Abstract
Ultraviolet (UV) radiation plays an important role in the pathogenesis of skin photoaging. Depending on the wavelength of UV, the epidermis is affected primarily by UVB. One major characteristic of photoaging is the dehydration of the skin. Membrane-inserted water channels (aquaporins) are involved in this process. In this study we demonstrated that UVB radiation induced aquaporin-3 (AQP3) down-regulation in cultured human skin keratinocytes. Kanglaite is a mixture consisting of extractions of Coix Seed, which is an effective anti-neoplastic agent and can inhibit the activities of protein kinase C and NF-κB. We demonstrated that Kanglaite inhibited UVB-induced AQP3 down-regulation of cultured human skin keratinocytes. Our findings provide a potential new agent for anti-photoaging. The related molecular mechanisms remain to be further elucidated.
Keywords: Kanglaite, photoaging of skin, aquaporin-3
Introduction
Skin aging is a degenerative process including intrinsic aging which is characterized by dryness, generalized wrinkling and thin appearance. Solar ultraviolet (UV) radiation is the most significant extrinsic factor which causes photoaging of the skin, manifesting as deep wrinkling, severe roughness and dryness. The photoaging of the skin partly overlaps and superimposes the intrinsic dryness (1). Solar UV reaching earth is comprised of UVA (320–400 nm in wavelength) and UVB (280–320 nm). UVB is mostly absorbed by the epidermis and predominantly affects keratinocytes (1,2).
Water movement across the plasma membrane occurs via two pathways: diffusion through the lipid bilayer and via aquaporins (AQPs) (1,3–5). The AQPs act primarily as water-selective pores and facilitate water transport across cell plasma membranes (6). There are at least 13 mammalian AQPs (AQP0-AQP12), which have been divided into two groups on the basis of their permeability. AQP 1, 2, 4, 5 and 8 are primarily water-selective transporters; AQP 3, 7, 9 and 10 transport water, glycerol and other small solutes (7,8).
It has been demonstrated that AQP3 expressed in the basal layer of the epidermis and the deficiency reduce the stratum corneum hydration and glycerol content (4,8,9). After exposure to UV radiation, AQP3 down-regulation reduces the stratum corneum hydration; the deficient water conditions damage the function of the skin, leading to dryness and wrinkle formation (1,10). However, the functions of AQP3 in human skin keratinocytes remain to be further elucidated.
Adequate photoprotection is essential to prevent UV-related damage. Photoprotective agents, such as polyphenols and baicalin, have been demonstrated to be effective in photoprotection via influencing pertinent cell signaling pathways (11). Kanglaite is a mixture consisting of extractions from Coix seed, a Chinese herb, which has been demonstrated to be effective in anticancer treatment via inhibition of COX-2, MMP9, protein kinase C and NF-κB (12,13). We carried out this study to investigate whether Kanglaite has any protective effects against UVB-induced AQP3 down-regulation in cultured human skin keratinocytes.
Materials and methods
UV light apparatus
The UV radiation apparatus used in the study was the Waldmann UV801KL (Waldmann GmbH Co., Germany). The UVB wavelength was 285–350 nm (peak 312 nm). As previously described, (5,15) before UVB radiation, cultured human skin keratinocytes were washed with 1 ml PBS buffer and then changed to 0.5 ml PBS in each well. The cells were radiated at the desired intensity without a plastic dish lid. After UVB radiation, the cells were returned to incubation in basal medium with treatments for various times prior to harvest.
Chemicals and reagents
Rabbit anti-AQP3 was obtained from Chemicon (Temecula, CA). Monoclonal mouse anti-β-actin was obtained from Sigma (St. Louis, MO). Kanglaite was obtained from the Zhejiang Kanglaite Pharmaceutical Co. (Hangzhou, China).
Cell culture
As previously described (5,14), spontaneously immortalized human keratinocytes (HaCaT) were maintained in DMEM medium (Sigma) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), penicillin/streptomycin (1:10; Sigma) and 4 mM L-glutamine (Sigma), in a CO2 incubator at 37°C. For Western blotting, cells were reseeded in 6-well plates at a density of 0.5×106 cells/ml with fresh complete culture medium.
