An abscess is a localized collection of purulent fluid that can have a significant impact on the care and clinical outcome of a patient. It is at times a relatively benign event, potentially treatable with antibiotic medication alone. However, not uncommonly, abscess formation can be a life-altering event if it leads to sepsis, a spectrum of severe systemic illness resulting from hematogenous spread of infection and an important cause of morbidity and mortality.1 Sepsis is among the 10 leading causes of death in the United States2 and results in a rapid cascade of potential life-threatening events that can include bacteremia, cardiac decompensation, acute respiratory distress syndrome (ARDS), hemodynamic compromise, and organ failure. Sepsis typically results in prolonged hospitalization or even death. In addition, treatment of sepsis is a major component of health care expenditure. The total annual cost for the hospitalization of patients with severe sepsis in the United States has been estimated at $16.7 billion, and it is postulated that this figure has probably risen since these data were published in 2006.3
Historically, intra-abdominal abscesses were treated with operative drainage that was associated with significant morbidity and mortality.4 In the last 2 decades, advances in image-guided percutaneous drainage have provided a safe and effective alternative to operative debridement.5 Presently, most would consider image-guided percutaneous abscess drainage (IGPAD) as the treatment of choice because it offers a relatively simple, minimally invasive option with the goal of averting the development of sepsis, reducing length of hospital stay (LOS), and reducing the cost of treatment. Indeed in some cases, IGPAD can be performed successfully on an outpatient basis.6 Radiologists possess knowledge of anatomy, familiarity with most types of drainage procedures, and expertise in procedural tools and techniques, and therefore they are well suited to perform IGPAD with a high rate of technical and clinical success. For example, computed tomography (CT)-guided abscess drainage has been shown to provide definitive treatment for 70 to 90% of abdominal abscesses.6 IGPAD can be a relatively simple and short procedure; however, as with any seemingly simple procedure it can be made complicated by improper techniques. This article reviews basic techniques and aims to offer 11 helpful points to simplify the technique, minimize procedure time, and maximize technical and clinical success rates.
Preprocedural evaluation includes the attainment of informed consent from the patient or the designated health care proxy, and preprocedural planning includes a thorough review of appropriate imaging studies and appropriate laboratory parameters. Although IGPAD is considered to be associated with only a moderate risk of bleeding,7 serum coagulation parameters should be adequate to proceed with the procedure. In my practice, platelet count should be at least 50,000/µL and international normalized ratio (INR) should be <1.5. In addition, a serum hemoglobin level of at least 9.0 g/dL is optimal, particularly in high-risk cases. Aspirin need not be held but thienopyridines such as clopidogrel should ideally be withheld for 5 days prior to the procedure. Withholding of low molecular weight heparin may depend on the particular agent and its associated half-life. I recommend withholding these agents for two to four half-lives prior to the procedure, although exceptions may be made in particularly urgent cases. If time permits, coagulopathy and severe thrombocytopenia can be corrected with transfusions using fresh-frozen plasma and platelets, respectively, as well as other factors.
Ultrasound (US) and CT are the most commonly used imaging modalities to guide IGPAD, and fluoroscopy is often also used to guide serial dilatation and catheter placement following successful needle access.
The techniques of IGPAD are well known. Either the Seldinger or the trocar technique is used, depending on the size and location of the abnormality. With the trocar technique, the collection is initially accessed using a small gauge needle and contents are aspirated to verify needle placement. Then, parallel to this needle, a coaxial combination of a catheter, stiffening cannula, and sharp stylet is advanced directly into the collection. With the Seldinger technique, initial access to the cavity is gained using a small 21- or 22-gauge needle, followed by 0.018-in wire conversion to 0.035- or 0.038-in wire with the use of a Cope (Cook Medical, Bloomington, IN), Neff (Cook), or AccuStick (Boston Scientific, Natick, MA) coaxial catheter introduction system. Seldinger technique is often used for small deep, high-risk, and difficult-to-access collections, and the trocar technique is often used for large and superficial collections. Anecdotal evidence shows there is more patient pain associated with the trocar technique, and direct, nontarget catheter placement with a large-bore catheter using the trocar technique may result in greater morbidity than initial nontarget access with a skinny needle using the Seldinger technique. However, trocar technique offers the advantages of speed and avoidance of extra-cavitary leakage of abscess contents associated with serial access tract dilatation.
Rarely, the viscosity of the cavity content may prevent successful fluid aspiration. Aborting the placement of a drainage catheter may be premature in such cases, with the operator believing that the cavity does not contain any fluid but it is instead an “undrainable” phlegmon, hematoma, or other pathology. An alternative and useful test is to perform the wire test. Successful passage of the initial guidewire, especially if it assumes the shape of the cavity, implies that the content of the cavity is at least partly fluid. In most such instances, fluid can be successfully aspirated upon the introduction of the drainage catheter or a dilator with multiple side holes such as a biliary type catheter and a Yueh needle (Cook Medical, Bloomington, IN), respectively. In other cases, advancement of a catheter into an apparently undrainable collection can be followed by instillation of tissue plasminogen activator (TPA) fibrinolytic therapy to facilitate complete drainage.
Antibiotic Prophylaxis
The authors of the Society of Interventional Radiology (SIR) standards of practice guidelines for adult antibiotic prophylaxis consider percutaneous abscess drainage a dirty procedure and, as such, routine preprocedural prophylactic antibiotic administration is recommended.15 In general, organisms encountered include skin flora (gram-positive organisms) and intracavitary pathogens (typically gram-negative bacteria). The authors could not reach a consensus for the first-choice antibiotic agent, but because abscesses are typically polymicrobial, broad-spectrum antibiotic agents are warranted in the absence of existing culture data.15 Two reasonable drug regimens would include a second- or third-generation cephalosporin or ampicillin/sulbactam (vancomycin or clindamycin in case of penicillin allergy) for gram-positive coverage, plus an aminoglycoside for gram-negative coverage. Of course, an antibiotic regimen should continue following abscess drainage.
