Abstract
Background
Bocaviruses are classified as a genus within the Parvoviridae family of single-stranded DNA viruses and are pathogenic in some mammalian species. Two species have been previously reported in dogs, minute virus of canines (MVC), associated with neonatal diseases and fertility disorders; and Canine bocavirus (CBoV), associated with respiratory disease.
Findings
In this study using deep sequencing of enriched viral particles from the liver of a dog with severe hemorrhagic gastroenteritis, necrotizing vasculitis, granulomatous lymphadenitis and anuric renal failure, we identified and characterized a novel bocavirus we named Canine bocavirus 3 (CnBoV3). The three major ORFs of CnBoV3 (NS1, NP1 and VP1) shared less than 60% aa identity with those of other bocaviruses qualifying it as a novel species based on ICTV criteria. Inverse PCR showed the presence of concatemerized or circular forms of the genome in liver.
Conclusions
We genetically characterized a bocavirus in a dog liver that is highly distinct from prior canine bocaviruses found in respiratory and fecal samples. Its role in this animal’s complex disease remains to be determined.
Keywords: Canine bocavirus 3, Episome, Coinfection
Background
Parvoviruses consist of small non-enveloped, autonomously replicating, single-stranded DNA viruses with genome length between 4.5 - 5.5 kb [1]. Bocavirus, a genus of the family Parvoviridae, is characterized by the presence of a third major ORF named NP1. Bocaviruses are known to infect multiple mammalian species including humans [2], cows [3], pigs [4-6], gorillas [7], chimpanzees [8], California sea lions [9], dogs [10-13], cats [11], bats [14], and pine martens [15]. Bocavirus infections can cause respiratory and gastrointestinal symptoms in young animals and humans, but are also often subclinical in adults [2,16]. While many bocaviruses were initially identified in feces or respiratory secretion they can also be found in blood [2,16].
Minute virus of canines (MVC) was the first known bocavirus infecting dogs. MVC was isolated in 1967 in the feces of a clinical healthy dog, and later recognized as causing neonatal diseases and fertility disorders in dogs [16]. The second species of dog bocavirus (Canine bocavirus, CBoV) was identified in 2011 in the respiratory samples from diseased and healthy dogs [10]. One genotype of CBoV was associated with respiratory disease as it showed higher prevalence in diseased animals than healthy controls [10]. Variants of this CBoV were also detected in fecal, nasal, urine and blood samples collected from dogs in Hong Kong [11].
In this study, an infectious etiology was suspected for a dog with severe hemorrhagic gastroenteritis, necrotizing vasculitis, granulomatous lymphadenitis and anuric renal failure. The clinical and post-mortem workups for infectious causes in this case included negative test results for Canine Parvovirus 2, Canine Enteric Coronavirus, Canine distemper virus, Salmonella, Campylobacter, Clostridium perfringens enterotoxin A gene, Cryptosporidium, and Giardia. Special stains of histologic specimens revealed no detectable bacteria or other known infectious agents. Using deep sequencing, we characterized viral sequences present in the dog’s liver revealing a third species of canine bocavirus described here.
Results and discussion
A sequence of 300 bases showing sequence similarity to bocaviruses (BLASTx E < 10-3) was initially identified by 454 pyrosequencing. The sequence was extended by degenerate PCR targeting conserved bocavirus regions, yielding a ~2.5 kb partial genome sequence. The tissue nucleic acids were further analyzed using the MiSeq Illumina platform generating 16 contigs composed of 133 reads with similarities to bocaviruses (BLASTx E < 10-3), which allowed the amplification of all three ORFs. The virus was highly divergent from the other two known canine bocaviruses, MVC and CBoV, and was provisionally named Canine bocavirus 3 (CnBoV3) [10,11,13].
