Abstract
Dietary nitrate (NO3−) supplementation, via its reduction to nitrite (NO2−) and subsequent conversion to nitric oxide (NO) and other reactive nitrogen intermediates, reduces blood pressure and the O2 cost of submaximal exercise in humans. Despite these observations, the effects of dietary NO3− supplementation on skeletal muscle vascular control during locomotory exercise remain unknown. We tested the hypotheses that dietary NO3− supplementation via beetroot juice (BR) would reduce mean arterial pressure (MAP) and increase hindlimb muscle blood flow in the exercising rat. Male Sprague–Dawley rats (3–6 months) were administered either NO3− (via beetroot juice; 1 mmol kg−1 day−1, BR n= 8) or untreated (control, n= 11) tap water for 5 days. MAP and hindlimb skeletal muscle blood flow and vascular conductance (radiolabelled microsphere infusions) were measured during submaximal treadmill running (20 m min−1, 5% grade). BR resulted in significantly lower exercising MAP (control: 137 ± 3, BR: 127 ± 4 mmHg, P < 0.05) and blood [lactate] (control: 2.6 ± 0.3, BR: 1.9 ± 0.2 mm, P < 0.05) compared to control. Total exercising hindlimb skeletal muscle blood flow (control: 108 ± 8, BR: 150 ± 11 ml min−1 (100 g)−1, P < 0.05) and vascular conductance (control: 0.78 ± 0.05, BR: 1.16 ± 0.10 ml min−1 (100 g)−1 mmHg−1, P < 0.05) were greater in rats that received BR compared to control. The relative differences in blood flow and vascular conductance for the 28 individual hindlimb muscles and muscle parts correlated positively with their percentage type IIb + d/x muscle fibres (blood flow: r= 0.74, vascular conductance: r= 0.71, P < 0.01 for both). These data support the hypothesis that NO3− supplementation improves vascular control and elevates skeletal muscle O2 delivery during exercise predominantly in fast-twitch type II muscles, and provide a potential mechanism by which NO3− supplementation improves metabolic control.
Key points
Inorganic nitrate (NO3−) supplementation with beetroot juice (BR) in humans lowers blood pressure and the O2 cost of exercise and may improve exercise tolerance following its reduction to nitrite (NO2−) and nitric oxide (NO).
The effect of inorganic NO3− supplementation with BR on skeletal muscle blood flow (BF) and vascular conductance (VC) within and among locomotory muscles during exercise is unknown.
Inorganic NO3− supplementation with BR in rats resulted in lower exercising mean arterial pressure, lower blood [lactate], and higher total skeletal muscle hindlimb BF and VC during submaximal treadmill running.
The greater BF and VC was found in muscles and muscle parts containing primarily type IIb + d/x muscle fibres.
These data demonstrate that inorganic NO3− supplementation improves vascular control and elevates skeletal muscle O2 delivery during exercise predominantly in fast-twitch type II muscles, and provide a potential mechanism by which NO3− supplementation improves metabolic control.
Introduction
It is now recognized that nitric oxide (NO) functions as a major contributor to skeletal muscle vascular and metabolic control (reviewed by Joyner & Tschakovsky, 2003). NO is produced endogenously by the reduction of l-arginine to l-citrulline via three distinct nitric oxide synthase (NOS) isoforms: constitutively expressed endothelial NOS (eNOS) and neuronal NOS (nNOS), as well as inducible NOS (iNOS) (reviewed by Stamler & Meissner, 2001). In addition, there is emerging evidence that dietary inorganic nitrate (NO3−) delivered, for example, via ingested beetroot juice (BR) can be reduced to nitrite (NO2−) and, subsequently, NO and other reactive nitrogen intermediates and impact haemodynamic and muscle metabolic function (Larsen et al. 2007; Bailey et al. 2009). These effects have been divorced from other active BR constituents (i.e. antioxidants; Lansley et al. 2011a,b) and, crucially, the reduction of NO2− to NO is potentiated by hypoxic and acidic conditions (Cosby et al. 2003), which may be present during muscular exercise. In contrast, hypoxic conditions impair NOS function and therefore compromise NO bioavailability from that pathway under the very conditions when NO is requisite to balance O2 delivery to O2 utilization in skeletal muscle (Ferreira et al. 2006a,b; Hirai et al. 2010).
In humans, acute (2–3 h) and chronic (3–6 days) dietary NO3− ingestion via sodium NO3− salt (Larsen et al. 2007) or BR (Bailey et al. 2009; Vanhatalo et al. 2010a; Kenjale et al. 2011; Lansley et al. 2011a,b) reduces blood pressure, lowers submaximal exercise oxygen uptake (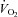 ) and has been shown to enhance exercise tolerance. In addition, BR ameliorates the muscle metabolic perturbations found during exercise when breathing a hypoxic inspirate (Vanhatalo et al. 2011), improves muscle oxygenation in peripheral artery disease patients (Kenjale et al. 2011) and improves human mitochondrial efficiency as measured using the P/O ratio (Larsen et al. 2011).
) and has been shown to enhance exercise tolerance. In addition, BR ameliorates the muscle metabolic perturbations found during exercise when breathing a hypoxic inspirate (Vanhatalo et al. 2011), improves muscle oxygenation in peripheral artery disease patients (Kenjale et al. 2011) and improves human mitochondrial efficiency as measured using the P/O ratio (Larsen et al. 2011).
Collectively, these investigations suggest that augmented dietary NO3− might serve to maintain or even increase skeletal muscle blood flow (BF; and hence O2 delivery) in the presence of reduced O2 demand, which may be expected to enhance metabolic control via increases in intramyocyte 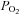 . However, we are unaware of any measurements of BF and vascular conductance (VC) within and among skeletal muscles during locomotory exercise. Indeed, within the running rat model it is possible to determine the impact of BR on vascular control across discrete muscle fibre-type populations. Such information is essential for resolving the effect of BR on O2 delivery to O2 utilization matching within and across muscles, which may have important metabolic consequences. Accordingly, the purpose of the present investigation was to test the hypotheses that ingesting BR for 5 days would, in the face of increased plasma [NO3−], [NO2−] and lowered mean arterial pressure (MAP): (1) increase BF and VC in locomotory muscles across the spectrum of both high and low oxidative capacities, and (2) thereby presumably increase the O2 delivery to O2 utilization ratio, thus reducing blood [lactate]. Results from the present investigation may provide mechanistic links between changes in plasma [NO3−] and [NO2−] and improved muscle oxygenation and metabolic function following NO3− supplementation (Kenjale et al. 2011; Vanhatalo et al. 2011).
