 ± 0.23) × 10−3 | 13.3 ± 3.00 | NA |
| 9 | 3 | ATP | L-Val | L-Leu | NADPH | 1.32 ± 0.63 | (9.73 ± 5.51) × 10−3 | 2.26 ± 1.67 | 3.53 ± 2.21 |
| 10 | 1 | NADPH | L-Val | L-Tyr | ATP | 0.71 ± 0.05 | (2.15 ± 0.40) × 10−5 | 551 ± 110 | 2.16 ± 0.68 |
| 11 | 2 | NADPH | L-Val | L-Phe | ATP | 1.53 ± 0.08 | (1.68 ± 0.29) × 10−5 | 1510 ± 272 | 9.70 ± 8.00 |
| 12 | 4 | NADPH | L-Val | L-4Aph | ATP | 1.30 ± 0.08 | (1.18 ± 0.23) × 10−5 | 1830 ± 381 | 2.04 ± 0.65 |
| 13 | 3 | NADPH | L-Val | L-Leu | ATP | 0.38 ± 0.02 | (5.12 ± 1.15) × 10−6 | 1220 ± 284 | 1.84 ± 0.61 |
Due to the relaxed substrate specificity of A2, we evaluated L-Phe, L-4Aph, and L-Leu (Table 5, entries 2–4) with fixed saturating concentrations of L-Val, ATP, and NADPH and observed formation of the respective pyrazinones 2–4. The kcat/KM values for L-Phe, L-4Aph, and L-Leu are 2.8, 17, and 302-fold lower than that of L-Tyr, which is the best A2 substrate. The 2.8-fold difference in kcat/KM for L-Tyr and L-Phe almost precisely matches the 3:1 observed product ratio of aureusimines A (1) and B (2).2 The decrease in kcat/KM is driven exclusively by an increase in KM values for L-Phe, L-4Aph, and L-Leu as all three substrates exhibit approximately 3-fold greater kcat values. Surprisingly, despite being an extremely poor substrate for A2, L-Leu is incorporated into a aureusimine-C (3), demonstrating that this substrate is efficiently processed by the downstream C and R domains.3 Although direct comparison of exchange kinetics and steady-state turnover kinetics is not valid, we did observe close correlation in the kcat/KM values. This suggests the ATP–PPi exchange assay, commonly used to assess A-domain substrate specificity is an excellent surrogate for the overall complete NRPS reaction. By contrast, the KM values obtained from the ATP–PPi exchange assay were up to 60–fold higher than observed in the product formation assay. The kcat value in the product formation assay for L-Tyr is the lowest of all A2 substrates examined, which was unexpected since L-Tyr displays the highest kcat in the ATP–PPi exchange assay. This indicates that transfer of the tyrosinyl-AMP intermediate to T2, condensation with L-Val~S-T2 or the reductive release of the final product is much slower than with L-Phe or the other substrates.
When ATP was examined as the varied substrate at fixed concentrations of L-Val and NADPH, but employing different A2 substrates (L-Tyr, L-Phe, L-4Aph, and L-Leu; Table 5, entries 6–9) the observed KM’s ranged from 0.1 mM (with L-Tyr as the non-varied amino acid) to 9.7 mM (with L-Leu as the non-varied amino acid). The KM values for ATP thus vary over two orders of magnitude and parallel the trend in amino acid KM’s. This is expected since the amino acid and ATP are linked through the kinetic mechanism, where binding of the amino acid drives binding of ATP through formation of the acyl–adenylate and further downstream central complexes. Since ATP is abundant, estimated to be present at 3 mM in cells, these differences in KM’s may be less important in vivo.22 When NADPH was examined as the varied substrate at fixed concentrations of L-Val and ATP, but employing different A2 substrates (L-Tyr, L-Phe, L-4Aph, and L-Leu; Table 5, entries 10–13), the KM and kcat/KM values ranged less than five-fold.
