Abstract
Microcystins (MCs), a cyclic heptapeptide hepatotoxins, are mainly produced by the bloom-forming cyanobacerium Microcystis, which has become an environmental hazard worldwide. Long term consumption of MC-contaminated water may induce liver damage, liver cancer, and even human death. Therefore, in addition to removal of MCs in drinking water, novel strategies that prevent health damages are urgently needed. Sulforaphane (SFN), a natural-occurring isothiocyanate from cruciferous vegetables, has been reported to reduce and eliminate toxicities from xenobiotics and carcinogens. The purpose of the present study was to provide mechanistic insights into the SFN-induced antioxidative defense system against MC-LR-induced cytotoxicity. We performed cell viability assays, including MTS assay, colony formation assay and apoptotic cell sorting, to study MC-LR-induced cellular damage and the protective effects by SFN. The results showed that SFN protected MC-LR-induced damages at a nontoxic and physiological relevant dose in HepG2, BRL-3A and NIH 3 T3 cells. The protection was Nrf2-mediated as evident by transactivation of Nrf2 and activation of its downstream genes, including NQO1 and HO-1, and elevated intracellular GSH level. Results of our studies indicate that pretreatment of cells with 10 μM SFN for 12 h significantly protected cells from MC-LR-induced damage. SFN-induced protective response was mediated through Nrf2 pathway.
Keywords: Microcystin-LR (MC-LR), Sulforaphane (SFN), NF-E2-related factor 2 (Nrf2), Phase II enzymes, Glutathione (GSH), Chemoprevention
Introduction
Microcystins (MCs) are a family of cyclic heptapeptide hepatotoxins produced by freshwater species of microcystis. MCs have been implicated in the development of liver cancer, necrosis and even deadly intrahepatic bleeding (Carmichael, 1994). A common feature of these monocyclic peptide toxins is that they share a distinctive 20-carbon amino acid, 3-amino-9-methoxy -2,6,8-trimethyl-10-phenyldeca -4,6-dienoic acid (Adda). The Adda component has been known to be a key player for the toxicity of these compounds (Fig. 1A). Also, some intoxication episodes caused by toxic cyanobacterial booms have been reported. Yu et al (Ueno et al., 1996) have found a close correlation between the incidence of Primary Liver Cancer (PLC) in Haimen City, Jiangsu Province and MCs in drinking water through a two-year (1993–1994) epidemiological survey, and hypothesized that the MCs in the drinking water is one of the risk factors for the high incidence of PLC in Haimen city. In 1996, an incident, which caused the death of 50 people, occurred in Caruaru, Brazil due to the use of microcystin contaminated hemodialysis water (Jochimsen et al., 1998). Mounting evidence shows that many domestic animals, fish and humans had been taken ill or killed by drinking water with cyano-toxin in some cases (Falconer, 2001). Among the Microcystins, Microcystin-LR (MC-LR) is the most toxic MCs variant, and is also the most commonly encountered in a contaminated aquatic system. Due to its high toxicity, the World Health Organization (WHO) has established a safe guideline with the recommended upper limit of 1 μg L−1 of MC-LR in the drinking water for human daily consumption (WHO, 1998).
Fig. 1.
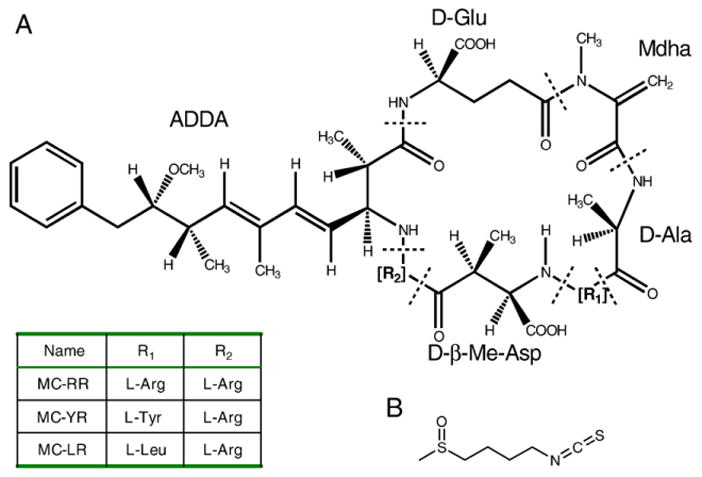
Chemical structure of microcystins (A) and sulforaphane (B).
In China, eutrophication in many lakes has become a serious environmental threat. It is estimated that about 85% of lakes are eutrophicated and the rate is still increasing. The dominant species in these lakes are Microcystins-producing microcystis spp.. Many freshwater lakes, streams and reservoirs along the Changjiang (Yangtze) River basin are heavily polluted by the increasing economic development activities. In 2007, a huge blue-green algae bloom in China’s third-largest lake (Lake Tai) appeared, which was responsible for the contaminated drinking water supplies in Wuxi city. The lake pollution poses a greater nationwide problem; cell density of the cyanobacteria bloom has reached up to more than 2 billion per liter in some of the biggest lakes in China, such as Lake Dianchi, Lake Chao and Lake Tai.
Clearly, the most effective way to reduce microcystin contamination in drinking water is to inhibit the growth of Microcystis in water resources. Sophisticated water treatment to reduce and remove microcystin is another option. However, these operations are both technically and economically challenging, especially in developing countries such as China. Therefore, novel strategies to prevent MC-induced health hazards have become important options and are urgently needed. Recently, Vitamin E has been implicated in the protection against chronic exposure to MC-LR through reducing lipid peroxidation and limiting toxin-induced ALT leakage and GST activity inhibition (Gehringer et al., 2003).
