Abstract
Background
Polycystin-2 (PC2), encoded by the gene that is mutated in autosomal dominant polycystic kidney disease (ADPKD), functions as a calcium (Ca2+) permeable ion channel. Considerable controversy remains regarding the subcellular localization and signaling function of PC2 in kidney cells.
Methods
We investigated the subcellular PC2 localization by immunocytochemistry and confocal microscopy in primary cultures of human and rat proximal tubule cells after stimulating cytosolic Ca2+ signaling. Plasma membrane (PM) Ca2+ permeability was evaluated by Fura-2 manganese quenching using time-lapse fluorescence microscopy.
Results
We demonstrated that PC2 exhibits a dynamic subcellular localization pattern. In unstimulated human or rat proximal tubule cells, PC2 exhibited a cytosolic/reticular distribution. Treatments with agents that in various ways affect the Ca2+ signaling machinery, those being ATP, bradykinin, ionomycin, CPA or thapsigargin, resulted in increased PC2 immunostaining in the PM. Exposing cells to the steroid hormone ouabain, known to trigger Ca2+ oscillations in kidney cells, caused increased PC2 in the PM and increased PM Ca2+ permeability. Intracellular Ca2+ buffering with BAPTA, inositol 1,4,5-trisphosphate receptor (InsP3R) inhibition with 2-aminoethoxydiphenyl borate (2-APB) or Ca2+/Calmodulin-dependent kinase inhibition with KN-93 completely abolished ouabain-stimulated PC2 translocation to the PM.
Conclusions
These novel findings demonstrate intracellular Ca2+-dependent PC2 trafficking in human and rat kidney cells, which may provide new insight into cyst formations in ADPKD.
Keywords: Polycystin-2, Protein trafficking, Calcium signaling, Kidney cells, Autosomal dominant polycystic kidney disease
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most commonly inherited monogenetic disease, affecting more than 1 in 1000 live births, causing renal failure [1]. ADPKD is caused by mutation in two associated proteins, polycystin-1 or −2 (PC1 and PC2), which are essential for the formation and maintenance of a proper structure of the renal tubule. These mutations in PC1 and PC2 are responsible for approximately 85% and 15% of all ADPKD cases, respectively [2]. It is well established that PC2 acts as an ion channel permeable to calcium ions (Ca2+) [3]. Interestingly, loss of PC2 channel function and subsequent impaired Ca2+ signaling may contribute to ADPKD pathogenesis [4]. A controversial aspect of PC2-mediated signaling in ADPKD is whether this protein is expressed in the endoplasmic reticulum (ER) or in the plasma membrane (PM). The amount of PC2 present in the ER, where it functions as a Ca2+-activated intracellular Ca2+ release channel [3], is significantly larger than the amount of PC2 expressed in the PM, where it functions as a non-selective cation channel [5,6]. In addition, PC2 has also been documented in the primary cilium of kidney epithelial cells, where it contributes to the mechano-sensing machinery by mediating Ca2+ entry in response to flow rate changes [7]. Nevertheless, the exact function and mechanism of PC2 activation in the cilium and/or other subcellular organelles remain largely unknown.
Ca2+ signaling is a vital mechanism in many cell types, controlling diverse cellular processes, such as: secretion, mechano-transduction, cell death, gene expression, or proliferation (for review see [8]). Under certain conditions, via a sophisticated interplay between Ca2+ channels and transporters located in the PM and/or on the membrane of internal organelles, such as the ER, sustained oscillatory Ca2+ signaling can occur [9]. These Ca2+ oscillations encode important information in their frequency and amplitude, which is decoded by cells using different Ca2+-effectors, such as protein kinases, phosphatases, proteases or transcription factors, which in turn regulate adaptive cellular responses. Intracellular Ca2+ signaling mishandling is involved in the pathogenesis or progression of several disease conditions and particularly in kidney disease, where intracellular Ca2+ signaling appears to be linked to cystic formation during ADPKD [10].
In the current study, we investigated the dynamic nature of PC2 localization in primary human and rat kidney proximal tubule cells. We found that PC2 translocates from a basal, reticular-like localization to a PM localization when cells were challenged with agents that raise the basal concentration of intracellular Ca2+. PC2 trafficking was inhibited either by intracellular Ca2+ release blockers or by inhibitors of key Ca2+-dependent proteins. Taken together, these results demonstrate that PC trafficking in kidney cells is regulated by intracellular Ca2+ and may contribute to the general understanding of ADPKD.
