Abstract
Lyme disease is a major human health problem which continues to increase in incidence and geographic distribution. As a vector-borne zoonotic disease, Lyme disease may be amenable to reservoir targeted strategies for control. We have previously reported that a vaccinia virus (VV) based vaccine expressing outer surface protein A (OspA) of Borrelia burgdorferi, the causative agent of Lyme disease, protects inbred strains of laboratory mice against infection by feeding ticks and clears the ticks of infection when administered by gavage. Here we extend these studies to develop an effective bait formulation for delivery of the VV based vaccine and test its characteristics under simulated environmental conditions. We show that this vaccine is efficacious in decreasing acquisition of B. burgdorferi by uninfected larval ticks as well as in decreasing transmission from infected ticks to its natural reservoir, Peromyscus leucopus, when fed to mice in oral baits. Using live, in vivo imaging techniques, we describe the distribution of vaccinia virus infection after ingestion of the baited vaccines and establish the use of in vivo imaging technology for optimization of bait delivery. In summary, a VV based OspA vaccine is stable in an oral bait preparation and provides protection against infection for both the natural reservoir and the tick vector of Lyme disease.
Keywords: Vaccinia virus, Borrelia burgdorferi, Lyme disease, Reservoir-targeted vaccine, Tick, OspA
1. INTRODUCTION
Lyme disease is the most common vector-borne disease in the United States. It is caused by infection with the spirochete, Borrelia burgdorferi, which is transmitted by an Ixodes tick vector. Despite efforts to control the incidence of human Lyme disease, its incidence and geographic distribution have continued to grow over the last decade. In addition to interventions targeting at human risks, there has been growing interest in targeting the vector and reservoir hosts to control disease. Because there is no vertical transmission of B. burgdorferi, larval ticks acquire the bacteria by feeding on infected rodents and birds. In the U.S., the white-footed mouse (Peromyscus sp) is the major reservoir host in most areas [1, 2]. The infected larvae molt into nymphs which also feed on small rodents and birds and transmit B. burgdorferi to new hosts to perpetuate the cycle of infection [3]. Nymphal ticks molt to adult ticks which may also transmit infection, but more typically feed on larger animals such as deer which are not competent hosts for B. burgdorferi. Humans are incidentally infected by B. burgdorferi as a result of bites by infected nymph and adult ticks.
Previous studies have shown that immunization of mice with recombinant outer surface protein A (OspA) of B. burgdorferi both prevents acquisition of spirochetes by feeding larval ticks and protects uninfected mice from B. burgdorferi infection during feeding of infected ticks [4, 5]. OspA is upregulated when the bacteria is within the tick host and may be important for its ability to colonize and persist in ticks [6]. In OspA vaccinated mice, anti-OspA antibodies are taken into the tick during feeding resulting in prevention of colonization of an uninfected tick or the killing of the spirochetes inside infected ticks [7-10]. An OspA based human vaccine was previously approved by United States Food and Drug Administration for human use [11]. Although the vaccine had a protective efficacy of 76% in human adults, it was removed from the market by the manufacturer due to low sales. Contributing to the low sales were the incomplete protection, the need for frequent booster vaccinations and a concern, that was subsequently shown to be unfounded, that antibodies to OspA may be involved in the development of autoimmune arthritis.
Other approaches to limit Lyme disease have included reducing tick numbers by spraying acaricides on vegetation as well as application of acaricides directly on tick hosts such as mice and deer [12]. While each of these approaches has been shown to be successful in limited settings, none is effective enough to be likely to significantly reduce the incidence of human Lyme disease as a stand-alone intervention. Integrated management strategies combining several different approaches are currently being examined and may show promise in reducing carriage in wildlife reservoirs. The development of a reservoir targeted vaccine to prevent transmission and acquisition of B. burgdorferi by ticks would be an important component of an integrated strategy. The most successful example of a reservoir targeted vaccine strategy is the use of a vaccinia virus (VV) vectored rabies vaccine for raccoons and foxes [13-17]. Tsao et al. have previously shown that vaccination of wild Peromyscus with an OspA based vaccine administered by injection can decrease the carriage rate of B. burgdorferi in ticks the following season, providing support for the concept of a reservoir targeted vaccine for Lyme disease [18, 19]. We have previously reported development of a VV based reservoir-targeted vaccine for Lyme disease that was effective in protecting mice from B. burgdorferi infection when administered by gavage [20]. In this study, we present our progress in developing a baited vaccine suitable for distribution in the wild.
2. MATERIALS AND METHODS
2.1 Vaccinia virus, bacteria and mice strains
Vaccinia virus expressing OspA (VV-ospA) was constructed as previously described [20]. VV carrying firefly luciferase reporter gene (VV-FL) was constructed as described [21]. All strains of VV were grown and maintained in HeLa cells as previously described [22].
B. burgdorferi (strain N40 D10E9) was used for our experiments. The spirochetes were grown in Barbour-Stoenner-Kelly II (BSK) media at 37°C as previously described [23].
Peromyscus leucopus were obtained from the Peromyscus Genetic Stock Center (Columbia, SC) or from a colony of mice that has been outbred at Tufts University School of Veterinary Medicine. The mice from Tufts University School of Veterinary Medicine were derived from wild mice captured on Martha’s Vineyard, MA and were in their 12th generation. These mice were maintained by random inbreeding within cages containing 6-18 animals. All studies were performed on 6-8 week old mice.
For in vivo imaging of VV dissemination in mice, 6-8 weeks old Balb/c mice were used. All Balb/c mice were purchased from Charles River Laboratories (Boston, MA).
2.2 Ticks
Ixodes scapularis tick larvae were obtained from National Tick Research and Education Center, Oklahoma State University (Stillwater, OK). B. burgdorferi-infected nymphs were generated as previously described [20]. Briefly, uninfected larvae were allowed to feed on B. burgdorferi-infected SCID mice to repletion. The engorged larvae were collected and allowed to molt into nymphs in 4-6 weeks at room temperature and 95% relative humidity. Prevalence of B. burgdorferi infection in fed larvae was determined by culture and PCR of a portion of the recovered ticks from each batch [20]. Only batches with greater than 85% infection rates were used for subsequent studies.
2.3 Preparation of oral baits
The bait formulation was a proprietary mixture of natural food products developed by Foodsource Lures Corp. (Alabaster, AL). Dry, powdered formula was mixed with peanut butter flavoring as an attractant, with or without nut shells and water at 50°C. The ingredients were thoroughly mixed and allowed to cool to 40°C. The desired dose of recombinant VV was added to the semi-solid bait mix and cooled at room temperature to make solid bait cakes.
2.4 Stability of VV in oral baits
The stability of VV in the baits was determined under various conditions of temperature, humidity and freeze-thaw environment arising due to temperature variation between day and night. After exposure to the test condition, portions of VV-ospA laden bait cake were processed at different time points to determine the stability of virus. Different regions of the bait were sampled (outer surface, inner core) and results combined. VV titers per unit weight of bait was measured by crystal violet plaque assay as previously described [24]. Briefly, VV-laden baits were flash frozen in liquid nitrogen, powdered using a pestle and mortar and resuspended in MEM supplemented with 2.5% FBS. The suspension was trypsinized, serially diluted and appropriate dilutions were plated on BS-C-1 cells in a six-well plate. The titers were calculated by crystal violet staining after 2 days of incubation at 37°C.