Sulforaphane (Fig. 1B), a natural-occurring isothiocyanate (ITC) from cruciferous vegetables such as broccoli sprouts, is an effective cancer chemoprotective agent in cell culture and animal studies. Epidemiological studies have also shown that intake of dietary ITC is associated with reduced risk of human cancer in various types, including lung, breast, colon, and prostate (Spitz et al., 2000; Fowke et al., 2003; Lin et al., 1998; Joseph et al., 2004). Several mechanisms, including suppression of cytochrome P450 enzymes, induction of cell cycle arrest and apoptotic pathways, inhibition of angiogenesis and anti-inflammatory activity, have been proposed for chemoprevention activity of SFN. It is believed that a major mechanism by which SFN protects cells is through NF-E2-related factor 2 (Nrf2)-mediated induction of phase II enzymes that protect cells from oxidative stress and carcinogens (Dinkova-Kostova et al., 2002; Jeong et al., 2006). Nrf2, a transcription factor belonging to the cap’n’collar subfamily, is pivotal in regulating cellular responses to oxidative and electrophilic insults. Its target genes are crucial in maintaining cellular redox homeostasis and facilitating xenobiotic metabolism. SFN has been proposed to release Nrf2 from Keap1-Nrf2 cytoplasmic complex by covalently binding to some key cysteines in Keap1. Consequently, the released Nrf2 translocates into the nucleus, binds to the antioxidant response element (ARE), and activates transcription of ARE-dependent phase II enzymes (Dinkova-Kostova et al., 2002). Other studies also indicate that SFN stabilizes Nrf2 protein probably through its inhibition of Keap1-dependent proteasomal degradation (Kobayashi et al., 2004). In either case, Nrf2 is essential for SFN-induced antioxidative response. Nrf2-regulated phase II enzymes include NAD(P)H:quinone oxidoreductase 1 (NQO1) and Heme oxygenase-1(HO-1). NQO1 catalyzes the reduction of quinones, protecting cells against redox cycling and oxidative stress (Riley and Workman, 1992), and reduces the toxic metabolite of acetaminophen back to the parent compound in vitro (Moffit et al., 2007). HO-1 catalyzes the breakdown of heme into bilirubin, carbon monoxide, and iron. The significance of Nrf2 as a novel molecular target for cancer chemoprevention has been further confirmed in studies using Nrf2-null mice. Nrf2-null mice have reduced basal and induced levels of antioxidative enzymes and are more susceptible to toxicants and carcinogens (Hayes et al., 2000; Cho et al., 2002; Rangasamy et al., 2004; Kensler et al., 2007).
A wide variety of compounds isolated from dietary sources or plants are capable of activating Nrf2 pathway. However, the most attractive Nrf2 activators are those with less toxic effects. These agents can be classified into five categories: 1) phenolic antioxidants (caffeic acid, epigallocatechin-3-gallate, butylated hydroxyanisol); 2) dithiolethiones (oltipraz, 3H-1,2-dithiol-3-thione); 3) triterpenoids (CDDO-Im, Yates et al., 2009; oleanolic acid, Reisman et al., 2009); 4) diterpenoid (Oridonin, Du et al., 2008); and 5) isothiocyanates (SFN). The protective effects of SFN against Arsenic-induced cytotoxity through activation of the Nrf2-mediated defensive response have been demonstrated in the previous studies (Shinkai et al., 2006; Cornblatt et al., 2007). And SFN is available in people’s daily life and less toxic effects with low dose. We hypothesized that SFN, a powerful indirect antioxidant and strong Nrf2 activator, could prevent MC-LR, an electrophile and reactive oxygen species (ROS) inducer, induced cell cytotoxicity. In this study, we found that SFN is able to effectively protect MC-LR-induced cell damage in a variety of cell lines at non-toxic and physiological concentrations through the Nrf2-dependent defensive response, supporting a novel perspective in using SFN as a protective agent against MC-LR-caused cellular damage.
Materials and methods
Chemicals and reagents
Microcystin-LR was purified from our lab. Sulforaphane (SFN), tert-Butylhydroquinone (tBHQ) and all other reagents were the highest grade available from Sigma-Aldrich, unless otherwise noted. MC-LR, SFN, L-Buthionine-sulfoximine (BSO) and tBHQ were dissolved in dimethyl sulfoxide (DMSO) and stored at −20 °C until use.
Cells and culture conditions
HepG2 (Human hepatocellular liver carcinoma cell line), BRL-3A (rat hepatocyte cell line), and NIH 3 T3 (Mouse embryonic fibroblast cell line) cells were obtained from Cell Bank of Chinese Academic of Science and grown in DMEM medium supplemented with 10% (v/v) fetal bovine serum, 100 unit/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37 °C and 5% CO2.
Transient transfection of siRNA
For the small interfering RNA (siRNA) experiments, 20 nM of control siRNA (Qiagen) and Nrf2-specific siRNA (SI03246950, Qiagen) were used. Cells were incubated with siRNA or control siRNA in serum-free Opti-MEM without antibiotics using Oligofectamine transfection reagent (Invitrogen). After 4 h, Opti-MEM with 2× concentrated serum was added, resulting in a final concentration of 10% FBS. Cells were incubated for 48 h before treatment.
Cell lysates preparation and immunoblot analysis
After treatment, cells were lysed in a buffer containing 50 mM Tris-HCl, 10 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 1 mM DTT, for 20 min on ice. Cytosolic fractions were obtained as supernatant after centrifugation at 15,000×g for 10 min at 4 °C. The pellet was resuspended in nuclear extraction buffer (20 mM Hepes, pH 7.9, 0.5 M NaCl, 1 mM EDTA, 20% glycerol, 1 mM DTT) for 20 min on ice, followed by centrifugation at 15,000×g for 10 min at 4 °C. Proteins (20 μg) were loaded on a SDS-polyacrylamide gel, separated by electrophoresis, and electroblotted onto PVDF membranes (Millipore). Immunoblot analysis was performed with specific antibodies and enhanced chemoluminescence-based detection (Millipore). Antibodies against Nrf2, Keap1, NQO1, and HO-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β-actin was purchased from Sigma (St. Louis, MO).
