Abstract
Emergent percutaneous nephrostomy is a potentially lifesaving procedure with a high technical success rate, minimal morbidity, and long safety record that is often used in the setting of an obstructed and infected renal collecting system (i.e., pyonephrosis). This article discusses all aspects of the emergent placement of nephrostomy catheters including indications, techniques, results, and complications. Differences between emergent and nonemergent placement of percutaneous nephrostomy catheters are also addressed.
Keywords: percutaneous nephrostomy, pyonephrosis, obstructive uropathy, acute renal failure
Objectives: Upon completion of this article, the reader will be able to identify and discuss the indications for emergent percutaneous nephrostomy placement, preprocedural management, techniques in catheter placement, complications, and acceptable complications rates according to the Society of Interventional Radiology Standards of Practice Committee guidelines.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Case Presentation
A 66-year-old man with a past surgical history significant for radical cystectomy and ileal conduit for transitional cell carcinoma (TCC) 7 months ago presented to the emergency department with 2-day history of worsening left flank pain and fevers. Vital signs were as follows: temperature 103.5°F, blood pressure 104/58 mm Hg, pulse 85 beats/minute, respiratory rate 18/minute, and 98% oxygen saturation on room air. Physical examination confirmed left costovertebral tenderness; the lungs were clear and the abdomen was soft and nontender. Blood was drawn and sent for microbiology, blood count, chemistry, and coagulation panel. Urine from the urostomy was sent for urinalysis. A renal ultrasound was obtained, demonstrating left mild to moderate pelvocaliectasis (Fig. 1A). Pertinent laboratory values returned as follows: white blood cell count 14.4 × 109/L, potassium 4.7 mmol/L, serum creatinine 2.5 mg/dL, platelet count 274 × 109/L, partial thromboplastin time 30 seconds, international normalized ratio (INR) 1.2. The patient was started on empirical antibiotics (3.375 g piperacillin/tazobactam intravenous [IV]). Interventional radiology was consulted for an emergent percutaneous nephrostomy (PCN) catheter placement.
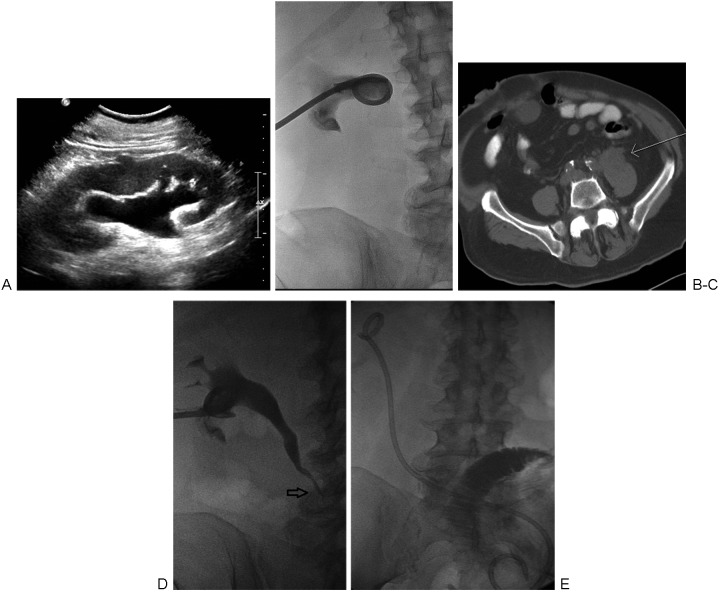
Figure 1.
(A) Ultrasound image demonstrating mild to moderate hydronephrosis of the left kidney. (B) Fluoroscopic spot image obtained following percutaneous placement of a 12F nephrostomy tube placement. Postplacement gentle injection of contrast was performed to confirm appropriate placement. (C) Axial computed tomography without contrast performed after percutaneous nephrostomy demonstrates a 4-cm retroperitoneal mass (arrow) causing the ureteral obstruction. (D) Antegrade nephrostogram performed 13 days later demonstrates a tapered narrowing of the midureter (arrow), corresponding to the location of the retroperitoneal mass. (E) The malignant stricture was traversed, and an internal ureteral stent was placed.
