Abstract
Obstetric hemorrhage from placenta accreta is associated with a high rate of maternal morbidity and mortality. Recently, balloon occlusion catheters have been used to control intraoperative bleeding during the surgical management of placenta accreta. In this article, we present a review of the literature reporting the use of balloon occlusion catheters in the management of placenta accrete, and a case presentation outlining the use of a Fogarty balloon occlusion catheter to achieve hemostasis in the preoperative management of placenta percreta.
Keywords: placenta accreta, pelvic hemorrhage, balloon occlusion, postpartum hemorrhage, interventional radiology
Objectives: Upon completion of this article, the reader will be able to explain the indications for embolization for placental abnormalities as well as describe the balloon occlusion technique useful for this condition.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Placenta accreta is an abnormality of placental implantation the carries a high rate of maternal morbidity and mortality secondary to obstetric hemorrhage. Placenta accreta is defined as abnormal adherence of the placenta to the uterine wall, whereby the chorionic villi directly attach to the myometrium due to the absence of the decidua basalis and the fibrinoid layer of Nitabuch.1 The abnormal placental implantation is further subdivided into three classifications according to the depth of penetration by the villi. In the most common form, placenta accreta, the placenta adheres to the myometrium without further invasion. When the placenta invades the myometrium, the process is referred to as placenta increta. Placenta percreta is the most advanced form, in which the placenta grows through the myometrium, encroaching on and sometimes penetrating the serosa. In placenta percreta, the placenta may adhere to structures adjacent to the uterus such as the bladder.1,2 Although placenta percreta accounts for <5% of all placenta accretas, it is the most serious form and carries the greatest risk of maternal death from hemorrhage.3
The reported incidence of placenta accreta ranges from 1 in 540 to 1 in 93,000 births, the incident varying with diagnostic method (clinical versus histopathological), time period reported, and population studied. There has been an almost 10-fold increase in the incidence of accreta reported recently compared with that reported in the 1950s, which is believed to be due to the increasing rate of cesarean deliveries, a known significant risk factor.3
Intraoperative ligation of the hypogastric or uterine arteries or preoperative catheterization with intraoperative balloon-occlusion of the hypogastric arteries are current methods employed to control intraoperative bleeding.2,3,4 Multiple case reports in the literature have documented the successful management of placenta accreta with preoperative placement of balloon occlusion catheters in the hypogastric arteries or other arteries supplying the uterus.3,4 Differences in the reported technique and outcomes merit discussion. In this article, we discuss 38 patients from the reported literature with placenta accreta, increta, and percreta managed with balloon occlusion catheters. First we present a case of a multiparous woman with placenta percreta who was managed with preoperative placement of Fogarty balloon occlusion catheters in the hypogastric arteries.
Case Report
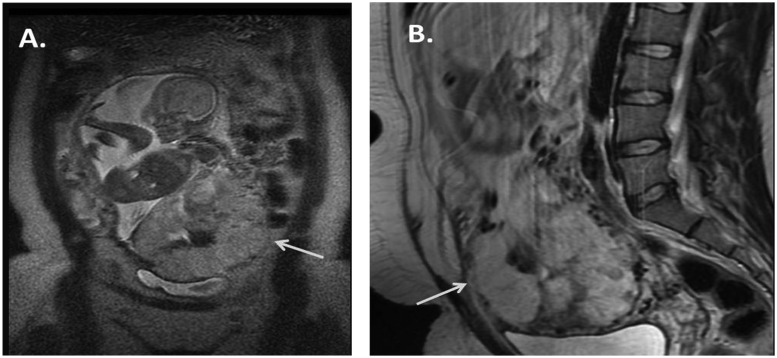
A 23-year-old G4P2102 woman with hypertension and sickle cell disease was admitted at 34 4/7 weeks of gestation with a 4-day history of spotty vaginal bleeding. The patient had a history of three prior cesarean deliveries, with the third pregnancy ending in uterine rupture and fetal demise. Ultrasound from an outside institution performed at 23 weeks demonstrated full placenta previa and suspected placenta accreta. Magnetic resonance imaging revealed complete placenta previa with the placenta severely thinning the myometrium, extending both anteriorly and inferiorly in the region of the bladder. Large vessels were also seen supplying the placenta in the region of the cervical os (Fig. 1). The patient was scheduled for cesarean delivery with likely hysterectomy.