Reductive Release
The reductase domain in AusA is a member of the short chain dehydrogenase/reductase (SDR) enzyme superfamily and contains the signature YXXXK catalytic motif as well as Rossman fold for NADPH binding (Figure S3, Supporting Information). AusA exhibits strict cofactor specificity for NADPH and is unable to use NADH (data not shown). To further assess the R-domain specificity and kinetics we employed a continuous kinetic assay to monitor consumption of NADPH by a decrease in absorbance at 360 nm (Materials and Methods). Incubation of AusA with all substrates and cofactors (L-Val, L-Tyr, NADPH, and ATP) resulted in a time-dependent decrease in the concentration of NADPH corresponding to 2.01 min−1 (Table 6, entry 1). The rate of aureusimine-A (1) production (kobs) under these conditions is 0.28 min−1 (Table 6, entry 1). To understand the origin of the substantially higher NADPH consumption relative to product formation, we then performed the assay under otherwise identical conditions, but excluding either L-Val or L-Tyr. In the absence of L-Tyr we did not observe any NADPH consumption (Table 6, entry 2). However, in absence of L-Val, we unexpectedly observed substantial NADPH consumption with a kobs of 1.64 min−1 (Table 6, entry 3). These results indicate the R-domain is able to rapidly turnover L-Tyr, presumably to either the corresponding aldehyde (i.e. tyrosinal) or alcohol (i.e. tyrosinol). To confirm the turnover products, we synthesized authentic standards of tyrosinol and a stabile oxime derivative of tyrosinal (see Supporting Information). Since tyrosinal is unstabile, the reaction products were directly treated with pentafluorobenzyl hydroxylamine to form a tyrosinal oxime derivative (Materials and Methods). LC-MS confirmed that L-Tyr is reduced to tyrosinal, but not further reduced to tyrosinol. We then excluded ATP from the reaction or added the A2-specific inhibitor Phe-AMS to demonstrate L-Tyr must be activated by A2 and tethered to T2. In both cases consumption of NADPH was not observed (data not shown). Taken together, these results confirm L-Tyr must be activated by A2 in order to undergo reduction and show the R-domain is able to turnover L-Tyr-S~T2 much more rapidly than the dipeptidyl substrate L-Val–L-Tyr-S~T2. These results in turn suggest dipeptide formation catalyzed by the C-domain is substantially slower than reductive release since the partition ratio of products (reduced tyrosine products versus cyclized dipeptides) is reflective of T2 occupancy. Finally, we note the observed consumption of NADPH with all substrates (kobs = 2.01 min−1) is nearly equal to the sum of the observed rates of aureusimine-A (1) formation (kobs = 0.28 min−1) and tyrosine turnover (kobs =1.64 min−1).
Table 6.
NADPH consumption assay
| Entry | Substrates | NADPH Consumption kobs, min−1 | Aureusimine-A Formation kobs, min−1 |
|---|---|---|---|
| 1 | L-Val, L-Tyr ATP, NADPH | 2.01 ± 0.05 | 0.28 ± 0.01 |
| 2 | L-Val, ATP, NADPH | 0 | 0 |
| 3 | L-Tyr, ATP, NADPH | 1.64 ± 0.01 | 0 |
DISCUSSION
Pyrazinones are relatively rare heterocyles in natural products and their reported activities range from antibacterial for simple 3,6-disubstituted analogues to antiviral for the more complex (bis)indole alkaloids. The naturally occurring 3,6-disubstituted pyrazinones include argvalin,23 arglecin,24 OPC-15161,25 flavacol and related hydroxylated metabolites,26 several 1-hydroxypyrazinones,27 peramine,28 and the (bis)indole alkaloid dragmacidins.29 Aureusimine-B was previously isolated from a Streptomyces sp. and although no antibacterial activity was observed, it was shown to inhibit calpain, a serine protease with modest activity (~ 1 μM).30.
