Abstract
We sought to characterize molecular expression and ionic conductances in a novel population of interstitial cells (PDGFRα+ cells) in murine bladder to determine how these cells might participate in regulation of detrusor excitability. PDGFRα+ cells and smooth muscle cells (SMCs) were isolated from detrusor muscles of PDGFRα+/eGFP and smMHC/Cre/eGFP mice and sorted by FACS. PDGFRα+ cells were highly enriched in Pdgfra (12 fold vs. unsorted cell) and minimally positive for Mhc (SMC marker), Kit (ICC marker) and Pgp9.5 (neuronal marker). SK3 was dominantly expressed in PDGFRα+ cells in comparison to SMCs. αSlo (BK marker) was more highly expressed in SMCs. SK3 protein was observed in PDGFRα+ cells by immunohistochemistry but could not be resolved in SMCs. Depolarization evoked voltage-dependent Ca2+ currents in SMCs, but inward current conductances were not activated in PDGFRα+ cells under the same conditions. PDGFRα+ cells displayed spontaneous transient outward currents (STOCs) at potentials positive to −60 mV that were inhibited by apamin. SK channel modulators, CyPPA and SKA-31, induced significant hyperpolarization of PDGFRα+ cells and activated SK currents under voltage clamp. Similar responses were not resolved in SMCs at physiological potentials. Single channel measurements confirmed the presence of functional SK3 channels (i.e. single channel conductance of 10 pS and sensitivity to intracellular Ca2+) in PDGFRα+ cells. The apamin-sensitive stabilizing factor regulating detrusor excitability is likely to be due to the expression of SK3 channels in PDGFRα+ cells because SK agonists failed to elicit resolvable currents and hyperpolarization in SMCs at physiological potentials.
Key points
SK currents have been recorded from detrusor smooth muscle cells, but current density at physiological holding potentials is negligible.
We discovered a new class of interstitial cell in the bladder that were identified using antibodies against platelet-derived growth factor receptor-α(PDGFRα+ cells).
SK3 channel transcripts and protein are highly expressed in PDGFRα+ cells in comparison to smooth muscle cells.
Current density attributable to SK-like currents is much higher in PDGFRα+ cells than in smooth muscle cells. Single channel currents, consistent with the conductance and Ca2+ sensitivity of SK3 channels were measured in PDGFRα+ cells.
The abundance of SK3 channels in PDGFRα+ cells in detrusor muscles suggests that PDGFRα+ cells, not SMCs, provide apamin-sensitive regulation of detrusor excitability.
Introduction
Overactive bladder is a highly prevalent condition, affecting approximately 33 million adults in the United States. Symptoms of overactive bladder include frequency, urgency, urge incontinence, or nocturia. As bladder volume increases, involuntary contractions of the detrusor muscle are often associated with overactive bladder. The mechanisms underlying these involuntary contractions have not yet been fully elucidated. Despite the considerable impact this condition has on a patient's quality of life, pharmacological treatments of overactive bladder, such as antimuscarinic agents, are inadequate and often associated with significant adverse side effects (Kennelly & DeVoe, 2008). In this study we explore a new pathway that might be possible to exploit for therapeutic benefit in overactive bladder.
Several classes of K+ channels may participate in the regulation of detrusor excitability during bladder filling. Two families of Ca2+-activated K+ conductances have been characterized in detrusor smooth muscle cells (SMCs): large-conductance (BK) and small conductance (SK) Ca2+-activated K+ channels. BK channels have been extensively studied in detrusor SMCs, and knocking out the pore-forming subunit of these channels enhances detrusor over-activity (Heppner et al. 1997; Herrera et al. 2001; Herrera & Nelson, 2002; Meredith et al. 2004). Targeting BK channels for therapeutic benefit, however would be problematic due to the side effects on other organs including the vascular system. SK channel expression in detrusor muscle has also been reported (Herrera & Nelson, 2002; Herrera et al. 2003; Parajuli et al. 2012), and electrical field stimulation of nerves evokes transient hyperpolarization responses that are blocked the SK channel antagonist, apamin. Apamin also increases the spontaneous contractile activity of detrusor muscles (Thorneloe et al. 2008), and ablation of SK channels (SK2 or SK3 KO mice) produces bladder overactivity. SK currents have been recorded from detrusor myocytes, but positive holding potentials were needed to resolve these currents. Therefore, current through these channels would be negligible at physiological resting membrane potentials (RMP) of detrusor cells (Herrera et al. 2003; Parajuli et al. 2012).
We have recently discovered a new class of interstitial cell in the bladder (Koh et al. 2012). We identified these cells with antibodies against platelet-derived growth factor receptor-α (PDGFRα), and refer to these cells as PDGFRα+ cells. PDGFRα+ cells are associated with varicose nerve processes in detrusor muscles (Koh et al. 2012). Thus, PDGFRα+ cells may be innervated and receive and transduce neurotransmitter signals. Little is yet known about the molecular and functional apparatus of PDGFRα+ cells that might facilitate neural responses. In this report, we investigated expression of SK channels in PDGFRα+ cells and SMCs using analysis of gene transcripts, protein and electrophysiological behaviour to compare expression levels and function of SK channels.