MTT assay
The cell proliferation effect of Kanglaite was determined by the MTT assay. The cells (4×103 cells/ml) were cultured on a 96-well plate in a DMEM medium with different concentrations of Kanglaite (0.5×10−4, 1×10−3, 5×10−3, 1×10−2, 5×10−2, 1×10−1 ml/ml) for 24 h. The cells were next washed with PBS and 200 μl of MTT (0.05 mg/ml) was added to each well, followed by incubation for 4 h at 37°C. The supernatant was removed, and 200 μl of dimethylsulfoxide was added to each well to dissolve the formazan product. Wells without cells were used as blank controls. Absorbance was determined at 570 nm, spectrophotometrically, using an ELISA reader (Tecan, Salzburg, Austria). The results are expressed as the percentage of control cells obtained from six experiments conducted under the same culture conditions.
RNA extraction, reverse transcription and PCR
Total-RNA from cells was extracted using the SV total-RNA Isolation System (Zhongshan, China) following the manufacturer’s instructions. The concentration of total-RNA was determined by measuring the optical density at 260 nm. Total-RNA (1 μg) was converted into first-strand cDNA using the ImProm-II Reverse Transcription system with random primers following the manufacturer’s instructions (Zhongshan). Parallel reactions for each RNA sample were run in the absence of reverse transcriptase to assess any genomic DNA contamination of the RNA.
For the semi-quantitative reverse transcription PCR experiment, the product was amplified using specific primers designed as described before (15,16): AQP3 forward, 5′-GCT GTC ACT CTG GGC ATC CTG-3′ and reverse primers, 5′-GCG TCT GTG CCA GGG TGT AG-3′, amplifying a 131-bp product and the GAPDH forward, 5′-TCC TGT GGC ATC CAC GAA ACT-3′ and reverse primers, 5′-GAA GCA TTT GCG GTG GAC GAT-3′, amplifying a 313-bp product.
The real-time quantitative PCR experiments were carried out in an Rotor-gene 3000 (Australia), using a SYBR-Green PCR Mastermix (Zhongshan). Each sample was analyzed iduplicate along with standard and no-template controls. The reaction contained 30 ng cDNA in 1 μl Mastermix, including pre-set concentrations of deoxyribonucleotide triphosphates, MgCl2, and buffers, along with 300 nM forward and reverse primers and the SYBR-Green reporter dye. The PCR parameters were 95°C for 2 min, 40 cycles at 95°C for 15 sec, 60°C for 1 min and 72°C for 30 sec (Fig. 1). RNA concentrations were determined by comparing cDNA-generated signals in samples with those generated from known amounts of cDNA. RNA levels were corrected with the GAPDH cDNA signal for variations in the amounts of input RNA. The product purity was confirmed using a dissociation standard curve.
Figure 1.
GAPDH and AQP3 real-time quantitative PCR proliferation curves.
Western blot analysis
As reported previously (5,14), cultured skin keratinocytes with or without treatment were washed with cold PBS and harvested by scraping into 100 μl of RIPA buffer. Cell lysates were incubated at 4°C for 30 min. Proteins (20 μg) were denatured in 5X SDS-PAGE sample buffer for 5 min at 95°C. Proteins were separated by 10 or 12% SDS-PAGE gels and transferred onto PVDF membranes (Millipore, Bedford, MA). Nonspecific binding was blocked with 10% dry milk in TBST for 1 h at room temperature. After blocking, membranes were incubated with specific antibodies in dilution buffer (2% BSA in TBS) overnight at 4°C. Blots were incubated with horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG at appropriate dilutions and room temperature for 1 h. Antibody binding was detected using the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences) following the manufacturer’s instructions and visualized by autoradiography with Hyperfilm.
Statistical analysis
The values in the figures were expressed as the means ± standard error (SE). The data in this study are representative of more than three different experiments. Repeated measures of one factor ANOVA was used to analyze the data. The SNK-q assay was performed between the treated groups. The Student’s t-test was performed to detect differences between the Kanglaite and vehicle groups. P<0.05 was considered significant.