Figure 1.
(A) Coronal magnetic resonance imaging (MRI) demonstrates segment of placenta extending outside of the visible myometrium in the region of the anterior inferior abdominal wall (arrow). (B) Sagittal MRI demonstrates the placenta severely thinning the myometrium with possible extension outside of it inferiorly in the region of the bladder (arrow).
On admission, consults were made to urology and interventional radiology for surgical planning. Specifically, the obstetrician requested preoperative occlusion balloon catheterization of the hypogastric arteries due to the high risk of bleeding. Of note, the obstetric department had performed a cesarean delivery on a woman with placenta percreta 1 month prior, using surgical ligation of the hypogastric arteries, which had resulted in significant blood loss requiring 17 units of packed red blood cells.
On the day after admission, the patient presented to the angiography suite for placement of occlusive balloon catheters to be used during the hysterectomy. Using a right femoral artery approach, a 4F over-the-wire Fogarty balloon catheter was positioned in the contralateral left internal iliac artery and inflated with 0.3 cc of iodinated contrast material to obtain occlusion of the artery (Figs. 2 and 3).
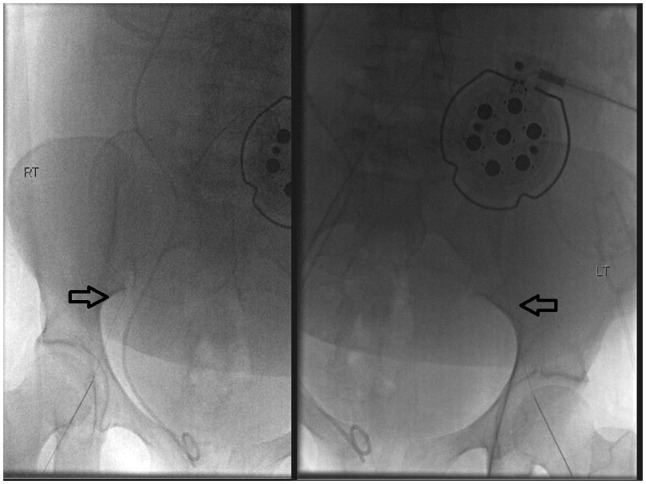
Figure 2.
Spot fluoroscopic images demonstrating bilateral common femoral artery access obtained using micropuncture 0.018 guidewire access (arrows). Note the fetal monitoring device overlying the pelvis; fetal monitoring was performed by the obstetrician present during the procedure.
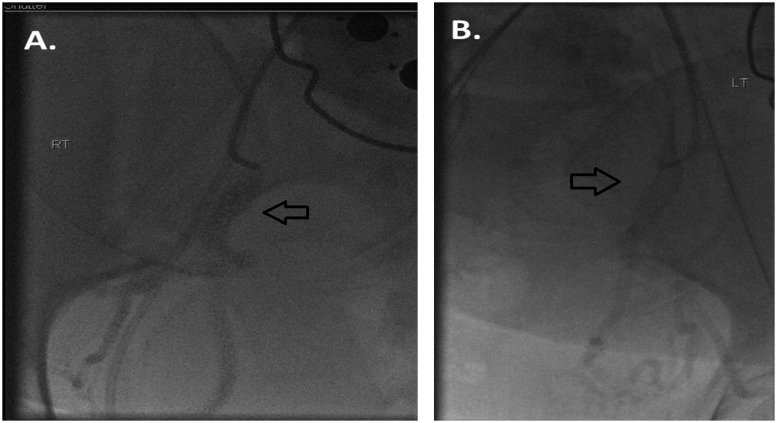
Figure 3.
(A) Following crossover access, a small test puff injection was given to document position within the right internal iliac artery. Contrast is seen within the anterior division of the internal iliac artery (arrow). (B) Following crossover access, a small test puff of contrast confirms positioning within the anterior division of the left internal iliac artery (arrow).
