Abstract
Approximately 100,000 cases of upper gastrointestinal bleeding (UGIB) require inpatient admission annually in the United States. When medical management and endoscopic therapy are inadequate, endovascular intervention can be lifesaving. These emergent situations highlight the importance of immediate competence of the interventional radiologist in the preangiographic evaluation as well as the endovascular treatment of UGIB. We describe a case of UGIB managed with endovascular embolization and detail the angiographic techniques used. The case description is followed by a detailed discussion of the treatment approach to UGIB, with attention to both nonvariceal and variceal algorithms.
Keywords: embolization, upper gastrointestinal hemorrhage, emergent, TIPS
Objectives: Upon completion of this article, the reader will be able to review the current diagnostic and treatment algorithms for acute upper gastrointestinal hemorrhage.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Case Presentation
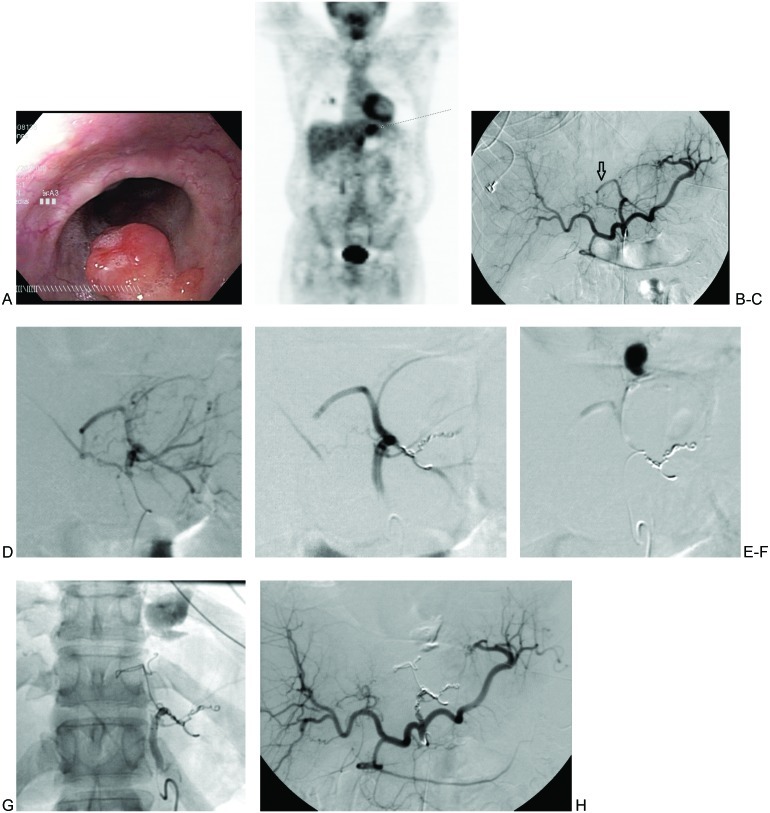
A 65-year-old man with a history of hypertension and alcohol abuse presented to the emergency department with acute onset of hematemesis. Initial laboratory work demonstrated his hemoglobin to be low at 11.6 g/dL. Liver function tests and coagulation parameters were normal. Nasogastric lavage findings corroborated the clinical suspicion of active upper gastrointestinal bleeding (UGIB). An urgent esophagogastroduodenoscopy was performed by the gastroenterology service that revealed a large diverticulum at the gastroesophageal junction that contained a mass lesion (Figs. 1A and 1B). No active bleeding was visualized, but the mucosa was noted to have stigmata of recent bleeding. An ulcerated tip seen on the diverticular mass lesion was postulated to represent an exposed vessel. A biopsy of the mass was performed. This was immediately followed by an injection of 10 cc of 1:10,000 epinephrine at the biopsy site to control postbiopsy bleeding. The gastroenterology service concluded that future bleeding arising from this mass lesion would not be amenable to endoscopy therapy.
Figure 1.
(A) Esophagogastroduodenoscopy image of the distal esophageal diverticular mass lesion. (B) Coronal positron emission tomography scan with focus of abnormal activity in epigastrium corresponding with diverticular mass lesion seen on endoscopy (arrow). (C) Celiac arteriogram without angiographic evidence of bleeding. Note replaced left hepatic artery arising from the left gastric artery (arrow). (D) Left gastric arteriogram with round area of neovascularity in the epigastrium corresponding with the mass lesion seen on endoscopy. Tumoral blush was visible on delayed imaging (not shown). Supplying branches arise from the left gastric artery, as well as from the replaced left hepatic artery to the left gastric artery. (E) Particle embolization of the mass yielded attenuation of the tumoral blush. Additional coil embolization was performed. A curvilinear vessel arising from the replaced left hepatic artery remain visualized. (F) Angiography of the superselected curvilinear branch noted in (E) demonstrates active extravasation with pooling. (G) Successful embolization of the curvilinear branch using coils that were extended into the replaced left hepatic and left gastric arteries. Resolution of tumoral blush and active extravasation. (H) Successful embolization the curvilinear branch using coils that were extended into the replaced left hepatic and left gastric arteries. Resolution of tumoral blush and active extravasation.
Diagnosis
UGIB caused by an invasive squamous cell carcinoma within a distal esophageal diverticulum.