Results
Effect of Kanglaite on proliferation in cultured human skin keratinocytes
Cultured skin keratinocytes were treated with 0, 5×10−4, 1×10−3, 5×10−3, 1×10−2, 5×10−2, 1×10−1 ml/ml Kanglaite. The results of the MTT assay showed proliferation rates of 0.093±0.008, 0.963±0.280, 1.140±0.201, 1.073±0.132, 1.055±0.233, 1.068±0.208 and 0.857±0.218, respectively. Kanglaite in all the examined concentrations exhibited no inhibitory effect on the proliferation of cultured skin keratinocytes (P>0.05).
UVB radiation down-regulates AQP3 mRNA in cultured human skin keratinocytes
Cultured skin keratinocytes were radiated with 1, 10, 20 mJ/cm2 UVB. Cells were collected after 24 h of culture. The effect of UVB radiation on gene expression was determined by means of real-time quantitative PCR. The results of a relative quantification analysis revealed that the UVB-induced AQP3 mRNA down-regulation was dose-dependent. The AQP3 mRNA expression was down-regulated after radiation with 1 mJ/cm2 UVB and the down-regulation was most obvious after 20 mJ/cm2 of UVB irradiation (F=19.88, P<0.0005) (Fig. 2A). The UVB-induced down-regulation of AQP3 mRNA was also time-dependent. The AQP3 mRNA expression was first found to be down-regulated at 6 h and was most obvious at 24 h after radiation with 10 mJ/cm2 UVB (F=25.30, P<0.0002) (Fig. 2B).
Figure 2.
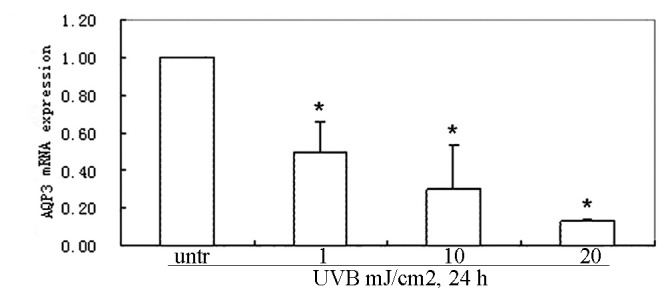
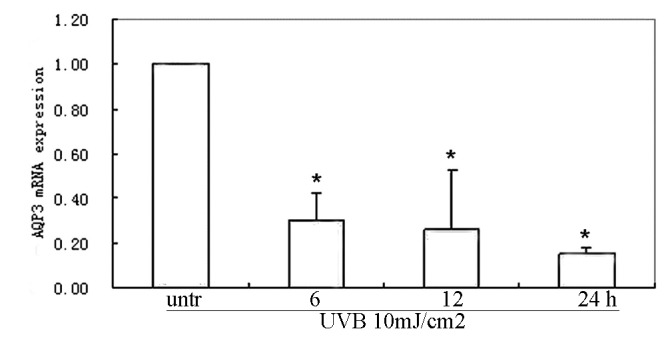
UVB induces down-regulation of AQP3 mRNA expression in a time- and dose-dependent manner in cultured skin keratinocytes (HaCaT cells). (A) HaCaT cells were treated with different UVB doses (1, 10 or 20 mJ/cm2) after 24 h of incubation. (B) HaCaT cells were treated with UVB (10 mJ/cm2) and the AQP3 gene expression was analyzed by real-time quantitative PCR at different times (6, 12 and 24 h) (B). The differences between the treated groups vs. the untreted group were analyzed by the SNK-q assay. The data represent the mean ± SE of three independent experiments. *P<0.05 vs. the untreated (untr) group.