The balloon was then deflated and continuous infusion was given through the catheter. The left femoral artery was accessed and a 4F over-the-wire Fogarty balloon catheter was positioned in the contralateral right internal iliac artery. The balloon was test-inflated with 0.3 cc of dilute iodinated contrast to achieve hemostasis (Fig. 4). The balloon was deflated and the catheter was placed on continuous infusion (Fig. 5). Both of the catheters were securely fastened in position using a bio-occlusive dressing.
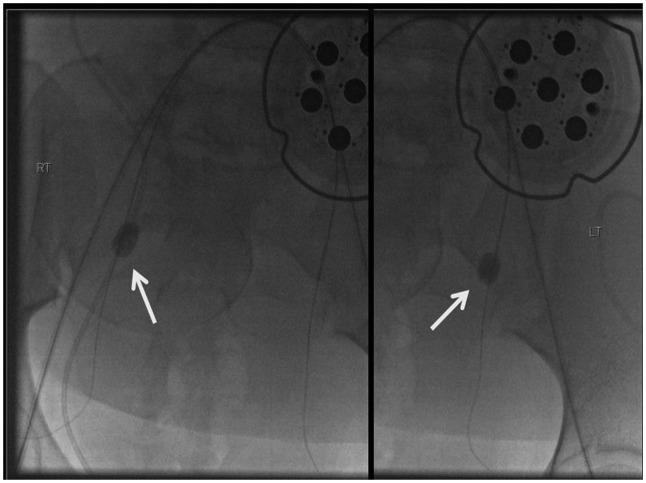
Figure 4.
French Fogarty balloons (arrows) are placed into position within the internal iliac arteries and inflated to confirm and document appropriate positioning.
Figure 5.
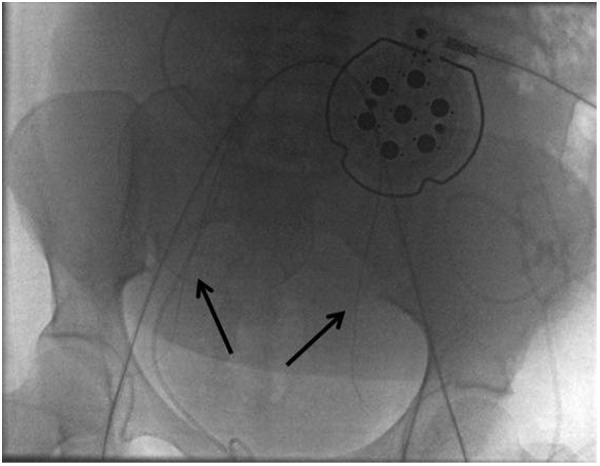
Final fluoroscopic image demonstrates the deflated over-the-wire balloons in position (arrows). Balloons were then secured to the patient using bio-occlusive dressing. The patient was taken to the operating room where the balloons were subsequently inflated immediately prior to delivery.
The patient was transferred to the operating room for cesarean delivery. On opening the abdomen, a protruding shiny surface placenta was visualized in the lower uterine segment, cranial to the bladder. Approximately 20 minutes after the incision was made, the infant was delivered by cesarean section and the cord was clamped. Both occlusion balloon catheters were inflated to their previously measured amounts. Hysterectomy was performed with careful dissection of the planes to preserve the bladder without disrupting the placenta. An intraoperative urology consult was obtained for surgical repair of an inadvertent cystotomy that occurred during the hysterectomy. Before the urology service initiated repair of the bladder, the balloons were deflated. Prior to deflating the balloons, the estimated blood loss was 3000 cc. After balloon deflation and during the bladder repair, there was an additional 1500 cc of estimated blood loss. The patient received a total of 4 units of packed red blood cells, 5 L of crystalloid, and 1250 cc of 5% albumin during surgery.
After closing the anterior abdomen, both balloon catheters were removed. Both sheaths were removed, and hemostasis was achieved by manual compression of both groins.