2.5 Mouse vaccination
Peromyscus mice were vaccinated with VV-ospA or a control (VV-vp37) vaccine delivered via gavage or offered by oral baits that were placed in cages for the mice to ingest ad libitum. For vaccination with oral baits, only one mouse was kept per cage and each cage was supplied with approximately 2g of bait containing 2×108 pfu of VV-ospA or control virus (VV-vp37). All mice consumed the baits within 48 hrs. Gavaged mice were administered 2×108 pfu of VV-ospA or control vaccine (VV-vp37) suspension in MEM.
2.6 Determination of antibody response
The antibody response in mice was determined by endpoint titer ELISA using a protocol similar to that described previously [24]. Briefly, 96-well plates (Costar, Corning, NY) were coated with 2 μg/ml of MBP-OspA diluted in PBS and incubated overnight at 4 °C. The MBP-OspA was removed and the plates were blocked with 1% BSA dissolved in PBS. After removing the blocking reagent, the top well of each lane was loaded in duplicate with serum dilutions. The samples were serially diluted in equal volume of PBS. Following overnight incubation at 4 °C, the samples were aspirated and the plates were washed 0.1% Tween 20 in PBS. The plates were incubated with anti-Peromyscus HRP-linked IgG secondary antibody (1:25000 dilution in PBS) (KPL, Inc. Gaithersburg, MD) for 1 hour at room temperature. The secondary antibody was aspirated, washed and incubated with Sure Blue TMB peroxidase substrate (KPL, Inc. Gaithersburg, MD) followed by addition of equal volume of TMB stop solution (KPL, Inc. Gaithersburg, MD). Absorbance was measured at 450 nm. Serum from unvaccinated mice was used as the negative control. The mean of absorbance values of unvaccinated mouse serum was set as cut-off. The reciprocal of highest dilution above the cut-off was defined as endpoint antibody titer of each serum sample.
2.7 Determination of VV-ospA efficacy
Efficacy of the VV-ospA vaccine was determined for both inhibition of larval tick acquisition and for inhibition of transmission to uninfected mice by infected nymphal ticks.
To test the efficacy against transmission to uninfected mice, mice were provided VV-ospA baits or control (VV-vp37) baits. Antibody response was determined 4 weeks post vaccination. On week 5 post-vaccination, mice were challenged by allowing 6-7 B. burgdorferi infected nymphal ticks to feed to repletion as previously described [20]. The fed nymphs were collected and assayed for B. burgdorferi infection by culturing their homogenate in BSK media at 37°C. The mice were assayed for B. burgdorferi infection by culture of an ear punch in BSK media at week 2 and cultures of the ear, bladder and heart culture at week 4 post-challenge. Presence of B. burgdorferi was determined by observing the cultures by darkfield microscopy. A mouse was defined as infected with B. burgdorferi if one or more organ cultures were found positive by darkfield microscopy.
To determine the vaccine efficacy in preventing B. burgdorferi acquisition by larvae, Peromyscus mice were infected with B. burgdorferi by allowing 6-7 infected nymphal ticks to feed on these mice. Infection was confirmed by culture of an ear punch in BSK at week 2-3 after tick infestation. The infected mice were vaccinated with VV-ospA or control by bait feeding and serum antibody response to OspA was determined 4 weeks post-vaccination by ELISA. Ixodes larvae were fed on VV-ospA and control vaccinated mice. The fed ticks were collected and observed for 5 days. After 5 days, the ticks were homogenized and cultured in BSK media [20]. The presence of B. burgdorferi was determined by observing the cultures under microscope. All animal procedures were reviewed and approved by the Tufts University Institutional Animal Care and Use Committee.
2.8 Bioimaging of VV infection
Balb/c mice were given a single dose of 108 pfu of VV-FL [21] via oral gavage or fed as oral bait. At various time points, mice were administered luciferin substrate (150 mg/kg per mouse) by intraperitoneal injection and bioluminescence was measured using an IVIS200 imager (Xenogen Corp.). All images were captured after 5 minutes of exposure at a binning parameter of 8 (medium). The images were normalized to a uniform photon flux scale to minimize the variability between images taken at different time points.
To determine the effect of gastric pH on viral uptake via bait, the mice were administered ranitidine (125 mg/kg mouse, every 12 h) by intraperitoneal injection concurrent with placement of bait in the cage. Injections were continued twice daily until the bait was consumed (about 2 days). Bioluminescence at various time points was measured using IVIS200 imager.
2.9 Determination of viral titers in mouse tissue
Crystal violet plaque assayw were performed to determine the localization of VV infection in various mice tissues. Briefly, the harvested organs from a vaccinated mouse were dissected, homogenized and resuspended in 1 ml of MEM medium. The tissue homogenate was incubated with equal volume of 0.5% trypsin for 30 min at 37°C and appropriate dilutions were plated on BS-C-1 six well plates. The VV titers were determined by crystal violet titering assay as previously described [24].
2.10 Statistics
Fisher’s exact test was used to determine if significant differences in experiments that resulted in discrete data points (e.g. positive and negative results for B. burgdorferi infection of mice and ticks).
The Mann-Whitney U test was used to calculate statistical differences in experiments that resulted in continuous variables (e.g. luciferase intensity between control and infected mice). The tests used for each experiment is listed in the figure legend.
3. RESULTS
3.1 Stability of VV in oral bait
The bait formulation used in this study was developed by Foodsource Lures Corp. (Alabaster, AL). The bait formulation consists of a proprietary compound natural product and eliminates plasticizers or catalysts that may inactivate the vaccine. Prior work with this bait formulation has shown that it is durable in both wet and arid conditions and resists mold and insect infestation. The bait matrix withstands rain and will maintain scent attractants for weeks. The bait can be formulated with minimal heating compared with other similar baits which was important for maintaining viability of the viral vaccine. It has been previously shown to be palatable to wild Peromyscus [25]. The stability of VV in oral bait was determined at 25°C, at 37°C, and under freeze-thaw conditions (Figure 1). In all cases, baits were sampled from multiple locations (inner core, outer surface), however we did not see a consistent difference in viral titers related to location. At 25°C, titers of VV-ospA declined by approximately 1 log over 6 weeks when kept in a sealed conical tube (Figure 1A). Titers in the bait could be easily maintained above the minimum dosage required to generate a protective antibody response for this duration of time. To test the effects of desiccation on the vaccine titer, we allowed the baited vaccines to remain exposed to unhumidified air. Under these conditions, the bait became harder within few days due to loss of moisture. However, the VV titers were not significantly different compared to conditions where moisture was maintained (Figure 1B).
Figure 1.