Ideally, the antibiotic should be administered intravenously at or greater than 1 hour prior to the anticipated start of the procedure, the pharmacokinetics of the chosen antibiotic, notwithstanding. In the absence of preprocedural establishment of positive culture and sensitivity studies, the antibiotic may be tailored to the most common pathogen found in the particular disease and organ system. The most common bacteria found in intra-abdominal abscesses are gram-negative rods and anaerobes, particularly Escherichia coli, Bacteroides fragilis, and Enterococcus species.8,9,10 Pyogenic liver abscesses are most often caused by Enterobacter species and anaerobes.8,11 The most common organisms in reported series of splenic abscesses have been aerobic microbes, particularly Streptococci and E. coli.4 However, geographic variations and population differences have been reported,5,12,13 with Llenas-Garcia et al reporting a higher incidence of M. tuberculosis in their series. Lee et al reported the most common pathogens in splenic abscesses were Streptococcus viridians (27.8%) and Klebsiella pneumonia (22.2%) in 18 study patients.14
Imaging Guidance
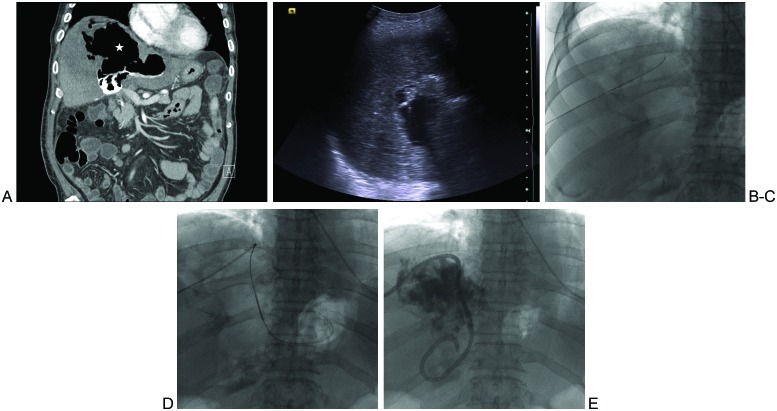
Many considerations should be made when choosing the imaging modality to guide abnormal fluid collection drainages. Each imaging modality offers unique advantages and disadvantages. Conventional fluoroscopy fails to provide internal body detail, limiting its use to the drainage of large superficial fluid cavities or intraorgan cavities containing a sufficient amount of air that can be used for targeting and as an adjunctive modality to US and CT. (Figs. 1A–E) Fluoroscopic guidance can also be used when clear anatomical landmarks are available to ensure accurate needle placement. A combination of initial US or CT guidance for the placement of the access needle and guidewire followed by fluoroscopic guidance for the wire and catheter manipulations and completion of the procedure can be useful for difficult drainages such as small or relatively deep cavities. In general, the combination of sonographic and fluoroscopic guidance is the most dynamic method because it provides multiplanar real-time visualization of needle advancement and direct visualization of dilator and catheter placement.
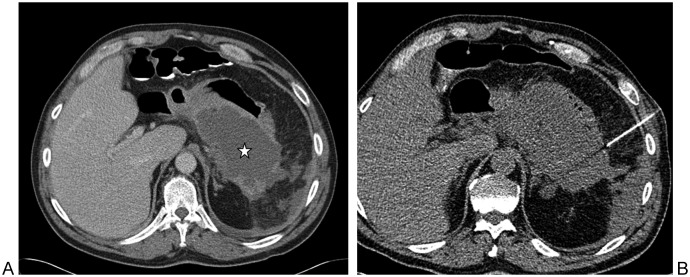
Figure 1.
Intrahepatic air-fluid cavity in patient with an orthotopic liver transplant and recurrent fevers. (A) Coronal image from computed tomography of the abdomen performed immediately following T-tube cholangiogram shows a large cavity containing mostly air (star) and a small amount of contrast inferiorly. (B) Ultrasound- and (C) fluoroscopic-guided needle and then wire access to the intra-hepatic cavity via an inferolateral approach. (D) Angled catheter-wire manipulation within the cavity in attempt to optimize the position of the to-be-placed drainage catheter loop. (E) A multiple side hole biliary-type catheter is shown in its final resting position, with the distal loop left within the fluid-containing portion of the cavity.
Intracavitary air may prevent optimal visualization of an abscess using US guidance. CT may be used for air-containing cavities, for small or deep cavities, and for those with a potentially intervening hollow viscus or solid organ along the path of the access needle.
For accurate needle guidance and catheter placement, it is critical to measure, measure, measure and feel, feel, feel! The initial length of the needle access, from the skin to just within the cavity, should be measured and adhered to with each subsequent step during the procedure. Feeling comes with experience but it implies operator-dependent detection of ease or difficulty during wire, dilator, and catheter advancement, particularly during blind advancement when intermittent, nonflucroscopic CT guidance is the modality of choice. Devices should travel freely over firmly held guidewires; if sudden wire or device resistance is met or if the patient reports a concurrent significant increase in pain, then wire kinking, nontarget access, or wire/dilator perforation of the back wall of the abscess cavity should be suspected. A low threshold for repeat CT imaging is advisable for proper adjustment of the access device(s).
Care should also be taken when using fluoroscopy for guidewire and catheter manipulations. The internal extent of the wire should always be noted, and the length of each subsequently advanced piece of equipment should be made to mimic the same length and location. If initial wire entry in the cavity is without resistance, the wire should be advanced further into the cavity so as to mimic and document the shape and size of the cavity. If the wire advances without resistance, many operators will advance as much wire as possible into the cavity in an attempt to break up any potential loculations in the cavity. The aim is for a similar configuration of the final placed drainage catheter. As with all cavities, a specimen should be collected prior to any injection of contrast material, ideally immediately following needle access to the cavity. This is for optimal evaluation in the microbiology or other laboratory. Care should be taken, however, not to aspirate all of the contents prior to placement of the drainage catheter because this may lead to complete collapse of the cavity, precluding catheter placement. If a contrast examination of the cavity is desired during initial catheter placement, care must be taken not to overinject or overdistend the cavity. In general, the cavity should be completely evacuated once the catheter is placed, followed by the injection of no more than half of the aspirated volume. Although this may lead to suboptimal detection of associated pathology, such as fistula formation, the theoretical risk of injection-induced septicemia can be avoided. A formal contrast examination can be performed when the patient's clinical condition improves, usually after 24 to 48 hours of cavity drainage.