Treatment Options
Multiple current therapeutic options exist for the management of placental abnormalities. The least invasive therapy is to leave the placenta in situ postdelivery and allow for spontaneous regression. Alternatively, methotrexate can be administered to assist in regression.1,5 Surgical removal of the placenta is often undertaken with subsequent hysterectomy, but surgical management carries a high risk of bleeding.
The surgical approach to managing obstetric hemorrhage is intraoperative ligation of the uterine or internal iliac arteries. Surgical ligation is not usually initiated until significant intraoperative bleeding has occurred. At the point of ligation, visualization of the vessels can be difficult due to the hemorrhage, and the ligation can also be technically difficult due to distorted anatomy.1
Preoperative placement of internal iliac artery occlusion catheters with intraoperative arterial occlusion is a more recent method employed to control obstetric hemorrhage. With balloon catheter occlusion, the balloons are in place and need only to be inflated, utilizing less than a minute's time; this can be crucial in a moment of massive hemorrhage. Because delay is eliminated, it is presumed that the blood loss will also be limited. Preoperative placement of balloon occlusion catheters also allows for intraoperative Gelfoam (Pfizer, New York, NY) embolization if necessary.
Balloon Occlusion Techniques
Intraoperative balloon occlusion of the aorta, common iliac, hypogastric, and uterine arteries has been described in the literature. Due to the extensive collateral blood supply of the uterus, arising from branches such as the obturator, lumbar, sacral, rectal, and femoral arteries, occlusion proximal to the uterine arteries is desirable. However, occlusion of larger vessels such as the aorta and common iliac arteries runs the risk of ischemia to the extremities. In the reported cases of occlusion of the aorta and common iliac arteries, pressures were monitored in the lower extremities, as were serial lower extremity blood gases. In the reported case of aortic occlusion, occasional deflation of the balloon prevented distal extremity ischemia.
Balloon occlusion of the hypogastric (internal iliac) arteries is most commonly reported.4 Given the extensive collateralization in the pelvis, it is expected that some blood loss will occur despite the vessel occlusion.
Although both the axillary artery and femoral arteries have been used for preoperative arterial access, if the catheterization occurs prior to the cesarean delivery, then the femoral artery approach is preferable given the proximity and decreased risk of complications. However, if the cesarean delivery is in progress and access to the groins is limited, then an axillary approach is reasonable.6,7
The size of the balloon should be optimally tailored to the vessel being occluded because a poorly occluded vessel will yield incomplete hemostasis and increased hemorrhage. In our case, a Fogarty balloon catheter was used to achieve maximal wall tension while minimizing risk of rupture. During preoperative placement of the catheter, a test injection can be performed during balloon inflation to evaluate for the degree of hemostasis.
Indications, Contraindications, and Risk Factors
The main indication for preoperative temporary balloon catheter occlusion of the pelvic arteries prior to cesarean delivery is an increased risk of obstetric hemorrhage, such as in cases of placentas previa, accreta, increta, and percreta. In the cases reviewed, balloon occlusion was also performed in a case of uterine arteriovenous malformation (AVM).8 Few contraindications have been described in the use of balloon catheters. These include the inability preoperatively to select the optimal placement site of the occlusion balloon and the risk of continued bleeding secondary to extensive collateral circulation within the pelvis.4,9
In cases with subsequent embolization of the uterine arteries, complications reported include bladder ischemia, sepsis, and pelvic abscess.3 The cases managed with combined embolization and temporary balloon catheter occlusion had a greater reported complication rate than those treated with temporary balloon catheter occlusion alone.10
Outcomes
Review of the literature yields 38 reported patients treated with balloon catheter occlusion in the management of placenta accreta, increta, and percreta. Dubois et al first described the preoperative placement of internal iliac artery occlusion balloons in two patients with placenta.7 Since this publication in 1997, nine additional reports have been published describing a similar technique. In all the reported cases in the literature, no mortality was recorded. Estimated blood loss during delivery ranged from 600 to 10,000 cc, with patients requiring anywhere from 0 to 42 units packed red blood cell transfusion.