. However, we are unaware of any measurements of BF and vascular conductance (VC) within and among skeletal muscles during locomotory exercise. Indeed, within the running rat model it is possible to determine the impact of BR on vascular control across discrete muscle fibre-type populations. Such information is essential for resolving the effect of BR on O2 delivery to O2 utilization matching within and across muscles, which may have important metabolic consequences. Accordingly, the purpose of the present investigation was to test the hypotheses that ingesting BR for 5 days would, in the face of increased plasma [NO3−], [NO2−] and lowered mean arterial pressure (MAP): (1) increase BF and VC in locomotory muscles across the spectrum of both high and low oxidative capacities, and (2) thereby presumably increase the O2 delivery to O2 utilization ratio, thus reducing blood [lactate]. Results from the present investigation may provide mechanistic links between changes in plasma [NO3−] and [NO2−] and improved muscle oxygenation and metabolic function following NO3− supplementation (Kenjale et al. 2011; Vanhatalo et al. 2011).
Methods
Ethical approval
Nineteen young adult male Sprague–Dawley rats (3–4 months old; body mass = 416 ± 12 g) were used in the present investigation. Rats were maintained on a 12:12 h light–dark cycle with food and water available ad libitum. All experimental procedures were conducted under the guidelines established by The Journal of Physiology (Drummond, 2009) and approved by the Institutional Animal Care and Use Committee of Kansas State University. All rats were familiarized with running on a custom-built motor-driven treadmill for 5 min day−1 at a speed of 20 m min−1 up a 5% grade for ∼5 days.
BR supplementation
Rats were assigned randomly to receive either tap water (control; n= 11) or 5 days of BR supplementation (BR; n= 8) (NO3− dose; 1 mmol kg−1 day−1 diluted in 100 ml of tap water; Beet it™, James White Drinks, Ipswich, UK) with consumption monitored daily. Preliminary studies in our laboratory demonstrated this dose elevated plasma [NO3−] and [NO2−] to levels approximating those seen in humans following NO3− supplementation (Lundberg & Govoni, 2004; Bailey et al. 2009; Kenjale et al. 2011). Moreover, this dose is similar to NO3− doses administered to humans after accounting for the ∼7-fold greater resting metabolic rate in rats compared to humans (Musch et al. 1988).
Instrumentation and regional BF measurements
Rats were first anaesthetized using a 5% isoflurane/O2 mixture. Subsequently, while maintained on a 2–3% isoflurane/O2 mixture, a catheter (PE-10 connected to PE-50; Clay Adams Brand, Sparks, MD, USA) was placed in the ascending aorta via the right carotid artery. A second catheter (PE-10 connected to PE-50) was placed surgically in the caudal (tail) artery as described previously (Musch & Terrell, 1992). Both catheters were tunnelled subcutaneously to the dorsal aspect of the cervical region and exteriorized through a puncture wound in the skin. Following incision closure, anaesthesia was terminated and the animal was given 1–2 h to recover before initiation of the final experimental protocol (Flaim et al. 1984).
After recovery, the rat was placed on the treadmill and the caudal artery catheter was connected to a 1 ml syringe chambered in a Harvard infusion/withdrawal pump (model 907; Cambridge, MA, USA). The carotid artery catheter was then connected to a pressure transducer (P23ID; Gould Statham, Valley View, OH, USA) maintained at the same height as the animal and exercise was initiated. Treadmill speed was increased progressively over an ∼30 s period to a speed of 20 m min−1 (5% grade, ∼60%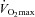 ; Musch et al. 1988). The rat continued to exercise for another 2.5 min until a total time of 3 min was reached. At the 3 min mark the pump connected to the caudal artery catheter was activated and withdrawal was initiated at a rate of 0.25 ml min−1. Simultaneously, HR and MAP were measured and recorded using the carotid artery catheter. The carotid artery catheter was then disconnected from the pressure transducer and 0.5–0.6 × 106 15 μm-diameter radiolabelled microspheres (57Co or 85Sr in random order; Perkin Elmer, Waltham, MA, USA) were injected into the aortic arch for determination of regional BF. Following the microsphere injection ∼0.2 ml of blood was sampled from the carotid artery catheter for determination of [lactate] (Nova Stat Profile M; Nova Biomedical, Waltham, MA, USA) after which exercise was terminated. Following a minimum 1 h recovery period, a second microsphere injection was performed while the rat sat quietly on the treadmill for determination of resting BF, HR and MAP. This experimental strategy (i.e. exercise before rest) mitigates potential influences of the pre-exercise anticipatory response on resting skeletal muscle BF measurements (Armstrong et al. 1989).
; Musch et al. 1988). The rat continued to exercise for another 2.5 min until a total time of 3 min was reached. At the 3 min mark the pump connected to the caudal artery catheter was activated and withdrawal was initiated at a rate of 0.25 ml min−1. Simultaneously, HR and MAP were measured and recorded using the carotid artery catheter. The carotid artery catheter was then disconnected from the pressure transducer and 0.5–0.6 × 106 15 μm-diameter radiolabelled microspheres (57Co or 85Sr in random order; Perkin Elmer, Waltham, MA, USA) were injected into the aortic arch for determination of regional BF. Following the microsphere injection ∼0.2 ml of blood was sampled from the carotid artery catheter for determination of [lactate] (Nova Stat Profile M; Nova Biomedical, Waltham, MA, USA) after which exercise was terminated. Following a minimum 1 h recovery period, a second microsphere injection was performed while the rat sat quietly on the treadmill for determination of resting BF, HR and MAP. This experimental strategy (i.e. exercise before rest) mitigates potential influences of the pre-exercise anticipatory response on resting skeletal muscle BF measurements (Armstrong et al. 1989).
Determination of regional BF and VC
Following the second microsphere infusion, rats were killed with a sodium pentobarbital overdose (≥50 mg kg−1, infused into the carotid artery catheter). The thorax was opened and placement of the carotid artery catheter was confirmed before the internal organs and individual muscles and muscle parts of the hindlimb were identified and excised. Upon removal, tissues were weighed and placed promptly into counting vials.