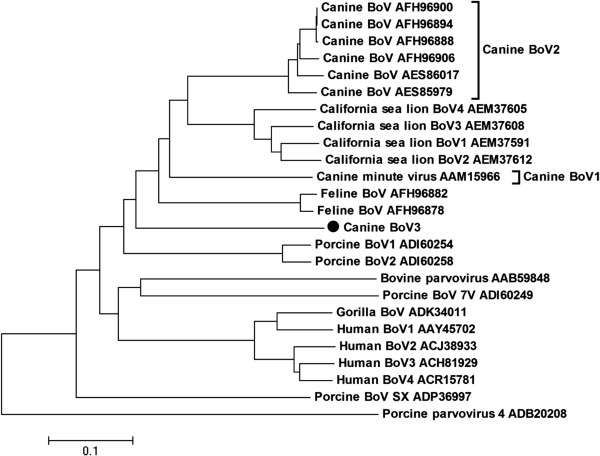
The nonstructural (NS1) protein encoded by ORF1 was 778 aa long, and contained motifs associated with rolling circle replication, helicase and ATPase. The NP1 protein encoded by the middle ORF3 was 194 aa long. The ORF2 encoded capsid proteins VP1 (689 aa) and proteolytically processed VP2 (560 aa). CnBoV3 shared 51%, 57%, 56% aa similarity with the NS1, NP1 and VP1 region of CBoV, respectively, and 49%, 52%, 57% aa similarity with the NS1, NP1 and VP1 regions of MVC. Phylogenetic analysis of the entire VP1 was performed to determine the relationship between CnBoV3 and other bocaviruses. CnBoV3 was phylogenetically distinct from the known dog bocaviruses and only loosely related to California sea lion bocaviruses, CBoV, feline bocavirus and MVC (Figure 1). Phylogenetic trees constructed by NS1 and NP1 region yielded similar topology (data not shown).
Figure 1.
Phylogenetic tree based on aligned amino acid sequences of full-length VP proteins of representative bocavirus species.
Bocaviruses are believed to replicate through the parvovirus rolling hairpin model, which generate replication intermediates of concatemers with head-to-head or tail-to-tail structure [17]. Recent experimental evidence showed the presence of head-to-tail concatemers or circularized genomes of human bocavirus (HBoV) 1&3 [18,19] and porcine bocaviruses [20], indicating that some bocaviruses may use a rolling - circle replication model.
Using inverse PCR with primers directed outward from the 5’ and 3’ extremities of the partial genome sequence we were able to amplify head-to-tail sequence of the CnBoV3 non-translated regions (NTR) between the VP termination and the NS1 initiation codons (Figure 2A). The generation of specific PCR products indicated the presence of concatemerized or circular forms of the genome in the liver [18].
Figure 2.
A) Inverse PCR was performed to obtain the NTR sequence between the VP termination and the NS initiation codon of episomal forms of CnBoV3. Primers (P1 and P2) were situated at VP and NS region respectively. Secondary structures (symbolic in the box) may have impeded the PCR resulting in several products of different length. B) Highly identical ‘rabbit ear’ structure of CnBoV3 and MVC. C) Alignments of NTR sequences obtained by sequencing different inverse PCR products directly (PCR1&2) and subcloning products (colony 1–4). Sequences outside the two arrows were identical. Underlined sequence shows location of “rabbit ear” sequence.
To confirm these results, multiple repeats of the inverse PCR were generated and directly sequenced as well as subcloned into a plasmid vector. PCR amplicons and plasmid inserts were Sanger sequenced using protocol for GC-rich/hairpin sequences. The resulting sequences were aligned and the length of the region between the VP stop and the NS start codon calculated. This region varied in length from 392 to 506 nt (Figure 2C). Mfold analyses of the longest NTR sequence (506 nt) showed that the long palindromic hairpin terminal repeats (e.g. approximately 150 nt at both ends in MVC or bovine parvovirus, BPV) were missing, but a typical “rabbit ear” structure was detected that was nearly identical to a structure in the MVC 5'NTR sequence (Figure 2B). These results suggested that inverse PCR may have been impeded by strong secondary DNA structures (Figure 2A &C, between two red arrows). None of the currently reported NTR regions of bocavirus obtained by inverse PCR contained both complete inverted terminal repeats seen in MVC or BPV [18-20].
Conclusions
In this study we genetically characterize a third species of canine bocavirus (CnBoV3), highly divergent from MVC [21] and CBoV [10,11], in the liver of a dog with severe disease. The presence of three distinct canine bocaviruses, phylogenetically interspersed with viruses from different mammalian hosts, may reflect their origins from different cross-species transmissions. The detection of CnBoV3 in liver tissue indicated that the virus had likely breached the mucosal barrier of the typical sites of bocavirus replication in the respiratory or enteric tracts. The detection of episomal forms by PCR indicated that replication may be occurring in hepatocytes or other liver cell types and that viremia was also likely present although the lack of available blood sample prevented direct testing. We also detected in that animal co-infection with a canine circovirus. Circovirus infection can lead to lymphocytic depletion and immunosuppression in the host [22]. Whether CnBoV3, canine circovirus, or their combination were involved in this dog’s severe symptoms requires further investigation.