Of the 11 articles reviewed, 5 were case reports, 3 were case series, and 3 were case comparisons, comparing patients treated with balloon occlusion of the pelvic arteries with patients treated with surgical ligation or other management.
Vessels occluded with balloon catheters included the aorta, common iliac, internal iliac, and uterine arteries. Patients were either catheterized preoperatively with intraoperative inflation of the balloons to achieve vessel occlusion or the balloon catheters were placed intraoperatively under emergent conditions. Some patients were managed with embolization of the uterine arteries after balloon occlusion via Gelfoam injection through the balloon catheter.7
Balloon diameters ranged from 8.5 mm to 25 mm, increasing in size with the vessel occluded. Some cases reported occlusion of the internal iliac artery with 8.5-mm balloons; others reported needing 20-mm-diameter balloons to occlude the internal iliacs (Table 1).
Table 1. Balloon Characteristics Reported in the Treatment of Obstetric Hemorrhage.
| Size | Artery Occluded | Brand | No. of Patients Treated | Reported Success |
|---|---|---|---|---|
| 8.5 mm, 5F | Anterior division IIA | Meditech | 2 | Yes |
| 8.5 mm, 5F | IIA | Meditech | 1 | No |
| 8.5 mm, 5F | IIA/Anterior division IIA/Uterine | Meditech | 7/2/1 | No difference |
| 8.5 mm, 6F | Anterior division IIA | Boston Scientific | 1 | Yes |
| 11.5 mm, 7F | Anterior division IIA (hypogastric) | Boston Scientific | 5 | Yes/relatively good |
| 11.5 mm, 7F | Anterior division IIA | Boston Scientific | 6 | No difference |
| 20 mm, 8F | IIA | Meditech | 1 | Yes |
| 20 mm (4 × 20) | R anterior division IIA/L uterine | Na | 1 | Yes |
| 25 mm, 5F | CIA | Goodtec | 1 | Yes |
| Aortic occlusion | Abdominal aorta | BVM Medical | 1 | Yes |
| 4F Fogarty | Hypogastric | 1 | Yes |
CIA, common iliac artery.
Estimated blood loss was ≤2000 mL in 15 cases, >2000 mL in 16 cases, >5000 mL in 3 cases, and equaled 10,000 in 1 case. Although massive transfusions were rare, one patient required 42 units of blood, one patient 15 units of blood transfusion, and another required 17 units.
Discussion
Opinions on the use of arterial balloon occlusion in the management of placental disorders are varied. Of the articles reviewed, seven of the articles, including case reports and case series, conclude that balloon catheter occlusion is an effective method to reduce obstetric hemorrhage in cases of placental abnormality. However, two studies comparing cases managed with and without pelvic artery balloon catheters claimed that there is no difference in blood loss.6,9 Also, a report of a patient with placenta percreta managed initially with methotrexate, and later requiring hysterectomy, reported serious morbidity despite balloon catheter occlusion of the internal iliac arteries.11
One case in which an 8.5-mm balloon was used to occlude the internal iliac artery reported that no change in blood flow was noted after inflation of the balloon.12 Given the fact that the internal iliac arteries are expected to be larger in caliber than the anterior division of the internal iliacs, which require up to 11.5 mm diameter for occlusion, it may be that the cases of unsatisfactory hemostasis are due to inadequate occlusion of the vessel.
The reported estimated blood loss in simple cesarean delivery without placental abnormality is 1000 mL. In a report of 56 cases of placenta accreta managed with surgical ligation, estimated blood loss was >2000 mL in 41 cases, 5000 mL in 9 cases, 10,000 mL in 4 cases, and 20,000 mL in 2 cases. Three patients required 70 units of blood.13
In this review, estimated blood loss was ≤2,000 mL in 15 cases, >2000 mL in 16 cases, >5000 in 3 cases, and equaled 10,000 in 1 case. One patient required 42 units of blood transfusion, one patient required 17 units of blood, and another required 15 units.