Radioactivity of each tissue was determined with a gamma scintillation counter (Packard Auto Gamma Spectrometer, model 5230; Downers Grove, IL, USA). Tissue BF was then calculated using the reference sample method (Musch & Terrell, 1992) and expressed as ml min−1 (100 g)−1. Adequate mixing of the microspheres was verified for each rat, demonstrated by a <15% difference in BF to the right and left kidneys and to the right and left hindlimb musculature. VC was calculated by normalizing BF to MAP and expressed as ml min−1 (100 g)−1 mmHg−1.
Blood sampling and measurement of plasma NO3− and NO2−
A blood sample was collected from control and BR group rats to assess differences in plasma [NO3−] and [NO2−]. Following instrumentation and before regional BF measurements ∼0.8 ml of blood was drawn from the caudal artery catheter and centrifuged at 5000 g at 4°C for 6 min. Plasma was subsequently extracted and immediately frozen at –80 °C for later analysis of [NO3−] and [NO2−].
All measurements of plasma NO3− and NO2− were performed within 30 min of thawing via chemiluminescence with an Ionic/Sievers NO analyser (NOA 280i; Sievers Instruments, Boulder, CO, USA). To obtain plasma NO2− levels and to avoid potential reduction of NO3−, potassium iodide in acetic acid was used as a reductant. This reductant possesses the ability to reduce NO2− to NO but is incapable of reducing higher oxides of nitrogen (i.e. NO3−), thus increasing the specificity for NO2−. Plasma NO3− concentrations were then obtained using the same apparatus with the stronger reductant vanadium chloride in hydrochloric acid at a temperature of 95°C. This stronger reductant reduces the sum of all nitrogen oxides with an oxidation state of +2 or higher (predominantly NO3− (μm)) but also includes NO2− and nitrosothiols (nm).
Statistical analysis
Plasma [NO3−] and [NO2−] were compared using unpaired Student's t tests. All other data were compared within (rest vs. exercise) and among (control vs. BR) groups using mixed two-way ANOVAs and Student–Newman–Keuls post hoc tests where appropriate. Pearson product-moment correlations and linear regressions were used to determine relationships between variables. Muscle fibre type composition was based on the percentage of type I, type IIa, type IIb and type IId/x fibres in the individual muscles and muscle parts of the rat hindlimb as reported by Delp & Duan (1996). Significance was set at P < 0.05 and values are expressed as mean ± SEM.
Results
There was no between-group difference in the total hindlimb muscle/body mass ratio (control: 8.8 ± 0.2, BR: 8.3 ± 0.2%, P > 0.05) despite modest differences in total body mass (control: 442 ± 14, BR: 384 ± 8 g, P < 0.05).
Effects of BR on plasma [NO3−] and [NO2−]
Plasma [NO3−] and [NO2−] were significantly greater in rats receiving BR when compared to controls (Fig. 1).
Figure 1. Effects of dietary NO3− supplementation with BR on plasma [NO3−] and [NO2−].
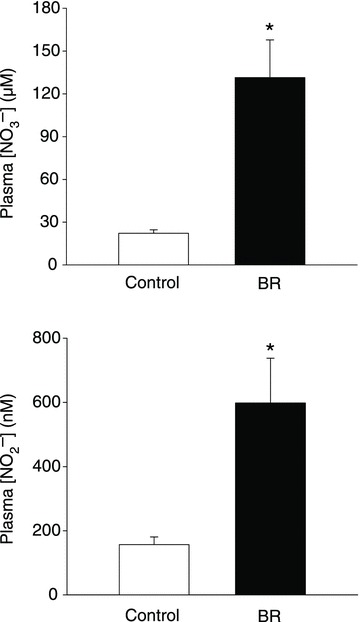
*P < 0.05 vs. control.
Effects of BR on HR, MAP and blood [lactate] at rest and during exercise
HR, MAP and blood [lactate] values are presented in Table 1. Rats receiving BR had significantly lower exercising but not resting MAP (P = 0.48) compared to controls. There were no differences in resting blood [lactate]. Exercising blood [lactate] was lower in the BR group than in the control group.
Table 1.
Effects of 5 days of BR supplementation on HR, MAP and blood [lactate] at rest and during exercise
| HR (beats min−1) | MAP (mmHg) | Blood [lactate] (mm) | ||||
|---|---|---|---|---|---|---|
| Control | BR | Control | BR | Control | BR | |
| Rest | 405 ± 8 | 409 ± 13 | 138 ± 3 | 132 ± 7 | 0.9 ± 0.1 | 0.7 ± 0.1 |
| Exercise | 525 ± 9† | 521 ± 6† | 137 ± 3 | 127 ± 4* | 2.6 ± 0.3† | 1.9 ± 0.2*† |
Data are mean ± SEM. *P < 0.05 vs. control, †P < 0.01 vs. rest.
Effects of BR on skeletal muscle BF and VC at rest and during exercise
There were no differences in total resting hindlimb BF (control: 16 ± 2, BR: 20 ± 4 ml min−1 (100 g)−1, P= 0.30) or VC (control: 0.12 ± 0.01, BR: 0.15 ± 0.02 ml min−1 (100 g)−1 mmHg−1, P= 0.20). There were no differences in resting BF or VC in any of the 28 individual hindlimb muscles or muscle parts (Table 2). Total exercising hindlimb muscle BF and VC were higher in BR-supplemented rats than in controls (Fig. 2). Specifically, BR resulted in greater BF in 17, and VC in 21, of the 28 individual hindlimb muscles or muscle parts compared to the control group (Table 3). All individual muscles and muscle parts demonstrating greater BF were comprised of ≥66% type IIb + d/x muscle fibres whereas VC was higher in muscles and muscle parts ranging from 14 to 100% type IIb + d/x muscle fibres. Relative differences in BF and VC with BR (i.e. percentage ΔBF and ΔVC, respectively) were significantly positively correlated with the percentage of type IIb + d/x muscle fibres in the individual hindlimb muscles and muscle parts (Fig. 3). Figure 4 illustrates the marked differences in percentage ΔBF and ΔVC for the extremes of muscle fibre type composition (i.e. all muscles composed of 100% and ≤20% type IIb + d/x muscle fibres) of the individual muscles and muscle parts of the hindlimb.
Table 2.