Kanglaite inhibits UVB-induced down-regulation of AQP3 mRNA in cultured human skin keratinocytes
Cultured skin keratinocytes were radiated with 10 mJ/cm2 UVB. Cells were collected after 24 h of incubation with 1, 2.5 or 5 μl/ml Kanglaite and the same concentrations of Kanglaite vehicles were used as controls. Real-time quantitative PCR was performed. Application of 1 μl/ml Kanglaite significantly up-regulated the AQP3 mRNA expression after 24 h incubation (F=−3.84 P=0.0184); 2.5, 5 μl/ml Kanglaite incubation significantly up-regulated the AQP3 transcripts by 8.92±1.04 and 20.20±2.25-fold, respectively. Significant up-regulation was observed in all 1, 2.5 and 5 μl/ml Kanglaite-treated groups vs. the Kanglaite vehicle groups (Fig. 3A). After 6, 12 or 24 h of incubation with 2.5 μl/ml Kanglaite and the vehicles, significant up-regulation of the AQP3 transcripts was only observed in the 24 h samples (F=−4.80, P=0.0086) (Fig. 3B). There were no significant changes in the 6 and 12 h samples between the Kanglaite and vehicle groups.
Figure 3.
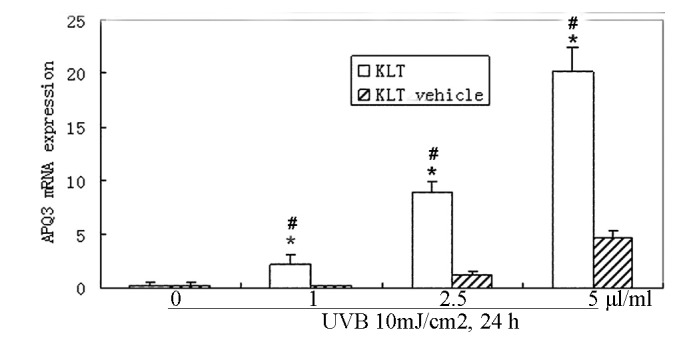
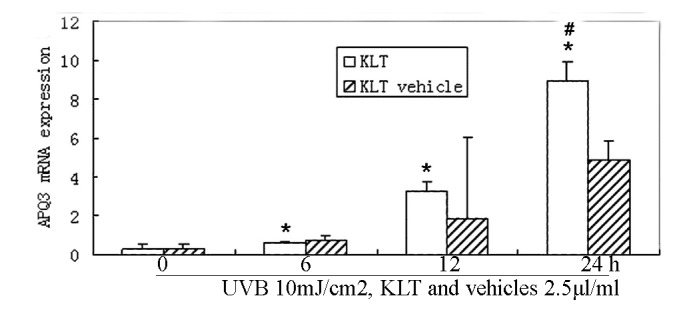
Kanglaite up-regulates the AQP3 gene in a dose-dependent manner in HaCaT cells. (A) HaCaT cells were treated with different doses of Kanglaite (KLT) (1, 2.5 or 5 μl/ml) and the same concentrations of Kanglaite vehicle for 24 h. AQP3 expression was analyzed by real-time quantitative PCR. The differences between the Kanglaite or vehicle treated groups vs. the UV-radiated groups were assessed by the SNK-q assay. The Student’s t-test was used to assess differences between the Kanglaite and vehicle groups. The data represent the mean ± SE of three independent experiments. *P<0.05 vs. the UV-radiated group. #P<0.05 vs. the Kanglaite vehicle group. (B) Kanglaite up-regulates the AQP3 gene in a time-dependent manner in HaCaT cells. HaCaT cells were treated with 2.5 μl/ml Kanglaite and the same concentrations of Kanglaite vehicle for 6, 12 and 24 h. AQP3 gene expression was analyzed by real-time quantitative PCR. The data represent mean ± SE of three independent experiments. *P<0.05 vs. UV-radiated group. #P<0.05 vs. the Kanglaite vehicle group.
UVB irradiation down-regulates AQP3 protein in cultured skin keratinocytes
Cultured skin keratinocytes were radiated with 1, 10 or 20 mJ/cm2 UVB. Cells were collected after 24 h of culture. Western blot analysis showed that UVB irradiation down-regulated the AQP3 protein expression in a dose-dependent manner. AQP3 protein expression began to decrease after 1 mJ/cm2 UVB radiation, and more significantly when radiated with 20 mJ/cm2 UVB compared to untreated samples (Fig. 4A). After being radiated with 10 mJ/cm2 UVB and cultured for 6, 12 or 24 h, AQP3 protein expression was again measured by Western blotting. The results showed that AQP3 began to decrease at 6 h and further decreased at 12 h. After 24 h culture, AQP3 expression began to return towards basal levels compared to that at 12 h (Fig. 4B).