Measurement of intracellular GSH
The intracellular GSH concentration was measured using Glutathione Assay kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All the procedures were followed according to the manufacturer’s instructions. All the experiments described in this section were performed in triplicates to obtain means and standard deviations.
Cell viability assay (MTS)
HepG2 and NIH 3 T3 cells were seeded in 96-well plates at a density of 5000 cells/well for 24 h before being treated with MC-LR for 24 h in the presence or absence of 10 μM SFN pretreatment for 12 h. The cell viability assay was performed using the CellTiter 96 Aqueous non-radioactive cell proliferation assay kit (Promega). The absorbance was read at 490 nm on an ELISA reader (Bio-rad, USA). The data were expressed as percent of cell viability compared with that of the control, which was treated with 0.1 % dimethyl sulfoxide (DMSO) and the percentage of cell survival was obtained. The values presented are means (n=3)±SD.
Colony Formation Assays
Cells were treated with 10 μM and 20 μM MC-LR for 24 h in the presence or absence of 10 μM SFN pretreatment for 12 h, trypsinized, and seeded in plates at 600 cells for HepG2 and 500 cells for NIH 3 T3 per plate. Colonies were stained with crystal violet and counted 2–3 weeks after seeding.
Detection of apoptotic cells
Apoptotic cell death was conducted with an Annexin V-FITC apoptosis detection kit (BD Pharmingen) according to the manufacturer’s protocol. A FACScan flow cytometer (Beckman) was used for detection of cell death.
Data analysis
At least three independent experiments were conducted for all analyses. Data were stored and analyzed on personal computers using Excel 2003 (Microsoft) and origin 7.5 (software). Values are expressed as means±standard deviation. A student’s t-test and the Mann-Whitney test were used to compare averaged values and P values of < 0.05 were considered statistically significance.
Results
Cytotoxicity of MC-LR on HepG2 and BRL-3A cells
To study the cytotoxicity of MC-LR to liver cells, we first characterized the concentration dependence of MC-LR-induced cell death in HepG2 cells, a cell line with a highly responsive Nrf2 pathway. As shown in Fig. 2A, treatment with MC-LR for 24 h reduced cell survival in a concentration-dependent manner. MC-LR concentration with substantial reduction in cell survival was 10 μM and IC50 concentration was at 21.9 μM. We repeated experiments on BRL-3A, a rat liver-derived cell line. MC-LR concentration with substantial reduction in cell survival was 5 μM and IC50 concentration was 7.5 μM. It seems that the BRL-3A cell is more sensitive in MC-LR-induced cell death than HepG2 cells.
Fig. 2. Cytotoxicity of MC-LRon HepG2 and BRL-3A cells.
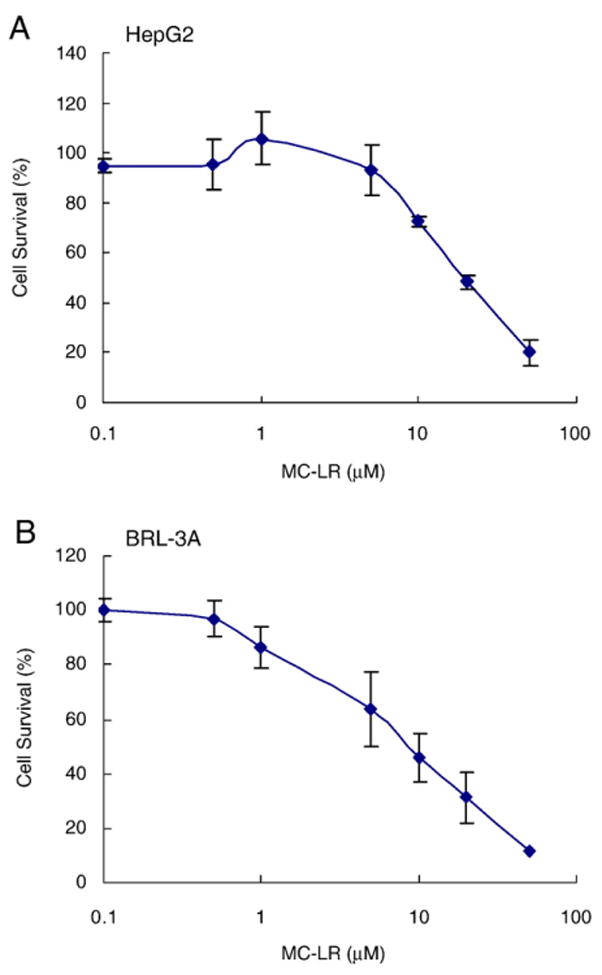
MC-LR is toxic to HepG2 (A) and BRL-3A (B) cells. Cells seeded in 96-well plates were treated with increasing concentrations of MC-LR for 24 h before the MTS assay.
SFN pretreatment blocks MC-LR-induced cell death
To demonstrate SFN-induced cytoprotection towards MC-LR-induced cell death, HepG2 cells were treated with 10 μM MC-LR for 24 h with and without pretreatment with SFN (1–20 μM) for 12 h. Results from MTS assay (Fig. 3A) show that MC-LR significantly reduced the cell viability to 64±1.13% (p<0.005). However, pre-treatment with 10 μM SFN for 12 h completely blocked MC-LR caused cell death (100.1±2.04%, p<0.005. The protection by SFN was also time- and dose-dependent (Figs. 3A and B). Substantial protection can be achieved by pretreatment with 1 μM SFN for 12 h or with 10 μM SFN for 6 h. Similar cytoprotection was also observed in BRL-3A cells (Supplemental Fig. 1). Since BRL-3A is more sensitive to MC-LR-induced cell death, only 5 μM MC-LR was used to induce toxicity.