Effects of BR supplementation on resting hindlimb muscle BF and VC
| BF (ml min−1 (100 g)−1) | VC (ml min−1 (100 g)−1 mmHg−1) | |||
|---|---|---|---|---|
| Control | BR | Control | BR | |
| Ankle extensors | ||||
| Soleus (9%) | 84 ± 15 | 102 ± 25 | 0.62 ± 0.11 | 0.75 ± 0.18 |
| Plantaris (80%) | 15 ± 2 | 10 ± 1 | 0.11 ± 0.01 | 0.08 ± 0.01 |
| Gastrocnemius, red (14%) | 42 ± 6 | 50 ± 15 | 0.31 ± 0.05 | 0.37 ± 0.10 |
| Gastrocnemius, white (100%) | 14 ± 2 | 10 ± 2 | 0.10 ± 0.02 | 0.08 ± 0.01 |
| Gastrocnemius, mixed (91%) | 14 ± 2 | 15 ± 3 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| Tibialis posterior (73%) | 17 ± 2 | 15 ± 4 | 0.12 ± 0.01 | 0.11 ± 0.02 |
| Flexor digitorum longus (68%) | 21 ± 3 | 10 ± 2 | 0.15 ± 0.02 | 0.07 ± 0.01 |
| Flexor halicus longus (71%) | 13 ± 2 | 10 ± 1 | 0.09 ± 0.01 | 0.07 ± 0.01 |
| Ankle flexors | ||||
| Tibialis anterior, red (63%) | 19 ± 3 | 19 ± 8 | 0.14 ± 0.02 | 0.13 ± 0.05 |
| Tibialis anterior, white (80%) | 19 ± 2 | 16 ± 3 | 0.14 ± 0.02 | 0.12 ± 0.02 |
| Extensor digitorum longus (76%) | 16 ± 2 | 14 ± 3 | 0.12 ± 0.01 | 0.10 ± 0.02 |
| Peroneals (67%) | 17 ± 3 | 18 ± 3 | 0.12 ± 0.02 | 0.13 ± 0.02 |
| Knee extensors | ||||
| Vastus intermedius (4%) | 43 ± 8 | 87 ± 18 | 0.32 ± 0.06 | 0.64 ± 0.26 |
| Vastus medialis (82%) | 14 ± 2 | 22 ± 7 | 0.10 ± 0.01 | 0.16 ± 0.05 |
| Vastus lateralis, red (35%) | 39 ± 6 | 78 ± 23 | 0.28 ± 0.04 | 0.57 ± 0.16 |
| Vastus lateralis, white (100%) | 15 ± 2 | 13 ± 2 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| Vastus lateralis, mixed (89%) | 16 ± 1 | 26 ± 7 | 0.12 ± 0.01 | 0.19 ± 0.05 |
| Rectus femoris, red (66%) | 22 ± 4 | 27 ± 11 | 0.16 ± 0.03 | 0.19 ± 0.07 |
| Rectus femoris, white (100%) | 15 ± 2 | 15 ± 4 | 0.11 ± 0.01 | 0.11 ± 0.02 |
| Knee flexors | ||||
| Biceps femoris anterior (100%) | 10 ± 1 | 10 ± 1 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| Biceps femoris posterior (92%) | 11 ± 1 | 13 ± 3 | 0.08 ± 0.01 | 0.10 ± 0.02 |
| Semitendinosus (83%) | 12 ± 2 | 16 ± 4 | 0.08 ± 0.01 | 0.12 ± 0.03 |
| Semimembranosus, red (72%) | 15 ± 2 | 24 ± 7 | 0.11 ± 0.02 | 0.18 ± 0.05 |
| Semimembranosus, white (100%) | 13 ± 2 | 11 ± 2 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| Thigh adductors | ||||
| Adductor longus (5%) | 115 ± 7 | 136 ± 12 | 0.84 ± 0.06 | 1.06 ± 0.12 |
| Adductor magnus & brevis (89%) | 15 ± 3 | 21 ± 5 | 0.12 ± 0.02 | 0.15 ± 0.04 |
| Gracilis (77%) | 16 ± 2 | 19 ± 3 | 0.11 ± 0.02 | 0.14 ± 0.02 |
| Pectineus (69%) | 17 ± 2 | 24 ± 6 | 0.12 ± 0.01 | 0.18 ± 0.04 |
Data are mean ± SEM. Values in parentheses indicate percentage type IIb + d/x according to Delp & Duan (1996). Control, n= 11; BR, n= 8.
Figure 2. Effects of dietary NO3− supplementation with BR on total hindlimb muscle BF and VC during submaximal locomotory exercise.
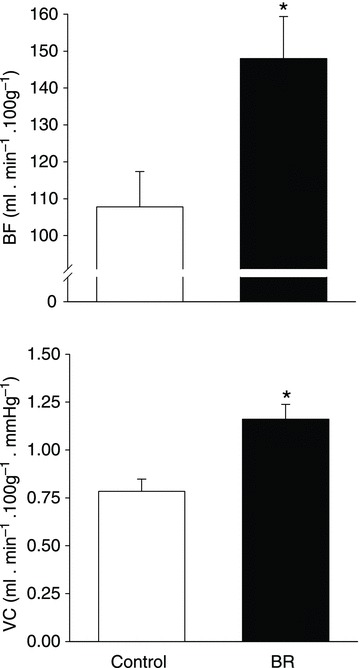
*P < 0.05 vs. control.
Table 3.