Approach
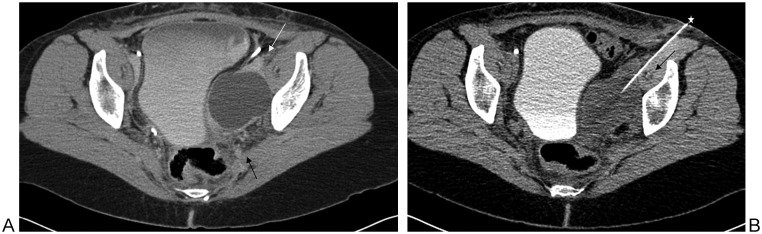
Preprocedural planning may be the most important step of the procedure to avoid potential complications. The course of the initial needle access on imaging foretells the ultimate path and safety of the placement of the final drainage catheter. If the access needle transgresses an important structure, such as a sizable artery, this can lead to the formation of a pseudoaneurysm and/or bleeding. In Fig. 2, careful planning of needle approach was required to avoid major vessels because a very small window was available, and ultimately, there were no associated vascular complications during catheter placement and subsequent removal of the drainage catheter (Figs. 2A, B). Alternatively, a transgluteal approach could have been chosen, as shown by the arrow. The operator was biased toward an anterior approach due to the fear of transgressing the sizable branches of the left internal iliac artery and vein along the projected posterior course.
Figure 2.
(A) An abnormal fluid collection, on a postoperative basis, is seen posterior to the left iliopsoas and the external iliac artery and vein (white arrow). Note the sizable branches of the left internal iliac vessels posteriorly (black arrow). (B) Chosen needle (star) path between the left external iliac vessels and the iliopsoas musculature within which lies the iliac circumflex artery and vein (black arrow). There was no associated vascular complication during the procedure and following removal of the drainage catheter. Alternatively, a transgluteal approach could have been chosen. The operator was biased toward an anterior approach due to the size of the coursing branches of the posterior left internal iliac artery and vein.
These suggested approach recommendations will minimize the risk of complications:
Use the safest, most direct, and shortest percutaneous route
Avoid intervening organs or vital anatomical structures
Avoid contamination of sterile areas
Aim for placing the drainage catheter in the most dependent portion of the cavity
Use an angled approach
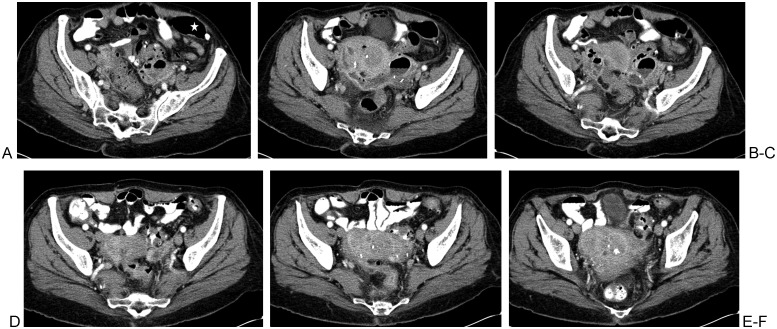
The use of the most direct and shortest percutaneous route minimizes the length of the internal catheter. Avoiding vital anatomical structures such as sizable arteries and veins lessens the bleeding risks and avoids pseudoaneurysm formation. Where it is acceptable to traverse the stomach or intestines for percutaneous biopsy of deep lesions,16 it is usually not acceptable to place external drainage catheters through a solid organ or hollow viscus. Exceptions do exist, such as the occasional placement of drains through the liver, the transgastric approach (endoscopically and percutaneously) to treat pancreatic fluid collections,17 the transrectal or transvaginal approach to drain pelvic abscesses, and the placement of cecostomy tubes for colonic decompression. Interloop abscesses cannot be drained from a percutaneous approach due to the lack of safe access (Figs. 3A–F); they are treated via needle aspiration and/or antibiotic therapy, or via open surgery.18
Figure 3.
Interloop diverticular abscess with no safe percutaneous route to accommodate drainage catheter placement. (A, B) An elongated air-fluid cavity is surrounded by multiple structures that do not afford safe passage of a drainage catheter; large intestine (star), left iliac bone, left iliac arteries, and veins. (C) Image shows a possibly safe percutaneous needle access into the abnormal air-fluid collection. Peristaltic bowel activity and variability in bowel positioning renders this possibility uncertain until the time of drainage. (D–F) Repeat computed tomography a few days later following an antibiotic regimen and bowel rest. There is complete resolution of the abscess cavity, presumably due to complete decompression into the fistulized large intestine.
When possible, the distal loop of the drainage catheter should be made to rest in the most dependent portion of the cavity to facilitate more effective evacuation of the abscess cavity contents. The suggestion of using an angled approach to catheter placement has a few rationales. An angled course of the needle allows for smoother coiling of the initial guidewire within the cavity. This in turn affords easier advancement of the wire and subsequent access devices including the final drainage catheter. After drain placement, fluoroscopic-guided abscessogram may become necessary in the future. An angled approach of the catheter has the added benefit of distancing the examiner's hand from the direct path of the fluoroscopic beam.
With the use of nondynamic, nonfluoroscopic imaging guidance for percutaneous placement of drainage catheters, another recommended suggestion is to measure at multiple points of the procedure. The distance from the skin to the entry point of the cavity should be measured. Guidewire length should also be measured, with a longer distance inserted to ensure adequate access to the cavity. Two measurements of the drainage catheter should be taken. The first distance (D1), the shortest, should be from the distal tip of the catheter, equaling the initial distance measured from the skin to the cavity. The second distance, D2, is measured from the most proximal distal side hole of the catheter, and the proximal catheter is marked. Given the distance of the catheter loop, D-loop, the catheter should be advanced at least through a distance of D1 plus D-loop, to ensure that the entire length of the distal catheter loop with side holes is completely embedded within the cavity, with no proximal side hole external to the cavity. The catheter should then be secured in place, with sutures or other securement devices, at the skin entry site.
Particularly difficult access locations in the abdomen include subphrenic fluid collections, posterior epigastric and peripancreatic fluid collections, and deep pelvic fluid collections.