Fig. 3.
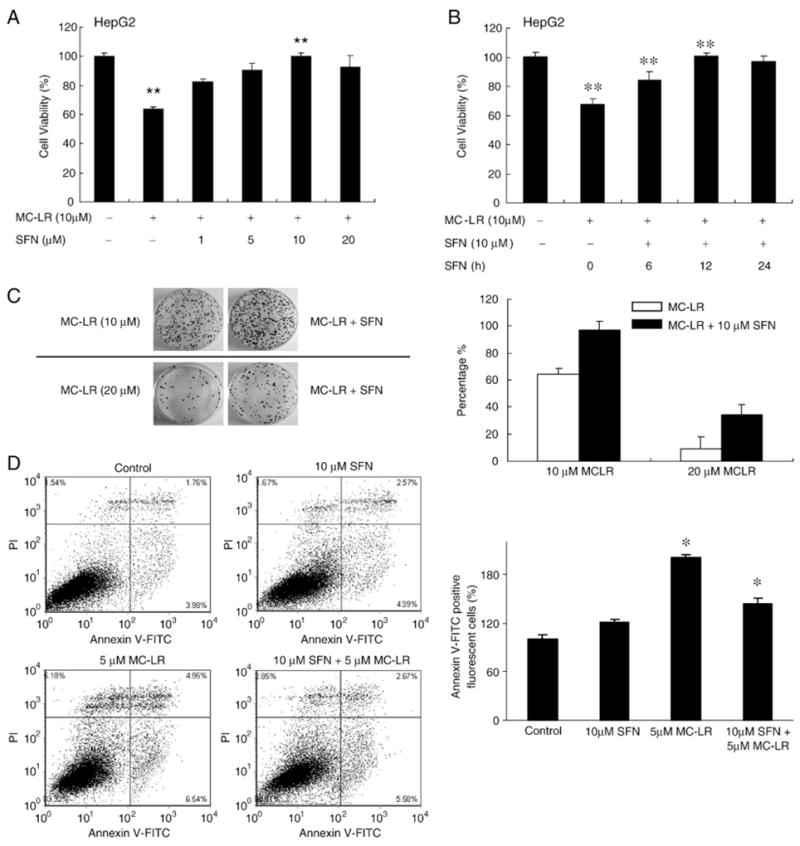
SFN protects HepG2 cells from MC-LR-induced cytotoxicity. (A) HepG2 cells were pretreated with the indicated concentrations of SFN for 12 h, and then treated with MC-LR for 24 h. (B) is the assay results of different pretreatment times. 0 h means cells were treated with SFN and MC-LR at the same time. The cell viability was determined by MTS assay. Each value represents the mean±S.D. of three determinations. **, p<0.01. (C) Colony formation assay confirms cytoprotection by SFN. Left, colony formation images in HepG2 clones after 2 weeks. Cells were pretreated with or without 10 μM SFN for 12 h and then treated with MC-LR for another 24 h, as indicated in materials and methods. Right, percentage of viable HepG2 cells after 2 weeks with MC-LR treatment for 24 h in the presence or absence of SFN. Error bars indicate the S.D. (n=3). (D) SFN pretreatment inhibits MC-LR-induced apoptotic cell death. Cell death in HepG2 cells with or without pretreatment with 10 μM SFN for 12 h and then treated with MC-LR for another 24 h. Apoptotic cell death was detected using Annexin V-FITC staining and flow cytometry. The bar graph was the proportion of early apoptotic cells plus late apoptotic cells comparing with control; the mean±SD was calculated from experiments run in triplicate (right).
Additionally, SFN (10 μM) and MC-LR (10 μM) were mixed and incubated at 37 °C for 24 h, then analyzed by reverse-phase HPLC. No additional peaks were detected and the size of the original peaks were not changed (data not shown), ruling out possibilities that the inhibition of cell death was due to reaction between SFN and MC-LR and thus lowered cellular uptake of MC-LR.
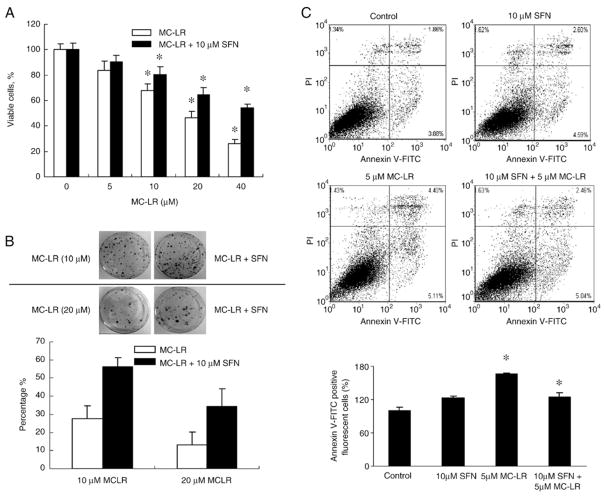
To study whether SFN induces cytoprotection against MC-LR toxicity in normal cells, we treated NIH 3 T3 cells, a non-cancerous cell line used to show cell transformation by carcinogens (Sutherland et al., 1985), with up to 40 μM MC-LR for 24 h with and without 10 μM SFN pretreatment for 12 h. The cell viability assay (Fig. 4A) shows that SFN was able to protect against all of MC-LR concentrations. Interestingly, the SFN protection was more obvious at higher MC-LR concentrations. SFN pretreatment increased viability of cells treated with 40 μM MC-LR from 24% to 53%.
Fig. 4.