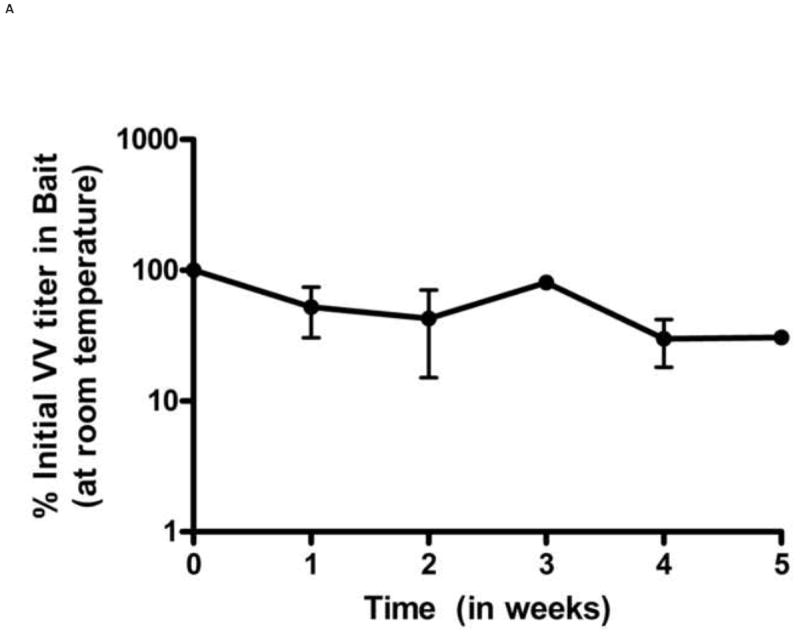
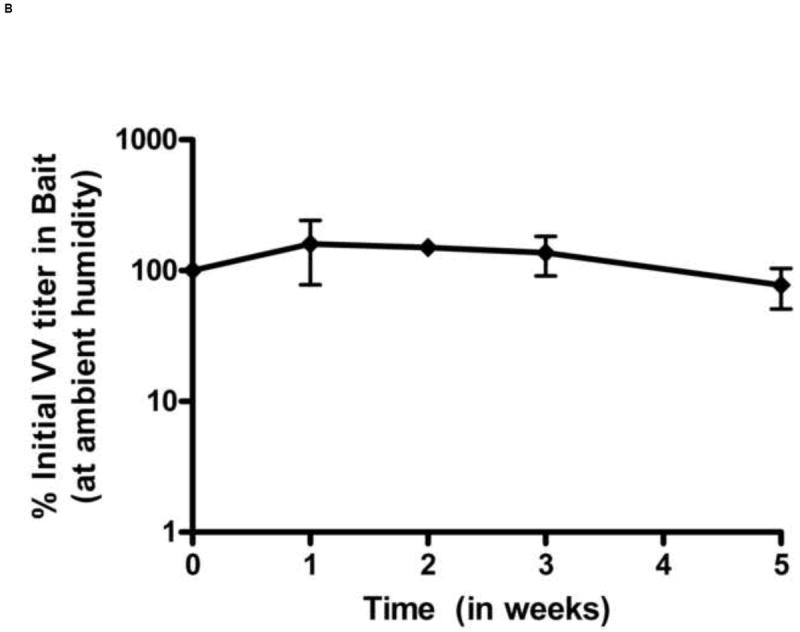
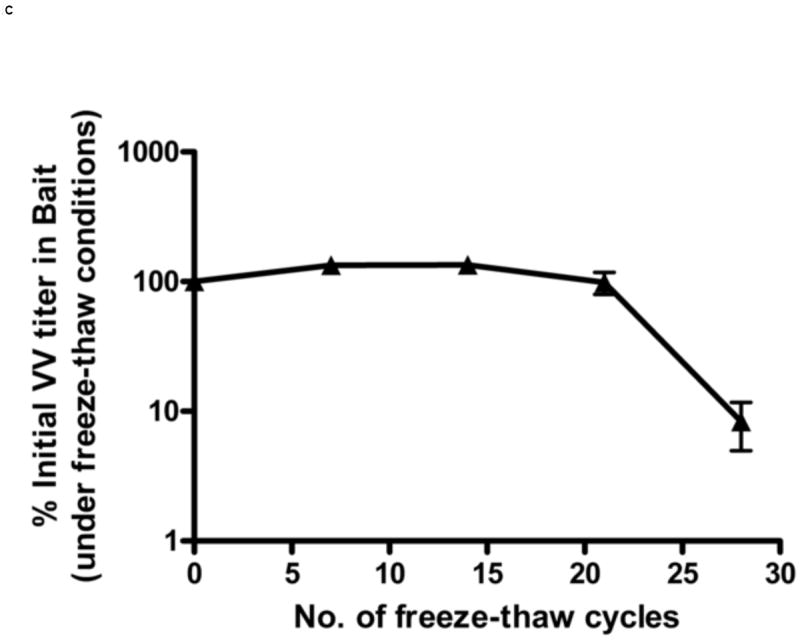
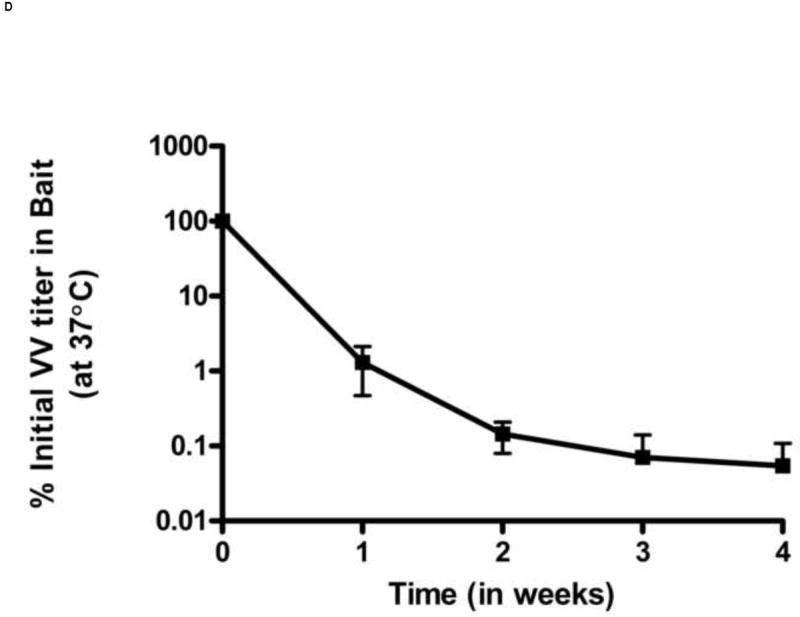
Stability of VV in oral baits. The stability was determined by titering VV in baits by crystal violet plaque staining at different time points. Titers per unit weight of bait were measured at each time point. VV stability in baits was determined under following different conditions: constant room temperature (A), ambient humidity (B), effect of freeze-thaw cycles (C) and 37°C (D). Each data point represents results of 3 independent experiments. Error bars represent standard error of the means. In figure 1D, the lower error bars for weeks 3 and 4 (not shown) extend to 0% as virus infection was not observed in two experiments.
We also tested the effects of multiple freeze-thaws on viral titers. Because larval ticks feed in late Spring and Summer, the vaccine is likely to be deployed beginning in late Winter/early Spring when temperatures in endemic areas frequently still reach freezing. The vaccine titers in baits subjected to multiple daily freeze thaws remained stable for up to 21 freeze thaw cycles and then began to decline (Figure 1C). By the end of 28 freeze-thaw cycles, the VV titers fell to approximately 10% of the initial titer in oral bait. Conversely, under conditions of high heat, the titers of VV-ospA declined rapidly. When the baits were kept at a constant 37°C temperature, the VV titer in bait decreased to 1% of its initial titer within one week.(Figure 1D).