Subphrenic Fluid Collections
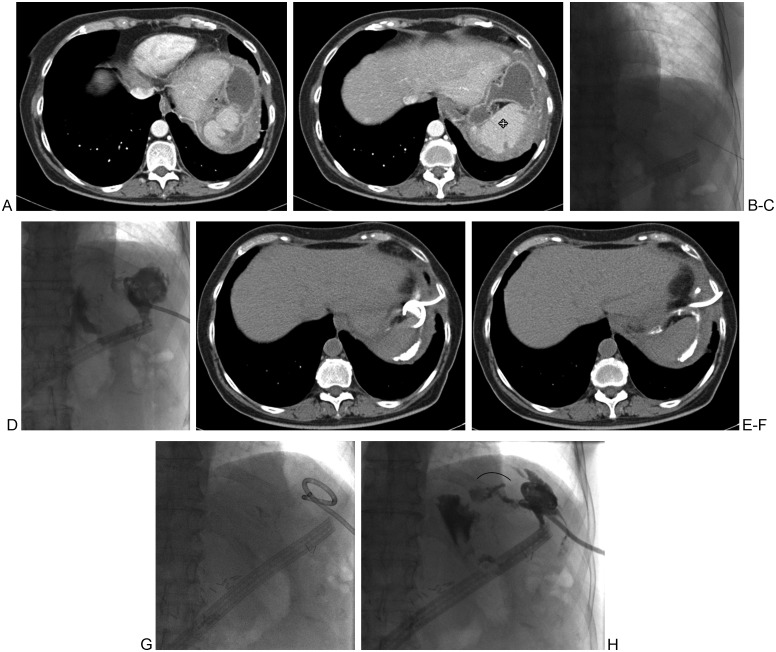
The difficulty of subphrenic collections is mainly caused by the inferior extent of the pleural space. Potential complications from transpleural access to the abscess cavity include pneumothorax, hydropneumothorax, or empyema. Potential drainage strategy may include angling the needle cranially from either an anterior subcostal approach below the 7th rib or a lateral approach below the 10th rib. Because each patient's body habitus is different, the interventionalist should tailor the approach based on individual patient imaging prior to the procedure and fluoroscopic evaluation and guidance, if possible, during all respiratory phases to avoid pleural transgression. Figs. 4A–F illustrate the avoidance of transgression of the left pleural space during placement of a drainage catheter to evacuate an abscess located superior to the spleen.
Figure 4.
A 59-year-old woman, 11 weeks following resection of an intraductal papillary mucinous neoplasm and subtotal pancreatectomy. (A, B) Two consecutive axial images show an abnormal fluid cavity anterior to the spleen (quad arrow). (C) An inferolateral approach is used, as shown on the fluoroscopic image, in an attempt to avoid traversing the inferolateral pleural space. (D) The complexity of the cavity with an associated fistula is visualized. (E, F) On post drainage day 20, repeat computed tomography imaging shows the course of the drainage catheter and resolution of the abscess cavity. (G, H) Abscessogram performed on the same day shows a mostly collapsed cavity but a persistent medial fistula (arc).
Posterior Epigastric and Peripancreatic Fluid Collections
Challenges in these locations include the interposition of stomach as well as small and large bowel. Potential access approaches can be transhepatic, posterior, or lateral. CT for guidance becomes a necessity to avoid traversing the stomach and intestines (Figs. 5A, B).
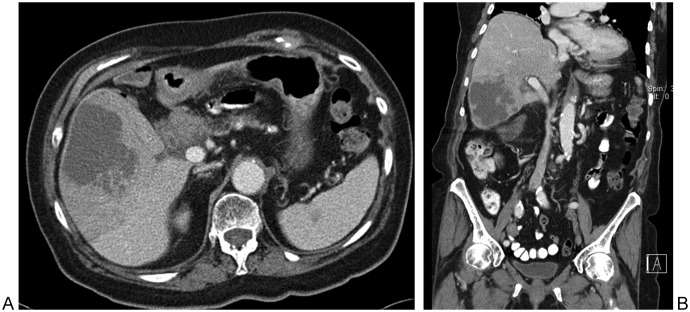
Figure 5.
Drainage of a peripancreatic fluid collection. (A) Diagnostic computed tomography (CT) image shows a sizable retrogastric fluid collection (star) in a patient with pancreatitis and fever. (B) Draping of the (now nondistended) stomach superiorly and superolaterally forced the use of CT for guidance for safe needle access into the pseudocyst. Intraprocedural CT image shows safe needle entry into the pseudocyst via an inferolateral approach, posterior to the posterior margin of the stomach.
Deep Pelvic Fluid Collections
Draining abscesses in the deep pelvis is limited by interposed vessels, bowel, and the urinary bladder. This can be mitigated by ancillary preprocedural steps such as placement of a Foley catheter for bladder decompression and overnight oral or intraprocedural rectal contrast administration for bowel opacification. Approaches that can be used are transvaginal, transrectal, and transgluteal. The first two approaches imply the use of endoluminal US, often followed by fluoroscopic guidance. The transvaginal approach is safe and effective, but it should be avoided in premenarchal and virginal patients. The transrectal route tends to be less painful than the transvaginal approach. Monitored anesthesia care is advised for both approaches.
The transgluteal approach to an abnormal cavity is used when there is no safe anterior abdominal access route and the transrectal and transvaginal approaches are not an option. Transgluteal drainage is best performed under CT guidance because neighboring structures such as the rectosigmoid colon are inadequately visualized with sonographic guidance. In addition, the complex anatomy of the greater sciatic foramen (GSF) encourages the use of CT guidance. Butch et al reported the ideal catheter placement to be the lower portion of the GSF, at the level of the sacrospinous ligament.20 This is as adjacent to the sacrum as possible and inferior to the piriformis muscle. This location avoids the vascular and neural elements located cephalad at the level of the piriformis muscle. The superior and inferior gluteal arteries and veins, and the internal pudendal artery are located in the cephalad aspect of the GSF, as is the sciatic nerve that runs immediately posterior to the ischial spine. Catheters adjacent to or transgressing the piriformis muscle or the sacral plexus have a higher incidence of persistent pain,20 which usually resolves upon removal of the drainage catheter. Thus the access route is chosen as close to the sacrum as possible (Figs. 6A–M)
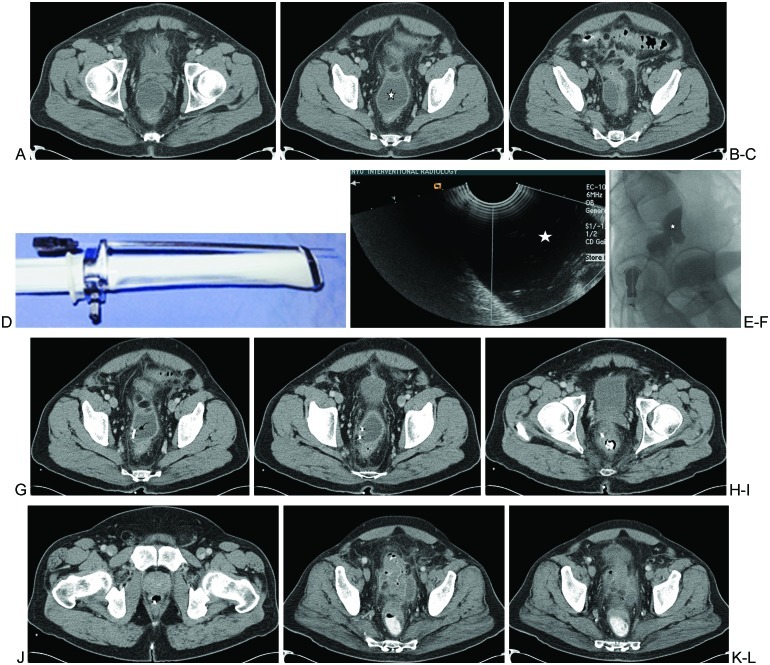
Figure 6.