Effects of BR supplementation on exercising hindlimb muscle BF and VC
| BF (ml min−1 (100 g)−1) | VC (ml min−1 (100 g)−1 mmHg−1) | |||
|---|---|---|---|---|
| Control | BR | Control | BR | |
| Ankle extensors | ||||
| Soleus (9%) | 296 ± 42 | 312 ± 33 | 2.14 ± 0.30 | 2.43 ± 0.23 |
| Plantaris (80%) | 207 ± 15 | 247 ± 15* | 1.50 ± 0.10 | 1.94 ± 0.10* |
| Gastrocnemius, red (14%) | 452 ± 44 | 500 ± 39 | 3.27 ± 0.98 | 3.93 ± 0.29* |
| Gastrocnemius, white (100%) | 42 ± 7 | 66 ± 11* | 0.30 ± 0.05 | 0.51 ± 0.08* |
| Gastrocnemius, mixed (91%) | 149 ± 12 | 209 ± 17* | 1.08 ± 0.08 | 1.64 ± 0.11* |
| Tibialis posterior (73%) | 118 ± 17 | 133 ± 17 | 0.85 ± 0.12 | 1.05 ± 0.14 |
| Flexor digitorum longus (68%) | 99 ± 14 | 103 ± 15 | 0.71 ± 0.09 | 0.81 ± 0.11 |
| Flexor halicus longus (71%) | 75 ± 10 | 86 ± 9 | 0.54 ± 0.06 | 0.67 ± 0.06 |
| Ankle flexors | ||||
| Tibialis anterior, red (63%) | 343 ± 35 | 368 ± 31 | 2.47 ± 0.23 | 2.88 ± 0.20 |
| Tibialis anterior, white (80%) | 119 ± 14 | 161 ± 19* | 0.85 ± 0.09 | 1.26 ± 0.13* |
| Extensor digitorum longus (76%) | 55 ± 7 | 80 ± 10* | 0.39 ± 0.05 | 0.62 ± 0.07* |
| Peroneals (67%) | 128 ± 11 | 166 ± 7* | 0.93 ± 0.08 | 1.31 ± 0.06* |
| Knee extensors | ||||
| Vastus intermedius (4%) | 359 ± 39 | 348 ± 40 | 2.60 ± 0.27 | 2.75 ± 0.31 |
| Vastus medialis (82%) | 114 ± 18 | 163 ± 30 | 0.82 ± 0.12 | 1.28 ± 0.25* |
| Vastus lateralis, red (35%) | 388 ± 43 | 449 ± 43 | 2.81 ± 0.28 | 3.56 ± 0.37* |
| Vastus lateralis, white (100%) | 33 ± 5 | 45 ± 8 | 0.24 ± 0.03 | 0.35 ± 0.06* |
| Vastus lateralis, mixed (89%) | 168 ± 21 | 227 ± 16* | 1.22 ± 0.14 | 1.77 ± 0.14* |
| Rectus femoris, red (66%) | 224 ± 33 | 310 ± 30* | 1.62 ± 0.23 | 2.45 ± 0.26* |
| Rectus femoris, white (100%) | 101 ± 13 | 178 ± 31* | 0.72 ± 0.08 | 1.39 ± 0.23* |
| Knee flexors | ||||
| Biceps femoris anterior (100%) | 50 ± 8 | 77 ± 14* | 0.36 ± 0.05 | 0.61 ± 0.11* |
| Biceps femoris posterior (92%) | 79 ± 8 | 130 ± 10* | 0.58 ± 0.06 | 1.03 ± 0.08* |
| Semitendinosus (83%) | 56 ± 6 | 75 ± 12* | 0.40 ± 0.04 | 0.58 ± 0.09* |
| Semimembranosus, red (72%) | 119 ± 14 | 174 ± 15* | 0.86 ± 0.10 | 1.37 ± 0.11* |
| Semimembranosus, white (100%) | 33 ± 6 | 61 ± 11* | 0.24 ± 0.04 | 0.48 ± 0.09* |
| Thigh adductors | ||||
| Adductor longus (5%) | 316 ± 38 | 329 ± 45 | 2.28 ± 0.27 | 2.58 ± 0.34 |
| Adductor magnus & brevis (89%) | 83 ± 8 | 108 ± 15* | 0.60 ± 0.05 | 0.85 ± 0.12* |
| Gracilis (77%) | 42 ± 15 | 57 ± 9* | 0.30 ± 0.03 | 0.45 ± 0.07* |
| Pectineus (69%) | 54 ± 8 | 81 ± 13* | 0.39 ± 0.06 | 0.64 ± 0.10* |
Data are mean ± SEM. Values in parentheses indicate percentage type IIb + IId/x muscle fibres according to Delp & Duan (1996). Control, n= 11; BR, n= 8. *P < 0.05 vs. control. All 28 muscles and muscle parts of the hindlimb demonstrated elevated exercising BF and VC compared to rest within control and BR groups (P < 0.05 for all).
Figure 3.
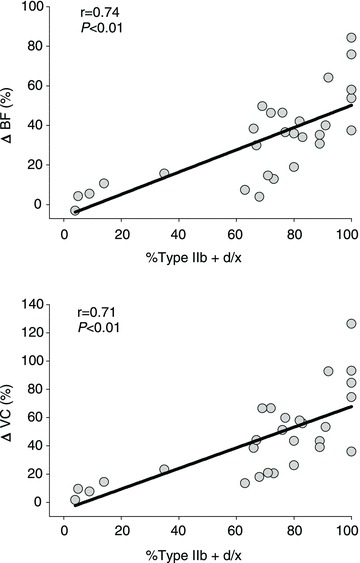
Relationship between the relative differences in total hindlimb muscle BF and VC (percentage ΔBF and ΔVC, respectively) with dietary NO3− supplementation with BR during submaximal locomotory exercise and the percentage of type IIb + d/x fibres found in the individual muscles and muscle parts of the rat hindlimb according to Delp & Duan (1996)
Figure 4. Relative differences in BF and VC (percentage ΔBF and ΔVC, respectively) for NO3− supplemented rats compared to controls during submaximal locomotory exercise for all hindlimb muscles and muscle parts comprising 100% type IIb + d/x fibres (continuous lines and symbols) and ≤20% type IIb + d/x fibres (dashed lines and open symbols) according to Delp & Duan (1996).
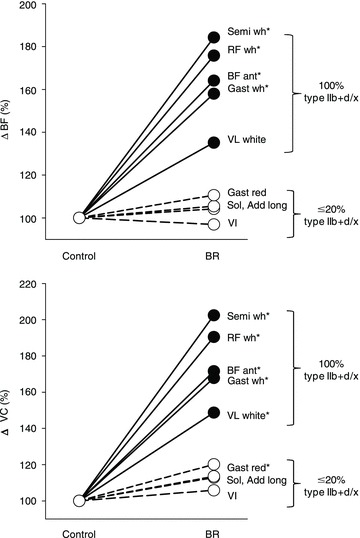
*P < 0.05 vs. control. Semi wh, white portion of the semitendinosus; RF wh, white portion of the rectus femoris; BF ant, anterior portion of the biceps femoris; Gast wh, white portion of the gastrocnemius; VL white, white portion of the vastus lateralis; Gast red, red portion of the gastrocnemius; Sol, soleus; Add long, adductor longus; VI, vastus intermedius.
Effects of BR on renal and splanchnic BF and VC at rest and during exercise
Renal and splanchnic BF and VC values are presented in Table 4. Renal VC was significantly higher in rats receiving BR than in controls at rest (P < 0.05). Liver VC was greater during exercise in BR-supplemented rats than in controls (P < 0.05).
Table 4.