3.2 Antibody response in mice
To determine whether VV-ospA was effective when administered orally to its natural host, Peromyscus leucopus mice were immunized with 2×108 pfu of VV-ospA administered by oral gavage or offered ad libitum for ingestion in 2-3 gm oral baits. The dose was selected based on our previous experience with VV-based vaccine [20, 24]. Thirty percent of the mice given the baited vaccine consumed the vaccine within 24 hrs and 90% had consumed the entire vaccine by 48 hrs. Serum samples were collected at different time points and antibody titer to OspA was determined by endpoint ELISA. Anti-OspA antibody titers were not significantly different in mice vaccinated either via oral gavage or bait fed at 4 weeks post-vaccination (Figure 2).
Figure 2.
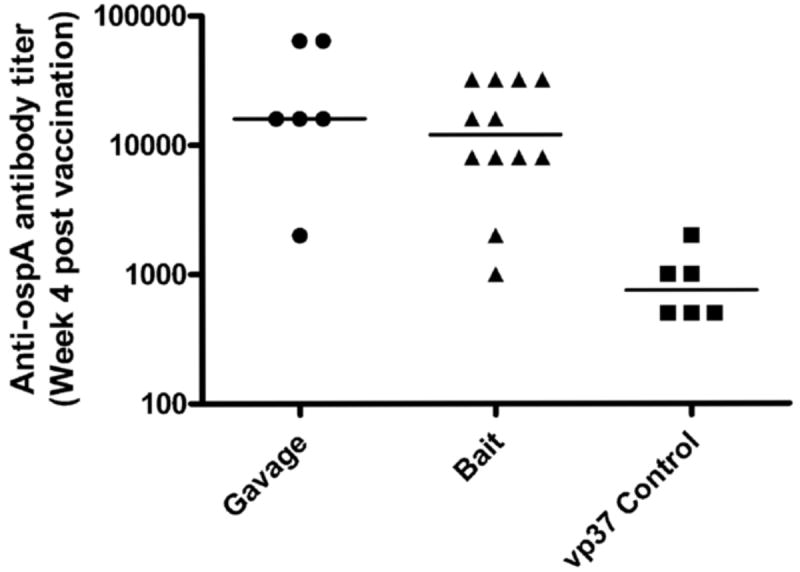
Antibody responses to VV-ospA administered by gavage and oral bait. Anti-OspA endpoint antibody titer in Peromyscus mice on week 4 post vaccination is shown. VV-ospA was administered to mice via oral gavage (circles) or fed as oral baits (triangles). The control mice (squares) were vaccinated with an irrelevant VV strain (VV-vp37) either via gavage or bait-fed. Each data point represents the antibody response in a single mouse and the horizontal bars represent mean antibody response of the group.
We determined the stability of anti-OspA antibody response in Peromyscus mice immunized via feeding with VV-ospA laden baits or control baits. The VV-ospA orally vaccinated Peromyscus mice reached titers of greater than 8000 (the minimum titer required for 100% protection against transmission of B. burgdorferi to uninfected mice) by week 4 post-vaccination, with antibody titers peaking at 12 weeks and remaining stable for at least 10 more weeks before beginning to decrease (Figure 3). Antibody levels remained above control through week 35 (furthest timepoint tested).
Figure 3.
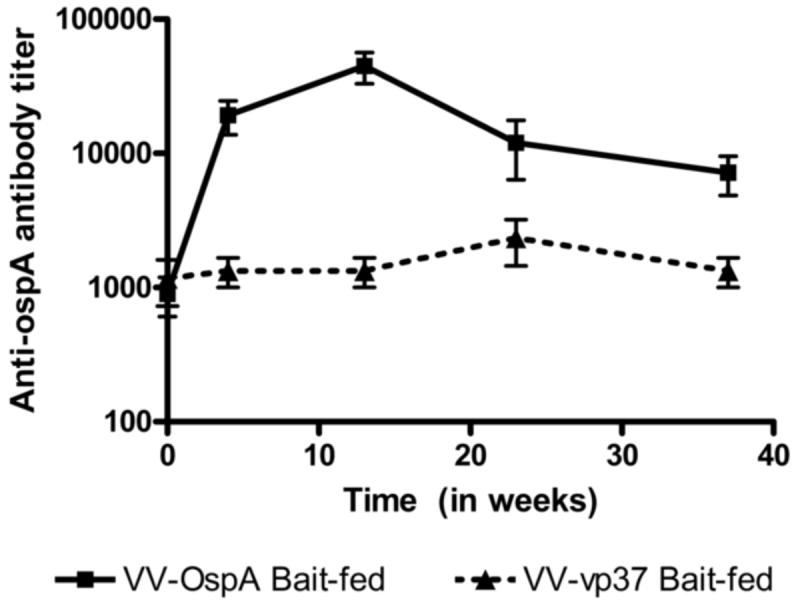
Kinetics of anti-OspA antibody response post-bait immunization. Peromyscus mice were fed with baits laden with 2×108 pfu VV-ospA or control (VV-vp37). The geometric mean and standard error (SEM) of serum antibody titer of at least five VV-ospA-vaccinated (square, solid line) and three control-vaccinated mice (triangle, dotted line) are shown.
3.3 Efficacy of VV-ospA in prevention of B. burgdorferi transmission to uninfected mice
To determine the efficacy of VV-ospA administered by oral baits in protecting uninfected mice against transmission of B. burgdorferi from infected ticks, Peromyscus mice were vaccinated with VV-ospA or control (VV-vp37) via bait feeding. The antibody response in mice was determined 4 weeks post-vaccination to ensure successful vaccination of the mice. Six B. burgdorferi-infected nymphs were allowed to feed on each mouse. The mice were tested for spirochete infection by culture of multiple organs which we have found to be more sensitive than PCR in detecting infection in our hands [20]. Only 16% of VV-ospA vaccinated mice became infected with B. burgdorferi compared with 75% of VV-vp37 vaccinated mice (p < 0.005) (Table 1).
Table 1.
Efficacy of VV-ospA bait vaccine in prevention of B. burgdorferi transmission to Peromyscus leucopus. Mice were fed with baits laden with 2×108 pfu VV-ospA or control (VV-vp37). The mice were challenged by feeding on them about 5-6 B. burgdorferi-infected Ixodes scapularis nymphs, on week 5 post-vaccination. The presence of spirochetes in a mouse was tested by culturing ear punches, bladder, and heart at various time points. Statistical analyses (Fisher’s exact test*) showed a significant degree of protection from spirochete infection in VV-ospA vaccinated mice vs. controls.
| Total Mice | # Mice Infected | % Mice Infected* | |
|---|---|---|---|
| VV-ospA | 12 | 2 | 16.16 |
| VV-vp37 | 12 | 9 | 75 |
p < 0.005
As expected, protection was correlated with antibody titer. The two VV-ospA-vaccinated mice that acquired B. burgdorferi infection upon tick feeding had low anti-OspA antibody titers (endpoint titer < 8000) post-vaccination.