Transrectal abscess drainage. (A–C) Multiple computed tomography (CT) images of the pelvis demonstrate a prerectal abscess cavity (star). (D) The transrectal probe with a securely attached needle is demonstrated. (E) Transrectal access is demonstrated into the hypoechoic fluid cavity (star). (F) With the patient in the left lateral decubitus position, with legs bent at the knee, the oblique lateral fluoroscopic projection image shows injected contrast within the bilobed cavity (star). (G–J) Images from a follow-up CT of the pelvis shows the pigtail drainage catheter in place (curved arrow). (K, L) CT of the pelvis performed following successful evacuation and treatment of the prerectal abscess and following removal of the drainage catheter.
Feld et al reported no superinfection of noninfected collections drained via a transvaginal or transrectal approaches to deep pelvic fluid cavities.19
Choose Drainable Collections
Initial failure of drainage of an abscess can be due to nonliquefaction of the contents. Acute hematomas, and occasionally acute peripancreatic fluid collections, pseudocysts, and phlegmon, are the most common examples. A phlegmon can be difficult to differentiate from an abscess on CT scan, and an attempt at drainage may become necessary if the patient exhibits clinical symptoms of infection.
Occasionally, especially in the absence of appropriate clinical information, an abscess can be mistaken for a neoplasm on CT imaging. Furthermore, a neoplasm may contain a superinfected necrotic center and be indistinguishable from tumor by clinical presentation as well as imaging evaluation. In these scenarios, fine-needle aspiration with or without core biopsy of the lesion should be performed for pathologic characterization to avoid upstaging the patient via spillage of malignant cells (Figs. 7A, B).
Figure 7.
(A) Axial and (B) coronal computed tomography images of a patient with a large liver lesion. Note the small poorly characterized splenic lesion. Percutaneous needle aspiration of the hypodense part of the liver lesion revealed no fluid. Core biopsy performed yielded a pathologic diagnosis of cholangiocarcinoma.
Abscesses <3 cm are often treated with antibiotic therapy alone.21 These can be sampled or aspirated with a needle, if only for the assessment of optimal antibiotic coverage. In addition, depending on location, cavities <3 cm may not be amenable to percutaneous placement of a drainage catheter. From a technical standpoint, there is insufficient space within such small cavities to afford exchanges of wires, dilators, and the formation of the distal catheter loop. Wire advancement is often problematic in such small diametral spaces. Forcible wire advancement can lead to perforation of the cavity wall that is opposite to the point of needle entry, risking spread of infection to an adjacent space.
Not all fluid collections are meant to be drained (Figs. 8A, B). Drainage of peripancreatic fluid collections should be reserved in case of associated symptoms or clinical suspicion of related infection.22 As mentioned earlier, cavities <3 cm in diameter need not be drained because they are typically treatable with antibiotic therapy alone. Lastly, due to a lack of safe percutaneous access, not all fluid collections are drainable percutaneously (Figs. 3A–F).
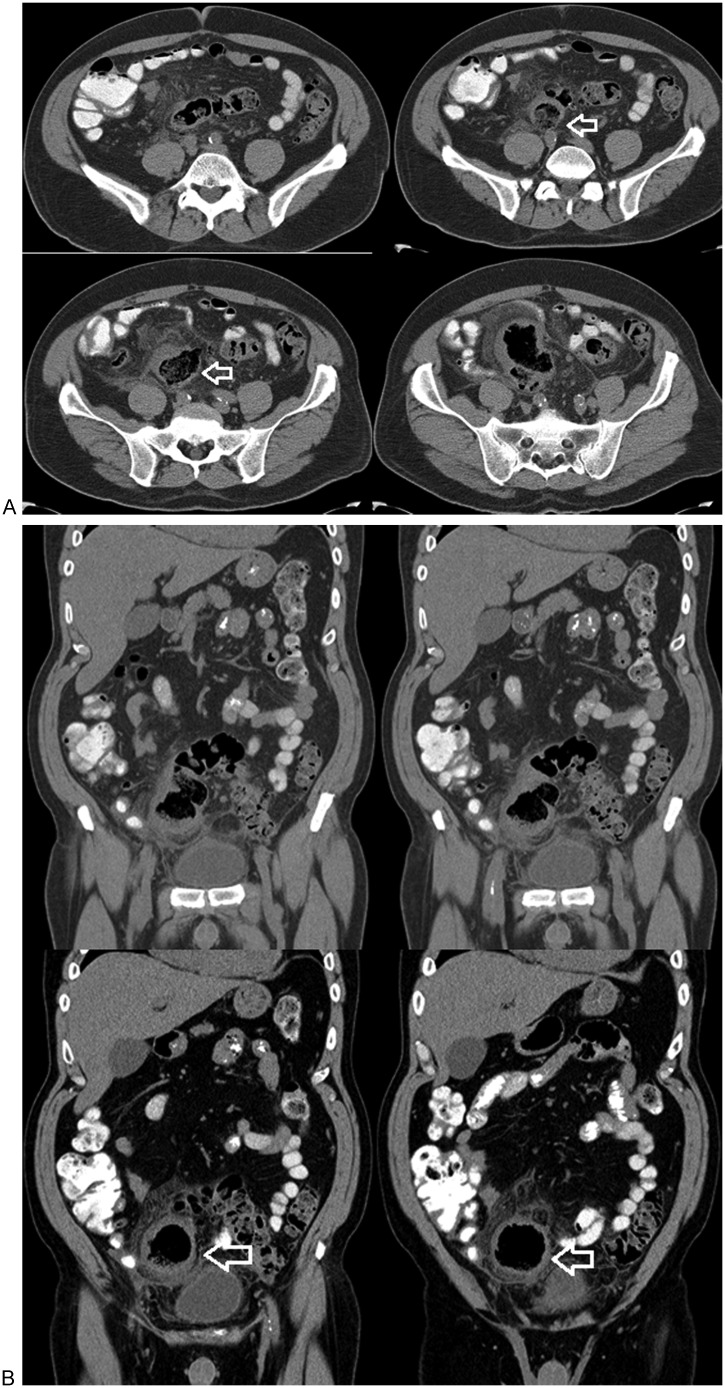
Figure 8.