A related dipeptide-derived class of natural products includes the diketopiperazines (DKPs), which can be produced nonribosomally or by cyclodipeptide synthases.31 NRPS-mediated synthesis of DKPs typically use a dimodule similar to AusA, but lacking a C-terminal domain to catalyze peptide release. In the absence of a C-terminal release domain, cyclization to afford a DKP generally occurs spontaneously, especially when proline is present as the first amino acid. The structurally related pyrazines are another important class of natural products. Based on the chemical precedence of Sperry and Badrinarayanan32 and recent biochemical characterization from Tang and co-workers of a fungal NRPS,33 it seems plausible that 2,5-disubstituted symmetrical pyrazines of bacterial and fungal origin may employ a similar biosynthetic logic using a NRPS with the C–A–T–R architecture to convert an amino acid to an amino aldehyde. Spontaneous dimerization of the amino aldehyde and subsequent oxidation would provide the pyrazine derivative. Dimerization may alternatively be enzyme catalyzed, as observed during the biosynthesis of saframycin by the monomodular NRPS SfmC.34
We utilized a novel strategy to assess the substrate specificity of each A-domain within the context of full-length AusA using selective A-domain acyl-adenylate mimics (Val-AMS for A1 and Phe-AMS for A2) to inhibit the other A-domain. Previous characterization of NRPSs with multiple A-domains has been performed primarily through excision of each A-domain from the NRPS and expression as a stand-alone protein. This not only limits kinetic evaluation to the adenylate-forming half reaction, but also does not allow characterization of the A-domain in its normal context where protein-protein interactions may modulate activity. Walsh and Balibar characterized the trimodular NRPS GliP involved in gliotoxin biosynthesis that contains two A-domains by separately mutating the conserved aspartic residue in each A-domain active site that ion-pairs with the amine of the amino acid substrate.35 The resulting mutation abolished A-domain activity and enabled kinetic evaluation in the context of the large trimodular NRPS. Another technique to characterize A-domain substrate specificity was reported by Kelleher and co-workers and involves direct characterization of the loaded substrate on the cognate T-domain using Fourier transform mass spectrometry.36 Our strategy employing A-domain inhibitors provides a complimentary method to characterize A-domain specificity in multidomain NRPSs containing more than one A-domain. A limitation is that it requires a priori knowledge of the substrate specificity from bioinformatic analysis.
AusA uses a reductase (R) domain for release of a dipeptidyl aldehyde that reacts intramolecularly with the N-terminus to afford a 6-membered imine, which spontaneously oxidizes to the final pyrazinone product. Interestingly, the domain architecture of AusA, A1–T1–C–T2–A2–R, is found in the NRPS genes involved in biosynthesis of the benzodiazepine anthramycin, but the loading and extension modules are separate proteins.37 The R-domain in the anthramycin NRPS is also predicted to catalyze a two-electron reductive release to afford an aldehyde, which undergoes spontaneous condensation with an internal amine to afford a 7-membered hemiaminal. However, the resultant hemiaminal in anthramycin biosynthesis does not dehydrate to an imine, likely as a result of ring strain. In the absence of an internal nucleophile to rapidly capture the product aldehyde, further reduction to an alcohol is favored (an overall four-electron reduction) since aldehydes are more reactive than thioesters. This is illustrated in the R-domain catalyzed four-electron reductive release of lyngbyatoxin,38 myxochelin,16 and mycobacterial glycopeptidolipids,39 all of which lack appropriately positioned hydroxy or amino groups to intramolecularly condense with the intermediate aldehyde.11 Gokahle and co-workers demonstrated aldehyde reduction is approximately 10-times faster than thioester reduction during their study of the R-domain involved in reductive release of the mycobacterial glycopeptidolipids. The mechanism for reduction is nonprocessive, requiring the initially formed aldehyde product to dissociate from the enzyme to allow for cofactor exchange of NAD(P) for NADPH. Rebinding of aldehyde followed by a second reduction provides the primary alcohol final product.39
The in vitro reconstitution of complete NRPS pathways40 has been reported for only a handful of natural products including cyclooctadepsipeptide PF1022,41, 42 pacidamycin,43 antimycin,44 beauvericins,45 and the siderophore synthesis pathways involved in production of enterobactin,46 yersiniabactin,47 pyochelin,48 vibriobactin,49 myxochelin,16 and pseudomonine.50 Biosynthesis of the indole alkaloid terrequinone, which uses a noncanonical monomodular NRPS for dimerization of a tryptophan derivative, was also reconstituted in vitro.51, 52 A focus of many earlier studies was aimed at uncovering novel enzyme activities as well as providing fundamental insight into the logic of NRPS biosynthesis, often using single turnover experiments employing radiolabeled substrates. The only available kcat values for product formation from fully reconstituted native pathways are for enterobactin, yersiniabactin, and pyochelin – and these range from 1.4 min−1 for yersiniabactin46 to 140 min−1 for enterobactin.46 Stachelhaus and Hahn have also reported the reconstitution of two excised NRPS modules from the tyrocidin NRPS pathway that produced a diketopiperazine with a kobs of 0.94 min−1.53 Surprisingly, KM’s for small molecule substrates and cofactors determined by measuring product formation have only been reported for salicylic acid in the synthesis of yersiniabactin.46
The lack of Michaelis–Menten kinetic parameters with respect to product formation can be attributed to several factors including lack of assay sensitivity, product inhibition, and/or the complexity of the multi-protein systems. Consequently, the results obtained herein that provide simple steady-state kinetic parameters (Table 5) of all substrates and cofactors including non-natural substrates involved in pyrazinone formation are notable. We found the KM values for the amino acid substrates were up to 60-fold lower in the product formation versus the classic ATP–PPi exchange assay that is most commonly used to assess A-domain substrate specificity. Significantly, the KM value for ATP in the product formation assay changed more than 100-fold depending on the amino acid employed. While this result is readily reconciled since the amino acid and ATP are kinetically linked through acyl-adenylate formation, it highlights the importance of a more careful examination of reaction kinetics. We believe the kinetic parameters obtained herein provide a useful foundation for future studies using AusA as a model NRPS and highlight the utility of this simple dimodular NRPS for kinetic characterization.