Figure 4.
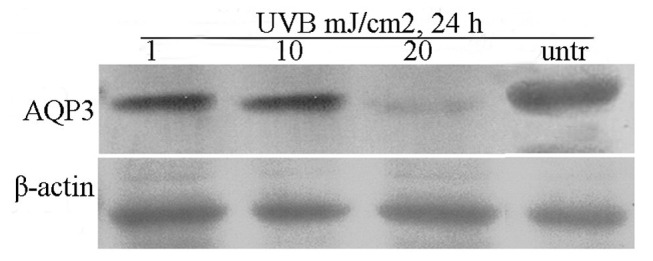
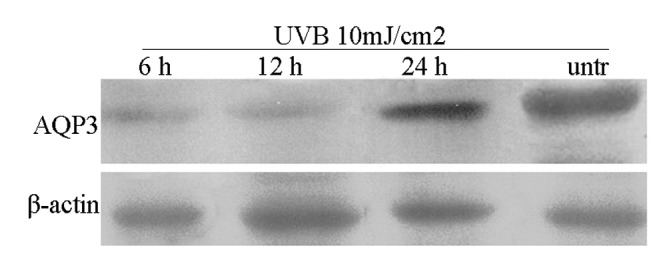
UVB induces AQP3 down-regulation in a time- and dose-dependent manner in HaCaT cells. (A) Cells were treated with different UVB doses (1, 10 or 20 mJ/cm2) and AQP3 expression was analyzed by Western blotting after 24 h. (B) HaCaT cells were treated with UVB (10 mJ/cm2) and AQP3 was analyzed by Western blotting at different times (6, 12 or 24 h). All experiments were repeated at least three times and similar results were obtained.
Kanglaite inhibits UVB-induced down-regulation of AQP3 protein expression in cultured skin keratinocytes
To analyze the effect of Kanglaite on AQP3 protein expression after UVB irradiation, cultured skin keratinocytes were radiated with 10 mJ/cm2 UVB and incubated with 1, 2.5, 5 μl/ml Kanglaite and Kanglaite vehicles, respectively. Application of 1 μl/ml Kanglaite significantly up-regulated AQP3 protein expression after 24 h incubation; 2.5, 5 μl/ml Kanglaite also significantly up-regulated the AQP3 protein expression (Fig. 5A). After 6, 12 or 24 h of incubation with 2.5 μl/ml Kanglaite and the vehicles, significantly up-regulated AQP3 protein expression was observed in the Kanglaite-treated groups. No significant changes in AQP3 protein expression were observed in the vehicle-treated groups (Fig. 5B).
Figure 5.
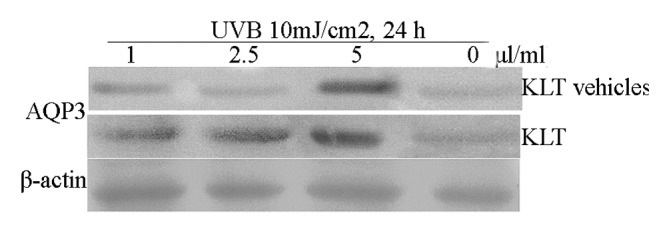
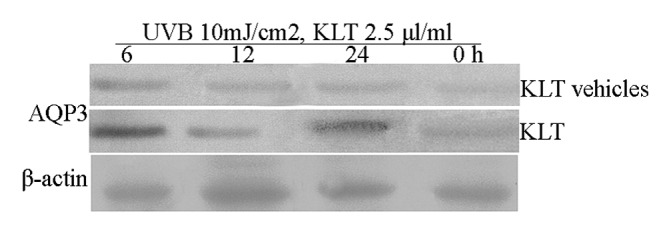
Kanglaite up-regulates AQP3 in a time- and dose-dependent manner in HaCaT cells. (A) HaCaT cells were treated with different doses of Kanglaite (1, 2.5 or 5 μl/ml) and the same concentrations of Kanglaite vehicles for 24 h or (B) treated with Kanglaite (2.5 μl/ml) for different times (6, 12, 24 h). AQP3 expression was analyzed by Western blotting. All experiments were repeated at least three times and similar results were obtained.