Methods
Cells cultures
Primary cultures of rat proximal tubule (rPT) cells were prepared as described previously [11]. Briefly, kidneys from 20-day-old female Sprague Dawley rats were used to prepare rPT cells. Cells were cultured in supplemented DMEM (20 mM Hepes, 24 mM NaHCO3, 10 mg/ml penicillin, 10 mg/ml streptomycin and 10% fetal bovine serum (FBS)) on glass coverslips for 48–72 h in 5% CO2 at 37°C. Cells were starved in 1% FBS and cultured in the absence of antibiotics for 24 h before the experiment. Primary cultures of human proximal tubule (hPT) cells were prepared as described. Briefly, approximately 10 g of fresh renal tissue, obtained from human nephrectomy samples, was dissected, and the obtained cortex was minced and then enzymatically digested with 1 mg/ml of type 4 collagenase. The resulting suspension was filtered and then centrifuged at 200 x g for 5 min. The pellet was washed three times with ice-cold Hank’s balanced salt solution and finally resuspended in DMEM/Ham’s Nutrient Mixture F12, supplemented with 10% FBS, insulin-transferrin-selenium, hydrocortisone and antibiotics. The final cell suspension was then plated onto coverslips coated with type IV collagen. All experiments were ethically approved by the Swedish Ethical Committee North, numbers 183/03 (rat) and 03–143 (human).
Reagents
Reagents and concentrations were as follows: ouabain (1 μM for hPT cells and 100 μM for rPT cells since rodents are more resistant to ouabain than humans [12], Sigma-Aldrich), ATP (25 μM, Sigma-Aldrich), bradykinin (20 nM, Sigma-Aldrich), ionomycin (1 μM, Sigma-Aldrich), thapsigargin (1 μM, Sigma-Aldrich), bis(2-aminophenoxy)ethane tetraacetic acid (BAPTA, 10 μM, Molecular Probes), 2-aminoethoxydiphenyl borate (2-APB, 100 μM, Sigma-Aldrich), KN-93 (10 μM, Sigma-Aldrich), LY294002 (10 μM, Sigma-Aldrich), wortmanin (5 μM, Sigma-Aldrich), FK506 (20 nM, Sigma-Aldrich), Go6983 (1 μM, Sigma-Aldrich), calphostin (5 μM, Sigma-Aldrich), and cycloheximide (CHX, 100 μM, Sigma-Aldrich). Cells were treated with inducers of Ca2+ signaling (ouabain, ATP, bradykinin) 3 h prior immunostaining and with inhibitors 15 min prior Ca2+ signaling-inducers.
Immunocytochemistry
Immunocytochemistry of PC2 in hPT and rPT cells was performed according to standard protocol. Cells were fixed with 4% paraformaldehyde for 15 min and then permeabilized for 10 min with 0.3% Triton X-100. After blocking with 1% BSA for 1 h, cells were incubated overnight at 4°C with anti-PC2 polyclonal antibodies (generously provided by Dr. Stefan Somlo, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA and Dr. Jing Zhou, Renal Division, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA) against amino acids 103 to 203 (YCB9) [13] or amino acids 44 to 62 [7] on the N-terminus, respectively. Both antibodies showed similar PC2 pattern in proximal tubule cells (Additional file 1: Figure S1). All images presented here are using the antibody against YCB9. Cells were incubated for 1 h at room temperature with Alexa488 fluorescent secondary antibody (1:500, Molecular Probes). Staining with only secondary antibody was used as control (Additional file 2: Figure S2). Slides were scanned with similar exposure time using a Leica TCS SP inverted confocal laser scanning microscope equipped with a 40x/1.4 NA oil-immersion objective.
Calcium imaging
For Ca2+ experiments cells were loaded with 5 μM Fura-2/AM (Invitrogen) at 37°C for 1 h. Calcium measurements were performed at 37°C in a heated chamber (QE-1, Warner Instruments) with a cooled CCD camera (ORCA-ERG, Hamamatsu) mounted on an upright microscope (Axioskop 2 FS, Zeiss) equipped with a 40x/0.8 NA water dipping lens. Excitation at 340 and 380 nm was carried out with a monochromator (Polychrome IV, TILL Photonics). Devices were controlled and data were recorded and analyzed with the computer software MetaFluor (Molecular Devices). All experiments were performed in physiological buffer (100 mM NaCl, 4 mM KCl, 25 mM NaHCO3, 1.5 mM CaCl2, 1.1 mM MgCl2, 1 mM NaH2PO4, 10 mM D-glucose, and 20 mM HEPES, pH 7.4). An oscillating cell was defined as a cell that displayed two or more Ca2+ peaks, of which each peak’s amplitude was at least 10% over baseline. PM permeability was measured using the Fura-2 fluorescence quenching technique with Mn2+. Fura-2 was then excited at the Ca2+-dependent wavelength, 340 nm, and at the Ca2+-independent wavelength, 360 nm. The PM Ca2+ permeability was measured as the fluorescence decrease after adding 0.1 mM MnC12 to the recording medium.
Statistics
PM PC2-positive cells were counted and expressed as the percentage over the total number of cells. The percentage of PM-positive cells corresponding to the control condition was assigned a value of 1, and the different treatments were normalized over this value in order to express them as fold-values over control. Data are presented as mean ± SEM or as a representative result of at least three independent experiments. One-way ANOVA with a Bonferroni post hoc test was used and significance was accepted at P < 0.05.