Methods
Preparation of detrusor PDGFRα+ cells and SMCs
Animals maintaining and all experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Use and Care Committee at the University of Nevada. Three types of mice (3–6 weeks) were used for this study. C57BL/6, Pdgfratm11(EGFP)Sor/J (both purchased from Jackson Laboratory, Bar Harbor, ME, USA) and smMHC/Cre/eGFP mice (donated by Dr Michael Kotlikoff, Cornell University) were anaesthetized by isoflurane (AErrane; Baxter, Deerfield, IL, USA) and killed by cervical dislocation. The abdomens were opened and the bladders were removed to Ca-free Hank's solution (Table 2, solution I) and cut open to rinse free of urine. The urothelial layer was peeled away and the detrusors were cut into 8∼10 pieces, which were digested in solution I (Table 2) containing 1 mg ml−1 papain and 1 mg ml−1 dithioerythritol (Both from Sigma, St Louis, MO, USA). After 20–25 min, the papain-digested detrusor pieces would be rinsed briefly, and further digested in solution I containing 5 mg ml−1 collagenase type II (Sigma) and 100 μm CaCl2 for 25–30 min. Both digestions were performed at 37°C. The digested tissue sections were gently triturated using a fire-polished glass Pasteur pipette. For SMCs, aliquots of the resulting cell suspensions were transferred to a 0.5 ml chamber and allowed to adhere for 10–15 min, then cells were thoroughly rinsed in solution contained Ca2+ (2 mm) and physiological concentrations of other ions (CaPSS, Table 2, solution II). For PDGFRα+ cells, the resulting cell suspensions were rinsed with culture medium SMGM (Clonetics Corp., San Diego, CA, USA), gently triturated and plated onto murine collagen-coated (2.5 μg ml−1, Falcon/BD) glass coverslips in 35 mm culture dishes. PDGFRα+ cells were maintained in a humidified atmosphere of 95% O2/5% CO2 at 37°C and were used for recordings within 6–8 h after plating.
Table 2.
Solutions
| Components (mm) | I (Ca-free Hank's) | II (CaPSS) | III (K-rich) | IV (Cs-rich) | V (high-K) |
|---|---|---|---|---|---|
| NaCl | 125 | 135 | |||
| CsCl | 135 | ||||
| KCl | 5.36 | 5 | 30 | 150 | |
| MgCl2 | 1.2 | ||||
| CaCl2 | 2 | 3.15 | 3.15 | ||
| Hepes | 11 | 10 | 10 | 10 | 10 |
| Glucose | 10 | 10 | 10 | 10 | 10 |
| Sucrose | 2.9 | ||||
| NaHCO3 | 15.5 | ||||
| Na2HPO4 | 0.336 | ||||
| K2HPO4 | 0.44 | ||||
| K aspartate | 110 | ||||
| CPD | 2.5 | 2.5 | |||
| MgATP | 3 | 3 | |||
| Na2GTP | 0.1 | 0.1 | |||
| EGTA | 5 | 5 | 5 |
Various intracellular free Ca2+ concentrations (10 nm to 1 μm) were used in solution III and V by adjusting the concentrations of EGTA and CaCl2 calculated with Maxchelator software (http://maxchelator.stanford.edu). pH 7.4 for solution II, pH 7.2 for other solutions.
Electrophysiological recordings
Whole cell currents and membrane potentials were recorded using whole cell voltage- and current-clamp techniques. Cells were placed in a 0.5 ml chamber mounted on an inverted microscope (Nikon, Japan). PDGFRα+ cells, isolated from Pdgfratm11(EGFP)Sor/J mice, were identified by the fluorescence of eGFP in nuclei. SMCs, isolated from either C57BL/6 or smMHC/Cre/eGFP mice, were rod-shaped and identified in dispersions of muscles from the latter mice by the fluorescence of eGFP in the cytosol. For single channel recordings in PDGFRα+ cells, on-cell and excised inside-out configurations of the patch-clamp techniques were used. Pipette tip resistances were: 3–4 MΩ for SMCs, 4–6 MΩ for PDGFRα+ cells, and 8–10 MΩ for single channel recordings. An Axopatch 200B amplifier with a CV-4 headstage (Molecular Devices, Sunnyvale, CA, USA) was used. All data were analysed using pCLAMP software (Axon Instruments, USA) and Graphpad Prism (v. 3.0, Graphpad Software Inc., SanDiego, CA, USA). Channel open probability (Po) was determined by dividing channel activity (NPo) derived using the pCLAMP software by the number of channels (N) as in the respective patch. All recordings were made at room temperature of ∼23°C.
Solutions and chemicals
All solutions used were summarized in Table 2. All drugs and reagents including apamin, CyPPA (N-cyclohexyl-N-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine) and SKA-31 (naphtho[1,2-d]thiazol-2-ylamine) were purchased from Sigma.