After obtaining consent and determining the patient was appropriate for moderate sedation, the patient was brought to the fluoroscopy suite and placed prone on the fluoroscopy table. Repeat vital signs showed the blood pressure dropped to 88/51 mm Hg and pulse rate remained at 87 beats per minute. Normal saline bolus was started. Under local anesthesia and using the Seldinger technique, a 12F PCN catheter was placed into the left kidney under ultrasound and fluoroscopic guidance (Fig. 1B). A total of 20 cc of pus was extracted and sent for culture and sensitivities. Blood pressure improved to 120/70 mm Hg at the conclusion of the procedure. The patient defervesced over the ensuing hospital stay and was discharged 2 days later. Cultures from the sample obtained during the procedure grew Escherichia coli. Follow-up computed tomography (CT) demonstrated a 4-cm irregular retroperitoneal mass causing obstruction at the mid-left ureter (Fig. 1C). Biopsy of the mass showed TCC. Follow-up antegrade pyelogram (Fig. 1D) was performed 13 days later during placement of an internal ureteral stent (Fig. 1E).
Introduction
PCN catheter insertion was first described by Goodwin et al in 19551 as an emergency procedure to relieve urinary obstruction.2 Subsequently, the safety and efficacy of this procedure has been established using a variety of different imaging modalities including various combinations of CT, fluoroscopy, and ultrasound. The scope of PCN catheter placement has been expanded, and currently nonemergent clinical scenarios including relieving urinary obstructions, diverting urine from a collecting system or ureteral leak, and accessing the collecting system for diagnostic and therapeutic procedures outnumber emergent indications.
Emergent nephrostomy is most commonly performed in the clinical setting of pyonephrosis, which can be defined as the presence of pus in an obstructed renal collecting system.3 Pyonephrosis may present with a classic triad of fever, flank pain, and hydronephrosis, or simply hydronephrosis and sepsis. In the appropriate clinical setting, pyonephrosis should also be suspected when imaging shows a complex appearance to a dilated collecting system. Due to the high risk of sepsis from pyonephrosis and the associated morbidity and mortality, early recognition and treatment of this condition is of paramount importance.
Pyonephrosis can occur in any age group because any patient with urinary obstruction may develop a superimposed infection. In children, urinary obstruction is commonly due to a ureteropelvic junction obstruction. In adults, urinary tract stones is the cause in >50% of cases of urinary obstruction.4 An intraluminal or extraluminal tumor is another important cause of urinary obstruction with an associated high morbidity. When pyonephrosis is present, antibiotics with gram-negative coverage are usually the first line of treatment because E. coli is the most common pathogen that is isolated. Other infectious agents including fungus and tuberculosis can also be present. The general rule is that prompt drainage is needed to prevent the risk of sepsis when “pus is under pressure.”3
Both PCN catheters and retrograde ureteral internal stents have been shown to be equally effective in relieving an obstructed renal collecting system, with similar complication rates.5 The desired method for drainage is often based on the experience of the urologist and/or interventional radiologist. Many arguments can be made to support the use of PCN catheters, including those listed here. First, a wide variety of catheter sizes can be placed (8F to 12F) depending on the characteristics of the fluid being drained. Second, the catheters can be easily irrigated depending on drainage characteristics to prevent malfunction. Third, the urine output of the kidney can be measured. Excessive ureteral manipulation can be avoided, decreasing the risk for sepsis or rupture.5 Fourth, the procedure can also be done with local anesthesia and moderate sedation, which eliminates the need for an anesthesiologist and risks associated with general anesthesia.3 Fifth, PCN catheter placement is often easier to schedule in the interventional radiology department, which can have flexibility in the daily schedule when compared with difficulties that arise with scheduling cases in busy operating rooms. For these reasons, in our hospitals, PCN catheter placement is the initial invasive treatment of choice for patients with suspected pyonephrosis.
Operators at some institutions attempt to perform retrograde ureteral stenting via cystoscopy as the primary invasive therapeutic procedure. Proponents of the placement of internal double-J ureteral stents via cystoscopy state that the patients are more comfortable without the burden of a catheter exiting the flank. There is also a lower potential for bleeding complications because the vascular kidney capsule is not punctured.5 Even though retrograde ureteral stenting by cystoscopy is attempted initially at such facilities, PCN catheter placement is typically pursued if ureteral stenting fails to place the stent or, if once a stent is placed, it fails to alleviate the symptoms.