SFN induces antioxidative protection in NIH 3 T3 cells. (A) SFN pretreatment improves cell viability suppressed by MC-LR. Cell survivals in NIH 3 T3 cells with or without pretreatment with 10 μM SFN for 24 h and then treated with MC-LR for another 24 h were measured by the MTS assay. (mean±S.D. of three experiments; *, p<0.05). (B) Colony formation assay confirms cytoprotection by SFN. Top, colony formation images in NIH 3 T3 clones after 3 weeks. Cells were pretreated with or without 10 μM SFN for 12 h and then treated with MC-LR for another 24 h, as indicated in materials and methods. Bottom, percentage of viable NIH 3 T3 cells after 3 weeks with MC-LR treatment for 24 h in the presence or absence of SFN. Error bars indicate the S.D. (n=3). (C) SFN pretreatment inhibits MC-LR-induced apoptotic cell death. Cell death in NIH 3 T3 cells with or without pretreatment with 10 μM SFN for 12 h and then treated with MC-LR for another 24 h. Cell death was detected using Annexin V-FITC staining and flow cytometry. The bar graph was the proportion of early apoptotic cells plus late apoptotic cells comparing with control; the mean±SD was calculated from experiments run in triplicate (bottom).
To further confirm the protection, we performed a colony formation assay using HepG2 and NIH 3 T3 cells. Results (Figs. 3C, 4B) show that treatment with 10 and 20 μM MC-LR dramatically reduced the number of colonies formed. However, pretreatment of cells with 10 μM SFN for 12 h more than doubled the colony numbers at both MC-LR concentrations.
Additionally, apoptotic cell death by MC-LR with and without SFN pretreatment was also quantified using Annexin V-FITC staining assay in HepG2 and NIH 3 T3 cells. Fig. 3D shows that treatment in HepG2 cells with 5 μM MC-LR for 24 h increased the percentage of apoptotic cells, whereas pretreatment followed by cotreatment with 10 μM SFN reduced apoptotic cell death (5.74 in control, 11.5 in MC-LR treated, 8.25 in cotreated). Fig. 4C also shows that the proportion of apoptotic cells was decreased by cotreatment with 10 μM SFN and 5 μM MC-LR in NIH 3 T3 cells (5.74 in control, 9.51 in MC-LR treated, 7.50 in cotreated). It should be noted that SFN alone at 10 μM only induced apoptosis slightly.
SFN activates Nrf2-mediated phase II enzymes and GSH
SFN is known to protect cells from oxidative stress or electrophilic attack by activating Nrf2-mediated signaling pathway (Prestera and Talalay, 1995; Dinkova-Kostova et al., 2002). To understand whether SFN-induced cytoprotection against MC-LR is also done through this pathway, we treated HepG2 cells with SFN and examined the Nrf2 levels in both cytoplasmic and nuclear fractions. Results (Fig. 5A) show that Nrf2 was rapidly enriched in the nuclear fraction after 1 h treatment with 10 μM SFN and the enrichment lasted for at least 8 h. Separate experiments indicate that substantial enrichment of Nrf2 in nucleus was observed in cells treated with as low as 5 μM SFN for 2 h (Fig. 5B). The Nrf2 enrichment was dose-dependent up to 20 μM. In contrast, the band intensity of Nrf2 in the cytoplasmic fraction of cell lysate was clearly reduced in agreement with the translocation followed by accumulation of Nrf2 in nucleus. The level of Keap1 in both fractions were not significantly changed, consistent with a previous study showing that SFN was unable to induce a switch of ubiquitination/degradation from Nrf2 to Keap1 (Zhang et al., 2005). SFN-induced NQO1 and HO-1 protein level increases were also observed by immunoblots (Fig. 5C).
Fig. 5.
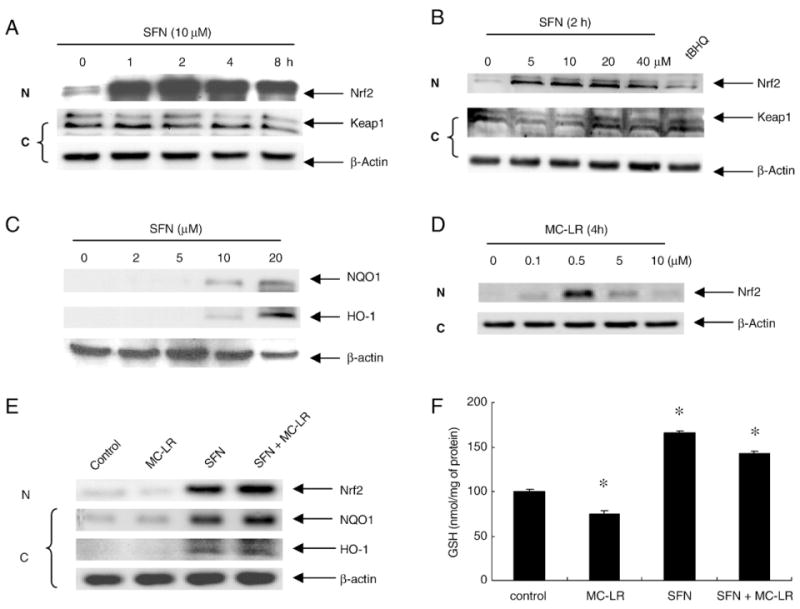
SFN effects on activation of Nrf2 pathway in HepG2 cells. (A) and (B), Nrf2 is enriched in nucleus after SFN treatment. (A) Time-course study. Nuclear (N) and cytoplasmic fractions (C) were extracted from cell lysate of HepG2 treated with 10 μM of SFN. (B) Dose-response study. The cells were treated with 0, 5, 10, 20, 40 μM of SFN for 2 h. tBHQ, a known Nrf2 pathway inducer, was used as a positive control. (C) SFN induces NQO1 and HO-1 expression. Cytoplasmic fractions were probed for NQO1 and HO-1. (D) Increase in nuclear Nrf2 protein in response to MC-LR. HepG2 cells were treated with MC-LR (0, 0.1, 0.5, 5, 10 μM) for 4 h. Cytosolic and nuclear fractions were prepared from each cell line and subjected to western blot analysis with anti-Nrf2 and β-actin antibody respectively. (E) HepG2 cells treated with 10 μM of MC-LR, 10 μM of SFN, and (SFN+MC-LR) for 4 h. Nuclear and cytoplasmic fractions were extracted from cell lysate. Nuclear fractions were probed for Nrf2, and cytoplasmic fractions were probed for HO-1 and NQO1. (F) Intracellular GSH was enhanced by SFN alone and (SFN + MC-LR) treatment. Cells treated with MC-LR alone, SFN alone and (SFN + MC-LR) for 12 h. Each value represents the mean±S.D. of three determinations. *, p<0.05.