Immunohistochemistry
Bladder muscles were denuded and stretched out on Sylgard dish. Tissue was fixed in paraformaldehyde saline solution (4% w/v in 0.1 m PBS; 10 min at 4°C). After fixation, preparations were washed in phosphate-buffered saline (PBS; 0.01 m, pH 7.4) and non-specific binding was reduced by incubating tissues with 1% bovine serum albumin (BSA) for 1 h. The tissues were incubated with primary antibody (anti-PDGFRα, 1:100, R&D Systems, Cat#AF1042, Minneapolis, MN, anti-SK3, Alomone labs, Cat#APC-025, Jersusalem, Israel) diluted in 0.05% Triton-X (Sigma Aldrich, St. Louis, MO) for 48 h at 4°C and with secondary antibodies (Alexa Fluor 488 donkey anti-goat IgG, 1:1000 and Alexa Fluor 594 donkey anti-rabbit IgG 1:1000, Invitrogen, Grand Island, NY, USA) for 1 hr at room temperature. Specimens were mounted on microscope slides and coverslipped with Aqua-mount mounting medium (Lerner laboratories, Pittsburgh, PA). Specimens were examined with a confocal microscope (Zeiss LSM510 Meta; Carl Zeiss, Thornwood, NY, USA and Olympus Fluoview FV1000, Center Valley, PA, USA). Micrographs were constructed using Zeiss LSM 5 Image Examiner software and Olympus FV-10 Viewer, and are composites of Z-series scans (0.2–0.5 μm optical sections) through a depth of 4–40 μm and were arranged in Adobe Photoshop.
Cell purification, RNA isolation, reverse-transcription PCR and quantitative PCR
eGFP-PDGFRα+ cells and eGFP/SMCs were purified by fluorescence-activated cell sorting (FACS; Becton Dickinson FACSAria using the blue laser (488 nm) and the GFP emission detector; 530/30 nm). Cells were further purified by hand collecting of fluorescent cells to maximize purity for molecular tests. Total RNA was isolated from PDGFRα+ cells and eGFP/SMCs using illustra RNAspin Mini RNA Isolation kit (GE Healthcare, Little Chalfont, UK), and first-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. PCR was performed with specific primers (Table 1) using Go-Taq Green Master Mix (Promega Corp., Madison, WI, USA). PCR products was analysed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers as PCR using Fast Syber green chemistry (Applied Biosystems, Foster City, CA, USA) on the 7900HT Real Time PCR System (Applied Biosysytems). Regression analysis of the mean values of three multiplex qPCRs for the log10 diluted cDNA was used to generate standard curves. Unknown amounts of messenger RNA (mRNA) were plotted relative to the standard curve for each set of primers and graphically plotted using Microsoft Excel. This gave transcriptional quantification of each gene relative to the endogenous Gapdh standard after log transformation of the corresponding raw data.
Table 1.
Primer sequences used for PCR
Statistical analyses
All data were expressed as means ± SEM. All statistical analyses were performed using Graphpad Prism. Student's paired or non-paired t test were used to compare groups of data and differences were considered to be significant at P < 0.05.
Results
Molecular and protein expression of SK channels in PDGFRα+ cells
Expression of Ca2+-activated K+ channels was compared in sorted PDGFRα+ cells, SMCs and unsorted cells from enzymatically-dispersed detrusor muscles (without suburothelium). Sorted cells were checked microscopically to verify that FACS enriched cells contained with eGFP. Expression of Gapdh (housekeeping), Kit (ICC), Myh11 (SMC), Pgp9.5 (neurons) was monitored to establish relative purification of PDGFRα+ cells and SMCs by FACS (Fig. 1A and B). Sorted PDGFRα+ cells were minimally positive for Kit (ICC marker) and Pgp9.5 (neuronal marker). PDGFRα+ cells were also minimally positive for Mhc (SMC marker; 0.16 ± 0.01; normalized value to Gapdh, n= 4) and greatly reduced compared to unsorted cells (6.18 ± 0.52; normalized value to Gapdh, n= 4, Fig. 1A). In contrast sorted SMCs revealed strong expression of Mhc (7.16 ± 0.62; normalized value to Gapdh, n= 4). We analysed major K+ channel families that encode Ca2+-activated K+ conductances; Kcnn1, Kcnn2, Kcnn3 (SK1–3), Kcnn4 (IK),Kcnma1 (BK) in PDGFRα+ cells, SMCs, and unsorted cells. Sorted PDGFRα+ cells demonstrated the higher expression of Pdgfra. Of the Ca2+-activated K+ conductances, Kcnn3 (SK3) was highly enriched in PDGFRα+ cells in comparison to expression in SMCs and unsorted cells. αSlo (BK marker) was more highly expressed in SMCs (n= 4, Fig 1B). Gene expression by quantitative RT-PCR (qPCR) was normalized against Gapdh (housekeeping gene).
Figure 1. End product gel and quantitative PCR of Ca2+-activated K+ channels in unsorted, sorted SMC and sorted PDGFRα+ cells.