Results
Subcellular localization of PC2 is dynamically regulated
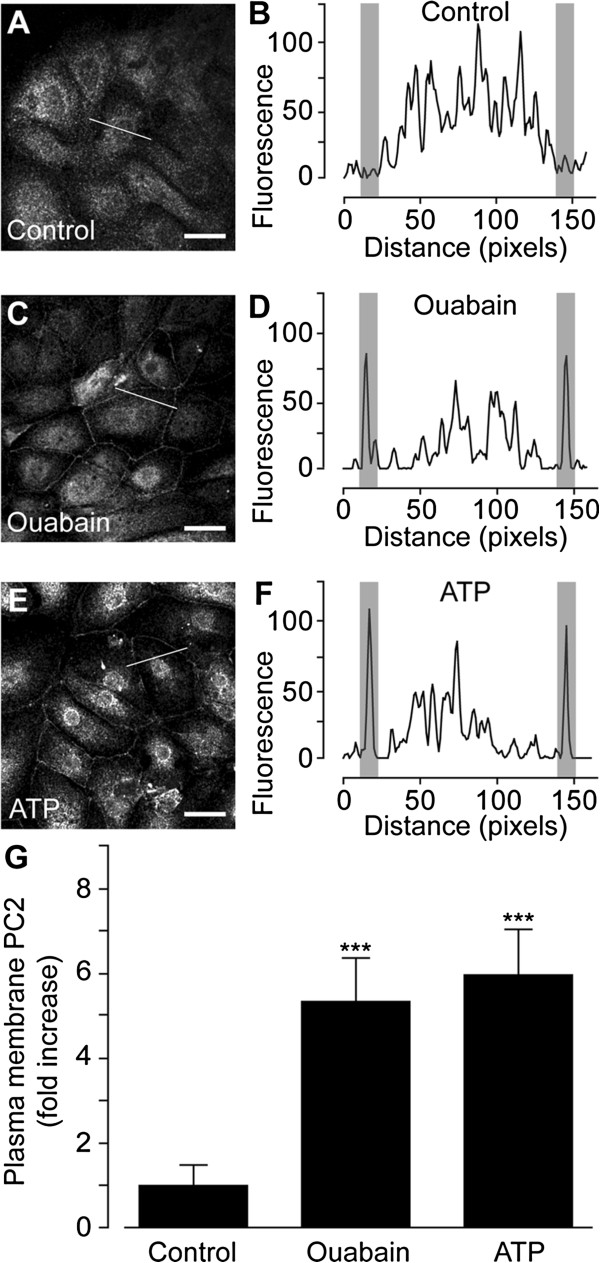
The subcellular localization of the PC2 protein was studied using immunocytochemistry in human primary proximal tubule (hPT) cells prepared from nephrectomy patients, as described in Materials and methods. In un-stimulated hPT cells PC2 exhibited a cytoplasmic/reticular staining (Figure 1A), in good agreement with a previous report [3]. Plotting the localization profile along a line through an individual cell confirmed the cytoplasmic/reticular localization pattern (Figure 1B). To test the dynamic nature of PC2 localization, hPT cells were challenged with the steroid ouabain, known to induce intracellular Ca2+ oscillations in rat kidney cells [14,15]. When hPT cells were exposed to ouabain, a strong PC2 localization in the PM was observed (Figure 1C and D). To test whether this effect was due to an increase of cytosolic Ca2+, hPT cells were treated with nucleotide adenosine triphosphate (ATP), a well-established inducer of cytosolic Ca2+ signaling in renal cells [16]. hPT cells exposed to ATP exhibited a clear translocation of PC2 towards the PM (Figure 1E and F). Quantification analysis of n single-cells from N independent preparations showed that the number of hPT cells positive for PC2 in the PM was significantly increased 5.3 ± 1.0-fold by ouabain and 6.0 ± 1.1-fold by ATP (control n = 76 N = 6, ouabain n = 159 N = 7, ATP n = 70 N = 4) (Figure 1G).
Figure 1.
PC2 trafficking to the PM in human proximal tubule (hPT) cells. (A-F). Immunocytochemistry of PC2 in hRT cells treated with control (A), 1 μM ouabain (C), or 25 μM ATP (E). Scale bars, 20 μm. Localization profile of PC2 along a line (as indicated) through the center of a single hPT cell treated with control (B), ouabain (D), or ATP (F). Gray areas represent PM regions. (G) Quantitative analysis of a number of PM PC2-positive hRT cells following indicated treatments. Values are the mean ± SEM, and ***P < 0.001 vs. control.