3.4 Efficacy of VV-ospA in prevention of B. burgdorferi acquisition by larvae ticks from infected mice
A major mechanism of action of a reservoir targeted OspA vaccine is through the prevention of acquisition of B. burgdorferi by larval ticks from infected mice. Because OspA is not expressed by B. burgdorferi in the mouse host, antibodies to OspA do not clear an infected mouse of B. burgdorferi infection; however, antibodies to OspA have been shown to prevent transmission from the infected mouse back to uninfected ticks [4, 5, 26]. Larval ticks were fed on B. burgdorferi infected mice vaccinated with VV-OspA or control. We observed a significant reduction in B. burgdorferi transmission from infected mice to tick larvae during feeding upon VV-ospA vaccination compared with control vaccine (Table 2).
Table 2.
Efficacy of VV-ospA vaccine to prevent acquisition of B. burgdorferi by ticks. B. burgdorferi-infected Peromyscus mice were vaccinated with VV-ospA or control vaccine via bait-feeding. Uninfected tick larvae were allowed to feed on these mice to repletion. Presence of the spirochetes in fed larvae was tested by culturing crushed ticks in BSK media. Statistical analyses (Fisher’s exact test†) showed a significant difference in acquisition of the infection by larvae feeding on VV-ospA vaccinated mice vs. control vaccinated mice.
| Total Fed larvae | # Larvae Infected | % Larvae Infected† | |
|---|---|---|---|
| VV-ospA | 43 | 10 | 23.29 |
| VV-vp37 | 53 | 45 | 84.90 |
p < 0.0001
3.5 Dissemination of VV in mice
Dissemination of VV after oral inoculation has not been well established and may vary between different strains of VV. To determine the dissemination of VV in mice we used a recombinant VV (strain WR) with a luciferase reporter (VV-FL) inserted into the same vp37 locus as ospA. Balb/c mice were used for imaging as the lighter coat color causes less interference with the luciferase activity signals. Mice were infected with 108 pfu of VV-FL given by oral gavage or fed with oral baits. At various time points, the bioluminescence signals were captured using an IVIS200 imager.
When VV-FL was administered via oral gavage, we observed a higher level of VV infection as compared to bait-fed mice. Although there was significant variability from mouse to mouse in the strength of the luciferase signal, gavaged mice generated robust signals from the region of the brain and nasal associated lymphoid tissues (NALT) by Day 3 which was never seen in orally infected mice (Figure 4A and 4B). The bioluminescence data were confirmed by direct titering of VV from specific organs. The mean VV-FL titer in brain and NALT was 6.7 × 103 pfu/g and 1.12 × 105 pfu/g respectively. However, no virus could be recovered from spleen, mesenteric lymph nodes or Peyer’s patches. A proportion of the gavaged mice had no luciferase signals from the brain or NALT and only a small amount of signal from the region of the abdomen. In these mice we could not detect any VV by tissue culture in the brain or NALT.
Figure 4.
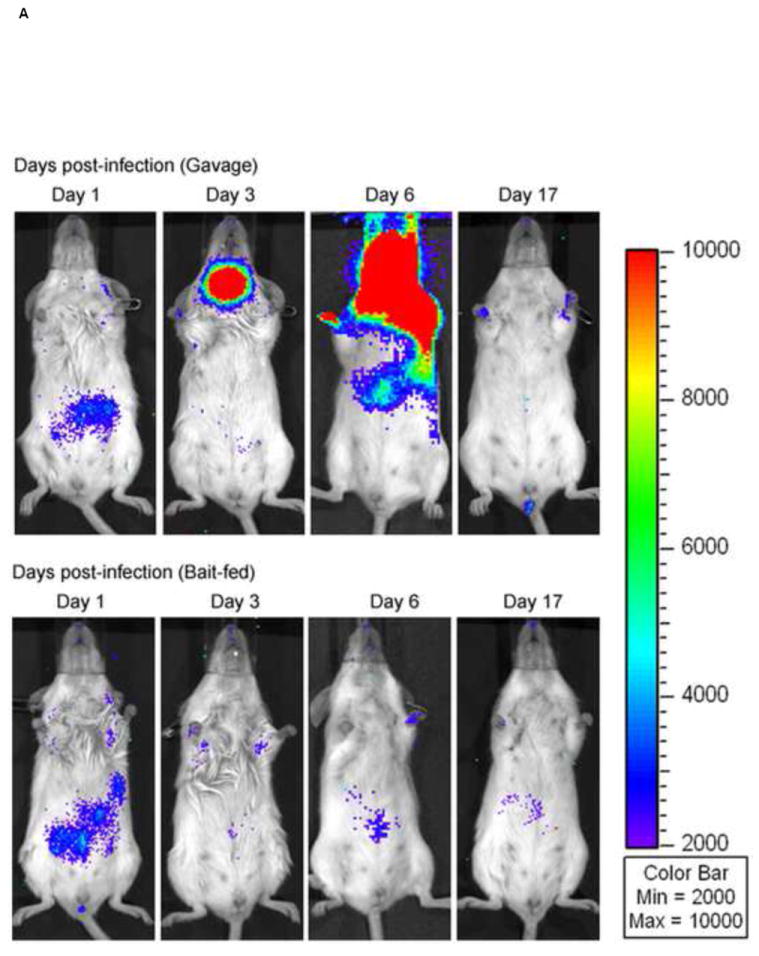
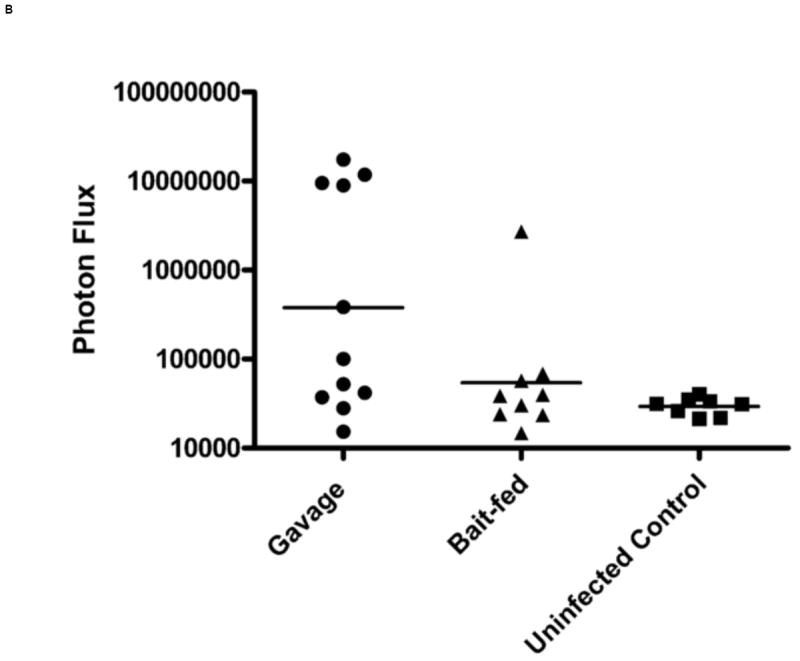
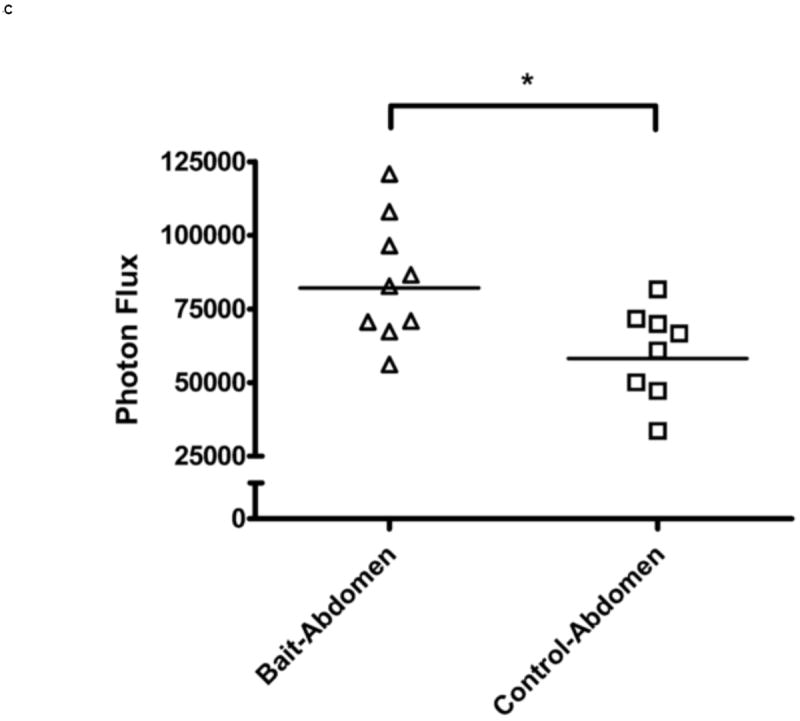
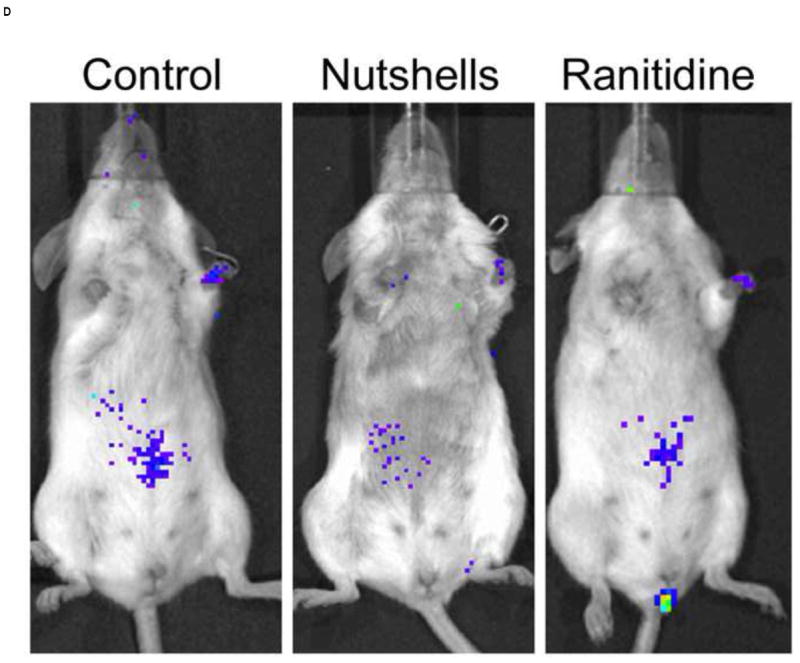
In vivo imaging of VV-FL administered by gavage and oral baits. Mice were infected with 108 pfu of VV-FL by oral gavage or fed as oral baits and bioluminescence images were captured using IVIS 200 imager at different time points post-infection. All images were normalized to an uninfected negative control. (A) Representative panel of bioluminescence images describing kinetics of VV-FL dissemination in Balb/c mice is shown. During VV-FL infection via gavage (upper panel), a strong localization of virus was observed around brain and NALT by Day 3. These are representative images of the strong response phenotype post gavage route infection. The Bait-fed mice (lower panel) generated