In the reported case requiring 42 units of blood transfusion, surgical maneuvers including aortic compression were initially attempted to obtain hemostasis, but ultimately an aortic balloon occlusion catheter was placed just below the renal arteries to maintain hemostasis.12 In the case demanding 15 units transfusion, the placenta was initially left in utero and methotrexate was administered postpartum to aid in spontaneous placental regression. Postpartum day 7, the patient began to have vaginal bleeding, and manual removal of the placenta was attempted after placement of bilateral internal iliac artery occlusive balloon catheters. A 3-cm perforation of the lower uterine segment resulted in increased hemorrhage, and ultimately, a hysterectomy was performed.14
It is important to consider that estimated blood loss is a subjective measurement, although being treated as an objective measurement for the purposes of this article. Also, blood transfusion, although a definite objective measurement, does not account for the amount of crystalloid fresh-frozen plasma, and other resuscitative measures taken. The single reported case of balloon occlusion used during an operation for a uterine AVM reported complete termination of blood flow after balloon catheter inflation.8 The remainder of the cases reported either a decrease in blood flow or no perceived change in blood flow. It is difficult to ascertain on a case-by-case basis the benefits of balloon catheter occlusion because the actual hemorrhage that would have occurred had balloon occlusion not been performed is unknown. Also, comparison against other cases of placental abnormality, although stated, are limited because the degree of placental invasion largely affects blood loss, as does the technique of the surgeon and the associated operative complications such as tear of the myometrium during the procedure.
If the defined goal is reduction in hemorrhage versus prevention of hemorrhage, occlusion of the hypogastric arteries seems reasonable in controlling for the internal iliac collaterals without compromising blood flow to the extremities. Anecdotally, in the case of balloon catheter occlusion presented here, the obstetrician specifically requested placement of the balloon catheter due to severe morbidity and excessive blood loss in a case of placenta percreta managed with surgical ligation just months prior. The obstetrician was very satisfied with the results, given the 3000 cc estimated blood loss during the period of balloon catheter occlusion, in comparison with the life-threatening blood loss experienced by his prior patient.
Conclusions
Placenta accreta is an extremely serious condition that carries a high rate of maternal morbidity and mortality from obstetric hemorrhage. Intraoperative ligation of the hypogastric or uterine arteries or preoperative catheterization with intraoperative balloon-occlusion of the hypogastric arteries are current methods employed to control the intraoperative bleeding.2,3,4
Multiple case reports in the literature document the successful management of placenta accreta with preoperative placement of balloon occlusion catheters in the hypogastric arteries or other arteries supplying the uterus (Table 2).
Table 2. Patient Characteristics and Reported Outcomes for Balloon Occlusion Procedures Performed for Obstetric Hemorrhage.
| Patient No. | Diagnosis | Gestational Age | Artery Occluded | Surgery | EBL | Units Transfused | Complications |
|---|---|---|---|---|---|---|---|
| 1 | Previa/Percreta | 37 weeks | IIA | CD/TAH | 1600 mL | 0 | – |
| 2 | Accreta | 36 weeks | IIA | CD/TAH | 1100 mL | 0 | – |
| 3 | Percreta (?) | 30 weeks | IIA | CD/TAH | 2000 mL | 2 U | – |
| 4 | Previa/Possible accreta | 36 weeks | IIA | CD/TAH | 2500 mL | 2 U | – |