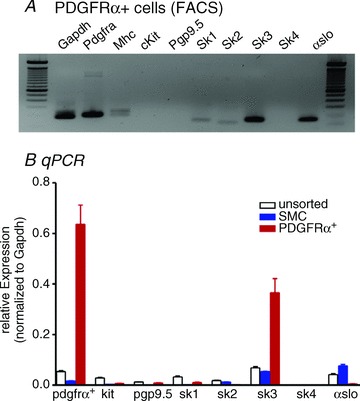
A, sorted PDGFRα+ cells were minimally positive for Mhc (smooth muscle cell marker), Kit (ICC marker) and Pgp9.5 (neuronal marker). SK3 and αslo were detected in sorted PDGFRα+ cells. B, Pdgfra was highly enriched (12 fold vs. unsorted cell) in PDGFRα+/eGFP cells. SK3 was dominantly expressed in PDGFRα+ cells in comparison to SMC and unsorted cells. αslo (BK marker) was more highly expressed in SMC. Relative expressions of all transcripts were normalized to Gapdh.
Immunohistochemistry was employed to verify the expression and determine cellular localization of SK3 protein in murine detrusor muscles. We have reported previously that PDGFRα antibodies label a population of interstitial cells in murine bladder (PDGFRα+ cells) (Koh et al. 2012). PDGFRα+ cells were immunopositive for SK3 channels, while SK3 channel expression was not resolved in SMCs (Fig. 2).
Figure 2. Immunohistochemistry of PDGFRα and SK3 channels in murine detrusor.
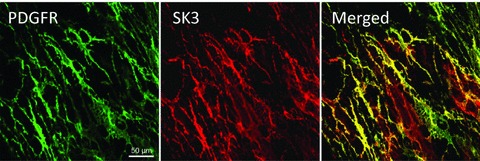
Whole-mount of the tunica muscularis in murine detrusor, displays immunoreactivity of PDGFRα+ cells and SK3 channels. These proteins were co-localized in PDGFRα+ cells in detrusor muscles.
Characterization of ionic conductance(s) in PDGFRα+ cells
PDGFRα+ cells could be recognized in a mixed population of dispersed detrusor cells due to expression of eGFP (Koh et al. 2012). Freshly isolated PDGFRα+ cells were used for electrophysiological experiments. Whole-cell voltage clamp techniques were employed to characterize voltage-dependent conductances in SMCs and PDGFRα+ cells. Cells were held at −80 mV and stepped to a range of potentials (−70 to +60 mV) in 10 mV increments for 400 ms or depolarized with voltage ramp protocols from −80 to +80 mV. Outward currents were measured in cells using external CaPSS solution (Table 2, solution II) and K+-rich internal solution containing 100 nm free Ca2+ (Table 2, solution III, ECl=−40 mV). Inward currents were recorded using a Cs+-rich internal solution and the same external CaPSS solution (Table 2). SMCs and PDGFRα+ cells expressed voltage-dependent outward currents (Fig. 3A and B). In SMCs, the outward current generated at positive potentials showed noisy fluctuations suggesting BK channels were activated (Fig. 3A). In contrast, PDGFRα+ cells did not show BK-like currents at positive potentials (Fig. 3B). Depolarization evoked voltage-dependent Ca2+ currents in SMCs as previously described (Fig. 3C), (Zhu et al. 2008) but inward current conductances were not resolved under the same conditions in PDGFRα+ cells (Fig. 3D, see arrow). Summary I-V curves showing outward currents are in Fig. 3E (SMCs, n= 8 from 5 mice; PDGFRα+ cells, n= 9 from 6 mice), and inward currents are shown in Fig. 3F (SMCs, n= 5 from 4 mice; PDGFRα+ cells, n= 9 from 6 mice).
Figure 3. Comparison of current–voltage relationships of a smooth muscle cell (SMC) and a PDGFRα+ cell.
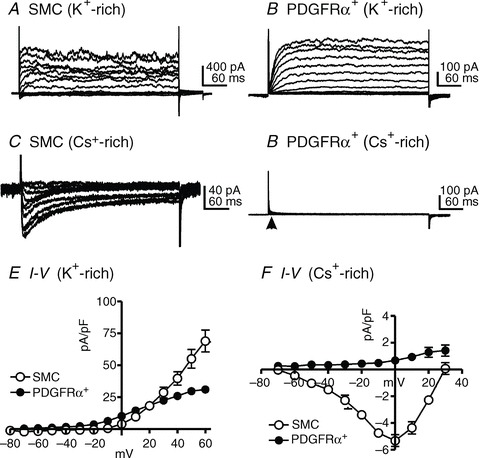
SMCs and PDGFRα+ cells expressed voltage-dependent outward currents (A and B). Depolarization evoked voltage-dependent Ca2+ currents in SMCs (C), but no inward current conductances were apparent under the same conditions in PDGFRα+ cells (D, see arrow). E and F demonstrate the summarized data of outward currents (E) and inward currents (F) from both types of cells.