a low level of VV infection as compared to gavage infected mice. Localization of brain and NALT was rarely observed post-bait-feeding. (B) Quantitative comparison of VV-FL infection in mice infected via gavage (circles) or bait-fed (triangles) is shown. Luciferase activity was quantified using LiveImage software. Each data point represents photon flux in the region of interest (ROI) of a single mouse on Day 5 post-infection. For comparing gavage vs. bait-fed infection, ROI was drawn around the brain and NALT region of each mouse. During VV-FL administration via gavage route, almost half of the mice generated a robust infection around brain and NALT which was not observed via bait-fed infection. (C) Quantitative measure of VV-FL infection via bait-feeding (open triangles) on Day 5 post-infection is shown. A significantly higher luciferase activity was measured in infected mice as compared to uninfected controls (open squares) (p < 0.05, Mann Whitney U test). The ROI for quantification of bait-fed infection was drawn around the abdominal region. (D) Effect of variations in bait feeding on uptake of VV. Balb/c mice were infected with VV-FL via feeding with baits that were mixed with broken nutshells (center). In another experiment, ranitidine was administered (i.p.) prior to offering mice with VV-FL laden ordinary baits to determine the effect of gastric pH neutralization on virus uptake via bait (right). Representative bioluminescence images of the two experiments on Day 5 post infection are shown. Control mice (left) were fed with ordinary bait laden with VV-FL without ranitidine administration. No significant difference in mean intensity was observed either using altered bait formulation with nutshells (center) or by ranitidine administration prior bait feeding.
In bait fed mice, we observed signals predominantly around the abdominal region (Figure 4C). These signals were far lower in intensity than those seen in the brain and NALT of gavaged mice. The abdominal signals also appeared to disappear sooner. While in case of gavage infection the virus was cleared by day 17 post-infection (n= 6), in bait-fed mice we observed clearance of the virus by day 12-14 post infection (n=6). Virus could not be cultured from the NALT, brain, spleen, mesenteric lymph nodes or Peyer’s patches of mice fed VV in the oral baits.
3.6 Manipulation of bait formulation to improve infection with VV
Although antibody titers were similar between gavaged and bait fed animals, the difference in productive infection as judged by live animal imaging and viral cultures of organs suggested that there may be opportunities to further optimize the bait formulation to increase productive infection—either resulting in the development of higher antibody titers or in allowing a decreased dose of VV to be used in the vaccines. We tested two modifications for increasing productive infection with VV administered by oral baits. Previous studies have suggested that oral trauma, such as might be sustained during oral gavage, may increase infection with VV. We created oral trauma, simulating what is encountered in wild mice eating a natural diet by adding broken nut shells to the baits. However, we did not observe any significant increase in luciferase signal by IVIS imaging in mice fed baits containing nut shells versus those fed regular baits (Figure 4D).
VV is known to be inactivated at low pH [27]. To determine whether differences between gavage and orally administered VV were due to buffers in the gavage fluid that may neutralize gastric acid, we administered the histamine 2 receptor antagonist, ranitidine, by intraperitoneal injection prior to bait feeding. The drug was administered twice daily and the bait feeding was monitored during this time. No change in luciferase signal was observed in ranitidine treated mice versus control mice (Figure 4D).