| 5 | Complete accreta, suspected percreta | 35 weeks, 4 days | IIA | CD/TAH | 4000 mL | 7 U | – |
| 6 | Complete previa, probable percreta | 34 weeks | Anterior division IIA | CD/TAH | 1500 mL | 3 U | Slow return of bowel motility, mild fever |
| 7 | Accreta | NA | Infrarenal abdominal aorta | CD/TAH | 2 U | – | |
| 8 | Previa/Percreta/Bladder invasion | 36 weeks | Anterior division IIA | CD/TAH | 1500 mL | 0 | – |
| 9 | Percreta | 30 weeks | Anterior division IIA | CD/TAH | 2000 mL | 0 | – |
| 10 | Percreta | 34 weeks | CIA | NA | 800 mL | 500 cc | – |
| 11 | Previa/Percreta | 34 weeks | IIA | CD/TAH | 3000 mL | 4 U | – |
| 12 | Previa | 31 weeks | L uterine artery, R anterior division IIA | CD/TAH | 600 mL | 0 U | Ileus |
| 13 | Previa/Percreta Delivered at 30 weeks, placenta delivered 7 days later |
30 weeks | IIA | CD/Manual transplacental removal | 1400 cc | 2 U/15 U | R Salpingo-oophorectomy; intentional anterior and unintentional posterior cystotomy; 3 cm uterine rupture |
| 14 | High-flow retroplacental AVM | NA | IIA | 2000 cc | 0 U | – | |
| 15 | Percreta adherent to bladder | NA | IIA | CD/TAH | 5000 cc | 10 U | – |
| 16 | Percreta | NA | IIA | CD/TAH | 4000 cc | 6 U | – |
| 17 | Percreta | NA | IIA | NA | 1000 cc | 0 U | – |
| 18 | Accreta | NA | IIA | CD/TAH | 5000 cc | 5 U | – |
| 19 | Accreta | NA | IIA | CD | 800 cc | 0 U | – |
| 20 | Accreta | NA | IIA | CD | 2600 cc | 2 U | – |
| 21 | Percreta and complete previa | 38 weeks | Infrarenal abdominal aorta | CD/TAH | 42 U | Vesicovaginal fistula | |
| 22 | Accreta | 36 weeks | IIA | CD/TAH | 5500 cc | 4 U | – |
| 23 | Accreta | 37 weeks | IIA | CD/TAH | 5200 cc | 6 U | – |
| 24 | Accreta | 36 weeks | Uterine artery | CD/TAH | 6700 cc | 5 U | – |
| 25 | Accreta | 38 weeks | IIA | CD | 1100 cc | None | – |
| 26 | Accreta | 36 weeks | IIA | CD/TAH | 2700 cc | 2 U | – |
| 27 | Previa/Focal accreta | 28 weeks | IIA | CD/TAH | 2200 cc | 17 U | – |
| 28 | Previa/Increta | 34 weeks | IIA | CD/TAH | 2500 cc | 6 U | – |
| 29 | Previa/Accreta | 36 weeks | IIA | CD/TAH | 7000 cc | 9 U | Cystostomy/bladder repair |
| 30 | Previa/Percreta | 30 weeks | IIA | CD/TAH | 2500 cc | 7 U | Cystostomy/bladder repair, pelvic hematoma |
| 31 | Previa/Accreta | 31 weeks | IIA | Curettage of lower uterine segment | 1000cc | 0 U | – |
| 32 | Previa/Percreta | 36 weeks | IIA | CD/TAH | 1500 cc | 0 U | – |
| 33 | Previa | 33 weeks | IIA | CD | 3950 cc | 5.4 U (mean) | – |
| 34 | Previa | 35 weeks | IIA | CD | 3100 cc | 5.4 U (mean) | – |
| 35 | Accreta | 35 weeks | IIA | CD/TAH | 4000 cc | 5.4 U (mean) | – |
| 36 | Previa | 32 weeks | IIA | CD/TAH | 3400 cc | 5.4 U (mean) | – |
| 37 | Previa | 35 weeks | IIA | CD/TAH | 4300 cc | 5.4 U (mean) | – |
| 38 | Percreta | 42 weeks | IIA | CD/TAH | 10,000 cc | 5.4 U (mean) | – |
| 39 | Accreta | 33 weeks | IIA | CD | 3200 cc | 5.4 U (mean) | – |
EBL, estimated blood loss; CD/TAH, cesarean delivery/total abdominal hysterectomy; NA, not applicable; CIA, common iliac artery; AVM, arteriovenous malformation.
The case presented here reviewed a specific situation in which a novel approach used Fogarty balloon catheters, which were placed into the bilateral internal iliac arteries preoperatively in a patient who underwent caesarean delivery and ultimately hysterectomy. No complications were encountered.
The use of balloon catheters in treating hemorrhage after delivery has been shown to be quite effective with few complications in various case reports to date, including the one presented here. It is important for practicing interventional radiologists to understand the indications and technical considerations in the use of balloon catheters in the treatment of postpartum hemorrhage secondary to placenta accreta.