Discussion
Some intracellular and extracellular proteins are involved in skin photoaging through regulation of cell signaling pathways such as JNK, ERK, p38 MAP kinase and PI3K/AKT kinases (14,17). Antioxidants and botanical agents such as polyphenols, epigallocathechin-3-gallate, flavonoids, isoflavonoids and all-trans retinoic acid have been demonstrated to be able to block certain pathways to exert their protective effect on skin photoaging (14,18–21).
Dehydration is one of the major events of skin photoaging. Previous studies have shown that AQP3 plays an important role in the regulation of water permeability (22). UV radiation down-regulates AQP3 expression in cultured skin keratinocytes, then decreases water permeability of keratinocytes. Meanwhile the reduction of water permeability is also due to the reduced glycerol transport through AQP3 (14).
UV-induced H2O2, oxidized lipid hydroperoxides and ROS also induce AQP3 down-regulation via activating the MEK/ERK pathway in cultured skin keratinocytes (23). The MEK/ERK pathway is one of the most important pathways in mediating the UV-induced skin photoaging and skin cancer (5,14,24).
In the present study, the results showed that UVB radiation down-regulated AQP3 mRNA and protein expression in a dose and time-dependent manner in cultured skin keratinocytes, which was in accordance with previous reports (5,14).
trans-Zeatin (tZ), retinoids and nicotinamide can up-regulate skin AQP3 expression (17,19,25). In a previous study it was found that tZ inhibits UV-induced MEK-ERK activation. tZ up-regulates AQP3 expression in a dose and time-dependent manner (5). In another study, UV radiation was shown to down-regulate AQP3 expression in cultured skin keratinocytes via reactive oxygen species mediated MEK/ERK pathways. All-trans retinoic acid inhibits the UV-induced AQP3 down-regulation and increases the water permeability of cultured keratinocytes (14). This may provide us the new agents with protective effects against UV-induced photoaging.
The Coix seed has long been used in traditional Chinese medicine for treatment of various diseases, particularly cancer and skin HPV infection. Kanglaite injection is an acetone extract of herbal medicine Coix seed using high performance liquid chromatography pharmaceutical technology. The injection has been approved for the treatment of lung, hepatic, colon, prostate, and esophageal cancer via inhibiting tumor cell mitosis at the boundary of the G2/M phase and inducing apoptosis through activation of the Fas/FasL pathway. Kanglaite treatment results in a significant down-regulation of PTGS2 mRNA, the gene which encodes COX-2 (12). Kanglaite significantly inhibits the growth of human MDA-MB-231 breast cancer cell via inhibiting NF-κB signaling and protein kinase C activity (13).
In this study, we found that Kanglaite inhibits UVB-induced down-regulation of AQP3 expression. The mode of action of Kanglaite is unlike certain ingredients of some cosmetics products which claim to increase epidermal AQP3 expression, and in fact, high AQP3 level may be associated with high risk of skin tumors (7,26). Our findings may provide a new agent with protective effects against UV-induced photoaging and may contribute to potential therapeutic strategies for the treatment and prevention of skin photoaging. The mechanism of the inhibitory effect of Kanglaite needs to be further investigated.