Indications
There are several indications for PCN placement (emergent or routine), and the indications are broadly categorized into obstruction with infection, obstruction without infection, urinary diversion for leaks/hemorrhagic cystitis, and accessing the collecting system for diagnostic (e.g., Whitaker test) and therapeutic procedures (e.g., percutaneous nephrolithotomy). These indications encompass >95% of the PCN placements that are performed.4 Within these broad categories of indications, emergent PCN catheters are usually placed in a subset of these patients. As noted in the introduction, the main indication for emergent PCN placement is to relieve an obstructed and infected collecting system (i.e., pyonephrosis) due to the risk of rapidly developing sepsis. In our hospitals, pyonephrosis is treated emergently regardless of the time of day or night. In general, we insert PCN catheters in patients with urinary obstruction and without infection during the daytime hours and on weekends, but rarely at night. In general, if either of these two emergent clinical situations is not present, a PCN is performed electively. In our hospitals, certain exceptions are considered, such as if the patient has a renal transplant or a solitary kidney, in which case more urgent drainage may be indicated.
Patient Preparation
Several laboratory values including platelet count and an INR should be obtained prior to the procedure. Our departments follow the consensus guidelines presented by the Society of Interventional Radiology (SIR) Standards of Practice Committee, which consider an acceptable INR to be ≤1.5, activated partial thromboplastin time ≤1.5 times control, and an adequate platelet count to be ≥50,000/mm3.6 Funaki and Vatakencherry reported a series of 140 PCN placements and found the risk of bleeding to be between 3.7% and 4.7%, which is near the threshold limits described by the SIR.4,7 If needed, the patient can be transfused with fresh-frozen plasma or with platelets before, during, or after the procedure to decrease bleeding risk. Blood cultures, complete blood count, blood chemistry, urinalysis, and urine cultures are usually already obtained by the referring clinical service. If present, hyperkalemia can be addressed medically or via dialysis as the clinical situation of the patient dictates.
The choice of preprocedural antibiotic is determined on whether or not the patient is septic. Because most patients who are referred to interventional radiology for emergent PCN catheter placement are presenting with urosepsis, antibiotic therapy likely has already been initiated by the referring service. The coverage typically includes broad-spectrum coverage for both gram-negative and gram-positive organisms. If antibiotics have not yet been administered, a broad-spectrum antibiotic such as ampicillin/sulbactam 1.5 to 3 g IV) may be given.8 If the patient is allergic to penicillin, a fluoroquinolone, such as levofloxacin (500 mg IV), can be administered. Other considerations for penicillin allergic patients include vancomycin or clindamycin with the addition of gentamycin. If a PCN catheter is placed in a patient who is not septic, our departmental policy is to administer a first-generation cephalosporin (e.g., cefazolin 1 g IV) or a fluoroquinolone (e.g., levofloxacin 500 mg IV) for the penicillin-allergic patient. It is important to remember that if a fungal infection is suspected, an antifungal medication should also be given as prophylaxis.9 For the uroseptic patient, antibiotics are continued for many days or weeks after PCN placement; the treatment regimen should be tailored to the culture and sensitivities of the urine specimen obtained during the procedure.
PCN catheters are placed under moderate sedation and local anesthesia with 1% lidocaine. Moderate sedation is achieved using a standard protocol of 25 to 50 mcg aliquots of fentanyl and 0.5 to 1 mg aliquots of midazolam. If the patient is allergic to contrast, a premedication regimen of 32 mg of methylprednisolone orally 12 hours and 2 hours before the procedure and 50 mg of diphenylhydramine orally or IV 1 hour before the procedure is administered.10 Although the goal of the procedure is to inject the contrast only into the collecting system, intra-arterial and IV contamination occurs with high frequency, which necessitates the need for premedication in the presence of a severe contrast allergy. If the patient's medical condition requires treatment that cannot be delayed by the premedication regimen, the procedure can be performed without contrast or with alternative contrast agents such as gadolinium or carbon dioxide.