References
- 1.Bodner L J, Nosher J L, Gribbin C, Siegel R L, Beale S, Scorza W. Balloon-assisted occlusion of the internal iliac arteries in patients with placenta accreta/percreta. Cardiovasc Intervent Radiol. 2006;29(3):354–361. doi: 10.1007/s00270-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 2.Sinha P, Oniya O, Bewley S. Coping with placenta praevia and accreta in a DGH setting and words of caution. J Obstet Gynaecol. 2005;25(4):334–338. doi: 10.1080/01443610500119739. [DOI] [PubMed] [Google Scholar]
- 3.Chou M M, Hwang J I, Tseng J J, Ho E S. Internal iliac artery embolization before hysterectomy for placenta accreta. J Vasc Interv Radiol. 2003;14(9 Pt 1):1195–1199. doi: 10.1097/01.rvi.0000086532.86489.97. [DOI] [PubMed] [Google Scholar]
- 4.Kidney D D, Nguyen A M, Ahdoot D, Bickmore D, Deutsch L S, Majors C. Prophylactic perioperative hypogastric artery balloon occlusion in abnormal placentation. AJR Am J Roentgenol. 2001;176(6):1521–1524. doi: 10.2214/ajr.176.6.1761521. [DOI] [PubMed] [Google Scholar]
- 5.Dildy G A III. Postpartum hemorrhage: new management options. Clin Obstet Gynecol. 2002;45(2):330–344. doi: 10.1097/00003081-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Shrivastava V Nageotte M Major C Haydon M Wing D Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta Am J Obstet Gynecol 20071974402, e1–e5 [DOI] [PubMed] [Google Scholar]
- 7.Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol. 1997;176(3):723–726. doi: 10.1016/s0002-9378(97)70582-9. [DOI] [PubMed] [Google Scholar]
- 8.Fuller A J, Carvalho B, Brummel C, Riley E T. Epidural anesthesia for elective cesarean delivery with intraoperative arterial occlusion balloon catheter placement. Anesth Analg. 2006;102(2):585–587. doi: 10.1213/01.ane.0000189551.61937.ea. [DOI] [PubMed] [Google Scholar]
- 9.Levine A B, Kuhlman K, Bonn J. Placenta accreta: comparison of cases managed with and without pelvic artery balloon catheters. J Matern Fetal Med. 1999;8(4):173–176. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<173::AID-MFM7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Ojala K MD, Perälä J, Kariniemi J, Ranta P, Raudaskoski T, Tekay A. Arterial embolization and prophylactic catheterization for the treatment for severe obstetric hemorrhage. Acta Obstet Gynecol Scand. 2005;84(11):1075–1080. doi: 10.1111/j.0001-6349.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 11.Butt K MD, Gagnon A, Delisle M F. Failure of methotrexate and internal iliac balloon catheterization to manage placenta percreta. Obstet Gynecol. 2002;99(6):981–982. doi: 10.1016/s0029-7844(02)02020-3. [DOI] [PubMed] [Google Scholar]
- 12.Bell-Thomas S M, Penketh R J, Lord R H, Davies N J, Collis R. Emergency use of a transfemoral aortic occlusion catheter to control massive haemorrhage at caesarean hysterectomy. BJOG. 2003;110(12):1120–1122. [PubMed] [Google Scholar]
- 13.Weeks S M, Stroud T H, Sandhu J, Mauro M A, Jaques P F. Temporary balloon occlusion of the internal iliac arteries for control of hemorrhage during cesarean hysterectomy in a patient with placenta previa and placenta increta. J Vasc Interv Radiol. 2000;11(5):622–624. doi: 10.1016/s1051-0443(07)61615-7. [DOI] [PubMed] [Google Scholar]
- 14.Paull J D, Smith J, Williams L, Davison G, Devine T, Holt M. Balloon occlusion of the abdominal aorta during caesarean hysterectomy for placenta percreta. Anaesth Intensive Care. 1995;23(6):731–734. doi: 10.1177/0310057X9502300616. [DOI] [PubMed] [Google Scholar]