We also determined the effect of ranitidine administration during bait feeding on antibody response against VV-ospA vaccine. For this purpose, Peromyscus mice were administered ranitidine and allowed to consume the VV-ospA baits. The control mice fed on VV-ospA baits without ranitidine administration. Anti-OspA antibody titers in mice sera were determined by endpoint ELISA 4-weeks post vaccination. No significant difference in anti-OspA antibody titers was observed between the two groups of mice (data not shown).
4. DISCUSSION
Here, we have reported protective efficacy of a vaccinia based, reservoir-targeted Lyme vaccine delivered by oral bait. We have shown that the vaccine, administered orally to Peromyscus mice, confers protection against both transmission of B. burgdorferi to vaccinated, uninfected mice as well as protection against acquisition of spirochete by larval ticks feeding on infected, vaccinated mice. We have further shown that we can create a vaccine that maintains sufficient titers of vaccinia to provide single dose vaccination for over 1 month when exposed to conditions simulating those that will likely be encountered during distribution. This vaccine can be delivered in a package which can easily be consumed by a mouse in hours. Of note, in previous studies, we have not seen an adverse effect on mice of administering doses with viral titers up to 2 logs higher than our target dose suggesting that there is unlikely to be significant harm to the animal for consuming a dose higher than is necessary to generate a protective antibody response [20]. We have also previously found that consumption of multiple doses over time does not result in adverse response and may in fact boost the antibody response [20].
Although baits for reservoir targeted use of vaccinia virus based vaccines have been previously developed for use with raccoons and foxes, significant differences between these animals and mice, the target animals for Lyme, required us to redesign the bait formulation. The vaccine for raccoons and foxes wraps bait material around a small sachet containing the vaccinia virus in a liquid vehicle. Because mice are significantly smaller and cannot swallow the sachet intact, the sachet method is unlikely to result in significant uptake of vaccine. As a result, we tested a vaccine fabrication process that incorporated VV-ospA directly into a liquid retaining bait formulation. In a separate study, this bait formulation has been shown to be attractive to wild mice as well as laboratory-reared animals [25]. A one-time placement of baits at 5 nest boxes over a 1 hectare area results in greater than 50% uptake of the bait in mice suggesting that penetration of the vaccine can be sufficient to provide to a significant proportion of the mouse population in the baiting area, particularly when baiting is carried out over an entire season.
Protection with the baited vaccine was not as high as we have previously reported for gavage administration of the VV-OspA in inbred C3H mice, which was up to 100% [20]. Failures of the vaccine in this study were all related to failure to develop sufficient antibody responses to OspA. The reason for the failure to develop an antibody response to OspA in some of the bait fed animals is unknown. We did not observe significant differences in the time it took mice to completely consume the vaccine between responders and non-responders although more subtle differences in the timing of uptake may have been missed by our observations. It is also possible that distribution of VV-OspA in the baits was uneven. Although random sampling of the baits did not show appreciable differences in viral concentration in different baits, it is certainly possible that these baits, which were fabricated under non-commercial laboratory conditions, had significantly lower vaccine dosages in some baits due to insufficient mixing. Genetic differences between responders and non-responders are a less likely explanation given that we tested mice from two separately maintained sources of outbred Peromyscus and we found no differences in the rate of response in each.
The use of in vivo imaging to track the virus as it established infection provided several interesting insights into the vaccine. The strain of VV used to construct both VV-OspA and VV-FL is a mouse adapted, neurotropic strain of VV. Administered by gavage, it has previously been shown to localize in gut associated lymphoid tissue (GALT) and spleen within 1 day post infection [28]. Intranasal administration of the VV-FL strain used in our experiments revealed dissemination to lung, brain, liver, and spleen [21]. Our studies with the luciferase expressing VV confirmed that mice administered this strain by gavage developed productive infection of the brain and NALT. However, when ingested orally from baits, the virus showed no propensity to involve the brain and NALT and was limited to a low grade infection of the intestinal tract. Of note, the mice taking oral baits developed similar antibody titers as those receiving gavage despite low level of productive viral infection that was undetectable by culture in NALT, brain, mesenteric lymph nodes and Peyer’s patches. This low grade infection may have another advantage for the use of this vaccine in the wild as low viral titers would be much less likely to be passed to other animals (e.g. predators that consume an animal soon after bait ingestion).
We also used in vivo imaging to assist in rapidly assessing the impact of various manipulations of the vaccine baits on productive viral infection. Based on the differences between responses in gavaged and bait fed mice, we tested changes to the consistency as well as the effects of decreasing gastric acidity. Although none of our manipulations resulted in improved antibody titers to OspA, the in vivo imaging system allowed us to rapidly assess the impact of our manipulations on productive viral infection and correlate it to the antibody response. As our goal was the development of improved vaccine formulations and not in discovering the mechanisms of infection, we did not extensively pursue the study of the effects of gastric pH on viral infection as, if the administration of ranitidine intraperitoneally at concentrations known to inhibit gastric acidity did not improve infection, it seemed unlikely that incorporation of an antacid into the bait would be successful. However, it remains possible that other, more effective means of neutralizing the effects of pH on the virus (e.g. microencapsulation of the vaccine) may prove effective in allowing a lowered dose of the vaccine to be effective.
In summary, we have developed a VV vectored, B. burgdorferi OspA based mouse reservoir targeted vaccine that appears efficacious in preventing tick transmission and acquisition of B. burgdorferi. We have shown that we can successfully formulate this vaccine into bait that can be effective for greater than 4 weeks under expected conditions. We anticipate that a distribution strategy will be based on replacement of bait vaccine into nest boxes on a 2-4 week schedule during peak tick feeding season. Of note we have shown that this vaccine can be protective after a single dose, giving it an advantage over non-virally vectored OspA vaccines which require multiple doses to develop protective immunity [18, 29-32]. However, a major disadvantage of vaccinia vectored vaccines compared with protein vaccines is the potential for infection in non-target species (particularly humans) and the potential for recombination with other wild orthopox viruses. The long safety track record for use of the vaccinia vectored rabies vaccine in the wild provides some reassurance for the safety of a vaccinia vectored reservoir targeted Lyme vaccine. Both a strength (from a safety standpoint), and a weakness (from an efficacy standpoint), of vaccinia based oral vaccines is that vaccinia does not appear to be passed from animal to animal and each animal must consume the vaccine to become protected [20]. Future field studies will be necessary to determine the efficacy and the optimal distribution strategies of the vaccine and to answer questions about how nesting and communal behaviors of the mice in the wild may affect the uptake of the vaccine. Successful development of a reservoir targeted vaccine against B. burgdorferi would be an important tool in an integrated strategy for the control/eradication of Lyme disease.
Highlights.
>We have developed a reservoir-targeted baited vaccine against Lyme disease. >The vaccine is stable in oral baits under various environment-simulated conditions. >A single dose of vaccine protects Peromyscus mice against infected tick bites. >A single dose vaccine protects ticks from infection during feeding on carrier mice. >Live, in vivo imaging was used to optimize bait composition.