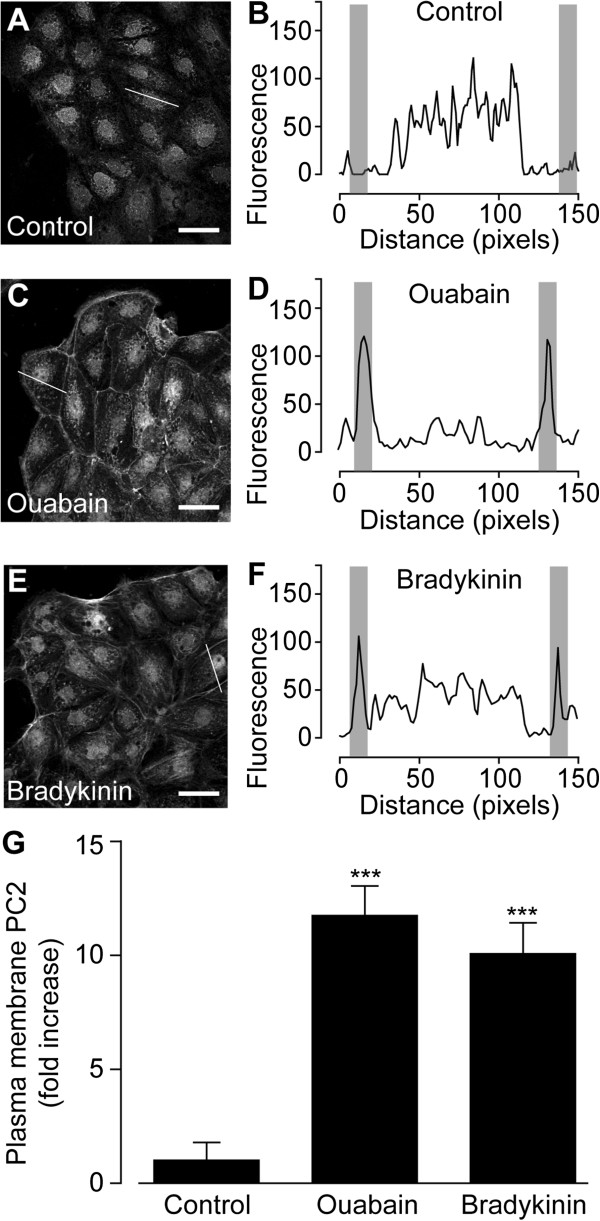
Next, the dynamic localization pattern of PC2 in rat kidney cells was investigated. Immunocytochemistry experiments in primary cultures of rat proximal tubule (rPT) cells showed a cytoplasmic/reticular localization pattern of PC2 in un-stimulated cells (Figure 2A and B), in accordance with results from hPT cells. This basal PC2 localization was clearly translocated towards the PM when rPT cells were treated with ouabain (Figure 2C and D) or bradykinin (Figure 2E and F). Both ouabain and bradykinin are reported to induce a Ca2+ response in rPT cells [15]. Quantitative analysis showed that the number of rPT cells positive for PC2 in the PM was significantly increased 11.7 ± 2.1-fold by ouabain and 10.1 ± 2.2-fold by bradykinin (control n = 175 N = 8, ouabain n = 123 N = 7, bradykinin n = 104 N = 5) (Figure 2G).
Figure 2.
PC2 trafficking to the PM in rat proximal tubule (rPT) cells. (A-F) Immunocytochemistry of PC2 in rPT cells treated with control (A), 100 μM ouabain (C), or 20 nM bradykinin (E). Scale bars, 20 μm. Localization profile of PC along a line (as indicated) through the center of a single rPT cell treated with control (B), ouabain (D), or bradykinin (F). Gray areas represent PM regions. (G) Quantitative analysis of a number of PM PC2 positive rPT cells following indicated treatments. Values are the mean ± SEM and ***P < 0.001 vs. control.
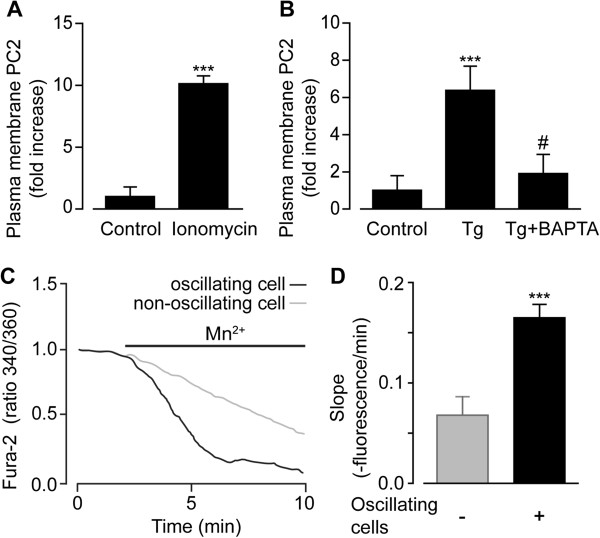
To test whether an increase of the cytosolic Ca2+ concentration was sufficient to induce PC2-trafficking towards the PM, rPT cells were exposed to various well-known agents affecting the cellular Ca2+ machinery. First, a Ca2+ influx was induced by using the Ca2+ ionophore ionomycin. Ionomycin caused a significant 10.1 ± 0.6-fold increase of PC2 PM positive rPT cells (control n = 175 N = 8, ionomycin n = 139 N = 8) (Figure 3A). In another approach, a transient Ca2+ release from the ER was induced by using thapsigargin, an inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, which caused a raise in cytosolic Ca2+. Thapsigargin stimulated a significant 6.4 ± 1.3-fold increase of PC2 translocation to the PM in rPT cells (thapsigargin n = 73 N = 6) (Figure 3B). The stimulatory effect of thapsigargin on PC2 trafficking was 1.9 ± 1.0-fold and reversed in cells pre-incubated with BAPTA (thapsigargin + BAPTA n = 76 N = 7), an intracellular Ca2+ chelator (Figure 3B).