Supplementary Material
Acknowledgments
We thank Mrs. Kathryn Nelson for carefully proofreading the manuscript and Prof. David H. Sherman (University of Michigan, Ann Arbor, MI, USA) for generously providing plasmid pSFP.
We gratefully acknowledge NIH Grant GM-068440 (to A. M. G.) and AI-070219 (to C.C.A.).
Abbreviations
- A-domain
adenylation domain
- L-4Aph
L-4-aminophenylalanine
- β-Phe
β-aminophenylalanine
- C-domain
condensation domain
- L-Cle
L-cycloleucine
- Mpa
α-methylphenylalanine
- nrp
nonribosomal peptide
- nrps
nonribosomal peptide synthetase
- Phe-AMS
5′-O-[N-(phenylalanyl)sulfamoyl]adenosine
- R-domain
reductase domain
- T-domain
thiolation domain
- TE
thioesterase
- L-Tle
L-tert-leucine
- Val-AMS
5′-O-[N-(valinyl)sulfamoyl]adenosine
Footnotes
ASSOCIATED CONTENT
Supporting Information. Synthesis of pyrazinones 1–4, Val-AMS 5, Phe-AMS 6, tyrosinol S8, tyrosinal oxime S10, alignment and homology model of the A1 domain of AusA, active-site titration of AusA, alignment of the R-domain, and saturation curves for all substrates shown in Tables 4 and 5. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt MA, Wang W, Roux CM, Beasley FC, Heinrichs DE, Dunman PM, Magarvey NA. Staphylococcus aureus nonribosomal peptide secondary metabolites regulate virulence. Science. 2010;329:294–296. doi: 10.1126/science.1188888. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann M, Fischbach MA. A family of pyrazinone natural products from a conserved nonribosomal peptide synthetase in Staphylococcus aureus. Chem Biol. 2010;17:925–930. doi: 10.1016/j.chembiol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt MA, Wang W, Roux CM, Beasley FC, Heinrichs DE, Dunman PM, Magarvey NA. Clarification of “Staphylococcus aureus nonribosomal peptide secondary metabolites regulate virulence”. Science. 2011;333:1381. doi: 10.1126/science.1188888. [DOI] [PubMed] [Google Scholar]
- 5.Sun F, Cho H, Jeong DW, Li C, He C, Bae T. Aureusimines in Staphylococcus aureus are not involved in virulence. PLoS One. 2010;5:e15703. doi: 10.1371/journal.pone.0015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secor PR, Jennings LK, James GA, Kirker KR, Pulcini ED, McInnerney K, Gerlach R, Livinghouse T, Hilmer JK, Bothner B, Fleckman P, Olerud JE, Stewart PS. Phevalin (aureusimine B)production by Staphylococcus aureus biofilm and impacts on human keratinocyte gene expression. PLoS One. 2012;7:e40973. doi: 10.1371/journal.pone.0040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieber SA, Marahiel MA. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem Rev. 2005;105:715–738. doi: 10.1021/cr0301191. [DOI] [PubMed] [Google Scholar]
- 8.Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 9.Gulick AM. Conformational dynamics in the Acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem Biol. 2009;4:811– 827. doi: 10.1021/cb900156h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer AC, Burkart MD. The ubiquitous carrier protein--a window to metabolite biosynthesis. Nat Prod Rep. 2007;24:750–773. doi: 10.1039/b603921a. [DOI] [PubMed] [Google Scholar]
- 11.Du L, Lou L. PKS and NRPS release mechanisms. Nat Prod Rep. 2010;27:255–278. doi: 10.1039/b912037h. [DOI] [PubMed] [Google Scholar]
- 12.Edwards DJ, Gerwick WH. Lyngbyatoxin biosynthesis: sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J Am Chem Soc. 2004;126:11432–11433. doi: 10.1021/ja047876g. [DOI] [PubMed] [Google Scholar]