Effects of BR supplementation on BF and VC to the kidneys and organs of the splanchnic region measured at rest and during exercise
| At rest | During exercise | |||||||
|---|---|---|---|---|---|---|---|---|
| BF (ml min−1 (100 g)−1) | VC (ml min−1 (100 g)−1 mmHg−1) | BF (ml min−1 (100 g)−1 | VC (ml min−1 (100 g)−1 mmHg−1 | |||||
| Control | BR | Control | BR | Control | BR | Control | BR | |
| Kidney | 434 ± 33 | 566 ± 44 | 3.22 ± 0.30 | 4.30 ± 0.25* | 421 ± 42 | 460 ± 51 | 3.04 ± 0.28 | 3.62 ± 0.39 |
| Stomach | 84 ± 7 | 91 ± 18 | 0.61 ± 0.06 | 0.66 ± 0.11 | 67 ± 13 | 59 ± 12† | 0.49 ± 0.10 | 0.45 ± 0.08† |
| Adrenals | 577 ± 85 | 664 ± 67 | 4.25 ± 0.68 | 5.22 ± 0.69 | 400 ± 63 | 540 ± 142 | 2.87 ± 0.44 | 4.30 ± 1.19 |
| Spleen | 339 ± 49 | 447 ± 104 | 2.47 ± 0.36 | 3.26 ± 0.69 | 62 ± 14† | 108 ± 27† | 0.44 ± 0.10† | 0.85 ± 0.22† |
| Pancreas | 118 ± 10 | 179 ± 66 | 0.86 ± 0.07 | 1.26 ± 0.43 | 110 ± 15 | 172 ± 74 | 0.80 ± 0.11 | 1.31 ± 0.53 |
| Small intestine | 313 ± 20 | 297 ± 36 | 2.30 ± 0.18 | 2.22 ± 0.22 | 240 ± 26† | 255 ± 40 | 1.73 ± 0.18 | 2.00 ± 0.32 |
| Large intestine | 124 ± 13 | 147 ± 15 | 0.91 ± 0.10 | 1.11 ± 0.08 | 127 ± 16 | 155 ± 22 | 0.92 ± 0.10 | 1.20 ± 0.15 |
| Liver‡ | 37 ± 14 | 32 ± 4 | 0.27 ± 0.10 | 0.25 ± 0.04 | 17 ± 3 | 34 ± 9 | 0.12 ± 0.02 | 0.26 ± 0.07* |
Data are mean ± SEM. *P < 0.05 vs. control; †P < 0.05 vs. rest. ‡Arterial, not portal, BF and VC.
Discussion
The principal novel finding of this investigation was that 5 days of BR supplementation in healthy rats elevated markedly plasma [NO3−] and [NO2−] and augmented total hindlimb muscle BF and VC during submaximal locomotory exercise, with targeted increases in the type IIb + d/x muscles and muscle parts. That the changes in exercising muscle BF were evident despite a reduction in exercising MAP demonstrates, for the first time, that dietary NO3− serves as a powerful controller of muscle O2 perfusion presumably following its reduction to NO2− and NO in vivo. These results are important from several perspectives, in particular because elevations in BF and therefore O2 delivery have the potential to raise 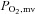 and hence the O2 driving pressure across the capillary–myocyte interface (as per Fick's Law). This ultimately enhances oxidative function, thereby reducing glycolytic metabolism dependence, as supported by reduced exercising blood [lactate] (Table 1).
and hence the O2 driving pressure across the capillary–myocyte interface (as per Fick's Law). This ultimately enhances oxidative function, thereby reducing glycolytic metabolism dependence, as supported by reduced exercising blood [lactate] (Table 1).
Effects of BR on plasma [NO3−], [NO2−] and MAP
Crucially, both plasma [NO3−] and [NO2−] (Fig. 1) rose to levels approximating what has been shown previously in humans following NO3− supplementation (Bailey et al. 2009; Vanhatalo et al. 2010a; Kenjale et al. 2011; Masschelein et al. 2012). While there were no differences in resting MAP between groups there was an ∼10 mmHg (Table 1) lower MAP during exercise in rats receiving BR compared to controls. The exercising MAP data presented herein are particularly interesting given that the effects of NO3− supplementation have been primarily studied in humans at rest. Interestingly, rats given BR had significantly higher resting renal VC (Table 4) suggesting that dietary NO3− reduces basal vasomotor tone and may play a cardioprotective role in renal vascular diseases as proposed previously (Lundberg et al. 2008; Tsuchiya et al. 2010; Carlström et al. 2011).
Effects of BR on exercising inter- and intra-muscular hindlimb BF and VC
The most striking result of the present investigation was the higher exercising BF and VC in BR rats compared to control rats. Recent studies performed in humans have shown an apparent increase in skeletal muscle blood volume estimated using near-infrared spectroscopy following NO3− or NO2− supplementation (Cosby et al. 2003; Bailey et al. 2009; Kenjale et al. 2011; Masschelein et al. 2012). However, muscle blood volume is not a measurement of BF per se and therefore, to our knowledge, this is the first study investigating the effects of NO3− supplementation on inter- and intra-muscular BF and VC at rest and during exercise.
The augmented BF and VC in the present investigation was observed predominantly in fast-twitch type IIb + d/x muscles, illustrating a fibre type selective effect of dietary NO3− supplementation on vascular control (Figs 3 and 4). This could be due in part to the lower 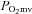 observed during contractions in muscles composed of primarily type II vs. type I fibres (Behnke et al. 2003; McDonough et al. 2005; Ferreira et al. 2006c). Cosby et al. (2003) demonstrated that NO2− reduction to NO is potentiated in low O2 environments via deoxyhaemoglobin, deoxymyoglobin and/or xanthine oxidoreductase. As a result, the reduction of NO2− to NO within the microvasculature of predominantly glycolytic type II muscles is probably amplified following NO3− supplementation, thereby increasing NO-mediated vasodilation in those muscles. Additionally, sympathetic adrenergic vasoconstriction occurs to a greater extent within more glycolytic type II compared to more oxidative type I muscles (Behnke et al. 2011) and the attenuation of skeletal muscle sympathetic vasoconstriction (i.e. functional sympatholysis) within glycolytic muscles during contractions (Thomas et al. 1994) is mediated at least in part by NO (Thomas & Victor, 1998; Dinenno & Joyner, 2004). This probably contributes to the observed muscle fibre type selective increases in BF and VC seen presently with BR during exercise but not at rest (Table 3).
observed during contractions in muscles composed of primarily type II vs. type I fibres (Behnke et al. 2003; McDonough et al. 2005; Ferreira et al. 2006c). Cosby et al. (2003) demonstrated that NO2− reduction to NO is potentiated in low O2 environments via deoxyhaemoglobin, deoxymyoglobin and/or xanthine oxidoreductase. As a result, the reduction of NO2− to NO within the microvasculature of predominantly glycolytic type II muscles is probably amplified following NO3− supplementation, thereby increasing NO-mediated vasodilation in those muscles. Additionally, sympathetic adrenergic vasoconstriction occurs to a greater extent within more glycolytic type II compared to more oxidative type I muscles (Behnke et al. 2011) and the attenuation of skeletal muscle sympathetic vasoconstriction (i.e. functional sympatholysis) within glycolytic muscles during contractions (Thomas et al. 1994) is mediated at least in part by NO (Thomas & Victor, 1998; Dinenno & Joyner, 2004). This probably contributes to the observed muscle fibre type selective increases in BF and VC seen presently with BR during exercise but not at rest (Table 3).