Treatment/Equipment
After admission, the patient had an acute onset of melena and his hemoglobin continued to decrease, reaching a nadir of 8.7 g/dL. He was transfused with 6 units of packed red blood cells (pRBCs) and 1 unit of platelets. He was brought to the angiography suite where emergent angiography was performed.
The right common femoral artery was accessed using a micropuncture system (Cook Medical Inc., Bloomington, IN). A 5F vascular sheath (Terumo, Elkton, MD) was placed. Using a 15-mm J wire and a 5F RC1 catheter (Cook), the celiac artery was accessed under fluoroscopic guidance. A celiac arteriogram was performed that revealed a replaced left hepatic artery with questionable tumoral blush in the epigastrium (Fig. 1C), although no active hemorrhage was visualized. A 5F VS1 catheter (Cook) and a glidewire (Terumo, Somerset, NJ) were used to select the left gastric artery, and another angiogram was performed. This confirmed tumoral blush in the epigastrium, as well as active contrast extravasation from tumoral branches arising from the gastrohepatic trunk and replaced left hepatic artery (Fig. 1D). Using a coaxial system, a large branch arising from the left gastrohepatic trunk was selected with a 3F Renegade STC microcatheter (Boston Scientific, Natick, MA) and Transend EX Floppy 0.014-inch microwire (Boston Scientific). After confirming catheter positioning with superselective angiography, embolization was performed with 500 to 700 μm Embospheres (Merit Medical, South Jordan, UT) and 2 mm × 8 cm Interlock VortX Diamond microcoils (Boston Scientific) (Fig. 1E–F). A curvilinear branch arising from the replaced left hepatic artery was similarly superselected, and angiography of this vessel also demonstrated active extravasation with pooling of contrast (Fig. 1G). Embolization of this branch was performed using 8 mm × 20 cm Interlock VortX Diamond microcoils. Coiling was extended proximally into the gastrohepatic trunk. Postembolization angiography of the left gastric artery confirmed absence of tumoral blush, resolution of active contrast extravasation, and successful occlusion of the gastrohepatic trunk (Fig. 1H).
Sixteen months later, the patient presented again with anemia (hemoglobin 7.1 g/dL). He had not undergone further treatment of his cancer in the interim. Bleeding was controlled at that time with medical management and radiation therapy. No further endovascular intervention was required.
Discussion
Background
UGIB is defined by a bleeding source proximal to the ligament of Treitz. UGIB accounts for 76% of gastrointestinal (GI) bleeding events.1 The incidence of UGIB in the United States is estimated at 102 per 100,000 per year, with a mortality rate of 5% based on data from 1991.2 UGIB can be divided into arterial (nonvariceal) and venous (variceal) etiologies; this is an important point of differentiation and a major branch in the management decision tree. Acute variceal bleeding is associated with a high early mortality rate of up to 30%.3,4 Causes of upper GI arterial bleeding include peptic ulcer disease (up to 40%), Mallory-Weiss tear (15%), hemorrhagic gastritis, pancreatitis-related pseudoaneurysms, neoplasm, hemobilia, aortoduodenal fistula, and trauma. Venous causes include variceal bleeding secondary to portal venous hypertension (e.g., due to cirrhosis or Budd-Chiari syndrome) or splenic vein thrombosis.
Symptoms
Typical UGIB symptoms include hematemesis or melena. Hematochezia, although commonly attributed to lower GI sources, can also occur depending on the bleeding rate and transit time of blood through the bowel. Patients with variceal bleeding are likely to present with clinical signs of cirrhosis, painless hematemesis, and a greater degree of hemodynamic instability. Nonvariceal bleeding is more commonly associated with coffee-ground emesis and nonsteroidal anti-inflammatory drug use.
Treatment Algorithm
Current therapeutic algorithms call for immediate medical stabilization followed by endoscopic diagnosis and intervention. Refractory cases should be referred for either transvenous or transarterial endovascular intervention, depending on the source of bleeding identified at endoscopy. Although surgery is generally considered the last option, endovascular intervention can be reattempted following failed surgery. An exception to this scheme is hepatobiliary bleeding, for which there is no role for endoscopy beyond diagnosis.
Medical
Medical management is the first line treatment of UGIB. Stabilization of blood pressure and fluid resuscitation are the immediate goals. Patients with hemodynamically significant bleeding should be immediately typed and crossed to allow for transfusion of blood products as necessary. The goal of transfusion in this setting is to raise the hemoglobin level to 7 g/dL. Although correction of coagulopathy is an important component of medical management, it should not delay early endoscopy.5
Placement of a nasogastric tube can help confirm an upper GI source of bleeding. If a variceal bleed is suspected, an esophageal balloon catheter can be placed to tamponade esophageal varices. These tubes have been reported to have variable success in achieving hemostasis, but they are a good option in stabilizing patients in the emergent setting prior to interventional procedures.
High-dose intravenous proton pump inhibitors (PPIs) have been shown to reduce the rate of rebleeding, the need for surgery, and mortality in high-risk patients with nonvariceal UGIB. The use of portal pressure-reducing medications such as somatostatin, octreotide, or vasopressin can improve outcomes in patients with variceal UGIB.
Endoscopy
Endoscopy should be the initial diagnostic intervention because it allows for localization of bleeding and determination of etiology via visual inspection and biopsy. It can be particularly helpful in differentiating arterial and venous sources of hemorrhage. Beyond its diagnostic utility, endoscopy also offers several therapeutic options. Endoscopic hemostasis can be achieved via thermocoagulation, sclerosant injection, or clips/banding. Epinephrine injection alone, as used in the case presentation here, is suboptimal. Endoscopic clip placement, even if ineffectual, can aid subsequent endovascular intervention by directing the interventional radiologist to the area of concern.