SK currents and channels in PDGFRα+ cells
When PDGFRα+ cells were dialyzed with 200 nm[Ca2+]i, STOCs were often observed (Fig. 4A), especially when cells were held at potentials less negative than −60 mV. Cells were depolarized by a ramp potential protocol from −80 mV to +80 mV (see green arrows). The ramp–evoked currents were compared between (Fig. 4Ba, black line trace in Fig. 4C) and during STOCs (Fig. 4Bb, red line trace in Fig. 4C). Responses to the ramp potentials demonstrate that STOCs were due to voltage-independent outward currents. The current density during STOCs at −40 mV was 12.5 ± 1.6 pApF−1 (n= 15). Apamin (1 μm, SK channel blocker) inhibited STOCs by 76.5 ± 6.1% (Fig. 4D, n= 6, P < 0.05). Ramp depolarization evoked voltage-independent outward currents at peaks of STOCs (Fig. 4D inset (a)) and apamin inhibited the voltage-independent outward currents (Fig. 4D inset (b)). We also tested the effect of Iberiotoxin (200 nm, BK channel blocker, n= 5, Fig. 4E) and TRAM34 (10 μm, intermediate Ca2+-activated K+ channel blocker, n= 4, data not shown) on STOCs. Neither drug affected the current-voltage relationship (Fig. 4E, inset).
Figure 4. Spontaneous transient outward currents (STOCs) in PDGFRα+ cells.
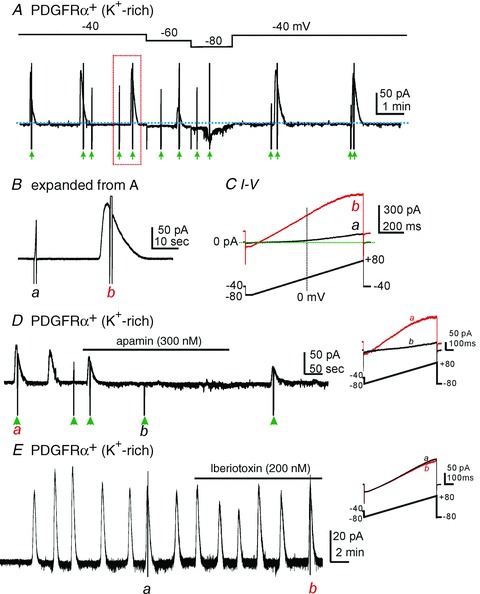
A, STOCs were observed in PDGFRα+ cells dialyzed with solutions containing 200 nm[Ca2+]i,. Green arrow heads denote ramp depolarizations from −80 to + 80 mV. B, expanded time scale from panel A (red dotted box). a and b denote ramp depolarizations. C, current responses to ramp depolarizations before (a) and during a STOC (b). Note reversal potential, lack of voltage-dependence at negative potentials, and inward rectification at positive potentials of current during STOC. These are classic signatures of SK currents (Soh & Park, 2001, 2002). Lower trace shows voltage-ramp protocol. D, apamin inhibited STOCs. Cell held at −40 mV. Green arrow heads denote ramp depolarizations. Inset shows current-voltage relationship during a STOC before (a) and after (b) apamin. E, iberiotoxin did not inhibit STOCs. Cell held at −40 mV. Inset shows current-voltage relationship during STOCs before (a) and after (b) Iberiotoxin. Lower traces in D and E insets denote voltage-ramp protocols.
We tested the effects of SK channel modulators (CyPPA and SKA-31) (Nielsen et al. 2011; Skarra et al. 2011; Parajuli et al. 2012) on PDGFRα+ cells. Cells were dialyzed with solutions containing low [Ca2+] (10 nm; which did not support STOC activity). CyPPA (10 μm) hyperpolarized cells from −31.4 ± 4.6 mV to −61.6 ± 2.9 mV under current clamp (I= 0, blue dot line, Fig. 5A, n= 14). In the same cells, CyPPA activated outward current at a holding potential of −40 mV (to simulate resting membrane potentials in detrusor muscles (Thorneloe & Nelson, 2004)) in voltage-clamp mode (Fig. 5A, red dotted line). CyPPA-activated current amplitude averaged 39.3 ± 7.1 pA (n= 19). Figure 5B shows currents evoked by ramp depolarization before (Fig. 5Ba, black trace) and in the presence of CyPPA (Fig. 5Bb, red trace). SKA-31 affected PDGFRα+ cells in the same manner as CyPPA. SKA-31 (10 μm) hyperpolarized cells from −34.4 ± 6.7 mV to −63.3 ± 6.5 mV in current clamp mode (I= 0; Fig. 4C, blue dotted line, n= 6) and activated outward currents in the same cells (47.0 ± 16.5 pA in amplitude at a holding potential of −40 mV) in voltage-clamp mode (red dotted line). Figure 5D shows currents evoked by ramp depolarizations before (Fig. 5Dc, black trace) and in the presence of SKA-31 (Fig. 5Dd, red trace).
Figure 5. Effects of CyPPA and SKA-31 on membrane potential and SK currents in PDGFRα+ cells.