The lack of BF differences within some of the highly oxidative muscles could potentially account for the disparities among NO3−-induced improvements in short-term high-intensity exercise (Bailey et al. 2009, Lansley et al. 2011a,b) but not long-duration exercise performance of highly trained endurance athletes (Cermak et al. 2012; Wilkerson et al. 2012). Any potential improvements in exercise performance following NO3− supplementation may be limited to exercise testing protocols that recruit fast-twitch type II muscle fibres. There may also be a BF-independent effect as supported by the faster rate and greater magnitude of muscle force development in mouse fast-twitch but not slow-twitch muscle following NO3− supplementation reported recently by Hernandez et al. (2012).
BR resulted in substantially higher hindlimb skeletal muscle BF and VC (Fig. 2) despite no reductions in BF or VC to renal or splanchnic organs during exercise compared to controls (Table 4), which may indicate a central effect, in which NO3− elevates cardiac output (and hence skeletal muscle BF) via increases in stroke volume. Dietary NO3− has previously been shown to attenuate ventricular dysfunction via improved cardiac contractility in doxorubicin-induced cardiomyopathy (Zhu et al. 2011). However, it seems more reasonable to suggest that the increases in BF seen herein result from a combination of peripheral and central components in which the increases in peripheral VC alleviate afterload, affording improvements in cardiac output and thus BF via an increase in stroke volume rather than a redistribution effect via vasoconstriction of the renal and splanchnic vascular beds. Therefore, the present data stand in stark contrast to the higher BF in the type IIb + d/x fibres of aged rats observed by Musch et al. (2004) given that the higher BFs in that report occurred concomitant with lower BF in slow-twitch muscles and splanchnic organs.
The elevated skeletal muscle BF with BR supplementation documented presently becomes particularly important when considering that elevating local O2 delivery (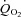 ) relative to demand (
) relative to demand (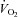 ) improves the
) improves the 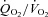 relationship thereby increasing the O2 pressure head (
relationship thereby increasing the O2 pressure head (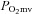 ) for blood–myocyte O2 flux as dictated by Fick's law of diffusion. Even if
) for blood–myocyte O2 flux as dictated by Fick's law of diffusion. Even if 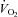 remains unchanged (and it is likely that it decreases via improvement in mitochondrial or muscle contractile efficiency, Larsen et al. 2007; Bailey et al. 2009; Vanhatalo et al. 2010a), the ∼38% increase in total hindlimb BF (Fig. 2) would be expected to increase mean
remains unchanged (and it is likely that it decreases via improvement in mitochondrial or muscle contractile efficiency, Larsen et al. 2007; Bailey et al. 2009; Vanhatalo et al. 2010a), the ∼38% increase in total hindlimb BF (Fig. 2) would be expected to increase mean 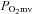 substantially. Accordingly, the reduced phosphocreatine (PCr) breakdown and improved exercise tolerance following BR reported by Jones and colleagues (Bailey et al. 2009; Vanhatalo et al. 2011) may have been mediated in part by elevated O2 driving pressures in the microvasculature which reduce PCr breakdown (Haseler et al. 1998; Vanhatalo et al. 2010b) and speed PCr recovery kinetics during hypoxia (Haseler et al. 1999). This mechanism is consistent with the lower blood [lactate] found herein with the BR group during exercise but remains to be tested specifically (Table 1).
substantially. Accordingly, the reduced phosphocreatine (PCr) breakdown and improved exercise tolerance following BR reported by Jones and colleagues (Bailey et al. 2009; Vanhatalo et al. 2011) may have been mediated in part by elevated O2 driving pressures in the microvasculature which reduce PCr breakdown (Haseler et al. 1998; Vanhatalo et al. 2010b) and speed PCr recovery kinetics during hypoxia (Haseler et al. 1999). This mechanism is consistent with the lower blood [lactate] found herein with the BR group during exercise but remains to be tested specifically (Table 1).
Experimental considerations and future directions
A major strength of the present investigation lies in the techniques used to measure inter- and intra-muscular BF and VC that, due to technical and ethical limitations, are unavailable in humans. In this regard, the measurements of BF and VC heterogeneity across the spectrum of varying muscle fibre type composition presented herein provide a unique perspective into the effects of dietary NO3− on skeletal muscle vascular control. This, in combination with the ability to measure both whole-body exercise performance (Copp et al. 2010a) and skeletal muscle microvascular function (e.g. 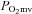 , Behnke et al. 2003), identifies the rat as a valuable research tool for future studies examining the mechanistic bases of the beneficial effects of dietary NO3− supplementation in humans. These data have significant clinical implications for a host of disease conditions associated with reduced NO bioavailability and concomitant vascular and metabolic dysfunction, which culminates typically in compromised exercise tolerance (e.g. chronic heart failure; reviewed by Poole et al. 2012). A prime example illustrating the potential clinical benefits of BR has already been demonstrated by Kenjale et al. (2011) who showed an ∼18% increase in peak walk time and time to claudication in peripheral artery disease patients following a single dose of BR.
, Behnke et al. 2003), identifies the rat as a valuable research tool for future studies examining the mechanistic bases of the beneficial effects of dietary NO3− supplementation in humans. These data have significant clinical implications for a host of disease conditions associated with reduced NO bioavailability and concomitant vascular and metabolic dysfunction, which culminates typically in compromised exercise tolerance (e.g. chronic heart failure; reviewed by Poole et al. 2012). A prime example illustrating the potential clinical benefits of BR has already been demonstrated by Kenjale et al. (2011) who showed an ∼18% increase in peak walk time and time to claudication in peripheral artery disease patients following a single dose of BR.