(A) Serial axial and (B) coronal reformatted images show an enlarged thick-walled cavity that is continuous with the colon (arrows). It apparently contains endoluminal fecal material and no fluid. Note the stranding of the adjacent fat. This was judged to be an inflamed giant sigmoid diverticulum.
Just Leave A Catheter In!
Simply stated, during aspiration procedures there is at times a tendency not to place, or to postpone the placement of, a drainage catheter. One recommendation is simply to leave a catheter in at the time of aspiration; it can always be removed. The exception to this recommendation is when the clinical suspicion of related infection is low and/or if the intent is solely diagnostic sampling of the cavity. Rajak et al showed that the clinical success of complete evacuation of abscesses is 100% with catheter placement and 60% with needle aspiration alone.23 In half of the patients in whom aspiration was performed and the material was thought not to be infected, the culture results were actually positive. Feld et al concluded that the character of the aspirated fluid is not a reliable indicator for infection and therefore should not be used for determining whether or not to place a catheter.19
In addition, the presence of a fistula should encourage catheter placement rather than mere aspiration. Kerlan et al showed that enteric fistulas were present in 36% of abdominal abscesses that were drained,24 and a catheter can facilitate resolution of both the abscess and the fistula. For liver abscesses, Rajak et al showed that percutaneous catheter drainage is more effective than needle aspiration. The latter, if limited to two attempts, has a high failure rate.23 Thomas et al reported that 43% of 14 patients had successful liver abscess treatment with aspiration and appropriate antibiotics without IGPAD25; abscesses <5 cm seemed to best respond to this treatment combination. However, 57% of those who had aspiration of the abscess initially went on to have drainage catheters placed within a 72-hour period.
Tailor the Drainage Catheter to the Contents of the Cavity
Catheter shape and size are important factors in the technical and clinical success of drainage procedures. Locking pigtail catheters are preferable over straight catheters. The latter may be more prone to premature and accidental dislodgement. Small cavities may best be treated with smaller diametral pigtail tubes such as the Dawson-Mueller catheter (Cook Medical, Bloomington, IN). This has a 10-mm pigtail diameter compared with the standard pigtail catheter with a 25-mm diameter (e.g., multipurpose drainage catheter, Cook Medical, Bloomington, IN). Drainage catheters with a distal hydrophilic coating can be advanced easier into thick-walled or fibrotic cavities, often without preceding percutaneous tract dilatation.
Simple serous contents can be efficiently drained with small-caliber catheters (8 to 10F). Complex cavities, such as bloody fluid, may require larger diameter catheters (>12F) for optimal drainage. Gobien et al reported no significant difference in the success or failure of percutaneous abscess drainage as a function of catheter size.26 However, other authors have suggested that catheter size may affect the ultimate clinical outcomes and duration of drainage, as well as complication rates and manipulations. Univariate analysis demonstrated catheter size to be associated with the risk of catheter failure, as shown by Cronin et al27 in a study of percutaneous drainage of peripancreatic fluid collections after distal pancreatectomy.
Fistulization: Delayed Demonstration
An abscess-related fistula may not be demonstrated at the time of initial drainage due to factors such as acute inflammation and underdistension of the abscess cavity to avoid systemic spread of infected contents. Yet, as stated earlier, a significant percentage of abdominal abscesses have associated fistulas. Interventional radiologists should resist the tendency of accepting blame for delayed demonstration of a fistula complicating an abscess because it typically represents an expected consequence of an adjacent infectious or inflammatory process. As documented by Ciftci et al, a fistula tends to prolong catheter treatment.28
Avoid the Temptation to Remove the Drainage Catheter Prematurely
Criteria for catheter removal include resolution of clinical signs of infection, daily patent catheter output <20 mL, no associated fistula,27 and repeat imaging showing resolution of the cavity, although the latter is not always necessary. Some investigators advocate the removal of the drainage catheter with daily output <10 mL.29 To maintain catheter patency, it is recommended to flush the catheter with saline volumes ranging from a few milliliters to 10 mL every 8 hours,27 depending on the size of the cavity and the capacity of the catheter. The use and the type of repeat imaging prior to the removal of drainage catheters is not universal. For complex cavities, CT may be the best surveillance imaging modality. For pediatric patients, repeat sonographic imaging is optimal due to the lack of ionizing radiation.
On follow-up imaging, complex cavities may not be completely evacuated despite an appropriately placed and patent drainage catheter. This is usually due to internal loculations, clots, or debris. Fibrinolysis with the use of tissue plasminogen activator (tPA) may be highly effective in achieving complete evacuation of the contents, and studies have demonstrated a good safety profile. In a retrospective study of 66 patients, Gervais et al showed complete drainage of organized pleural collections after treatment with tPA in 86% of patients without further surgical procedures.30 Treatment consisted of 4 to 6 mg tPA diluted in a volume of 0.9% saline measuring in 30 to 50% of the cavity volume. The tPA solution was administered twice daily with a 30-minute dwell time, repeated for 3 days. No treatment-associated hemorrhages occurred in patients receiving no anticoagulation or those on prophylactic anticoagulation.