- 13.Yin WB, Grundmann A, Cheng J, Li SM. Acetylaszonalenin biosynthesis in Neosartorya fischeri. Identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem. 2009;284:100–109. doi: 10.1074/jbc.M807606200. [DOI] [PubMed] [Google Scholar]
- 14.Maiya S, Grundmann A, Li SM, Turner G. The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem. 2006;7:1062–1069. doi: 10.1002/cbic.200600003. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt MA, Mok MC, Junop M, Magarvey NA. Heterologous expression and structural characterisation of a pyrazinone natural product assembly line. Chembiochem. 2012;13:2408– 2415. doi: 10.1002/cbic.201200340. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Weissman KJ, Muller R. Myxochelin biosynthesis: direct evidence for two- and four-electron reduction of a carrier protein-bound thioester. J Am Chem Soc. 2008;130:7554– 7555. doi: 10.1021/ja8025278. [DOI] [PubMed] [Google Scholar]
- 17.Vondenhoff GH, Van Aerschot A. Aminoacyl-tRNA synthetase inhibitors as potential antibiotics. Eur J Med Chem. 2011;46:5227–5236. doi: 10.1016/j.ejmech.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Francklyn CS, First EA, Perona JJ, Hou YM. Methods for kinetic and thermodynamic analysis of aminoacyl-tRNA synthetases. Methods. 2008;44:100–118. doi: 10.1016/j.ymeth.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copeland RA. Tight Binding Inhibitors, in Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists. Wiley; Hoboken, NJ: 2005. pp. 178–213. [PubMed] [Google Scholar]
- 20.Mitchell CA, Shi C, Aldrich CC, Gulick AM. Structure of PA1221, a nonribosomal peptide synthetase containing adenylation and peptidyl carrier protein domains. Biochemistry. 2012;51:3252–3263. doi: 10.1021/bi300112e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quadri LE, Weinreb PH, Lei M, Nakano MM, Zuber P, Walsh CT. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry. 1998;37:1585–1595. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 22.Neuhard J, Nygaard P. Cellular and Molecular Biology. Vol. 1. ASM Press; Washington, D. C: 1987. Escherichia coli and Salmonella typhimurium; pp. 445–473. [Google Scholar]
- 23.Tatsuta K, Fujimoto K, Yamashita M, Tsuchiya T, Umezawa S. Argvalin, a new microbial metabolite: isolation and structure. J Antibiot (Tokyo) 1973;26:606–608. doi: 10.7164/antibiotics.26.606. [DOI] [PubMed] [Google Scholar]
- 24.Tatsuta K, Tsuchiya T, Umezawa S, Naganawa H, Umezawa H. Revised structure for arglecin. J Antibiot (Tokyo) 1972;25:674–676. doi: 10.7164/antibiotics.25.674. [DOI] [PubMed] [Google Scholar]
- 25.Nakano Y, Kawaguchi T, Sumitomo J, Takizawa T, Uetsuki S, Sugawara M, Kido M. Novel inhibitors of superoxide anion generation, OPC-15160 and OPC-15161. Taxonomy, fermentation, isolation, physico-chemical properties, biological characteristics and structure determination. J Antibiot (Tokyo) 1991;44:52–58. doi: 10.7164/antibiotics.44.52. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki M, Maebayashi Y, Miyaki K. Isolation of a new type of pyrazine metabolite from Aspergillus ochraceus WILH. Chem Pharm Bull. 1972;20:2274–2276. [Google Scholar]
- 27.MacDonald JC. New analogues of aspergillic acid derived from methionine. Can J Biochem. 1972;50:543–549. doi: 10.1139/o72-075. [DOI] [PubMed] [Google Scholar]