To find out whether Nrf2 plays a role in SFN protection against MC-LR induced toxicities, we test the protein level of Nrf2 in nuclear fractions with MC-LR treatment alone in HepG2 cells. Fig. 5D shows that MC-LR enhanced the levels of Nrf2 protein in a dose-dependent manner and the highest induction was at 0.5 μM, but Nrf2 protein levels decreased with high doses of MC-LR (>5 μM). Treatment with high dose of MC-LR could inhibit Nrf2 activation and allow cells to undergo cell death probably due to Nrf2 regulation on cellular survival response. We further compared among treatment groups of MC-LR alone, SFN alone and (SFN+MC-LR) regarding Nrf2 activation, HO-1 and NQO1 expressions (Fig. 5E). The results show that treatment with SFN alone and (SFN + MC-LR) enriched Nrf2 in the nucleus. The protein levels of HO-1 and NQO1 were significantly induced by SFN alone and (SFN + MC-LR) treatment. The results suggest that Nrf2 activation by SFN pretreatment enhanced cellular survival response and blocked high dose MC-LR induced toxicities.
Previous studies report that SFN treatment triggers cellular GSH level elevation (Kim et al., 2003). To confirm this, we measured the level of intracellular GSH after treating HepG2 cells with MC-LR alone, SFN alone and (SFN+MC-LR). As shown in Fig. 5F, SFN treatment resulted in an increase of GSH level with a maximal 1.65-fold of the control at 10 μM for 12 h and cotreatment resulted in an increase with 1.43-fold. Our results confirm previous findings that SFN-induced cytoprotective responses may involve both phase II enzymes induction and antioxidant GSH elevation (Dinkova-Kostova et al., 2002; Kim et al., 2003).
To study whether SFN-induced antioxidative responses are cell line-specific, we treated two more cell lines, BRL-3A and NIH 3 T3, with 0, 1, 2, 5, 10 and 20 μM of SFN for 2 h. The nuclear fractions were extracted and blotted for Nrf2 analysis. The results show that SFN rapidly enriched Nrf2 in the nucleus in both cell lines. The enrichment was generally dose-dependent with the optimal SFN concentrations of 5 and 10 μM. Additionally, results also show that NQO1 and HO-1 protein levels and GSH levels were significantly induced by SFN treatment for 12 h (Supplemental Fig. 2). The results suggest that SFN-induced antioxidative response is unlikely to be cell line-specific.
Nrf2 plays an essential role in SFN-mediated protection against MC-LR-induced toxicity
To study the role of Nrf2 pathway in SFN-mediated protection against MC-LR-induced toxicity, we transiently knocked down Nrf2 in NIH 3 T3 and HepG2 cells by siRNA oligonucleotides. After 48 hours of transfection, cells were treated with either DMSO or 10 μM SFN for 4 h. Gene silencing against Nrf2 suppressed SFN-induced Nrf2 translocation and expression of NQO1 and HO-1 in NIH 3 T3 (Fig. 6A) and HepG2 (Supplemental Fig. 3A). The cell viability assay indicates that the selective silencing of Nrf2 abolished the cytoprotection by SFN against MC-LR-induced cell death in NIH 3 T3 (Fig. 6B) and HepG2 (Supplemental Fig. 3B). These results indicate that Nrf2 pathway plays a role in the SFN-induced cytoprotection. This notion was also supported by the assay assessing the proportion of apoptotic cells by MC-LR in NIH 3 T3 cells (Fig. 6C). Cells transfected with Nrf2-siRNA had more apoptotic cells when treated with a combination of SFN and MC-LR than MC-LR alone, contrary to the results indicated in Fig. 4C. Nrf2 is a key factor mediated the protective antioxidant response. Knockdown of Nrf2 mRNA inhibits the protection on oxidative stress, which was proved by MC-LR treated alone on Nrf2 cells and Nrf2-deficient cells (9.51 on NIH 3 T3 cells, 13.54 on NIH 3 T3-Nrf2 deficient cells). And more apoptosis was obtained with cotreatment of SFN and MC-LR in Nrf2-deficient cells (13.54 in MC-LR treated, 14.3 in cotreated). Therefore, SFN pretreatment aggravated MC-LR-induced cell death in Nrf2-deficient cells probably due to the loss of Nrf2-dependent defensive response.
Fig. 6.