Figure 3.
Intracellular Ca2+ release mediates PC2 translocation to the PM. (A-B) Quantitative analysis of a number of PM PC2-positive rPT cells following treatment with 1 μM ionomycin (A), 1 μM thapsigargin (Tg) (B), or thapsigargin with 10 μM BAPTA (B). (C) Single cell Ca2+ recording of two rPT cells loaded with Fura-2/AM treated with 100 μM ouabain followed by sequential addition of 2 mM Mn2+ to the recoding medium. (D) Statistical analysis of slope decrease in non-oscillating (gray bar) and oscillating cells (black bar) in response to ouabain after Mn2+ addition to the recording medium. Values are the mean ± SEM, *P < 0.05, ***P < 0.001 vs. control, and #P < 0.001 vs. treatment.
Taken together, these results demonstrate that cytosolic Ca2+ regulates PC2 trafficking in both human and rat kidney cells.
Ca2+ channels in the PM are more abundant in Ca2+ oscillating cells
A functional study of the PM Ca2+ permeability was then conducted using the manganese (Mn2+) quenching technique, based on the higher affinity Fura-2 has for Mn2+ than for Ca2+. When Mn2+ binds to Fura-2, it displaces Ca2+, resulting in a decreased fluorescence emission signal. Fura-2 preloaded rPT cells were first treated with ouabain and monitored using time-lapse Ca2+ imaging. Subsequent addition of Mn2+ to the recording medium induced a rapid decrease in the Fura-2 emission signal (Figure 3C). Interestingly, cells that were responding to ouabain with cytosolic Ca2+ oscillations showed a significantly steeper decrease in Fura-2 fluorescence compared to that of non-oscillating cells (Figure 3D). These results indicate an increased amount of Ca2+ channels present in the PM of cells exhibiting ouabain-induced Ca2+ oscillations.
Dynamic localization of PC2 is regulated by cytosolic Ca2+ signaling
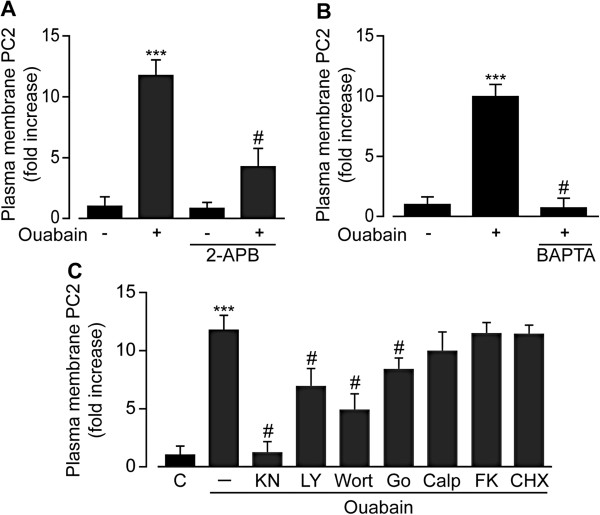
To further investigate the contribution of cytosolic Ca2+ signaling on PC2-trafficking, immunocytochemistry experiments were conducted on rPT cells stimulated with ouabain in the presence of intracellular Ca2+ signaling inhibitors. First, the InsP3R pathway was inhibited using 2-APB. In the absence of 2-APB, ouabain caused a significant 11.7 ± 1.3-fold increase of PC2 translocation to the PM (control n = 175 N = 8, ouabain n = 74 N = 4), while pre-incubation with 2-APB caused a significant 4.3 ± 1.5-fold decrease of ouabain-induced PC2 PM trafficking (ouabain n = 123 N = 7, ouabain + 2-APB n = 94 N = 4) (Figure 4A). Next, cytosolic Ca2+ was buffered using BAPTA. This treatment completely blocked the PC2 trafficking to the PM induced by ouabain (0.8 ± 0.7-fold, ouabain n = 123 N = 7, ouabain + BAPTA n = 56 N = 4) (Figure 4B). Together, these results suggest a pivotal role of InsP3R in triggering cytosolic Ca2+-dependent PC2 trafficking.
Figure 4.
PC2 trafficking to the PM depends on intracellular Ca2+ signaling and Ca2+-dependent kinases. (A-B) Quantitative analysis of PM PC2-positive rPT cells following treatment with 100 μM ouabain with 100 μM 2-APB (A), with 10 μM BAPTA (B), or with 10 μM KN-93, 10 μM LY294002, 5 μM wortmanin, 20 nM FK506, 1 μM Go6983, 5 μM calphostin, or 100 μM cycloheximide (C). Values are the mean ± SEM, ***P < 0.001 vs. control, and #P < 0.001 vs. treatment.