Patient Comfort
Intraprocedural patient comfort should always be a consideration for any drainage procedure for the sake of the patient and the operator. The interventional physician should not sacrifice comfort for speed in justifying not sedating the patient. The temptation of not considering factors that may affect the patient's comfort during the procedure should be avoided. Factors that may affect the patient's comfort should be considered including clinical history, current condition, and patient age. I employ the aid of an anesthesiologist for sedation for all pediatric patients. Teenage and young adult males are more prone to have vasovagal reactions, and they are less tolerant of pain, per my experience. The degree of anesthesia used, from light sedation to intubation, is usually discussed among the interventional and anesthesia physicians and, particularly in adult cases, the patient. Factors affecting decisions regarding appropriate anesthesia or sedation may include time since the last oral intake and the potential of intra- or postprocedural emesis (and risk of aspiration). Clinically unstable patients and many patients admitted to the intensive care unit should be under the care of an anesthesiologist during a drainage procedure.
Judicious but prudent use of local anesthesia is imperative if no intravenous sedation is to be administered. Generally, a benzodiazepine and a narcotic analgesic are administered for intraprocedural sedation. Midazolam and diazepam have similar duration of onset of action, but the former has a quicker peak, shorter duration of effect, and results in more amnesia. Fentanyl has a faster onset of action and quicker peak than morphine; in addition, it affords a shorter duration of effect and less nausea than morphine. Minimal and very infrequent cardiac depression and hypotensive effects are associated with fentanyl.31
Timing of Drainage
Where the indications and contraindications of drainage are well established, the timing of drainage is realistically open to personal discretion. Medical literature on this subject is mostly nonexistent. Although the clinical benefit of timely drainage cannot be debated, the question is whether or not the procedure can be deemed an emergency.
When initially detected by imaging during the night, drainage of an abscess can often be postponed to the following morning while covering the patient with empirical antibiotics and other resuscitative measures. During the night, it is often difficult to deem abscess drainage an emergency. Most interventional radiologists would advocate a preprocedural period of patient antibiotic administration and intravenous hydration prior to percutaneous drainage. Anecdotal evidence from experienced interventional radiologists would tend to favor this treatment approach and sequence. Transient bacteremia and pyrexia are well documented following abscess drainage,32 but sustained bacteremia leading to sepsis is less common. Its precipitation is believed to be due to mechanical agitation of a previously well-contained infectious cavity. The passage of a needle, and other equipment such as a wire and a drainage catheter, has the potential to provide temporary communication between the infected field and surrounding microvasculature, allowing passage of bacteria from the abscess into the bloodstream. It is therefore postulated that patients would benefit from a steady-state level of antibiotic concentration in the bloodstream for immediate defense against the systemic spread of infection. Experienced interventionalists with historical anecdotes of post-abscess evacuation bacteremia and/or sepsis in patients with inadequate antibiotic exposure prior to abscess drainage will attest to the need for a few hours of intravenous antibiotic administration (depending on the pharmacokinetics of the antibiotics) preceding such an intervention.
When the request for drainage is made on the night prior to a holiday or during the weekend, the decision of timing of the procedure may be contentious. Requesting medical and surgical personnel often use the threat of the patient becoming septic during the course of the delay to drainage as a reason for more immediate intervention. The name of the dissenting physician is often asked so it can be included in the patient's permanent record, an undesirable and contentious act that can be aimed to persuade the interventional physician for earlier intervention. At the same time, interventional physicians argue in favor of the remote possibility of this occurrence in patients who may have had this ongoing infection for at least a few days if not a few weeks. However, an argument can be made that timely drainage of an abscess can lead to more rapid improvement of patient clinical condition and decreased length of hospital stay. The final result of such encounters varies widely from institution to institution, and unfortunately very little to no scientific evidence supports either side of the argument.
Knowledge of Potential Complications
The advantage of awareness of the potential complications to any procedure cannot be understated. This makes the performing physician best able to avoid them. This is aided by knowledge of pertinent anatomy, with the avoidance of transgressing vital structures, especially nerves and arteries.
Generalized potential complications of fluid drainage include pain, sepsis, bleeding, peritonitis, and pseudoaneurysm formation. Complications tend to be site specific. Thomas et al reported the risk of sepsis following drainage of liver abscesses to be 26%.25 All seven affected patients began showing clinical signs of clinical deterioration within 15 to 30 minutes of catheter drainage, with an associated overall mortality rate of 7.4%. In this study, abscess size was not a predictor of postprocedural sepsis, and all patients had preprocedural intravenous antibiotic administration. It was noted that no patients who solely had needle aspiration of the abscess cavity developed sepsis.
A somewhat medial approach to the peritoneal cavity can lead to a rectus sheath hematoma via injury to the inferior epigastric artery.33 In addition to pain, pelvic bleeding and hematoma can complicate the drainage of deep pelvic abscesses.
Summary
Percutaneous drainage of abnormal fluid collections can be at times simple and at times challenging. The procedure has a significant impact on patient care and outcome, and its timing can affect hospital length of stay. Technical success of the procedure relies on many factors including choosing the appropriate imaging guidance, percutaneous approach, method of sedation, and drainage technique. Prior to the accumulation of clinical experience, avoiding procedure related complications is best afforded by the interventional physician's knowledge of the pertinent anatomy and anticipation of potential general and site specific complications.