Acknowledgments
The authors wish to thank Dr. Elizabeth Tenorio, Dr. Tanja Petnicki-Ocwieja and Dr. Meghan Marre for their helpful discussions.
The project was supported by National Institute of Allergy and Infectious Diseases grant R01AI068799 (L.T. Hu) and R41AI078631 (L.T. Hu) and by National Institute of Health grant P50CA093990 (G.D. Luker).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mather TN, Wilson ML, Moore SI, Ribeiro JM, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989 Jul;130(1):143–50. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 2.Donahue JG, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987 Jan;36(1):92–6. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 3.Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annual review of entomology. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 4.Fikrig E, Telford SR, 3rd, Barthold SW, Kantor FS, Spielman A, Flavell RA. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proceedings of the National Academy of Sciences of the United States of America. 1992 Jun 15;89(12):5418–21. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fikrig E, Barthold SW, Marcantonio N, Deponte K, Kantor FS, Flavell RA. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infection and immunity. 1992 Feb;60(2):657–61. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. Journal of clinical microbiology. 2000 Jan;38(1):382–8. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Silva AM, Fish D, Burkot TR, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infection and immunity. 1997 Aug;65(8):3146–50. doi: 10.1128/iai.65.8.3146-3150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva AM, Telford SR, 3rd, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996 Jan 1;183(1):271–5. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer MD, Wallich R, Simon MM. The outer surface protein A (OspA) of Borrelia burgdorferi: a vaccine candidate and bioactive mediator. Infection. 1996 Mar-Apr;24(2):190–4. doi: 10.1007/BF01713338. [DOI] [PubMed] [Google Scholar]
- 10.Pal U, Montgomery RR, Lusitani D, Voet P, Weynants V, Malawista SE, et al. Inhibition of Borrelia burgdorferi-tick interactions in vivo by outer surface protein A antibody. J Immunol. 2001 Jun 15;166(12):7398–403. doi: 10.4049/jimmunol.166.12.7398. [DOI] [PubMed] [Google Scholar]
- 11.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. The New England journal of medicine. 1998 Jul 23;339(4):209–15. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 12.Pound JM, Miller JA, George JE, Lemeilleur CA. The ‘ 4-poster’ passive topical treatment device to apply acaricide for controlling ticks (Acari: Ixodidae) feeding on white-tailed deer. Journal of medical entomology. 2000 Jul;37(4):588–94. doi: 10.1603/0022-2585-37.4.588. [DOI] [PubMed] [Google Scholar]
- 13.Brochier B, Blancou J, Thomas I, Languet B, Artois M, Kieny MP, et al. Use of recombinant vaccinia-rabies glycoprotein virus for oral vaccination of wildlife against rabies: innocuity to several non-target bait consuming species. Journal of wildlife diseases. 1989 Oct;25(4):540–7. doi: 10.7589/0090-3558-25.4.540. [DOI] [PubMed] [Google Scholar]
- 14.Brochier B, Kieny MP, Costy F, Coppens P, Bauduin B, Lecocq JP, et al. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991 Dec 19-26;354(6354):520–2. doi: 10.1038/354520a0. [DOI] [PubMed] [Google Scholar]
- 15.Matouch O, Vitasek J, Semerad Z, Malena M. Elimination of rabies in the Czech Republic. Developments in biologicals. 2006;125:141–3. [PubMed] [Google Scholar]
- 16.Muller T, Selhorst T, Potzsch C. Fox rabies in Germany - an update. Euro Surveill. 2005 Nov;10(11):229–31. [PubMed] [Google Scholar]
- 17.Rupprecht CE, Hanlon CA, Slate D. Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Developments in biologicals. 2004;119:173–84. [PubMed] [Google Scholar]
- 18.Tsao J, Barbour AG, Luke CJ, Fikrig E, Fish D. Vector borne and zoonotic diseases. 1. Vol. 1. Larchmont, NY: 2001. Spring. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks; pp. 65–74. [DOI] [PubMed] [Google Scholar]
- 19.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proceedings of the National Academy of Sciences of the United States of America. 2004 Dec 28;101(52):18159–64. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheckelhoff MR, Telford SR, Hu LT. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine. 2006 Mar 10;24(11):1949–57. doi: 10.1016/j.vaccine.2005.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luker KE, Hutchens M, Schultz T, Pekosz A, Luker GD. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005 Oct 25;341(2):284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Moss B, editor. Expression of proteins in mammalian cells using vaccinia viral vectors. New York: John Wiley & Sons Inc.; 1991. [Google Scholar]
- 23.Wang XG, Lin B, Kidder JM, Telford S, Hu LT. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J Bacteriol. 2002 Nov;184(22):6198–206. doi: 10.1128/JB.184.22.6198-6206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharya D, Mecsas J, Hu LT. Development of a vaccinia virus based reservoir-targeted vaccine against Yersinia pestis. Vaccine. 2010 Nov 10;28(48):7683–9. doi: 10.1016/j.vaccine.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telford SR, III, Cunningham JA, Waltari E, Hu LT. Nest box deployed bait for delivering oral vaccines to white footed mice. Tick and Tickborne Diseases. 2011 doi: 10.1016/j.ttbdis.2011.06.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fikrig E, Barthold SW, Flavell RA. OspA vaccination of mice with established Borrelia burgdorferi infection alters disease but not infection. Infection and immunity. 1993 Jun;61(6):2553–7. doi: 10.1128/iai.61.6.2553-2557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupprecht CE, Hamir AN, Johnston DH, Koprowski H. Efficacy of a vaccinia-rabies glycoprotein recombinant virus vaccine in raccoons (Procyon lotor) Reviews of infectious diseases. 1988 Nov-Dec;10(Suppl 4):S803–9. doi: 10.1093/clinids/10.supplement_4.s803. [DOI] [PubMed] [Google Scholar]
- 28.Gherardi MM, Esteban M. Mucosal and systemic immune responses induced after oral delivery of vaccinia virus recombinants. Vaccine. 1999 Mar 5;17(9-10):1074–83. doi: 10.1016/s0264-410x(98)00324-7. [DOI] [PubMed] [Google Scholar]
- 29.Gomes-Solecki MJ, Brisson DR, Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine. 2006 May 15;24(20):4440–9. doi: 10.1016/j.vaccine.2005.08.089. [DOI] [PubMed] [Google Scholar]
- 30.Luke CJ, Huebner RC, Kasmiersky V, Barbour AG. Oral delivery of purified lipoprotein OspA protects mice from systemic infection with Borrelia burgdorferi. Vaccine. 1997 Apr-May;15(6-7):739–46. doi: 10.1016/s0264-410x(97)00219-3. [DOI] [PubMed] [Google Scholar]
- 31.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice from Lyme borreliosis by oral vaccination with Escherichia coli expressing OspA. The Journal of infectious diseases. 1991 Dec;164(6):1224–7. doi: 10.1093/infdis/164.6.1224. [DOI] [PubMed] [Google Scholar]
- 32.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Long-term protection of mice from Lyme disease by vaccination with OspA. Infection and immunity. 1992 Mar;60(3):773–7. doi: 10.1128/iai.60.3.773-777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