Endoscopy performed within 24 hours of presentation is associated with improved patient outcomes and decreased hospital stay. However, the source of bleeding may not be seen in up to 24% of patients at the first endoscopic examination.6 Endoscopic treatment is reported to be effective in 85 to 90% of patients.7 However, repeat bleeding after endoscopic treatment occurs in 15 to 20%.8 Endoscopic predictors of rebleeding include visualized active bleeding, nonbleeding visible vessel, adherent clot, ulcer size >2 cm, and ulcer location in the posterior midgastric body or posterior duodenal bulb.5,9,10 Recurrent bleeding should be followed with a second endoscopic treatment before endovascular intervention is undertaken.11
There are no endoscopic interventions if a hepatobiliary source of hemorrhage is identified. The primary therapeutic option in such cases is endovascular treatment.
Computed Tomographic Angiography
When bleeding cannot be localized by endoscopy, computed tomographic angiography (CTA) may be considered before proceeding to conventional angiography. Compared with conventional angiography, CTA has the advantage of greater availability, speed, and noninvasiveness. It also does not suffer from bowel or respiratory motion artifact. No studies specific to UGIB have been performed, but a study by Yoon et al found CTA to have a sensitivity of 90.9%, a specificity of 99%, and an accuracy of 97.6% in localizing bleeds when considering both upper and lower GI hemorrhage.12 In an animal model, CTA was shown to detect bleeding rates as low as 0.3 mL/minute—better than conventional angiography.13 In addition to being able to localize a bleed, CTA also has the benefit of providing information on the vascular anatomy for preinterventional planning, as well as identifying extraluminal abnormalities.
CTA protocols are similar to those for endoleak evaluation. A noncontrast series is followed by an intravenous (IV) contrast-enhanced series in arterial and delayed phases. A positive CTA study will demonstrate hyperdense contrast material (>90 HU) within the bowel lumen on the arterial phase that increases on the delayed phase.14 Oral contrast should not be given because it will obscure IV contrast extravasation into the bowel lumen.
The primary limitation of CTA is the necessity of iodinated contrast. Typically 1.5 mL/kg (up to 150 mL) is administered. This can be prohibitive in patients with poor renal function. Radiation dose is another drawback to consider.
Angiography Indications
There are two indications for conventional angiography: The bleeding site cannot be identified by endoscopy or CTA, or the source of bleeding cannot be controlled endoscopically. Angiography is favored over surgery as the treatment of choice after failed endoscopic therapy, particularly in high-risk surgical patients.15 It is minimally invasive, associated with lower mortality,16 and there has been shown to be no difference in outcome between patients managed with surgery versus arterial embolization.17,18 As mentioned previously, arterial embolization is also the primary treatment of choice for bleeding into the pancreatic duct or biliary tree, neither of which can be managed endoscopically.
Preangiography
Prior to angiography, the patient's renal and coagulation statuses should be assessed. Elevated partial thromboplastin time and prothrombin/international normalized ratio, as well as thrombocytopenia, should be corrected. Embolization is less likely to succeed in the setting of coagulopathy.15
Angiography and Embolotherapy
Angiography should be targeted to the suspected site of bleeding. Review of imaging (such as CTA) prior to intervention can profoundly expedite cases by demonstrating vascular occlusions or anomalous anatomy. One should also consider the angle at which the mesenteric vessels arise from the aorta. This may suggest a benefit in using a brachial artery approach rather than the more commonly used femoral artery approach to gain better mechanical advantage when attempting to select the mesenteric vessels.
We begin with a mapping aortogram to obtain a survey of the vascular anatomy. This aids specifically in identifying the origins of the mesenteric vessels and in guiding subsequent catheter selection, ultimately saving valuable time in the treatment of a hemodynamically unstable patient. If recent imaging of the vascular anatomy is available, either as CTA or conventional angiogram, an initial aortogram can be skipped in favor of a selective mesenteric angiogram to decrease contrast load and time delay in patients with poor renal function. Contrast extravasation is rarely visible on the mapping aortogram. We use a 5F nonmarking pigtail catheter (Cook, Bloomington, IN) placed through a 5F vascular sheath for the aortogram.
Although not regularly used at our institution, 1 mg glucagon may be administered prior to angiography to limit peristalsis and respiratory motion artifacts on digital subtraction angiography (DSA). For similar reasons, breath hold during DSA is ideal, although not always feasible depending on the patient's condition and level of sedation.
We favor the use of Rosch celiac (RC-1) or visceral selective (VS1) catheters (Cook) to select the celiac artery and superior mesenteric artery (SMA). Alternatively, a Waltman loop technique using a 5F glide catheter (Terumo, Somerset, NJ) can be useful in negotiating steeply angled and tortuous mesenteric vessels. Power injection of contrast is used for all angiograms to optimize detection of active bleeding. Celiac artery and SMA angiogram injection rates are typically 5 cc/second for a total volume of 20 to 25 cc. Imaging is performed until the portal venous phase to document patency of the portal vein. This is important in cases that potentially require hepatic artery embolization such as hemobilia or in cases with variant arterial anatomy such as described in the case presentation here. Prolonged angiograms may also reveal varices that were not readily apparent by endoscopy. If there is hemobilia related to recent percutaneous biliary drain placement, removal of the tube over a guidewire may be necessary prior to angiography because the relative tamponade effect of the tube may obscure visualization of the bleeding vessel.