Access/Technique
Relevant Anatomy
The ideal puncture site into the kidney is via a posterior calyx using a subcostal approach. The posterior calyx is desirable because entry may traverse the Brodel avascular plane, which should help minimize the risk of bleeding complications. A posterior calyx entry also helps for subsequent dilations and catheter placement because these can be performed in a straight line. If an anterior calyx is entered, an acute angle will be encountered to reach the renal pelvis, which can make catheter placement more difficult. A subcostal entry is desirable to minimize the risk of pleural complications, which can be encountered with supracostal access, particularly if entering above the 11th rib. A supracostal approach has been described as having a significantly higher intrathoracic complication rate, including pneumothorax and pleural effusion, and higher overall complication rate when compared with subcostal access in patients undergoing percutaneous renal surgery.11
Existing imaging, such as CT or ultrasound, are reviewed preoperatively to avoid potential complications from inadvertent organ puncture.12 Injury to adjacent viscera near the kidneys has been reported rarely, with rates between 0% and 3%.13 The colon has been described as the organ most likely to be injured. Inadvertent puncture typically occurs due to a very lateral puncture when the colon is in its normal location or due to a puncture using standard anatomical landmarks when the colon is in an aberrant position such as in a retrorenal location.13,14
Standard Procedure for Initial Pelvocalyceal Access
The patient is placed prone on a fluoroscopy table. If performing a unilateral procedure, a pillow can be placed underneath the abdomen to elevate the ipsilateral side by 20 to 30 degrees. After prepping and draping in the usual sterile fashion, fluoroscopy is used to localize an appropriate subcostal puncture site. Ultrasound is used to localize the kidney, to identify the degree of hydronephrosis, and to localize a posterior calyx. The skin, the soft tissues in the expected trajectory, and very importantly the renal capsule in the expected region of puncture are anesthetized with 1% lidocaine. A small skin incision is made. The technique of entering the collecting system is again based on physician preference. Many techniques have been described including a direct trocar access, the Seldinger technique, and techniques using one needle (“single-stick”') or two needles (“double-stick”).7,15,16 In cases involving pyonephrosis, we use a single-stick technique. In most routine cases, we prefer a hybrid technique described here.
Under ultrasound guidance, a 21-gauge needle (AccuStick II Introducer System; Boston Scientific, Natick, MA) is advanced into a posterior calyx. Urine is aspirated through the small gauge needle. The volume of urine aspirated is ideally more than the volume of dilute contrast that is injected to opacify the collecting system to verify needle position (Fig. 2B). A small amount of air can be injected because air will rise to fill a posterior calyx. If the puncture site is deemed inappropriate, a second small gauge needle can be advanced toward an air-filled posterior calyx using fluoroscopic guidance.
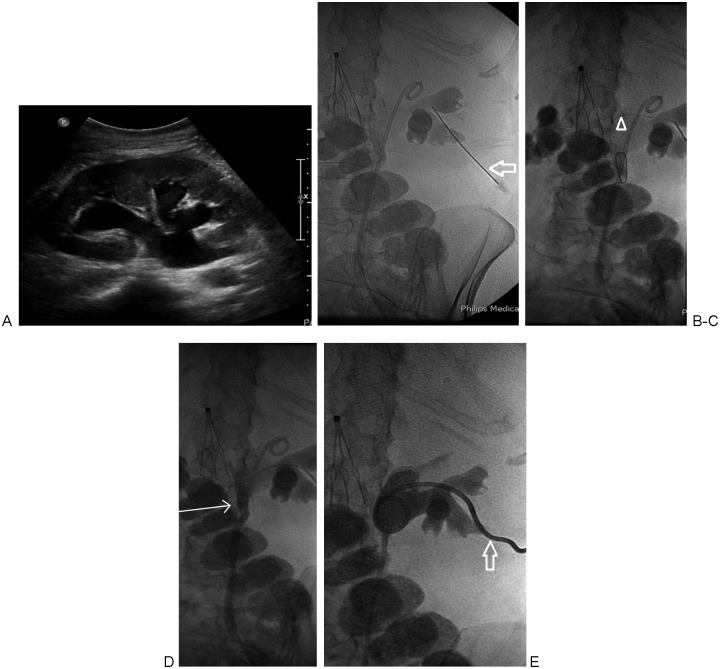
Figure 2.
(A) Ultrasound image demonstrating moderate pelvocaliectasis of the right kidney. (B) A 21-gauge needle access into posterior mid-pole calyx via a subcostal approach (arrow). Indwelling nonfunctioning ureteral stent is also noted. (C) An 0.018-inch guidewire has been advanced distally in the ureter (arrowhead). (D) Antegrade pyelogram via 6F dilator/sheath with radio-opaque tip (arrow) advanced into the proximal ureter. (E) Final image obtained following 8F percutaneous nephrostomy catheter placement (arrow).
If the patient has a fluoroscopically visualized target, such as a radiopaque kidney stone or an internal ureteral stent, the small gauge needle can be advanced under fluoroscopic guidance using a “down the barrel” technique by using 0 to 30 degrees of fluoroscopic angulation relative to the back. Once access into the collecting system is gained, air and contrast is injected in preparation for the second puncture.