- 28.Rowan DD, Hunt MB, Gaynor DL. Peramine, a novel insect feeding deterrent from ryegrass infected with the endophyte Acremonium loliae. J Chem Soc, Chem Commun. 1986:935–936. doi: 10.1007/BF01012099. [DOI] [PubMed] [Google Scholar]
- 29.Capon RJ, Rooney F, Murray LM, Collins E, Sim ATR, Rostas JAP, Butler MS, Carroll AR. Dragmacidins: new protein phosphatase inhibitors from a southern australian deep-water marine sponge, spongosorites sp. J Nat Prod. 1998;61:660–662. doi: 10.1021/np970483t. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez ME, White CB, Gregory J, Kydd GC, Harris A, Sun HH, Gillum AM, Cooper R. Phevalin, a new calpain inhibitor, from a Streptomyces sp. J Antibiot (Tokyo) 1995;48:1165–1167. doi: 10.7164/antibiotics.48.1165. [DOI] [PubMed] [Google Scholar]
- 31.Belin P, Moutiez M, Lautru S, Seguin J, Pernodet JL, Gondry M. The nonribosomal synthesis of diketopiperazines in tRNA-dependent cyclodipeptide synthase pathways. Nat Prod Rep. 2012;29:961–979. doi: 10.1039/c2np20010d. [DOI] [PubMed] [Google Scholar]
- 32.Badrinarayanan S, Sperry J. Pyrazine alkaloids via dimerization of amino acid-derived alpha-amino aldehydes: biomimetic synthesis of 2,5-diisopropylpyrazine, 2,5-bis(3-indolylmethyl)pyrazine and actinopolymorphol C. Org Biomol Chem. 2012;10:2126–2132. doi: 10.1039/c2ob06935k. [DOI] [PubMed] [Google Scholar]
- 33.Qiao K, Zhou H, Xu W, Zhang W, Garg N, Tang Y. A fungal nonribosomal peptide synthetase module that can synthesize thiopyrazines. Org Lett. 2011;13:1758–1761. doi: 10.1021/ol200288w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koketsu K, Watanabe K, Suda H, Oguri H, Oikawa H. Reconstruction of the saframycin core scaffold defines dual Pictet-Spengler mechanisms. Nat Chem Biol. 2010;6:408–410. doi: 10.1038/nchembio.365. [DOI] [PubMed] [Google Scholar]
- 35.Balibar CJ, Walsh CT. GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochemistry. 2006;45:15029– 15038. doi: 10.1021/bi061845b. [DOI] [PubMed] [Google Scholar]
- 36.Dorrestein PC, Blackhall J, Straight PD, Fischbach MA, Garneau-Tsodikova S, Edwards DJ, McLaughlin S, Lin M, Gerwick WH, Kolter R, Walsh CT, Kelleher NL. Activity screening of carrier domains within nonribosomal peptide synthetases using complex substrate mixtures and large molecule mass spectrometry. Biochemistry. 2006;45:1537–1546. doi: 10.1021/bi052333k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Phelan V, Ntai I, Farnet CM, Zazopoulos E, Bachmann BO. Benzodiazepine biosynthesis in Streptomyces refuineus. Chem Biol. 2007;14:691–701. doi: 10.1016/j.chembiol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Read JA, Walsh CT. The lyngbyatoxin biosynthetic assembly line: chain release by four-electron reduction of a dipeptidyl thioester to the corresponding alcohol. J Am Chem Soc. 2007;129:15762–15763. doi: 10.1021/ja077374d. [DOI] [PubMed] [Google Scholar]
- 39.Chhabra A, Haque AS, Pal RK, Goyal A, Rai R, Joshi S, Panjikar S, Pasha S, Sankaranarayanan R, Gokhale RS. Nonprocessive [2 + 2]e- off-loading reductase domains from mycobacterial nonribosomal peptide synthetases. Proc Natl Acad Sci USA. 2012;109:5681–5686. doi: 10.1073/pnas.1118680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattely ES, Fischbach MA, Walsh CT. Total biosynthesis: in vitro reconstitution of polyketide and nonribosomal peptide pathways. Nat Prod Rep. 2008;25:757–793. doi: 10.1039/b801747f. [DOI] [PubMed] [Google Scholar]