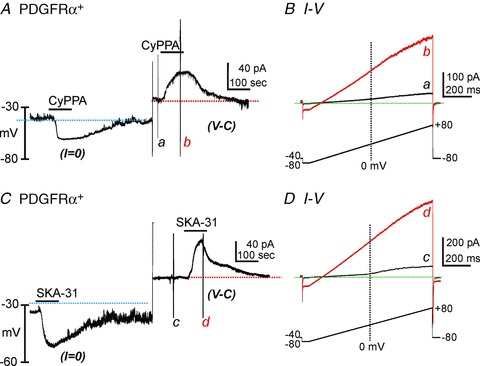
A, CyPPA (10 μm) induced membrane hyperpolarization under current clamp (I= 0, blue dot line). In the same cell, CyPPA activated outward current (current above red dotted line) at a holding potential of −40 mV under voltage-clamp mode (V-C). B, current responses to ramp-depolarizations before (a) and during (b) CyPPA. Inset denotes voltage-protocol. C, SKA-31(10 μm) induced membrane hyperpolarization under current clamp (I= 0, blue dot line). In the same cell, SKA-31 activated outward current (current above red dotted line) at a holding potential of −40 mV under voltage-clamp mode (V-C). D, current responses to ramp-depolarizations before (a) and during (b) SKA-31. Inset denotes voltage-protocol.
Expression of SK channels was also tested by recording single channel activity in inside-out patches excised from PDGFRα+ cells. Cells were bathed in solution V with 150 mm K+ to nullify cell resting potentials (Monaghan et al. 2011). Pipette solution was solution II with 5 mm K+. In inside-out patches, the amplitudes of single channel currents were 0.09 ± 0.01 pA, 0.14 ± 0.03 pA, 0.20 ± 0.03 pA and 0.29 ± 0.04 pA at −20, 0, +20 and +40 mV, respectively (Fig. 6A). Unitary current amplitudes were calculated from all-points amplitude histograms (right panels in Fig. 6B). The open probability (N*Po) of these channels increased from low levels to 0.94 ± 0.09 when [Ca2+] at the cytoplasmic surface of the patch was increased from 10−9 m to 3 × 10−7 m (Fig. 6C, n= 4). It was difficult to obtain precise amplitude histograms in the presence of high concentrations of cytoplasmic [Ca2+]. Therefore, we used ramp depolarizations during exposure of various concentrations of [Ca2+] to investigate the Ca2+ sensitivity of channels. Currents were measured at 0 mV and Plots were constructed from normalized current from 10−6 m[Ca2+]. The EC50 for [Ca2+] was calculated to be 98 nm (Fig. 6D). The large amplitude, whole-cell currents (Fig. 4) are due to a significant increase in the open probability of SK channels when cytoplasmic [Ca2+] was increased. The presence of multiple channels in patches confirms that SK channels are highly expressed in PDGFRα+ cells.
Figure 6. Single channel recordings from membrane patches excised from PDGFRα+ cells.
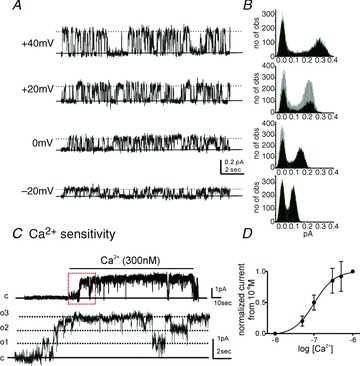
A, representative traces displayed single channel currents at −20 mV to +40 mV. Solid line denotes channel close and dotted line denotes channel opening. B, corresponding amplitude histogram from panel A. no of obs; numbers of observation. C, an increase in cytosolic [Ca2+] from 10nM to 300nM under excised patch increased open probability of SK channels. Lower panel display the expanded time scale from red box in upper panel. c; channel close, o; channel open, o1–o3; number of channel opening. D, plot of [Ca2+]vs. normalized current at 0 mV during ramp depolarization in 10−6 m[Ca2+] were constructed to describe Ca2+ sensitivity.
SK currents in smooth muscle cells
Expression studies suggested quite low expression of SK channel transcripts in SMCs in comparison to PDGFRα+ cells. Therefore, we also examined the effects of SK channel modulators during whole cell recording from detrusor SMCs to compare current densities. CyPPA (30 μm, Fig. 7A, n= 12) did not induce hyperpolarization of SMCs (−31.7 ± 0.5 mV vs. control −30.5 ± 2.3 mV). In the same SMC, CyPPA failed to activate resolvable outward currents at a holding potential of −40 mV (Fig. 7A, n= 9). The second SK channel modulator, SKA-31 (10 μm), also failed to induce hyperpolarization (current clamp) or activate outward currents in SMCs at −40 mV (voltage-clamp; Fig. 7B, n= 12). These data clearly demonstrate that PDGFRα+ cells have a much higher current density attributable to SK channels than SMCs, and suggest that at physiological potentials SMCs are therefore likely to contribute very little outward current at physiological potentials due to openings of SK channels.
Figure 7. CyPPA and SKA-31 had no effect on smooth muscle cells (SMCs) at physiological potentials.
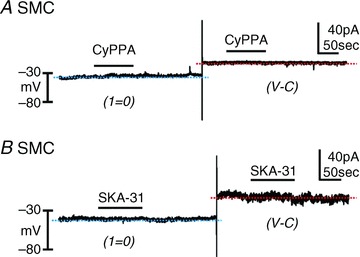
A, CyPPA had no resolvable effect on membrane potentials (I= 0, blue dotted line) and currents (voltage-clamp at −40 mV, red dotted line). B, SKA-31 did not cause hyperpolarization (I= 0, blue dotted line) or activate outward currents at a holding potential of −40 mV (voltage-clamp, red dotted line).