Ca2+-dependent protein kinases are involved in dynamic PC2 localization
To investigate the involvement of Ca2+-sensitive kinases on PC2 trafficking, selective chemical inhibitors were used. Under control conditions, ouabain induced a significant 12.4 ± 1.3-fold translocation of PC2 to the PM (ouabain n = 114 N = 5) (Figure 4C). A common target for Ca2+ signaling in mammalian cells is Ca2+/Calmodulin-dependent kinase (CaMK). Selective inhibition of CaMK with KN93 completely blocked the ouabain-induced PC2 trafficking to the PM (1.2 ± 0.9-fold, ouabain + KN93 n = 104 N = 7) (Figure 4C). The contribution of the Phosphatidylinositol 3-kinase (PI3K)/Akt pathway was next investigated. Pre-incubation with LY294002 or wortmanin also produced a significant decrease in ouabain-induced PC2 translocation (6.9 ± 1.5-fold, ouabain + LY294002 n = 72 N = 4 and 4.8 ± 1.4-fold, ouabain + wortmanin n = 92 N = 4, respectively) (Figure 4C). Inhibition of protein kinase C (PKC) by means of broad spectrum PKC inhibitors Go6983 or calphostin resulted in decreased levels of PC2 staining in the PM that reached statistical significance only in Go6983 pre-treated cells (8.4 ± 1.0-fold, ouabain + Go6983 n = 117 N = 6 and 9.9 ± 1.7-fold, ouabain + calphostin n = 67 N = 3) (Figure 4C). Finally, inhibition of the Ca2+-dependent protein-phosphatase calcineurin with FK506, had no effect on the PC2 translocation (11.4 ± 1.0-fold, ouabain + FK506 n = 124 N = 7) (Figure 4C).
The observed increase in the PC2 immunosignal from the PM could be due to di novo synthesis of PC2 proteins. To investigate this hypothesis, cells were pre-treated with cycloheximide (CHX) to stop the translation of new proteins. Pre-treatment of cells with CHX failed to stop increased PC2 trafficking to the PM following ouabain treatment (11.4 ± 0.8-fold, ouabain + CHX n = 51 N = 3) (Figure 4C).
Together, these results suggest a translocation of cellular PC2 to the PM via the intracellular Ca2+/CaMK pathway, with some involvement of the PI3K/Akt and PKC pathways.
Discussion
The notion that polycystin proteins act as cellular sensors that can modulate intracellular Ca2+ signaling is now well established [3]. Mounting evidence also supports the idea that mutations in the PC1 or PC2 gene perturb proper assembly, activity, and regulation of the polycystin proteins. Intriguingly, PC2 loss-of-function in modulating intracellular Ca2+ concentration may provide a possible explanation for the pathophysiology of ADPKD [17]. Here, we showed that the PC2 subcellular localization pattern is dynamically regulated in both human and rat kidney cells. Dependent on cytosolic Ca2+ increases, PC2 translocated from the cytosolic/ER compartment to the PM. When cells were challenged with ouabain, a treatment that has been shown to induce InsP3R-dependent Ca2+ oscillations in kidney cells [14,15], an increased PM PC2 localization and PM Ca2+ permeability were observed. The PM Ca2+ permeability was indirectly examined using Mn2+ quenching and electrophysiology recordings are required to determine absolute numbers. Pharmacological inhibition of key intracellular Ca2+ release components suppressed Ca2+-mediated PC2 trafficking, indicating a Ca2+-dependent translocation process. CaMK, a canonic Ca2+-activated kinase, was necessary for PC2 translocation, whereas the PI3K or PKC kinases contributed to a lesser extent. Mutations causing ADPKD have been reported altering the sub-cellular PC2 localization and/or function. For example, PC2 having the naturally occurring pathogenic mutant R742X resides in the PM [13], however, without having channel activity [18]. Another pathogenic missense mutation of PC2 is D511V [19], where a single amino acid in the third membrane-spanning domain is mutated, results in a loss of PC2 channel activity [3].
The subcellular localization pattern of the PC2 protein has been a long-lasting matter of controversy [20]. In various cell lines (LLCPK1, MDCK, HEK-293) and adult human kidney PC2 has been detected in ER where it function as a Ca2+ channel [13,21]. PC2 has also been detected in the basolateral and lateral cell membrane in adult human and rat kidney cells and collecting duct cells where it functions as cell-cell adhesion [6,21-25]. In primary cilium of human proximal tubule and mouse collecting duct cells PC2 is reported as a flow-sensitive channel [7,26,27]. These observations, and many others that report different PC2 localization [20], support the idea of a dynamic protein with its expression regulated by sub-cellular mechanisms. For example, PC2 contains an ER retention signal in its C-terminal sequence that inhibits trafficking to the cell surface [28]. PC2 deletion mutants for this ER retention signal constitutively translocate to the PM [18]. In ER Ca2+ stores, PC2 acts as a Ca2+-release channel that amplifies Ca2+ transients initiated by InsP3Rs [3]. Ca2+ releasing activity of PC2 is regulated by Ca2+ itself through a Ca2+-induced Ca2+ release mechanism [29] that requires direct association with InsP3R and regulates the physiological level of the intracellular Ca2+ concentration [30].