Angiography can detect bleeding rates as low as 0.5 mL/minute. The primary angiographic findings of bleeding are visualization of active contrast extravasation and contrast pooling in the venous phase. A review of studies by Loffroy et al found that angiographic evidence of active extravasation was seen in 54% of cases. Other indirect signs of bleeding on angiography include pseudoaneurysm, vessel spasm or cut-off, early venous filling, and hypervascularity. The presence of an abnormal blush may indicate an inflammatory process, which may represent a bleeding source.19
In theory, carbon dioxide (CO2) angiography is more sensitive than conventional angiography with iodinated contrast because the lower viscosity of CO2 should predispose it to extravasating through endothelial injuries. However, in practice CO2 imaging is degraded by fragmentation of the CO2 bolus and patient motion related to discomfort caused by the CO2 injection. It also has poorer spatial resolution, which may impair subsequent endovascular treatment.20
Many cases of UGIB are intermittent and thus produce negative angiography. Empirical or blind embolization of the vessels supplying the area of concern can be performed if no arterial abnormality is seen. A review of published series by Loffroy et al demonstrated that blind embolization is performed in 46% of endovascular cases. This technique is feasible due to the rich collateral circulation of the upper GI tract. The left gastric artery supplies the distal esophagus, cardia, and fundus, and there is collateralization with branches of the short gastric and right gastric arteries. The gastroduodenal artery (GDA) supplies the remainder of the stomach and duodenum. The SMA also provides duodenal supply via the pancreaticoduodenal arcades.19 No statistical difference in outcomes was shown between patients treated with empirical embolization versus embolization after angiographically demonstrated contrast extravasation.21,22 An alternative to blind embolization in cases of negative angiography is to target branches supplying the area of endoscopically placed clips.
Superselection of vessels may be necessary to demonstrate bleeding angiographically. This typically requires coaxial placement of a 3F microcatheter through a 5F catheter. The authors commonly employ a Renegade (Boston Scientific) or a Progreat (Terumo, Tokyo, Japan) microcatheter. The former is available in two types: HI-FLO and STC. The Renegade HI-FLO has a larger diameter (0.027 inches) and is best suited for cases where particulate agents are used. The slightly smaller diameter (0.021 inches) of the Renegade STC is preferred for the deployment of microcoils because the narrower lumen helps guard against intracatheter coil formation, particularly important when using detachable coils. Particles can also be administered through the Renegade STC, with the caveat that the smaller diameter can lead to aggregation of particles and occlusion of the catheter. In such cases, the catheter can be carefully flushed with a 1 cc saline-filled syringe. An advantage of the Progreat catheter (0.027”) is that it is available preloaded with a glidewire.
Once bleeding is identified on angiography, embolization should be performed both distal and proximal to the site of injury to prevent continued retrograde bleeding (“back door”). For example, GDA embolization performed for management of a duodenal ulcer may call for coil embolization distally into the right gastroepiploic artery, with extension proximally into the GDA. Adequate embolization is confirmed by SMA to exclude “back door” bleeding through the pancreaticoduodenal arcade. If bleeding persists, the inferior pancreaticoduodenal arcade should be superselected via an SMA approach, and embolization performed as distally as possible.
Distal catheterization can be limited by vasospasm and vessel tortuosity. The latter can be overcome by the use of soft-tipped microwires; the 0.014-inch Hi-Torque Balance Middle Weight (BMW) guidewire (Abbott Vascular, Santa Clara, CA) is a favorite of ours. The use of road mapping technique when attempting to subselect vessels can also be helpful. Vasospasm can be waited out or managed by infusion of 200 μg nitroglycerin into the affected artery. Although vasospasm may temporarily mask bleeding in the targeted vessel, it can occasionally be helpful by revealing bleeding from an adjacent artery.
Transarterial embolization is the first-line treatment for bleeding of hepatobiliary origin. The dual vascular supply of the liver (75% via the portal vein and 25% via the hepatic artery) permits embolization of hepatic artery branches without significant concern for infarction. However, portal vein patency should be confirmed by an extended celiac artery or SMA digital subtraction angiogram prior to embolization. Even with normal portal hepatic perfusion, arterial embolization can be complicated by abscess formation and biliary stenosis; the latter occurs because the bile ducts draw their supply primarily from the arterial rather than the portal system. Embolization with coils rather than particles is recommended to avoid ischemia of bile ducts and hepatocytes. Additionally, care should be taken to avoid embolization of the cystic artery.23 The current case presentation illustrates the importance of assessing portal vein patency even in cases of UGIB that are not hepatobiliary in origin. Although the replaced left hepatic artery was sacrificed to embolize the left gastric artery bleed successfully, the patient presented here did not experience any adverse sequelae of hepatic artery embolization.
Embolic Agents
There is no conclusive evidence to indicate that one embolic agent is superior to the others. In practice, operator familiarity with each agent and institutional availability largely determines what is used. Of note, however, is evidence that coils, when combined with polyvinyl alcohol particles or Gelfoam, are associated with lower bleeding recurrence rates compared with the use of coils alone.21 The effective use of particles and coils in combination is well illustrated in the case presentation. Regardless of the embolization agent used, postembolization angiography is important to assess for collateral filling after embolization. This should include an SMA.