Discussion
We provided immunohistochemical evidence previously showing the abundant distribution of PDGFRα+ cells in murine detrusor muscles (Koh et al. 2012). Another study has recently confirmed the presence of PDGFRα+ cells in human and guinea pig bladder muscles (Monaghan et al. 2012). Previous studies have also described PDGFRα+ interstitial cells in other visceral smooth muscles and shown these cells form gap junctions with SMCs (Horiguchi & Komuro, 2000; Kurahashi et al. 2011). In the present study we used animals with constitutive expression of eGFP in PDGFRα+ cells to identify these cells in the mixed cells obtained by enzymatic dispersions of detrusor muscles. PDGFRα+ cells were sorted by FACS to purify this population of cells and used for molecular characterizations and electrophysiological studies. We found that detrusor PDGFRα+ cells express small-conductance Ca2+-activated K+ channels, most prominently the SK3 isoform, and protein expression was verified by immunohistochemistry. Large amplitude outward currents and significant hyperpolarization occurred in PDGFRα+ cells in response to SK channel modulators, suggesting successful translation of transcripts into active SK channels in these cells. The single channel conductance of K+ channels in PDGFRα+ cells was small (<10 pS channel), and these channels were very sensitive cytoplasmic [Ca2+]. These properties are consistent with the expression of SK3 channels. In contrast expression of SK channels was low in SMC, SK3 protein was not resolved by immunohistochemistry, and no outward currents or hyperpolarizations were activated in these cells at physiological potentials. Thus, SK channel regulation of bladder excitability is likely to be mediated through PDGFRα+ cells rather than through SMCs.
SK channel mRNAs (SK1-SK4) were detected in murine, guinea pig and human detrusor SMCs previously (Herrera et al. 2003; Afeli et al. 2012; Parajuli et al. 2012), and SK3 protein was detected in extracts of detrusor muscles using Western analysis (Herrera et al. 2003; Parajuli et al. 2012). In the case of Western analysis, whole muscle extracts would have contained protein from SMCs and PDGFRα+ cells. Our experiments using qPCR on extracts of purified cell populations and immunohistochemical techniques demonstrated low expression of SK transcripts and SK3 protein in SMCs and abundant expression in PDGFRα+ cells. These expression data were consistent with electrophysiological measurements as described.
Studies of whole bladder muscles have shown that apamin, a bee venom extract that is a specific antagonist of SK channels, increases spontaneous contractility and EFS-evoked contractions of bladder muscles (Herrera et al. 2005; Parajuli et al. 2012). Mice with genetically deactivated SK channels displayed bladder overactivity (Herrera et al. 2003; Thorneloe et al. 2008). These observations suggest that SK channel openings, possibly in the form of the STOCs we observed in PDGFRα+ cells, tonically dampen detrusor excitability and stabilize contractility during bladder filling. It is possible that loss or defects in this stabilizing mechanism could convert otherwise normal neural regulation during filling and voiding to shift toward hyper-excitability and overactivity.
Close inspection of published data shows vanishingly little SK current density in SMCs (Herrera et al. 2003; Parajuli et al. 2012). Currents and hyperpolarization responses attributable to SK channels were resolved in previous studies only at membrane potentials well positive to the resting potentials of SMCs in situ. These potentials were imposed on cells for experimental purposes to increase the driving force on K+ ions and increase currents through K+ channels. SKA-31 (an SK channel modulator or agonist) increased whole-cell K+ currents from 2.6 to 3.0 pA pF−1 at +10 mV (i.e. 0.4 pA pF−1 increase). Under current-clamp mode, SKA-31 hyperpolarized guinea pig SMCs from −24.7 to −27.2 mV (net 2.5 mV hyperpolarization) (Parajuli et al. 2012). Detrusor SMCs sit at more negative potentials (at least −40 mV) in situ, and the transmembrane driving force for K+ ions would be far less than at +10 mV. In the present study, we tested holding potentials that simulate physiological conditions and demonstrated that PDGFRα+ cell generated STOCs, which were inhibited by apamin, and thus due to openings of SK channels. Summation of STOCs in PDGFRα+ cells in situ would tend to exert a stabilizing or hyperpolarizing influence on the SMC/PDGFRα+ cell syncytium. PDGFRα+ cells displayed high current density as a result of apamin-sensitive (i.e. SK) channels; STOCs had average current densities of ∼12.5 pA pF−1 at −40 mV (cell capacitance was ∼9.3 ± 1.0 pF). Outward currents activated by CyPPA were 4.7 ± 0.8 pA pF−1 and 5.1 ± 1.9 pA pF−1 by SKA-31. These are far greater current densities than reported in SMCs (i.e. 0.5 pA pF−1 at +10 mV) (Herrera et al. 2003). In our studies SMCs were not hyperpolarized by SK agonists, and these compounds activated no resolvable outward current at physiological holding potentials. Taken together, these data suggest that SK currents in PDGFRα+ cell might be responsible for the apamin-sensitive component of regulation of detrusor excitability.