PC2 has previously been reported to be present in the PM [22,25]. PC2 localization to the PM is then modulated by chemical chaperones, proteasome inhibitors, protein-protein interactions and phosphorylation, and also upon massive overexpression that eventually overrides the ER retention machinery [6]. Under certain conditions, for example when PC2 is truncated at or before Glu787 its product is detected in the PM [13]. Trafficking of PC2 in cells has also been suggested to occur through its physical interaction with PC1 via their C-termini, forming a heteromeric, non-selective cationic channel complex [31]. Efficient assembly of PC1 and PC2 appears to be essential for proper trafficking and channel activity [32]. These previous results and the findings presented here support the idea of a dynamically regulated subcellular localization of PC2.
Our results indicate that CaMK is an important regulator of PC2 trafficking to the PM. Indeed, phosphorylation has previously been demonstrated in PC2 trafficking [13]. Two evolutionarily conserved phosphorylation sites in the PC2 protein sequence were suggested to control its subcellular localization: serine residue 76 (Ser76)/Ser80 and Ser812, which are phosphorylated by glycogen synthase kinase 3 (GSK3) [33] and casein kinase 2 (CK-2) [34], respectively. We speculate that the PM PC2 portion might be phosphorylated differently than ER PC2. It has been shown that PKC-dependent phosphorylation at Ser801 is essential for a normal function of PC2 as an ER Ca2+ release channel [35]. Additionally, Never in mitosis A-related kinase 8 (Nek8), a serine/threonine kinase that is mutated in some cases of juvenile polycystic kidney disease, induced abnormal PC2 phosphorylation and trafficking to primary cilia in the kidney [36]. The Ca2+ dependent trafficking of PC2 reported herein might also play a role in the PC2 expression profile in cilia. Surprisingly little is known about the upstream physiological stimuli activating all these kinases to critically regulate PC2 trafficking. Whether the Ca2+ signaling pathway presented here is the missing upstream link in controlling CaMK, GSK-3, CK-2 or Nek8 in PC2 trafficking remains to be further examined.
Polycystin proteins are expressed in primary cilia of cultured renal epithelial cells, where they might function in transducing sensory information, such as shear stress during fluid flow [7], leading to Ca2+ influx through mechanically sensitive channels that reside in the ciliary membrane [37]. This Ca2+ signal is then amplified by Ca2+ release from internal ER/SR stores and spreads to neighboring cells through gap junctions. PC2 has been suggested to be a mechano-sensitive channel since mechano-transduction is abolished in the presence of a specific PC2 blocking antibody [7] and in epithelial cells isolated from PC1-deficient mice [7]. Intracellular Ca2+ release and PC2 trafficking to the PM may form a positive loop for Ca2+ influx and intracellular Ca2+ overload, a condition that has been reported previously in ADPKD progression [1,5]. However, the exact contribution of PC2 dynamic localization to the PM in ADPKD pathogenesis remains to be elucidated.
Our results show that endogenous agonists that increase cytosolic Ca2+ levels, such as ouabain, ATP, or bradykinin, induced PC2 trafficking to the PM. It was previously reported that ouabain triggers InsP3R-dependent intracellular Ca2+ oscillation in cultured rPT cells [14,15]. According to the results presented here, cells that exhibited ouabain-induced Ca2+ oscillations had increased PM Ca2+ permeability. Other reports have shown that cilia mechano-activation leads to ATP secretion, which acts as an auto/paracrine signal through purinergic receptor activation and intracellular Ca2+ signals [38], suggesting that both agonists may contribute to PC2 translocation to the PM under physiopathological conditions.
Conclusion
We conclude that PC2 subcellular localization is dynamically regulated through an intracellular Ca2+-dependent pathway, which in turn could be related to cystogenesis and ADPKD pathogenesis.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AM conceived the study and participated in its design, carried out the immunocytochemistry studies, cultured cells and drafted the manuscript. CI performed the statistical analysis and drafted the manuscript. SM carried out the manganese quenching experiments. AA conceived the study and participated in its design. PW participated in the design of the study and coordinated the collection of human tissue. PU designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
PC2 expression pattern in proximal tubule cells using two different antibodies. (A-D) Immunocytochemistry of PC2 in rat proximal tubule cells treated with control (A,C) or 100 μM ouabain (B,D) using anti-PC2 polyclonal antibodies against amino acids 103 to 203 (YCB9) or 44 to 62 on the N-terminus. Scale bars, 20 μm.
Immunocytochemistry negative control without primary PC2 antibody. (A-B) Immunocytochemistry staining in rat proximal tubule cells without (A) and with (B) anti-PC2 antibody present. Scale bars, 20 μm.
Contributor Information
Ayako Miyakawa, Email: ayako.miyakawa@ki.se.
Cristián Ibarra, Email: cristian.ibarra@ki.se.
Seth Malmersjö, Email: sethma@stanford.edu.
Anita Aperia, Email: anita.aperia@ki.se.
Peter Wiklund, Email: petrer.wiklund@karolinska.se.
Per Uhlén, Email: per.uhlen@ki.se.