A single-stick method has been described to utilize a single puncture into the posterior calyx under ultrasound guidance. After access into the collecting system is confirmed by the aspiration of urine, the tract is dilated over a wire and the nephrostomy catheter inserted without the use of contrast to opacify the collecting system. The single-stick method is quicker, does not have the risks associated with injection of contrast, and does not have significant difference in complication rates. Decreasing the risks associated with contrast injection, including worsening sepsis presumably caused by forcing bacteria from the collecting system into the bloodstream, is desirable in patients with pyonephrosis.7,15
The direct trocar technique involves obtaining access to the collecting system with a small gauge needle under ultrasound guidance. A pyelogram is performed after aspiration of a sufficient amount of urine. Following a similar trajectory, a drainage catheter placed on a metal stiffener with an associated trocar needle is advanced into the collecting system under ultrasound and/or fluoroscopic guidance. The tip of the trocar needle may not be particularly echogenic, making visualization under ultrasound more difficult. Once the needle is in the collecting system, the needle of the trocar system can be removed to aspirate urine. Over the metal stiffener, the catheter is advanced into the collecting system. This can be a very fast and effective method of PCN catheter placement, particularly in very dilated collecting systems.
If access is to be obtained into a nondilated collecting system (e.g., urine leak from ureteroenteric anastomosis or preoperative access for stone therapy), percutaneous puncture with ultrasound guidance is technically difficult.4,17 IV administration of 50 to 100 mL of nonionic IV contrast will opacify the collecting system facilitating fluoroscopic puncture. Carbon dioxide or a small amount of air has been described as an alternative contrast agent in patients with allergies to iodinated contrast.17
Antegrade Pyelography
During emergent PCN catheter placement, the objective is to inject as little contrast as possible to safely place the PCN catheter. Catheter placement is the primary objective, and this is performed at the expense of interrogating the entire collecting system for underlying pathology or physiological information. Just enough of the collecting system is opacified to determine the appropriateness of the puncture site and to safely deploy the catheter in the desired location. In the setting of pyonephrosis, the collecting system is usually already under pressure; if contrast is injected, the collecting system may become overdistended, which increases the risk of bacteremia and urosepsis. If urosepsis does occur, patients can become clinically unstable very quickly. Contrast is injected only after an equal or larger volume of urine is aspirated to minimize the risk of worsening bacteremia and urosepsis.
However during routine PCN catheter placement, antegrade pyelography can be performed once pelvocalyceal access is achieved (Fig. 2B). Proper PCN catheter deployment is facilitated when the collecting system can be clearly visualized. Pyelography is helpful to evaluate both the anatomical and functional characteristics of the collecting system. Underlying pathology, such as an obstructive calculus or tumor, can be diagnosed during pyelography. Also, urodynamics of the collecting system can potentially be evaluated.
Catheter Placement
Following optimal pelvocalyceal access, through the 21-g needle, a 0.018-inch guidewire (V18 control wire; Boston Scientific, Watertown, MA) is advanced into the ureter or coiled in the renal pelvis under fluoroscopic guidance (Fig. 2C). The needle is removed and the coaxial 4F and 6F dilator/sheath assembly (AccuStick II Introducer System; Boston Scientific) is advanced over the 0.018-inch guidewire. Once the 6F dilator/sheath is in the collecting system (Fig. 2D), a stiff 0.035-inch wire (Amplatz Superstiff guidewire; Boston Scientific) is again advanced into the ureter or coiled in the renal pelvis. The tract is dilated with 9F, 11F, and 13F dilators, and a 12F drainage pigtail catheter (Flexima ADPL, Boston Scientific) is coiled in the renal pelvis.7,16 Only a tiny amount of contrast is injected to confirm the catheter tip position (Fig. 1B). The catheter size depends on the clinical scenario; we tend to place larger catheters (e.g., 12F) in the setting of pyonephrosis, and smaller catheters (i.e., 8F to 10F) for all other clinical scenarios (i.e., obstruction without infection, diversions, etc.) (Fig. 2E). The catheter is either sutured to the skin or secured with an adhesive disc.
In the nonemergent setting, we recommend that the 0.018-inch wire be advanced distally into the ureter whenever possible. This allows for additional confirmation that the wire is actually in the collecting system instead of in the perirenal fat. When the wire is coiled in the perirenal fat, fluoroscopic images in certain projections may be deceiving, showing the wire superimposed on the renal pelvis. Additionally, advancing the wire down the ureter will lessen the likelihood of lacerating the renal pelvis with overaggressive wire coiling, overaggressive tract dilation, or overaggressive PCN catheter advancement.