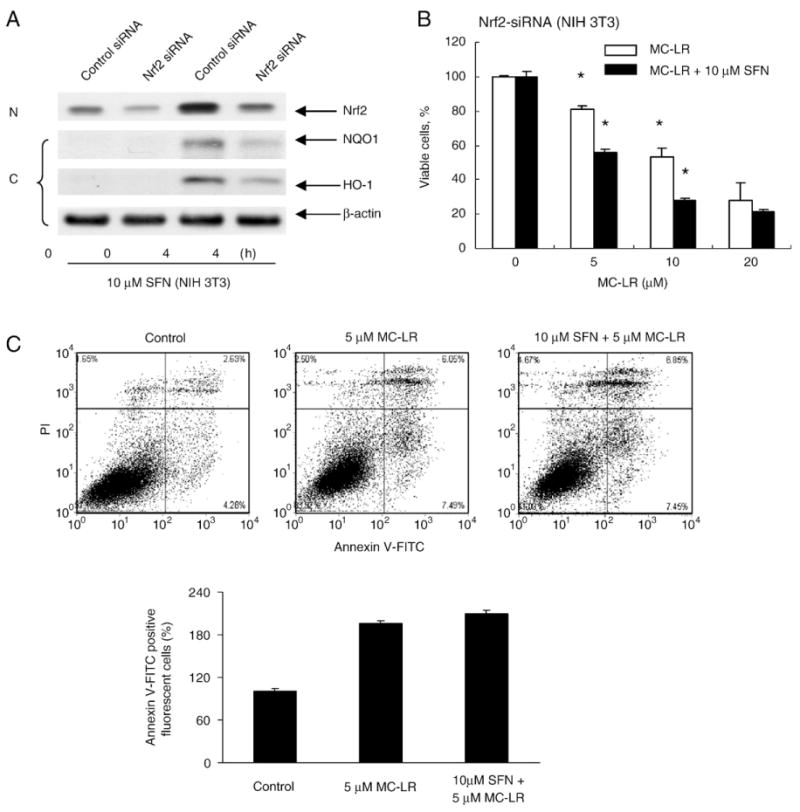
Nrf2 pathway is specifically responsible for SFN-induced cytoprotection. (A) Nrf2 siRNA is effective in silencing Nrf2 translocation and activation of target genes. NIH 3 T3 cells were transfected with either control oligos or Nrf2 siRNA. After transfection, cells were exposed to SFN for 4 h. Nuclear extracts were prepared to analyze the distribution of Nrf2, and cytoplasmic extracts were prepared to analyze the NQO1, HO-1 and β-actin expression. (B) Nrf2 silencing voids SFN-induced cytoprotection against MC-LR toxicity. NIH 3 T3 cells were transfected with Nrf2-siRNA for 48 h before treated with either MC-LR alone or a combination of MC-LR and SFN for 24 h. The cell survival rate was measured by the MTS assay. Error bars indicate the S.D. (n=3). *p<0.05. (C) The specificity of Nrf2 pathway in SFN-induced cytoprotection is confirmed by Annexin V-FITC assay. More apoptotic cells were observed in NIH 3 T3 cells transfected with Nrf2 siRNA and treated with SFN and MC-LR than those with only MC-LR.
Discussion
MC-LR, a main poison in the cyanobacerium Microcystis-contaminated lakes and rivers, poses an imminent threat to public health around the world. Chronically drinking water with trace amounts of MC-LR may cause severe organ damage to both animals and humans. In addition to sophisticated and costly water treatment to remove either Microcystis or its metabolic product-MCs, strategies to minimize its poisonous effects on animal and human health by enhancing detoxifying systems are very important, but rarely investigated. In this study, we demonstrated that pretreatment of a variety of cultured cells with SFN effectively induced antioxidative protection against cytotoxicity caused by MC-LR, providing a promising prospect that SFN may be used as a protective agent against MC-LR-contaminated drinking water.
Previous studies indicated that MC-LR, an electrophile, rapidly conjugates with GSH. In fact, the detoxification of MC-LR in the liver is known to occur via conjugation to glutathione with and without glutathione-S-transferases (GSTs) (Pflugmacher et al., 1998; Takenaka, 2001; Gehringer et al., 2004). Consequently, cellular GSH level is significantly reduced with elevated oxidative stress (Zegura et al., 2006). Therefore, the best approach to protect cells from MC-LR is to enhance antioxidative activities. It has been reported that SFN can effectively reduce cytotoxicity caused by a variety of compounds, like menadione, tert-butyl hydroperoxide, 4-hydroxynonenal, and arsenic trioxide (Gao et al., 2001; Shinkai et al., 2006; Yoon et al., 2008).
This study shows that SFN protects cells from MC-LR-induced toxicity through activation of the Nrf2 pathway. The Nrf2 signaling pathway has been reported to confer protection against chemical carcinogens-induced cytotoxicity (Shinkai et al., 2006; William and Thomas, 2008; Du et al., 2008). Here, we confirmed in several cell lines that SFN induced Nrf2 translocation to the nucleus and activated its downstream target genes encoding proteins, such as phase II enzymes NQO1 and HO-1. Induction of NQO1 and HO-1 has been established as a strong cellular defense against oxidative stress (Zhang, 2006). Also, we observed an intracellular GSH level increase after SFN treatment. Since γ-glutamylcysteine synthetase, a phase II enzyme regulated by Nrf2 pathway, is the rate-limiting enzyme in GSH biosynthesis, the GSH level increase may reflect the induction of γ-glutamylcysteine synthetase by SFN (Kong et al., 2001; Brook et al., 2001). Interestingly, SFN-induced antioxidative activities were abolished when Nrf2 was knocked down, indicating a specific role of Nrf2 pathway in SFN-induced cytoprotection against MC-LR. The discovery of the target pathway is significant because it provides a signal that leads to identify compounds with better efficacy. In this study, we found that the cytoprotection induced by SFN is evident at low concentration ranges of below 10 μM, which is physiologically attainable. Limited Nrf2 accumulation and cytoprotection were observed at high concentrations of SFN (40 μM for HepG2 cells, 20 μM for NIH 3 T3 cells), consistent with previous findings that SFN can induce substantial apoptosis in cells at high concentrations (Kong et al., 2001; Kim et al., 2003). Therefore, it is conceivable that lower SFN concentration may achieve optimal Nrf2-mediated antioxidative protection. Our data also show that substantial protection by SFN against MC-LR is time-dependent, and thus it is promising that long term protection against trace amounts of MC-LR in drinking water is achievable by daily consumption of SFN-rich vegetables. However, further preclinical and clinical studies on the protective activity of SFN on MC-LR toxicity are needed.