Acknowledgments
This study was supported by the Swedish Research Council, Åke Wibergs Stiftelse, Jeanssons Stiftelser, Magnus Bergvalls Stiftelse, the University of the Ryukyus Faculty of Medicine, Okinawa, Japan, the Japan Science and Technology Agency, and the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
References
- Harris PC. Molecular basis of polycystic kidney disease: PKD1, PKD2 and PKHD1. Curr Opin Nephrol Hypertens. 2002;11(3):309–314. doi: 10.1097/00041552-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4(3):191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun. 2004;322(4):1364–1373. doi: 10.1016/j.bbrc.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2 + −permeable nonselective cation channel. Proc Natl Acad Sci U S A. 2001;98(3):1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2 + −permeable cation channel in renal epithelia. Mol Cell Biol. 2003;23(7):2600–2607. doi: 10.1128/MCB.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Uhlen P, Fritz N. Biochemistry of calcium oscillations. Biochem Biophys Res Commun. 2010;396(1):28–32. doi: 10.1016/j.bbrc.2010.02.117. [DOI] [PubMed] [Google Scholar]
- Abdul-Majeed S, Nauli SM. Calcium-mediated mechanisms of cystic expansion. Biochim Biophys Acta. 1812;10:1281–1290. doi: 10.1016/j.bbadis.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature. 2000;405(6787):694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na, K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274(40):28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A. 2001;98(23):13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem. 2003;278(50):50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci. 2003;18:237–241. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- Stayner C, Zhou J. Polycystin channels and kidney disease. Trends Pharmacol Sci. 2001;22(11):543–546. doi: 10.1016/S0165-6147(00)01832-0. [DOI] [PubMed] [Google Scholar]
- Chen XZ, Segal Y, Basora N, Guo L, Peng JB, Babakhanlou H, Vassilev PM, Brown EM, Hediger MA, Zhou J. Transport function of the naturally occurring pathogenic polycystin-2 mutant, R742X. Biochem Biophys Res Commun. 2001;282(5):1251–1256. doi: 10.1006/bbrc.2001.4720. [DOI] [PubMed] [Google Scholar]
- Reynolds DM, Hayashi T, Cai Y, Veldhuisen B, Watnick TJ, Lens XM, Mochizuki T, Qian F, Maeda Y, Li L. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. J Am Soc Nephrol. 1999;10(11):2342–2351. doi: 10.1681/ASN.V10112342. [DOI] [PubMed] [Google Scholar]
- Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005;67(4):1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J Biol Chem. 2002;277(23):20763–20773. doi: 10.1074/jbc.M107788200. [DOI] [PubMed] [Google Scholar]
- Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11(5):814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- Obermuller N, Gallagher AR, Cai Y, Gassler N, Gretz N, Somlo S, Witzgall R. The rat pkd2 protein assumes distinct subcellular distributions in different organs. Am J Physiol. 1999;277(6 Pt 2):F914–F925. doi: 10.1152/ajprenal.1999.277.6.F914. [DOI] [PubMed] [Google Scholar]
- Ong AC, Wagner B. Detection of proximal tubular motile cilia in a patient with renal sarcoidosis associated with hypercalcemia. Am J Kidney Dis. 2005;45(6):1096–1099. doi: 10.1053/j.ajkd.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJ. Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet. 2002;11(1):59–67. doi: 10.1093/hmg/11.1.59. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current biology: CB. 2002;12(11):R378–R380. doi: 10.1016/S0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13(10):2508–2516. doi: 10.1097/01.ASN.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 2004;18(6):740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- Petri ET, Celic A, Kennedy SD, Ehrlich BE, Boggon TJ, Hodsdon ME. Structure of the EF-hand domain of polycystin-2 suggests a mechanism for Ca2 + −dependent regulation of polycystin-2 channel activity. Proc Natl Acad Sci U S A. 2010;107(20):9176–9181. doi: 10.1073/pnas.0912295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S, De Smedt H. Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J Biol Chem. 2010;285(24):18794–18805. doi: 10.1074/jbc.M109.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Guo L, Chen XZ, Segal Y, Peng JB, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye C. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca(2+) homeostasis in polycystic kidney disease. Biochem Biophys Res Commun. 2001;282(1):341–350. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and −2 produces unique cation-permeable currents. Nature. 2000;408(6815):990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC. Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet. 2006;15(9):1465–1473. doi: 10.1093/hmg/ddl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005;24(4):705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets AJ, Needham AJ, Gill SK, Ong AC. Protein kinase D-mediated phosphorylation of polycystin-2 (TRPP2) is essential for its effects on cell growth and calcium channel activity. Mol Biol Cell. 2010;21(22):3853–3865. doi: 10.1091/mbc.E10-04-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19(3):469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184(1):71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol (Oxf) 2009;197(3):241–251. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PC2 expression pattern in proximal tubule cells using two different antibodies. (A-D) Immunocytochemistry of PC2 in rat proximal tubule cells treated with control (A,C) or 100 μM ouabain (B,D) using anti-PC2 polyclonal antibodies against amino acids 103 to 203 (YCB9) or 44 to 62 on the N-terminus. Scale bars, 20 μm.
Immunocytochemistry negative control without primary PC2 antibody. (A-B) Immunocytochemistry staining in rat proximal tubule cells without (A) and with (B) anti-PC2 antibody present. Scale bars, 20 μm.