If further intervention is desired (e.g., nephroureterostomy, internal ureteral stent), the patient can return several days after decompression when stabilized (Fig. 1D, 1E). Excessive manipulation of an infected collecting system during the initial procedure can precipitate urosepsis.18
Postprocedural Care
Catheters are placed to a gravity drainage bag. Routine flushing of the catheter is only performed if output decreases or if output is very purulent or bloody, as such output may risk occluding the catheter. Urine has proteolytic enzymes that help break down blood clots, so obstruction due to hemorrhage is unusual.9 Patients who require emergent PCN catheter placement are typically very ill and will be admitted to the hospital or are already admitted. The patients are seen daily by the interventional radiology service. In addition to monitoring for defervescence and laboratory values (e.g., white blood cell count, potassium, serum creatinine), the amount and quality of urine output are noted. The skin at the puncture site is also evaluated.
We work closely with urologists to have an appropriate follow-up plan and typically bring the patients back in 1 to 2 weeks, after the patient stabilizes, to perform a diagnostic pyelogram. Depending on the clinical scenario, but particularly when the patient may need long-term stenting (e.g., malignant stricture, postoperative stricture in a nonsurgical candidate, fistula), we attempt to internalize the PCN or convert to a retrograde ureteral stent placed via the urostomy to allow for improved patient comfort. Patients who require long-term PCN catheters are scheduled to return every 6 to 12 weeks for routine catheter changes.
Results
When PCN catheters are placed emergently, initial technical success has been reported to be 98%.19 This is similar to the high technical success rate of 98 to 100% in a series reviewing both emergent and nonemergent PCN catheter placements.18 The risk of a technical failure for both emergent and routine procedures is higher in patients with nondilated collecting systems or in obese patients.18 However, PCN catheter placement rates in nondilated collecting systems can also be successful, with a success rate of 92 to 96%.9,17 The presence of pelvocaliectasis, which is common in most cases of emergent PCN catheter placement, contributes to the high technical success rate.
Complications
According to the SIR Standards of Practice Committee, an upper threshold of all complications during PCN placement should be set at 10%.4 Major complications include sepsis, hemorrhage, vascular injury, bowel transgression, and pleural complications,4 and they are categorized by outcome. These outcomes are for patients requiring therapy including minor hospitalization (<48 hours); patients requiring major therapy, including an unplanned increase in level of care and prolonged hospitalization (>48 hours); permanent adverse sequelae; and death. Minor complications require no therapy and are of no consequence, or they require nominal therapy of no long-term consequence (e.g., an overnight admission for observation only).4 When PCN catheters are placed emergently, Lee et al described a major complication rate of 6% and a minor complication rate of 28%.19 This major complication rate is within the expected range for the guidelines set forth by the Standards of Practice Committee by the SIR. As expected, more complications can occur when procedures are performed emergently afterhours. In the series by Lewis and Patel, a higher percentage of major complications during PCN placement occurred afterhours (5.7%) versus during workday hours (1.8%).20 The minor complication rate described by Lee et al of 28% is much higher than most series, which described minor complication rates to be lower, ranging from 15% to 25%.19 Finally, a very low mortality rate of 0.2% has been described with this procedure.9
The two most common and worrisome major complications include septic shock and hemorrhage, and the focus of the following discussion is on these two topics.
Septic Shock
One of the most feared complications of emergent PCN catheter placement is septic shock, which has a wide variety of presenting signs and symptoms. During emergent nephrostomy, many patients are already septic at the start of the procedure.18 Bacteria and endotoxins can be released from the urine and collecting system during the procedure with subsequent presenting symptoms of fever, chills, rigors, hypotension, and tachycardia.
The SIR Standards of Practice Committee describe the incidence of septic shock (defined by fever and chills with hypotension, requiring a major increase in level of care) at a reported rate of between 1% and 3%.4 The threshold guideline for this complication is <4%. PCN catheter drainage of pyonephrosis has been reported to have higher complication rates3; in this setting, septic shock is reported to occur in 7% to 9% of procedures. The threshold guideline for septic shock is also higher but remains <10%.4 In a series reported by Lee et al on emergent PCN placement, the incidence of sepsis was 3.6%.18,19 However, 100% of the patients in this report developed a transient increase in body temperature after the procedure.18,19 Higher rates of sepsis, from 4.5% up to 21%, have been reported; however, in many of these series, patients were already septic at the start of the procedure.18 As expected, in the emergency setting the risk of complications increases. In one series, this higher complication rate was attributed to a combination of the critical condition of the patients, less ancillary support, and/or the possibility of less experienced operators such as fellows or residents performing the procedure.