Materials and methods
A dog suffering from vomiting and hemorrhagic gastroenteritis was euthanized after a rapid disease course lasting seven days. Necropsy showed the presence of necrotizing vasculitis, granulomatous lymphadenitis and anuric renal failure. Clinical and post-mortem tests for multiple infectious agents of enteric disease were all negative. Liver Tissue was stored at −80°C until further processing. Tissue samples (~25 mg) were homogenized, filtered, and nuclease treated as previously described to enrich for nucleic acids within viral particles [23]. Nucleic acids were then extracted using the QIAamp viral RNA Mini kit (Qiagen), randomly amplified using random RT-PCR with randomized 3’ primers and nucleic acid libraries prepared as previously described [24] for sequencing using the Genome Sequencer FLX Instrument (454 Life Science, Roche). The pyrosequencing reads were sorted, trimmed, assembled and compared to the GenBank non-redundant databases as previously described [9]. Potential viral sequences were identified with translated protein sequence similarity matches (BLASTx to GenBank's NR database with E-value < 10-3) to known viral sequences. The presence of virus protein sequences detected by 454 pyrosequencing was confirmed by PCR and Sanger sequencing. Genome walking and degenerate PCR were used to amplify the viral genome. Enriched viral nucleic acids from the infected tissue was also handled using the ScriptSeq RNA-Seq library preparation kit (Epicentre) and sequenced by MiSeq system (illumina). The resulting near complete genome of CnBoV3 was deposited in GenBank with accession no. KC580640. Phylogenetic analyses based on aligned amino acid sequences from full-length VP proteins were generated by the neighbor joining method in MEGA [25], using amino acid p-distances, with 1,000 bootstrap replicates.
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
LL generated the data, interpreted the data and drafted the manuscript. PP selected and collected the animal sample and helped interpret results. CL and ME performed real-time PCR. LC, SN, ES and CC provided help with Illumina sequencing. CW and XD provided bioinformatics analyses of 454 and Illumina data. JQ assisted in the NTR analysis. ED directed the research and revised the draft. All authors read and approved the final manuscript.
Contributor Information
Linlin Li, Email: linlinli99013@gmail.com.
Patricia A Pesavento, Email: papesavento@ucdavis.edu.
Christian M Leutenegger, Email: Christian-Leutenegger@idexx.com.
Marko Estrada, Email: Marko-Estrada@idexx.com.
Lark L Coffey, Email: lcoffey@bloodsystems.org.
Samia N Naccache, Email: samianidal@gmail.com.
Erik Samayoa, Email: Erik.Samayoa@ucsfmedctr.org.
Charles Chiu, Email: charles.chiu@ucsf.edu.
Jianming Qiu, Email: jqiu@kumc.edu.
Chunlin Wang, Email: wangcl@stanford.edu.
Xutao Deng, Email: XDeng@bloodsystems.org.
Eric Delwart, Email: delwarte@medicine.ucsf.edu.
Acknowledgements
The work was supported by the Blood Systems Research Institute and NIH R01 HL105770 to ED. The Bernice Barbour Foundation and the UC Davis Center for Companion Animal Health supports the research of PP.