Coils
Coils are the most commonly used embolic agent, as well as the agent of choice at our institution. Coil placement is alternated with infusion of a slurry of gelatin sponge (Gelfoam, Pfizer, New York, NY) to create a “Gelfoam sandwich.” This technique helps expedite embolization and is especially useful in patients with underlying coagulopathy in whom thrombus is slow to develop on the fibered coil scaffolding. The Gelfoam sandwich technique also helps limit the number of coils needed to embolize larger and longer vessels such as the GDA.
Gelfoam
Use of Gelfoam alone provides a variable degree of short-term success because embolized vessels will recanalize over an indeterminate period of weeks to months. In theory this should cause a lesser degree of bowel ischemia compared with other embolic agents. However, because of the rich collateralization of the upper GI arterial system, there is less concern for bowel ischemia when treating UGIB. Thus permanent agents such as coils may be used without hesitation. Patients with prior bowel surgery (e.g., the Whipple procedure) are an exception to this rule. This population has altered vascular anatomy, and collateral circulation may be diminished or absent. In such cases, embolization with permanent agents may be relegated to the second option after Gelfoam because of the heightened concern for bowel ischemia.
Particles
Particle embolization, as described in the case presentation, is often used to control bleeding secondary to primary or metastatic GI tumors. Unlike with benign lesions of the GI tract, ischemia at the arteriole or capillary level may be beneficial when treating bleeding tumors. Use of particle sizes as small as 200 μm have been reported to be technically successful in the embolization of primary GI tumors and is not associated with bowel ischemia or postembolization syndrome.24 Utility has also been demonstrated in shrinking metastatic lesions prior to surgical resection.25 However, we advise against the use of particulate agents in the treatment of nontumoral UGIB due to the theoretical risk of organ necrosis.
Glue
In a study by Lee et al, N-butyl-2-cyanoacrylate, or “glue” (Trufill NBCA, Cordis Neurovascular, Miami Lakes, FL), was used successfully to achieve immediate hemostasis in 88% of cases of UGIB. In this series, recurrent bleeding was seen in only 7%.26 Unlike with microcoils or particles, the delivery microcatheter should be flushed with D5W solvent and wedged into the target vessel to prevent intracatheter polymerization and pericatheter flow of glue.27 Glue is then mixed with Ethiodol (Guerbet, Villepinte, France) in a 1:1 to 1:3 ratio to impart radio-opacity to the agent as well as to modify the degree of distal embolization desired. The end point of infusion is extravasation of the agent from the bleeding site or complete filling of the target vessel. Once injection is complete, the microcatheter should be removed immediately to prevent adherence to the catheter wall. Only a fraction of a milliliter of the NBCA-Ethiodol mixture is needed to achieve embolization. A single injection of glue can occlude both the main and collateral feeding vessels. Glue polymerizes within seconds and does not rely on an intact coagulation cascade to achieve hemostasis. As evidence of this, Jae et al reported an 83% success rate in patients with underlying coagulopathy and acute UGIB.27 It is this subset of patients for whom the authors recommend the use of this agent. Of note, a study by Lang found a high prevalence of duodenal stricture as a late complication of embolization using 6-cyanoacrylate.28 However, this was postulated to be secondary to the embolization technique in which terminal muscular branches were embolized, rather than use of the agent itself.
Vasopressin
Unlike for lower GI bleeding, vasopressin has not been shown to be effective for UGIB. The relatively larger vessels from which UGIB usually arises may not constrict to the same degree as the smaller branches associated with lower GI bleeding.29
Postangiography
If a patient is on aspirin, it should be resumed when the cardiovascular risks outweigh the risk of rebleeding. This determination should be made after consultation with all those involved in the care of the patient. In spite of the possible clopidogrel–PPI interaction, a PPI may be necessary in patients who developed acute GI bleeding while taking aspirin or clopidogrel.
Outcomes
A review of studies by Loffroy et al found that the overall technical and clinical successes of embolization in UGIB were 93% and 67% respectively, with a 33% rebleeding rate. Repeat embolization was successful in approximately half of these patients.19 The need for surgical intervention for patients with clinically unsuccessful arterial embolization is 15 to 20%.19,30
One study found UGIB to be more resistant to hemostasis (with a higher rate of early rebleeding) than lower GI hemorrhage.18 This was hypothesized to be secondary to refilling of injured vessels through collateral circulation distal to the point of embolization. It is also important to remember that embolization does not treat the underlying pathology of UGIB such as peptic ulcer disease. In these patients, gastric acid suppression and treatment of Helicobacter pylori are important adjuncts to prevent recurrence of bleeding. UGIB also tends to be more profuse than lower GI bleeding and is associated with greater risk factors (i.e., sicker patients), which may also contribute to treatment failure. Factors associated with clinical failure of arterial embolization include the use of anticoagulants, underlying coagulopathy, longer time interval between onset of bleed and embolization, increased number of pRBC transfusions, hypovolemic shock and/or vasopressor use, corticosteroids, and the use of coils as the lone embolic agent.18,19,30,31
The overall postembolization complication rate is 6 to 9%.30 Complications include access site hematoma, arterial dissection, contrast nephropathy, and nontarget embolization. Bowel ischemia or infarction can be caused by embolization too far distal in the vascular bed. This is of concern primarily when using particles or liquid embolic agents. Additionally, one must be cognizant that the normally rich collateral blood supply of the upper GI tract that protects against ischemia is compromised in patients who have had prior surgery or radiation therapy.