Bacteremia and septic shock can begin during or after the procedure. Careful monitoring is needed because patients can quickly become toxic and require emergent resuscitation and intubation. When this complication arises, IV fluids are aggressively administered. Rigors may be controlled with 25 to 50 mg meperidine, slowly pushed IV. An additional dose of broad-spectrum antibiotics, such as piperacillin/tazobactam or levofloxacin, may also be given immediately.
Hemorrhage
The rate of hemorrhage requiring transfusion following PCN placement is reported to be between 1% and 4%, with a desired threshold <4%.4 In a series of emergency PCN catheter placements, bleeding requiring transfusion occurred in 2.4%.18,19 Mild hematuria commonly occurs after PCN catheter placement and often resolves spontaneously after a few days. If bleeding is excessive, the catheter tip position is assessed to ensure the sideholes have not retracted into the parenchymal tract; in this setting, venous bleeding can be controlled by advancing the catheter more centrally. Exchange for a larger diameter catheter may tamponade a bleeding source in the parenchymal tract around the catheter. If the bleeding is heavy or pulsatile, an arterial injury should be considered, which may represent laceration of a renal artery branch, a bleeding pseudoaneurysm, or an arteriovenous fistula18 (Fig. 3A). Such arterial complications often require embolization using either coils, Gelfoam, or polyvinyl alcohol particles.18
Figure 3.
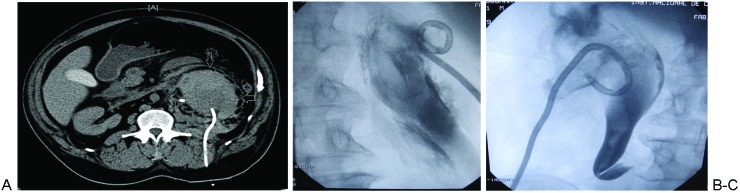
(A) Computed tomography obtained 12 hours after percutaneous nephrostomy catheter placement showing a subacute subcapsular renal hematoma (arrows).(B) Different patient than (A), demonstrating a nephrostomy catheter placed in Gerota's fascia utilizing direct trocar technique. The catheter lies outside of the renal collecting system. (C) Different patient than (A) and (B), with nephrostomy catheter placed in an extrarenal or intraparenchymal location utilizing direct trocar technique. Contrast still opacifies the collecting system, containing intraluminal clot, despite catheter position.
One hemorrhagic complication is placement of the catheter in the renal parenchyma or in Gerota's fascia (Fig. 3B and 3C). This tends to happen when the guidewire is not advanced distally enough in the ureter and the wire is coiled in what appears to be the dilated and distorted renal pelvis. Although a pyelogram may have been performed, a wire that is in coiling outside the kidney in Gerota's fascia may superimpose itself over the dilated renal pelvis and simulate appropriate intrapelvocalyceal placement. Although this can be diagnosed if the image intensifier or detector is rotated so the wire/catheter will project outside the collecting system, diagnosis may be not be so obvious if the nephrostogram is faint and the collecting system is severely dilated or distorted. For this reason, we recommend advancing wires distally into the ureter whenever possible.
Based on our anecdotal experience, extrarenal catheter placement is also associated with utilization of the direct trocar technique. Although urine may be aspirated once the trocar needle tip is in the collecting system, the full caliber of the catheter may not be fully in the collecting system. During advancement of the catheter over the metal stiffener, the catheter may buckle and form within the parenchyma or in Gerota's fascia. Additionally, movement of the kidney due to respirations may dislodge the tip of the catheter prior to advancement. After such intraparenchymal/extrarenal placement, a slight amount of urine may be still be aspirated and opacification of the collecting system upon contrast injection may still occur, due to the puncture already created in the collecting system (Fig. 3C).
Conclusion
The given case presentation of pyonephrosis is a very typical scenario when interventional radiologists are consulted for emergent PCN catheter placement. The case highlights that the goal of emergent PCN catheter placement is to decompress the collecting system rapidly with as little manipulation as necessary. It should be performed emergently when obstruction is present with a clinical suspicion of pyonephrosis. To minimize the risk of septic shock, diagnostic antegrade pyelography should not be performed at the time of initial catheter insertion in the emergent setting, and any diagnostic studies or additional ureteral manipulation should be deferred for several days to enable the urinary system to decompress. In general, a slightly higher rate of complications is encountered during emergent nephrostomy insertion, and it may be attributed to the underlying comorbidities often present in this patient population.