References
- 1. Berneburg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photoimmunol Photomed. 2000;16:239–244. doi: 10.1034/j.1600-0781.2000.160601.x. [DOI] [PubMed] [Google Scholar]
- 2. Young AR. Cumulative effects of ultraviolet radiation on the skin: cancer and photoaging. Semin Dermatol. 1990;9:25–31. [PubMed] [Google Scholar]
- 3. Zelenina M, Brismar H. Osmotic water permeability measurements using confocal laser scanning microscopy. Eur Biophys J. 2000;29:165–171. doi: 10.1007/pl00006645. [DOI] [PubMed] [Google Scholar]
- 4. Verdier-Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6:75–82. doi: 10.1111/j.1473-2165.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 5. Ji C, Yang Y, Yang B, et al. Trans-Zeatin attenuates ultraviolet induced down-regulation of aquaporin-3 in cultured human skin keratinocytes. Int J Mol Med. 2010;26:257–263. doi: 10.3892/ijmm_00000460. [DOI] [PubMed] [Google Scholar]
- 6. Hara-Chikuma M, Verkman AS. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol Cell. 2005;97:479–486. doi: 10.1042/BC20040104. [DOI] [PubMed] [Google Scholar]
- 7. Nakakoshi M, Morishita Y, Usui K, Ohtsuki M, Ishibashi K. Identification of a keratinocarcinoma cell line expressing AQP3. Biol Cell. 2006;98:95–100. doi: 10.1042/BC20040127. [DOI] [PubMed] [Google Scholar]
- 8. Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol. 2008;128:2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 9. Cao C, Sun Y, Healey S, et al. EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J. 2006;400:225–234. doi: 10.1042/BJ20060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roupe G. Skin of the aging human being. Lakartidningen. 2001;7:1091–1095. [PubMed] [Google Scholar]
- 11. Verschooten L, Claerhout S, Van Laethem A, Agostinis P, Garmyn M. New strategies of photoprotection. Photochem Photobiol. 2006;82:1016–1023. doi: 10.1562/2006-04-27-ir-884.1. [DOI] [PubMed] [Google Scholar]
- 12. Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo-or radio-therapy for cancer. Biosci Trends. 2010;4:297–307. [PubMed] [Google Scholar]
- 13. Woo JH, Li D, Wilsbach K, et al. Coix seed extract, a commonly used treatment for cancer in China, inhibits NF-kappa B and protein kinase C signaling. Cancer Biol Ther. 2007;6:2005–2011. doi: 10.4161/cbt.6.12.5168. [DOI] [PubMed] [Google Scholar]
- 14. Cao C, Wan S, Jiang Q, et al. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J Cell Physiol. 2008;215:506–516. doi: 10.1002/jcp.21336. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Bi Z, Chu W, Wan Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int J Mol Med. 2005;16:1117–1124. [PubMed] [Google Scholar]
- 16. Moon C, Rousseau R, Soria JC, et al. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol. 2004;75:128–133. doi: 10.1002/ajh.10476. [DOI] [PubMed] [Google Scholar]
- 17. Peus D, Vasa RA, Beyerle A, Meves A, Krautmacher C, Pittelkow MR. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J Invest Dermatol. 1999;112:751–756. doi: 10.1046/j.1523-1747.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 18. Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 19. Moon HI, Lee J, Kwak JH, Zee OP, Chung JH. Isoflavonoid from Viola hondoensis, regulates the expression of matrix metalloproteinase-1 in human skin fibroblasts. Biol Pharm Bull. 2005;28:925–928. doi: 10.1248/bpb.28.925. [DOI] [PubMed] [Google Scholar]
- 20. Fisher GJ, Talwar HS, Lin J, et al. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher GJ, Datta S, Wang Z, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002;34:192–200. [PubMed] [Google Scholar]
- 23. Kang S, Chung JH, Lee JH, et al. Topical N-acetylcysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol. 2003;120:835–841. doi: 10.1046/j.1523-1747.2003.12122.x. [DOI] [PubMed] [Google Scholar]
- 24. Kim MS, Kim YK, Eun HC, Cho KH, Chung JH. All-trans retinoic acid antagonizes UV-induced vegf production and angiogenesis via the inhibition of ERK activation in human skin keratinocytes. J Invest Dermatol. 2006;126:2697–2706. doi: 10.1038/sj.jid.5700463. [DOI] [PubMed] [Google Scholar]
- 25. Song X, Xu A, Pan W, et al. Nicotinamide attenuates aquaporin 3 overexpression induced by retinoic acid through inhibition of EGFR/ERK in cultured human skin keratinocytes. Int J Mol Med. 2008;22:229–236. [PubMed] [Google Scholar]
- 26. Verkman AS. A cautionary note on cosmetics containing ingredients that increase aquaporin-3 expression. Exp Dermatol. 2008;17:871–872. doi: 10.1111/j.1600-0625.2008.00698.x. [DOI] [PubMed] [Google Scholar]