Variceal Bleeding
Variceal sources of GI bleeding are distinct from arterial bleeding both in etiology and endovascular treatment. For these reasons, it is important to distinguish between nonvariceal and variceal sources of hemorrhage at the outset. Sources of variceal UGIB include gastroesophageal varices from portal venous hypertension, and gastric varices from splenic vein thrombosis. Active variceal hemorrhage accounts for about a third of all deaths related to cirrhosis.32 One should keep in mind, however, that 30% of patients with portal hypertension who present with UGIB actually have an arterial source of bleeding.33
Variceal bleeding stops spontaneously in only ∼50% of patients, which is considerably less than the rate seen with arterial UGIB.34,35,36 Following cessation of active hemorrhage, there is a high risk of recurrent hemorrhage. The greatest risk is within the first 48 to 72 hours, and over half of all early rebleeding episodes occur within the first 10 days.37 Each episode of bleeding carries a 30% mortality rate, with rates approaching 70 to 80% in patients with continued bleeding.38,39 The risk of rebleeding is high (60 to 70%) until the gastroesophageal varices are treated.40 Risk factors for early rebleeding include age >60 years, renal failure, large varices, and severe initial bleeding as defined by a hemoglobin level <8 g/dL at admission.37 The goals of management during an active bleeding episode are hemodynamic resuscitation, prevention and treatment of complications, and treatment of bleeding.
Endoscopic therapy is currently the definitive treatment of choice for active variceal hemorrhage and can be performed at the time of diagnostic endoscopy. Two forms of endoscopic treatment are commonly used: sclerotherapy and variceal band ligation. Urgent endoscopic and/or pharmacological treatments nevertheless fail to control bleeding in ∼10 to 20% of patients, and more definitive therapy such as portosystemic shunt creation must be immediately instituted.41 Although balloon tamponade is an effective way to achieve short-term hemostasis, due to complications of rebleeding following balloon deflation, its use is generally reserved for temporary stabilization until more definitive treatment can be instituted.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
Portal venous hypertension is most commonly attributable to cirrhosis and Budd-Chiari syndromes. Reduction of the portal-systemic venous gradient usually necessitates a transjugular intrahepatic portosystemic shunt (TIPS) creation with or without concomitant variceal embolization. A portosystemic gradient <12 mm Hg is associated with a lower risk of bleeding recurrence. At our institution, embolization of varices is not routinely performed at the time of TIPS unless it is in the setting of acute ongoing variceal bleeding. A retrospective study by Tesdal et al demonstrated that the incidence of rebleeding is lower in cases of TIPS with variceal embolization compared with TIPS alone.42 However, this study did not reveal a statistically significant difference in survival between the two cohorts. During TIPS, the authors routinely place 10-mm-diameter Viatorr stents (Gore, Newark, DE) and dilated them as needed to achieve the desired portosystemic gradient. This is typically achieved at 8 mm. If bleeding recurs in the short term, the stent is fully dilated to 10 mm and additional attempts at variceal embolization are made.
The model for end-stage liver disease, or MELD, is a scoring system for assessing the severity of chronic liver disease. It was initially developed to predict death within 3 months of surgery in patients who had undergone a TIPS procedure but was subsequently found to be useful in determining prognosis and in prioritizing patients for receipt of a liver transplant.43,44,45 Patients with poor hepatic reserve (MELD >17 to 19) have been shown to do poorly with the TIPS procedure.46,47,48,49
In cases of extrahepatic venous occlusion, recanalization of the portal vasculature (portal vein, splenic vein, or superior mesenteric vein) can be attempted. Following wire recanalization via either a transjugular or a transhepatic approach, a 3F infusion catheter is placed across the affected vascular segment and thrombolysis initiated. The protocol at our institution calls for an infusion of tPA at 0.5 mg/hr. Although catheter thrombolysis is effective only for acute clot, it is attempted in nearly all cases to optimize vessel patency prior to angioplasty and/or stent deployment. In addition to portal vascular reconstruction, embolization of varices can be performed via the same access route. After recanalization, the patient is placed on long-term anticoagulation to prevent rethrombosis. To this end, one may also consider placement of a TIPS to improve flow and thereby decrease the risk of thrombosis and, ultimately, variceal bleeding.
Balloon-Occluded Retrograde Transvenous Obliteration
Gastric varices represent a slightly different pathology and hemodynamic issue than esophageal varices. Most gastric varices are due to portal hypertension, whereas others are secondary to splenic vein thrombosis. Balloon-occluded retrograde transvenous obliteration (BRTO) is a highly effective and minimally invasive treatment for isolated gastric varices, particularly in patients who are not suitable candidates for TIPS due to poor hepatic reserve. BRTO is widely accepted in Japan with growing utilization worldwide. This technique uses an occlusion balloon to control the blood flow and delivery of sclerosant through prominent draining veins of portosystemic shunts (most commonly a gastrorenal shunt) contributing to the gastric varices. With the shunt outflow occluded, the goal is to fill the variceal complex sufficiently with a sclerosing agent and obliterate the gastric varices without refluxing into the systemic or portal circulation. Successful treatment relies on an understanding of the anatomy and hemodynamic patterns of the gastric varices. For a detailed discussion of BRTO, see the September 2011 issue (Volume 28, Number 3) of Seminars in Interventional Radiology.