References
- Tattersall P, Bergoin M, Bloom M, Brown K, Linden R, Tijssen P. In: Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Fauquet C, Mayo M, Maniloff J, Desselberger U, Ball L, editor. San Diego: Academic; 2005. pp. 353–369. [Google Scholar]
- Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Soderlund-Venermo M. Human bocavirus-the first 5 years. Rev Med Virol. 2012;22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- Chen KC, Shull BC, Moses EA, Lederman M, Stout ER, Bates RC. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986;60:1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WX, Li JS, Huang CP, Yao DP, Liu N, Cui SX, Jin Y, Duan ZJ. Identification and nearly full-length genome characterization of novel porcine bocaviruses. PLoS One. 2010;5:e13583. doi: 10.1371/journal.pone.0013583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Lan D, Li L, Wang C, Cui L, Zhang W, Hua X, Zhu C, Zhao W, Delwart E. Genomic characterization and high prevalence of bocaviruses in swine. PLoS One. 2011;6:e17292. doi: 10.1371/journal.pone.0017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Li L, Simmonds P, Wang C, Moeser A, Delwart E. The fecal virome of pigs on a high-density farm. J Virol. 2011;85:11697–11708. doi: 10.1128/JVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Mehta N, Esper F, Poljsak-Prijatelj M, Quan PL, Qaisar N, Delwart E, Lipkin WI. Identification and characterization of a new bocavirus species in gorillas. PLoS One. 2010;5:e11948. doi: 10.1371/journal.pone.0011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp CP, LeBreton M, Kantola K, Nana A, Diffo Jle D, Djoko CF, Tamoufe U, Kiyang JA, Babila TG, Ngole EM. Widespread infection with homologues of human parvoviruses B19, PARV4, and human bocavirus of chimpanzees and gorillas in the wild. J Virol. 2010;84:10289–10296. doi: 10.1128/JVI.01304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shan T, Wang C, Cote C, Kolman J, Onions D, Gulland FM, Delwart E. The fecal viral flora of California sea lions. J Virol. 2011;85:9909–9917. doi: 10.1128/JVI.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Mehta N, Dubovi EJ, Simmonds P, Govindasamy L, Medina JL, Street C, Shields S, Lipkin WI. Characterization of novel canine bocaviruses and their association with respiratory disease. J Gen Virol. 2012;93:341–346. doi: 10.1099/vir.0.036624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Yeung HC, Teng JL, Wu Y, Bai R, Fan RY, Chan KH, Yuen KY. Identification and characterization of bocaviruses in cats and dogs reveals a novel feline bocavirus and a novel genetic group of canine bocavirus. J Gen Virol. 2012;93:1573–1582. doi: 10.1099/vir.0.042531-0. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Kawakami K, Abe T, Mochizuki M. A minute virus of canines (MVC: canine bocavirus) isolated from an elderly dog with severe gastroenteritis, and phylogenetic analysis of MVC strains. Vet Microbiol. 2010;145:334–338. doi: 10.1016/j.vetmic.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D, Green B, Carmichael LE, Parrish CR. The canine minute virus (minute virus of canines) is a distinct parvovirus that is most similar to bovine parvovirus. Virology. 2002;302:219–223. doi: 10.1006/viro.2002.1674. [DOI] [PubMed] [Google Scholar]
- Wu Z, Ren X, Yang L, Hu Y, Yang J, He G, Zhang J, Dong J, Sun L, Du J. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J Virol. 2012;86:10999–11012. doi: 10.1128/JVI.01394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand JM, van Leeuwen M, Schapendonk CM, Simon JH, Haagmans BL, Osterhaus AD, Smits SL. Metagenomic analysis of the viral flora of pine marten and European badger feces. J Virol. 2012;86:2360–2365. doi: 10.1128/JVI.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteufel J, Truyen U. Animal bocaviruses: a brief review. Intervirology. 2008;51:328–334. doi: 10.1159/000173734. [DOI] [PubMed] [Google Scholar]
- Schildgen O, Qiu J, Soderlund-Venermo M. Genomic features of the human bocaviruses. Future Virol. 2012;7:31–39. doi: 10.2217/fvl.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Hornig M, Asokan A, Williams B, Henriquez JA, Lipkin WI. Bocavirus episome in infected human tissue contains non-identical termini. PLoS One. 2011;6:e21362. doi: 10.1371/journal.pone.0021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusebrink J, Schildgen V, Tillmann RL, Wittleben F, Bohmer A, Muller A, Schildgen O. Detection of head-to-tail DNA sequences of human bocavirus in clinical samples. PLoS One. 2011;6:e19457. doi: 10.1371/journal.pone.0019457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WZ, Huang CP, Duan ZJ. [Identification and characterization of porcine bocavirus episomes] Bing Du Xue Bao. 2012;28:418–423. [PubMed] [Google Scholar]
- Binn LN, Lazar EC, Eddy GA, Kajima M. Recovery and characterization of a minute virus of canines. Infect Immun. 1970;1:503–508. doi: 10.1128/iai.1.5.503-508.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segales J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. 2012;164:10–19. doi: 10.1016/j.virusres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Victoria JG, Kapoor A, Dupuis K, Schnurr DP, Delwart EL. Rapid identification of known and new RNA viruses from animal tissues. PLoS Pathog. 2008;4:e1000163. doi: 10.1371/journal.ppat.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria JG, Kapoor A, Li L, Blinkova O, Slikas B, Wang C, Naeem A, Zaidi S, Delwart E. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol. 2009;83:4642–4651. doi: 10.1128/JVI.02301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]