In summary, the inability of SK agonists to activate resolvable current in SMCs at physiological potentials suggests that apamin-sensitive regulation of detrusor excitability occurs in PDGFRα+ cells, not SMCs. The SK conductance in PDGFRα+ cells may provide a stabilizing factor that regulates detrusor excitability during bladder filling. The mechanisms responsible for generation and maintenance of STOCs and the ability of neurotransmitters and other agonists to regulate SK channel open probability are in need of further investigation. We believe this pathway might provide a novel means of controlling bladder over-activity in human patients that have no adequate remedy at present.
Acknowledgments
This work was supported by NIH/P20-RR18751 to SDK. Images were collected using a Zeiss LSM510 confocal microscope and a core morphology facility obtained with support from NIH1 S10 RR16871 and P01 DK41315.
Glossary
- BK
large-conductance Ca2+-activated K+ channel
- CyPPA
N-cyclohexyl-N-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine
- EC50
half maximal excitatory concentration
- eGFP
enhanced green fluorescent protein
- FACS
fluorescence-activated cell sorting
- KO
knock out
- PDGFRα
platelet-derived growth factor receptor alpha
- SK
small-conductance Ca2+-activated K+ channels
- SKA-31
naphtho[1,2-d]thiazol-2-ylamine
- SMC
smooth muscle cell
- smMHC
smooth muscle myosin heavy chain
- STOC
spontaneous transient outward current
Author contributions
All experiments were performed in the laboratories of the Department of Physiology and Cell Biology at the University of Nevada, Reno. Most of the data were collected and analysed by HL with the assistance of other authors. Immunohistochemistry and molecular study were performed by BHK and LEP. KMS, SDK and HL shared in the design of experiments, interpretation of the data and the writing of the manuscript. All authors approved the final version of the manuscript.
References
- Afeli SA, Rovner ES, Petkov GV. SK but not IK channels regulate human detrusor smooth muscle spontaneous and nerve-evoked contractions. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00615.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R402–R409. doi: 10.1152/ajpregu.00488.2004. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BK(Ca) channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Kennelly MJ, DeVoe WB. Overactive Bladder: Pharmacologic Treatments in the Neurogenic Population. Rev Urol. 2008;10:182–191. [PMC free article] [PubMed] [Google Scholar]
- Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM, Koh SD. Platelet-derived growth factor receptor-alpha cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med. 2012;16:691–700. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Monaghan K, Baker SA, Dwyer L, Hatton WC, Sik PK, Sanders KM, Koh SD. The stretch-dependent potassium channel TREK-1 and its function in murine myometrium. J Physiol. 2011;589:1221–1233. doi: 10.1113/jphysiol.2010.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan KP, Johnston L, McCloskey KD. Identification of PDGFRalpha Positive Populations of Interstitial Cells in Human and Guinea Pig Bladders. J Urol. 2012;188:639–647. doi: 10.1016/j.juro.2012.03.117. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, Rode F, Rahbek M, Andersson KE, Ronn LC, Bouchelouche K, Nordling J, Bouchelouche P. Effect of the SK/IK channel modulator 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591) on contractile force in rat, pig and human detrusor smooth muscle. BJU Int. 2011;108:771–777. doi: 10.1111/j.1464-410X.2010.10019.x. [DOI] [PubMed] [Google Scholar]
- Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther. 2012;340:114–123. doi: 10.1124/jpet.111.186213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh H, Park CS. Inwardly rectifying current-voltage relationship of smallconductance Ca2+-activated K+ channels rendered by intracellular divalent cation blockade. Biophys J. 2001;80:2207–2015. doi: 10.1016/S0006-3495(01)76193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh H, Park CS. Localization of divalent cation-binding site in the pore of a small conductance Ca2+-activated K+ channel and its role in determining current-voltage relationship. Biophys J. 2002;83:2528–2538. doi: 10.1016/S0006-3495(02)75264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarra DV, Cornwell T, Solodushko V, Brown A, Taylor MS. CyPPA, a positive modulator of small-conductance Ca2+-activated K+ channels, inhibits phasic uterine contractions and delays preterm birth in mice. Am J Physiol Cell Physiol. 2011;301:C1027–C1035. doi: 10.1152/ajpcell.00082.2011. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ES, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+ -activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1737–R1743. doi: 10.1152/ajpregu.00840.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT. Properties of a tonically active, sodium-permeable current in mouse urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2004;286:C1246–C1257. doi: 10.1152/ajpcell.00501.2003. [DOI] [PubMed] [Google Scholar]
- Zhu HL, Brain KL, Aishima M, Shibata A, Young JS, Sueishi K, Teramoto N. Actions of two main metabolites of propiverine (M-1 and M-2) on voltage-dependent L-type Ca2+ currents and Ca2+ transients in murine urinary bladder myocytes. J Pharmacol Exp Ther. 2008;324:118–127. doi: 10.1124/jpet.107.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]