References
- 1.Goodwin W E, Casey W C, Woolf W. Percutaneous trocar (needle) nephrostomy in hydronephrosis. J Am Med Assoc. 1955;157(11):891–894. doi: 10.1001/jama.1955.02950280015005. [DOI] [PubMed] [Google Scholar]
- 2.Reznek R H, Talner L B. Percutaneous nephrostomy. Radiol Clin North Am. 1984;22(2):393–406. [PubMed] [Google Scholar]
- 3.Yoder I C, Lindfors K K, Pfister R C. Diagnosis and treatment of pyonephrosis. Radiol Clin North Am. 1984;22(2):407–414. [PubMed] [Google Scholar]
- 4.Ramchandani P, Cardella J F, Grassi C J. et al. Quality improvement guidelines for percutaneous nephrostomy. J Vasc Interv Radiol. 2003;14(9 Pt 2):S277–S281. [PubMed] [Google Scholar]
- 5.Pearle M S, Pierce H L, Miller G L. et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160(4):1260–1264. [PubMed] [Google Scholar]
- 6.Malloy P C, Grassi C J, Kundu S. et al. Standards of Practice Committee with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20(7, Suppl):S240–S249. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Funaki B, Vatakencherry G. Comparison of single-stick and double-stick techniques for percutaneous nephrostomy. Cardiovasc Intervent Radiol. 2004;27(1):35–37. doi: 10.1007/s00270-003-0088-8. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesan A M, Kundu S, Sacks D. et al. Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures. Written by the Standards of Practice Committee for the Society of Interventional Radiology and Endorsed by the Cardiovascular Interventional Radiological Society of Europe and Canadian Interventional Radiology Association. J Vasc Interv Radiol. 2010;21:1611–1630. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Maher M M, Fotheringham T, Lee M J. Percutaneous nephrostomy. Semin Intervent Radiol. 2000;17:329–339. [Google Scholar]
- 10.ACR Committee on Drugs and Contrast Media. American College of Radiology Manual on Contrast Media 7th ed. Reston, VA: American College of Radiology; 2010 [DOI] [PubMed] [Google Scholar]
- 11.Munver R, Delvecchio F C, Newman G E, Preminger G M. Critical analysis of supracostal access for percutaneous renal surgery. J Urol. 2001;166(4):1242–1246. [PubMed] [Google Scholar]
- 12.Dyer R B, Regan J D, Kavanagh P V, Khatod E G, Chen M Y, Zagoria R J. Percutaneous nephrostomy with extensions of the technique: step by step. Radiographics. 2002;22(3):503–525. doi: 10.1148/radiographics.22.3.g02ma19503. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle D N, Yeh B M, Meng M V, Breiman R S, Stoller M L, Coakley F V. Risk of injury to adjacent organs with lower-pole fluoroscopically guided percutaneous nephrostomy: evaluation with prone, supine, and multiplanar reformatted CT. J Vasc Interv Radiol. 2005;16(11):1489–1492. doi: 10.1097/01.RVI.0000175331.93499.44. [DOI] [PubMed] [Google Scholar]
- 14.Zagoria R J, Dyer R B. Do's and don't's of percutaneous nephrostomy. Acad Radiol. 1999;6(6):370–377. doi: 10.1016/s1076-6332(99)80233-5. [DOI] [PubMed] [Google Scholar]
- 15.Agostini S, Dedola G L, Gabbrielli S, Masi A. A new percutaneous nephrostomy technique in the treatment of obstructive uropathy. Radiol Med (Torino) 2003;105(5-6):454–461. [PubMed] [Google Scholar]
- 16.Funaki B, Tepper J A. Percutaneous nephrostomy. Semin Intervent Radiol. 2006;23(2):205–208. doi: 10.1055/s-2006-941451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel U, Hussain F F. Percutaneous nephrostomy of nondilated renal collecting systems with fluoroscopic guidance: technique and results. Radiology. 2004;233(1):226–233. doi: 10.1148/radiol.2331031342. [DOI] [PubMed] [Google Scholar]
- 18.Millward S F. Percutaneous nephrostomy: a practical approach. J Vasc Interv Radiol. 2000;11(8):955–964. doi: 10.1016/s1051-0443(07)61322-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee W J, Patel U, Patel S, Pillari G P. Emergency percutaneous nephrostomy: results and complications. J Vasc Interv Radiol. 1994;5(1):135–139. doi: 10.1016/s1051-0443(94)71470-6. [DOI] [PubMed] [Google Scholar]
- 20.Lewis S, Patel U. Major complications after percutaneous nephrostomy—lessons from a department audit. Clin Radiol. 2004;59(2):171–179. doi: 10.1016/s0009-9260(03)00336-2. [DOI] [PubMed] [Google Scholar]