Teaching Points/Pearls
Medical management (resuscitation) is the first-line treatment in the management of upper GI hemorrhage. The patient's coagulation status should be assessed and corrected because embolization is less likely to succeed in the setting of a coagulopathy.
Endoscopy is the initial diagnostic intervention that can localize and determine the etiology of an upper GI bleed as well as offer several treatment options. Determination of arterial versus venous/variceal source is a major point of differentiation warranting correspondingly different endovascular treatment options (arterial embolization versus TIPS/BRTO).
BRTO may be an effective treatment for gastric varices in patients who are not suitable candidates for TIPS due to poor hepatic reserve (MELD >17 to 19).
References
- 1.Peura D A, Lanza F L, Gostout C J, Foutch P G. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92(6):924–928. [PubMed] [Google Scholar]
- 2.Longstreth G F. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90(2):206–210. [PubMed] [Google Scholar]
- 3.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43(1):167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Sanyal A J, Grace N D, Carey W. Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 5.Greenspoon J, Barkun A. A summary of recent recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Pol Arch Med Wewn. 2010;120(9):341–346. [PubMed] [Google Scholar]
- 6.Vreeburg E M, Snel P, de Bruijne J W, Bartelsman J F, Rauws E A, Tytgat G N. Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92(2):236–243. [PubMed] [Google Scholar]
- 7.Bjorkman D J, Zaman A, Fennerty M B, Lieberman D, Disario J A, Guest-Warnick G. Urgent vs. elective endoscopy for acute non-variceal upper-GI bleeding: an effectiveness study. Gastrointest Endosc. 2004;60(1):1–8. doi: 10.1016/s0016-5107(04)01287-8. [DOI] [PubMed] [Google Scholar]
- 8.Lim J K, Ahmed A. Endoscopic approach to the treatment of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7(3):123–129. doi: 10.1053/j.tvir.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Lau J Y, Sung J J, Lam Y H. et al. Endoscopic treatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;11(340):751–756. doi: 10.1056/NEJM199903113401002. [DOI] [PubMed] [Google Scholar]
- 10.Laine L, Peterson W L. Bleeding peptic ulcer. N Engl J Med. 1994;331(11):717–727. doi: 10.1056/NEJM199409153311107. [DOI] [PubMed] [Google Scholar]
- 11.Laine L Jensen D M Management of patients with ulcer bleeding Am J Gastroenterol 20121073345–360.; quiz 361 [DOI] [PubMed] [Google Scholar]
- 12.Yoon W, Jeong Y Y, Shin S S. et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239(1):160–167. doi: 10.1148/radiol.2383050175. [DOI] [PubMed] [Google Scholar]
- 13.Kuhle W G, Sheiman R G. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228(3):743–752. doi: 10.1148/radiol.2283020756. [DOI] [PubMed] [Google Scholar]
- 14.Yann G, Rodallec M H, Boulay-Coletta I. et al. Multidetector CT angiography in acute gastrointestinal bleeding: why, when and how. Radiographics. 2011;31:E35–46. doi: 10.1148/rg.313105206. [DOI] [PubMed] [Google Scholar]
- 15.Millward S F. ACR appropriateness criteria on treatment of acute nonvariceal gastrointestinal tract bleeding. J Am Coll Radiol. 2008;5(4):550–554. doi: 10.1016/j.jacr.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson L G, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008;19(10):1413–1418. doi: 10.1016/j.jvir.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Ripoll C, Bañares R, Beceiro I. et al. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004;15(5):447–450. doi: 10.1097/01.rvi.0000126813.89981.b6. [DOI] [PubMed] [Google Scholar]
- 18.Defreyne L, Vanlangenhove P, De Vos M. et al. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218(3):739–748. doi: 10.1148/radiology.218.3.r01mr05739. [DOI] [PubMed] [Google Scholar]
- 19.Loffroy R, Rao P, Ota S, De Lin M, Kwak B K, Geschwind J F. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33(6):1088–1100. doi: 10.1007/s00270-010-9829-7. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu C, Buckenham T M, Belli A M. Using CO2-enhanced arteriography to investigate acute gastrointestinal hemorrhage. AJR Am J Roentgenol. 1999;173(5):1399–1401. doi: 10.2214/ajr.173.5.10541128. [DOI] [PubMed] [Google Scholar]
- 21.Aina R, Oliva V L, Therasse E. et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12(2):195–200. doi: 10.1016/s1051-0443(07)61825-9. [DOI] [PubMed] [Google Scholar]
- 22.Padia S A, Geisinger M A, Newman J S, Pierce G, Obuchowski N A, Sands M J. Effectiveness of coil embolization in angiographically detectable versus non-detectable sources of upper gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20(4):461–466. doi: 10.1016/j.jvir.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Mirsadraee S, Tirukonda P, Nicholson A, Everett S M, McPherson S J. Embolization for non-variceal upper gastrointestinal tract haemorrhage: a systematic review. Clin Radiol. 2011;66(6):500–509. doi: 10.1016/j.crad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Kurihara N, Kikuchi K, Tanabe M. et al. Partial resection of the second portion of the duodenum for gastrointestinal stromal tumor after effective transarterial embolization. Int J Clin Oncol. 2005;10(6):433–437. doi: 10.1007/s10147-005-0503-z. [DOI] [PubMed] [Google Scholar]