The differences in total body mass between groups cannot account for the greater exercising blood flows in BR rats given: (1) the hindlimb mass/body mass ratios were not different between groups and blood flows were normalized to muscle mass; (2) data from other laboratories (Armstrong & Laughlin 1985) as well as a comparison between the present control data and previous data from our laboratory (Copp et al. 2010b) indicate that varying body masses elicit similar BF values at matched treadmill speeds; and (3) subsets of body mass-matched control (n= 5, 405 ± 8 g) and BR (n= 5, 398 ± 8 g, P= 0.52) rats from the present investigation confirm that BR results in significantly higher muscle BF versus control (control: 94 ± 13, BR: 155 ± 13 ml min−1 (100 g)−1, P= 0.01).
Conclusions
This study is the first to investigate the effects of dietary NO3− supplementation on total, and inter- and intra-muscular hindlimb BF and VC both at rest and during submaximal locomotory exercise. In healthy rats BR supplementation for 5 days elicited marked elevations of plasma [NO3−] and [NO2−] and lower exercising MAP compared to control rats. Moreover, BR resulted in a higher total hindlimb muscle BF and VC with targeted increases in the muscles and muscle parts comprising principally type II + d/x muscle fibres. These data provide compelling evidence that dietary NO3− increases muscle O2 delivery in a fibre type-dependent manner following its reduction to NO2− and NO in vivo. This investigation offers novel insight into the role of NO3− in vascular control and provides a mechanistic linkage between elevated plasma [NO3−] and augmented metabolic control found in humans during exercise (Bailey et al. 2009; Larsen et al. 2010; Kenjale et al. 2011; Vanhatalo et al. 2011).
Acknowledgments
The authors would like to thank K. Sue Hageman, Gabrielle E. Sims and Dr Tadakatsu Inagaki for excellent technical assistance. These experiments were funded by American Heart Association Midwest Affiliate (10GRNT4350011) and NIH (HL-108328) awards to D.C.P. and KSU SMILE award to T.I.M.
Glossary
- BF
blood flow
- BR
beetroot juice
- eNOS
endothelial nitric oxide synthase
- HR
heart rate
- iNOS
inducible nitric oxide synthase
- MAP
mean arterial pressure
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NO2−
nitrite
- NO3−
nitrate
- NOS
nitric oxide synthase
- PCr
phosphocreatine
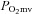
microvascular partial pressure of oxygen
- VC
vascular conductance
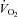
oxygen uptake
Author contributions
Conception and design of the experiments: S.K.F., C.T.H., S.W.C., D.M.H., T.I.M., D.C.P. Collection, analysis and interpretation of data: S.K.F., S.W.C., D.M.H., C.T.H., J.D.A., T.I.M. Drafting the article and revising it critically for important intellectual content: S.K.F., S.W.C., D.M.H., C.T.H., A.M.J., J.D.A., T.I.M., D.C.P. All authors approved the final version of the manuscript.
References
- Armstrong RB, Hayes DA, Delp MD. Blood flow distribution in rat muscles during preexercise anticipatory response. J Appl Physiol. 1989;67:1855–1861. doi: 10.1152/jappl.1989.67.5.1855. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J Exp Biol. 1985;115:201–213. doi: 10.1242/jeb.115.1.201. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Winyard PG, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr JM, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Armstrong RB, Delp MD. Adrenergic control of vascular resistance varies in muscles composed of different fiber types: influence of the vascular endothelium. Am J Physiol Regul Integr Comp Physiol. 2011;301:R783–790. doi: 10.1152/ajpregu.00205.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, Weitzberg E, Lundberg JO. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011;89:574–585. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- Cermak NM, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon L,JC. No improvement in endurance performance following a single dose of beetroot juice. Int J Sport Nutr Exerc Metab. 2012 doi: 10.1123/ijsnem.22.6.470. (in press, DOI: 10.1002/ppul.22556) [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol. 2010a;588:5077–5087. doi: 10.1113/jphysiol.2010.198382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol. 2010b;588:1321–1331. doi: 10.1113/jphysiol.2009.183723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol. 2006a;188:3–13. doi: 10.1111/j.1748-1716.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol. 2006b;186:223–232. doi: 10.1111/j.1748-1716.2006.01523.x. [DOI] [PubMed] [Google Scholar]
- Ferreira LF, McDonough P, Behnke BJ, Musch TI, Poole DC. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fiber types. Respir Physiol Neurobiol. 2006c;153:237–249. doi: 10.1016/j.resp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods. 1984;11:1–39. doi: 10.1016/0160-5402(84)90050-0. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol. 1998;85:1457–1463. doi: 10.1152/jappl.1998.85.4.1457. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol. 1999;86:2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai DM, Copp SW, Ferreira LF, Musch TI, Poole DC. Nitric oxide bioavailability modulates the dynamics of microvascular oxygen exchange during recovery from contractions. Acta Physiol. 2010;200:159–169. doi: 10.1111/j.1748-1716.2010.02137.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Tschakovsky ME. Nitric oxide and physiologic vasodilation in human limbs: where do we go from here. Can J Appl Physiol. 2003;28:475–490. doi: 10.1139/h03-035. [DOI] [PubMed] [Google Scholar]
- Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson J, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011a;43:1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, Dimenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011b;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Masschelein E, Van Thienen R, Wang X, Van Schepdael A, Thomis M, Hespel P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol. 2012;113:736–745. doi: 10.1152/japplphysiol.01253.2011. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol. 1988;65:964–970. doi: 10.1152/jappl.1988.65.2.964. [DOI] [PubMed] [Google Scholar]
- Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol. 1992;262:H411–419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Tomita S, Ishizawa K, Abe S, Ikeda Y, Kihira Y, Tamaki T. Dietary nitrite ameliorates renal injury in l-NAME-induced hypertensive rats. Nitric Oxide. 2010;22:98–103. doi: 10.1016/j.niox.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Bailey SJ, Blackwell JR, Dimenna FJ, Pavey T, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010a;299:R1121–1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, Dimenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power–duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol. 2010b;95:528–540. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard P, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol. 2011;589:5517–5528. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson DP, Hayward G, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50-mile time trial performance in well-trained cyclists. Eur J Appl Physiol. 2012;112:4127–34. doi: 10.1007/s00421-012-2397-6. 2012. [DOI] [PubMed] [Google Scholar]
- Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