- 41.Feifel SC, Schmiederer T, Hornbogen T, Berg H, Sussmuth RD, Zocher R. In vitro synthesis of new enniatins: probing the alpha-D-hydroxy carboxylic acid binding pocket of the multienzyme enniatin synthetase. Chembiochem. 2007;8:1767–1770. doi: 10.1002/cbic.200700377. [DOI] [PubMed] [Google Scholar]
- 42.Muller J, Feifel SC, Schmiederer T, Zocher R, Sussmuth RD. In vitro synthesis of new cyclodepsipeptides of the PF1022-type: probing the alpha-D-hydroxy acid tolerance of PF1022 synthetase. Chembiochem. 2009;10:323–328. doi: 10.1002/cbic.200800539. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Ntai I, Bolla ML, Malcolmson SJ, Kahne D, Kelleher NL, Walsh CT. Nine enzymes are required for assembly of the pacidamycin group of peptidyl nucleoside antibiotics. J Am Chem Soc. 2011;133:5240–5243. doi: 10.1021/ja2011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandy M, Rui Z, Gallagher J, Zhang W. Enzymatic synthesis of dilactone scaffold of antimycins. ACS Chem Biol. 2012 doi: 10.1021/cb300416w. org/10.1021/cb300416w. [DOI] [PubMed] [Google Scholar]
- 45.Matthes D, Richter L, Muller J, Denisiuk A, Feifel SC, Xu Y, Espinosa-Artiles P, Sussmuth RD, Molnar I. In vitro chemoenzymatic and in vivo biocatalytic syntheses of new beauvericin analogues. Chem Commun. 2012;48:5674–5676. doi: 10.1039/c2cc31669b. [DOI] [PubMed] [Google Scholar]
- 46.Gehring AM, Mori I, Walsh CT. Reconstitution and characterization of the Escherichia coli enterobactin synthetase from EntB, EntE, and EntF. Biochemistry. 1998;37:2648–2659. doi: 10.1021/bi9726584. [DOI] [PubMed] [Google Scholar]
- 47.Miller DA, Luo L, Hillson N, Keating TA, Walsh CT. Yersiniabactin synthetase: a four-protein assembly line producing the nonribosomal peptide/polyketide hybrid siderophore of Yersinia pestis. Chem Biol. 2002;9:333–344. doi: 10.1016/s1074-5521(02)00115-1. [DOI] [PubMed] [Google Scholar]
- 48.Patel HM, Walsh CT. In vitro reconstitution of the Pseudomonas aeruginosa nonribosomal peptide synthesis of pyochelin: characterization of backbone tailoring thiazoline reductase and N-methyltransferase activities. Biochemistry. 2001;40:9023–9031. doi: 10.1021/bi010519n. [DOI] [PubMed] [Google Scholar]
- 49.Keating TA, Marshall CG, Walsh CT. Reconstitution and characterization of the Vibrio cholerae vibriobactin synthetase from VibB, VibE, VibF, and VibH. Biochemistry. 2000;39:15522–15530. doi: 10.1021/bi0016523. [DOI] [PubMed] [Google Scholar]
- 50.Sattely ES, Walsh CT. A latent oxazoline electrophile for N-O-C bond formation in pseudomonine biosynthesis. J Am Chem Soc. 2008;130:12282–12284. doi: 10.1021/ja804499r. [DOI] [PubMed] [Google Scholar]
- 51.Schneider P, Weber M, Rosenberger K, Hoffmeister D. A one-pot chemoenzymatic synthesis for the universal precursor of antidiabetes and antiviral bis-indolylquinones. Chem Biol. 2007;14:635–644. doi: 10.1016/j.chembiol.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Balibar CJ, Howard-Jones AR, Walsh CT. Terrequinone A biosynthesis through L-tryptophan oxidation, dimerization and bisprenylation. Nat Chem Biol. 2007;3:584–592. doi: 10.1038/nchembio.2007.20. [DOI] [PubMed] [Google Scholar]
- 53.Hahn M, Stachelhaus T. Selective interaction between nonribosomal peptide synthetases is facilitated by short communication-mediating domains. Proc Natl Acad Sci USA. 2004;101:15585–15590. doi: 10.1073/pnas.0404932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.