- 25.Fidelman N, Freed R C, Nakakura E K, Rosenberg J, Bloom A I. Arterial embolization for the management of gastrointestinal hemorrhage from metastatic renal cell carcinoma. J Vasc Interv Radiol. 2010;21(5):741–744. doi: 10.1016/j.jvir.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Lee C W, Liu K L, Wang H P, Chen S J, Tsang Y M, Liu H M. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2007;18(2):209–216. doi: 10.1016/j.jvir.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Jae H J, Chung J W, Jung A Y, Lee W, Park J H. Transcatheter arterial embolization of nonvariceal upper gastrointestinal bleeding with N-butyl cyanoacrylate. Korean J Radiol. 2007;8(1):48–56. doi: 10.3348/kjr.2007.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang E K. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992;182(3):703–707. doi: 10.1148/radiology.182.3.1535883. [DOI] [PubMed] [Google Scholar]
- 29.Darcy M. Treatment of lower gastrointestinal bleeding: vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003;14(5):535–543. doi: 10.1097/01.rvi.0000064862.65229.8a. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren J A, Matsushima K, Lynch F C, Frankel H, Cooney R N. Angiographic embolization of nonvariceal upper gastrointestinal bleeding: predictors of clinical failure. J Trauma. 2011;70(5):1208–1212. doi: 10.1097/TA.0b013e318213faf1. [DOI] [PubMed] [Google Scholar]
- 31.Poultsides G A, Kim C J, Orlando R III, Peros G, Hallisey M J, Vignati P V. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008;143(5):457–461. doi: 10.1001/archsurg.143.5.457. [DOI] [PubMed] [Google Scholar]
- 32.Avgerinos A, Armonis A. Balloon tamponade technique and efficiency in variceal hemorrhage. Scaud J Gastroenterol Suppl. 1994;207:11–16. doi: 10.3109/00365529409104188. [DOI] [PubMed] [Google Scholar]
- 33.Lee E W, Laberge J M. Differential diagnosis of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7(3):112–122. doi: 10.1053/j.tvir.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Brett B T, Hayes P C, Jalan R.. Primary prophylaxis of variceal bleeding in cirrhosis. Eur J Gastroenterol Hepatol. 2001;13(4):349–358. doi: 10.1097/00042737-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350(16):1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 36.Infante-Rivard C, Esnaola S, Villeneuve J P. Role of endoscopic variceal sclerotherapy in the long-term management of variceal bleeding: a meta-analysis. Gastroenterology. 1989;96(4):1087–1092. doi: 10.1016/0016-5085(89)91627-2. [DOI] [PubMed] [Google Scholar]
- 37.de Franchis R, Primignani M. Why do varices bleed? Gastroenterol Clin North Am. 1992;21(1):85–101. [PubMed] [Google Scholar]
- 38.Smith J L, Graham D Y. Variceal hemorrhage: a critical evaluation of survival analysis. Gastroenterology. 1982;82(5 Pt 1):968–973. [PubMed] [Google Scholar]
- 39.de Dombal F T, Clarke J R, Clamp S E, Malizia G, Kotwal M R, Morgan A G. Prognostic factors in upper G.I. bleeding. Endoscopy. 1986;18 02:6–10. doi: 10.1055/s-2007-1018418. [DOI] [PubMed] [Google Scholar]
- 40.Graham D Y, Smith J L. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80(4):800–809. [PubMed] [Google Scholar]
- 41.Garcia-Tsao G, Sanyal A J, Grace N D, Carey W D. Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102(9):2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 42.Tesdal I K, Filser T, Weiss C, Holm E, Dueber C, Jaschke W. Transjugular intrahepatic portosystemic shunts: adjunctive embolization of collateral vessels in the prevention of variceal rebleeding. Radiology. 2005;236(1):360–367. doi: 10.1148/radiol.2361040530. [DOI] [PubMed] [Google Scholar]
- 43.Malinchoc M, Kamath P S, Gordon F D, Peine C J, Rank J, ter Borg P C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 44.Kamath P S, Wiesner R H, Malinchoc M. et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 45.Kamath P S, Kim W R. Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 46.Saxon R R. A new era for transjugular intrahepatic portosystemic shunts? J Vasc Interv Radiol. 2004;15(3):217–219. doi: 10.1097/01.rvi.0000116862.34422.a5. [DOI] [PubMed] [Google Scholar]
- 47.Ferral H, Gamboa P, Postoak D W. et al. Survival after elective transjugular intrahepatic portosystemic shunt creation: prediction with model for end-stage liver disease score. Radiology. 2004;231(1):231–236. doi: 10.1148/radiol.2311030967. [DOI] [PubMed] [Google Scholar]
- 48.Ferral H, Patel N H. Selection criteria for patients undergoing transjugular intrahepatic portosystemic shunt procedures: current status. J Vasc Interv Radiol. 2005;16(4):449–455. doi: 10.1097/01.RVI.0000149508.64029.02. [DOI] [PubMed] [Google Scholar]
- 49.Montgomery A, Ferral H, Vasan R, Postoak D W. MELD score as a predictor of early death in patients undergoing elective transjugular intrahepatic portosystemic shunt (TIPS) procedures. Cardiovasc Intervent Radiol. 2005;28(3):307–312. doi: 10.1007/s00270-004-0145-y. [DOI] [PubMed] [Google Scholar]