Supplementary Material
Acknowledgments
This work was supported by grants from “973” program (2008CB418000), Natural Science Foundation of China-Yunnan Project (U0833604), Chinese Academy of Sciences (KZCX1-YW-14-1) and National water pollution control and management technology major projects (2009ZX07527-005).
Abbreviations
- MCs
Microcystins
- MC-LR
Microcystin-LR
- SFN
Sulforaphane
- PLC
Primary Liver Cancer
- Nrf2
NF-E2-related factor 2
- GSH
Glutathione
- ARE
antioxidant response element
- ALT
Alanine aminotransferase
- ITC
isothiocyanate
- NQO1, NAD(P)H
quinone oxidoreductase 1
- HO-1
Heme oxygenase-1
- GSTs
glutathione-S-transferases
- tBHQ
tert-Butylhydroquinone
- siRNA
small interfering RNA
- DMSO
dimethyl sulfoxide
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.taap.2010.06.005.
References
- Brook JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by Sulforaphane. Cancer Epidemiol Biomark Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- Carmichael WW. The toxins of cyanobacteria. Sci Am. 1994;270:78–86. doi: 10.1038/scientificamerican0194-78. [DOI] [PubMed] [Google Scholar]
- Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- Cornblatt BS, Ye LX, Dinkova-Kostova AT, Erb M. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Villeneuve NF, Wang XJ, Sun ZH, Chen WM, Li JX, et al. Oridonin Confers Protection against Arsenic-Induced Toxicity through Activation of the Nrf2-Mediated Defensive Response. Environ Health Perspect. 2008;116:1154–1161. doi: 10.1289/ehp.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer IR. Toxic cyanobacterial bloom problems in Australian waters: risks and impacts on human health. Phycologia. 2001;40:228–233. [Google Scholar]
- Fowke JH, Chung FL, Jin F, Qi D, Cai Q, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- Gao X, Dinkova-Kostova AT, Talalay P. Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: the indirect antioxidant effects of sulforaphane. Proc Natl Acad Sci U S A. 2001;98:15221–15226. doi: 10.1073/pnas.261572998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehringer MM, Govender S, Shah M. An investigation of the role of vitamin E in the protection of mice against microcystin toxicity. Environ Toxicol. 2003;18:142–148. doi: 10.1002/tox.10110. [DOI] [PubMed] [Google Scholar]
- Gehringer MM, Shephard EG, Downing TG, Wiegand C, Neilan BA. An investigation into the detoxification of microcystin-LR by the glutathione pathway in Balb/c mice. Int J Biochem Cell Biol. 2004;36:931–941. doi: 10.1016/j.biocel.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, et al. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- Jochimsen EM, Carmichael WW, Jisi A. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. New Engl J Med. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, et al. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kim BR, Hu R, Keum YS, Hebbar V, Shen G, Nair SS, Kong AN. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003;63:7520–7525. [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong AN, Yu R, Hebbar V, Chen C, Owuor E, Hu R, et al. Signal transduction events elicited by cancer prevention compounds. Mutat Res. 2001;480–481:231–241. doi: 10.1016/s0027-5107(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, et al. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomark Prev. 1998;7:647–652. [PubMed] [Google Scholar]
- Moffit JS, Aleksunes LM, Kardas MJ, Slitt AL, Klaassen CD, Manautou JE. Role of NAD(P)H:quinone oxidoreductase 1 in clofibrate-mediated hepatoprotection from acetaminophen. Toxicology. 2007;230:197–206. doi: 10.1016/j.tox.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugmacher S, Wiegand C, Oberemm A, Beattie KA, Krause E, Codd GA, et al. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin LR: the first step in detoxification. Biochim BIOPHYS Acta. 1998;1425:527–533. doi: 10.1016/s0304-4165(98)00107-x. [DOI] [PubMed] [Google Scholar]
- Prestera T, Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1995;92:8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman SA, Aleksunes LM, Klaassen CD. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem Pharmacol. 2009;77:1273–1282. doi: 10.1016/j.bcp.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RJ, Workman P. DT-diaphorase and cancer chemotherapy. Biochem Pharmacol. 1992;43:1657–1669. doi: 10.1016/0006-2952(92)90694-e. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Sumi D, Fukami I, Ishii T, Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006;580:1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, et al. Dietary intake of isothiocyanates: evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomark Prev. 2000;9:1017–1020. [PubMed] [Google Scholar]
- Sutherland BM, Bennett PV, Freeman AG, Moore SP, Strickland PT. Transformation of human cells by DNAs ineffective in transformation of NIH 3 T3 cells. Proc Natl Acad Sci U S A. 1985;82:2399–2403. doi: 10.1073/pnas.82.8.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka S. Covalent glutathione conjugation to cyanobacterial hepatotoxin microcystin LR by F344 rat cytosolic and microsomal glutathione S-transferases. Environ Toxicol Pharmacol. 2001;9:135–139. doi: 10.1016/s1382-6689(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Nagata S, Tsutsumi T, Yu SZ. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sample in Haimen and Fusui, endemic areas of Primary Liver Cancer in China, by highly sensitive immunoassay. Carcinogenesis. 1996;17:1317–1321. doi: 10.1093/carcin/17.6.1317. [DOI] [PubMed] [Google Scholar]
- WHO. Addendum to Volume 2. Health Criteria and Other Supporting Information. Word Health Organization; Genveva, Switzerland: 1998. Guidelines for drinking-water quality. [Google Scholar]
- William OO, Thomas WK. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat Res/Rev Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75:2214–2223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Zegura B, Lah TT, Filipic M. Alteration of intracellular GSH levels and its role in microcystin- LR-induced DNA damage in human hepatoma HepG2 cells. Mutat Res. 2006;611:25–33. doi: 10.1016/j.mrgentox.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Sun ZH, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-Kelch Substrate Adaptor Protein for Cul3, Targets Keap1 for Degradation by a Proteasome-independent Pathway. J Biol Chem. 2005;280:30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.