References
- 1.Chalupka A N Talmor D The economics of sepsis Crit Care Clin 201228157–76., vi [DOI] [PubMed] [Google Scholar]
- 2.Martin G S, Mannino D M, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Martin G S, Mannino D M, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 4.Altemeier W A, Culbertson W R, Fullen W D, Shook C D. Intra-abdominal abscesses. Am J Surg. 1973;125(1):70–79. doi: 10.1016/0002-9610(73)90010-x. [DOI] [PubMed] [Google Scholar]
- 5.Gerzof S G, Robbins A H, Johnson W C, Birkett D H, Nabseth D C. Percutaneous catheter drainage of abdominal abscesses: a five-year experience. N Engl J Med. 1981;305(12):653–657. doi: 10.1056/NEJM198109173051201. [DOI] [PubMed] [Google Scholar]
- 6.Siewert B, Tye G, Kruskal J. et al. Impact of CT-guided drainage in the treatment of diverticular abscesses: size matters. AJR Am J Roentgenol. 2006;186(3):680–686. doi: 10.2214/AJR.04.1708. [DOI] [PubMed] [Google Scholar]
- 7.Patel I J, Davidson J C, Nikolic B. et al. Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 8.McDermott V G, Schuster M G, Smith T P. Antibiotic prophylaxis in vascular and interventional radiology. AJR Am J Roentgenol. 1997;169(1):31–38. doi: 10.2214/ajr.169.1.9207497. [DOI] [PubMed] [Google Scholar]
- 9.Dravid V S, Gupta A, Zegel H G, Morales A V, Rabinowitz B, Freiman D B. Investigation of antibiotic prophylaxis usage for vascular and nonvascular interventional procedures. J Vasc Interv Radiol. 1998;9(3):401–406. doi: 10.1016/s1051-0443(98)70290-8. [DOI] [PubMed] [Google Scholar]
- 10.Sieber P R, Rommel F M, Agusta V E, Breslin J A, Huffnagle H W, Harpster L E. Antibiotic prophylaxis in ultrasound guided transrectal prostate biopsy. J Urol. 1997;157(6):2199–2200. [PubMed] [Google Scholar]
- 11.Lorber B, Swenson R M. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975;55(6):1349–1354. doi: 10.1016/s0039-6109(16)40792-9. [DOI] [PubMed] [Google Scholar]
- 12.Johnson W C, Gerzof S G, Robbins A H, Nabseth D C. Treatment of abdominal abscesses: comparative evaluation of operative drainage versus percutaneous catheter drainage guided by computed tomography or ultrasound. Ann Surg. 1981;194(4):510–520. doi: 10.1097/00000658-198110000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemming A, Davis N L, Robins R E. Surgical versus percutaneous drainage of intra-abdominal abscesses. Am J Surg. 1991;161(5):593–595. doi: 10.1016/0002-9610(91)90907-u. [DOI] [PubMed] [Google Scholar]
- 14.Lee W S, Choi S T, Kim K K. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288–292. doi: 10.3349/ymj.2011.52.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatesan A M, Kundu S, Sacks D. et al. Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J Vasc Interv Radiol. 2010;21:1611–1630. doi: 10.1016/j.jvir.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Tseng H S, Chen C Y, Chan W P, Chiang J H. Percutaneous transgastric computed tomography-guided biopsy of the pancreas using large needles. World J Gastroenterol. 2009;15(47):5972–5975. doi: 10.3748/wjg.15.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan U, Vege S S, Chari S T, Baron T H. Minimally invasive techniques in pancreatic necrosis. Pancreas. 2009;38(8):867–875. doi: 10.1097/MPA.0b013e3181b3b237. [DOI] [PubMed] [Google Scholar]
- 18.Saluja S, Fields J M, Schwartz D S. et al. Percutaneous needle aspiration of small interloop abscesses in children. Pediatr Surg Int. 1998;13(7):528–530. doi: 10.1007/s003830050392. [DOI] [PubMed] [Google Scholar]
- 19.Feld R, Eschelman D J, Sagerman J E, Segal S, Hovsepian D M, Sullivan K L. Treatment of pelvic abscesses and other fluid collections: efficacy of transvaginal sonographically guided aspiration and drainage. AJR Am J Roentgenol. 1994;163(5):1141–1145. doi: 10.2214/ajr.163.5.7976890. [DOI] [PubMed] [Google Scholar]
- 20.Butch R J, Mueller P R, Ferrucci J T Jr. et al. Drainage of pelvic abscesses through the greater sciatic foramen. Radiology. 1986;158(2):487–491. doi: 10.1148/radiology.158.2.3941878. [DOI] [PubMed] [Google Scholar]
- 21.Jeffrey R B Jr, Federle M P, Tolentino C S. Periappendiceal inflammatory masses: CT-directed management and clinical outcome in 70 patients. Radiology. 1988;167(1):13–16. doi: 10.1148/radiology.167.1.3347712. [DOI] [PubMed] [Google Scholar]
- 22.Brun A, Agarwal N, Pitchumoni C S. Fluid collections in and around the pancreas in acute pancreatitis. J Clin Gastroenterol. 2011;45(7):614–625. doi: 10.1097/MCG.0b013e318213ef3e. [DOI] [PubMed] [Google Scholar]
- 23.Rajak C L, Gupta S, Jain S, Chawla Y, Gulati M, Suri S. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR Am J Roentgenol. 1998;170(4):1035–1039. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 24.Kerlan R K Jr, Jeffrey R B Jr, Pogany A C, Ring E J. Abdominal abscess with low-output fistula: successful percutaneous drainage. Radiology. 1985;155(1):73–75. doi: 10.1148/radiology.155.1.3975423. [DOI] [PubMed] [Google Scholar]
- 25.Thomas J, Turner S R, Nelson R C, Paulson E K. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? AJR Am J Roentgenol. 2006;186(5):1419–1422. doi: 10.2214/AJR.04.1914. [DOI] [PubMed] [Google Scholar]
- 26.Gobien R P, Stanley J H, Schabel S I. et al. The effect of drainage tube size on adequacy of percutaneous abscess drainage. Cardiovasc Intervent Radiol. 1985;8(2):100–102. doi: 10.1007/BF02552867. [DOI] [PubMed] [Google Scholar]
- 27.Cronin C G, Gervais D A, Castillo C F, Mueller P R, Arellano R S. Interventional radiology in the management of abdominal collections after distal pancreatectomy: a retrospective review. AJR Am J Roentgenol. 2011;197(1):241–246. doi: 10.2214/AJR.10.5447. [DOI] [PubMed] [Google Scholar]
- 28.Ciftci T T, Akinci D, Akhan O. Percutaneous transhepatic drainage of inaccessible postoperative abdominal abscesses. AJR Am J Roentgenol. 2012;198(2):477–481. doi: 10.2214/AJR.11.6680. [DOI] [PubMed] [Google Scholar]
- 29.Sandhu J. Fairfax, VA: Society of Cardiovascular & Interventional Radiology; 1997. Tutorial 8. Drainage of deep pelvic abscesses including transgluteal, transrectal, and transvaginal approaches; pp. 85–100. [Google Scholar]
- 30.Gervais D A, Levis D A, Hahn P F, Uppot R N, Arellano R S, Mueller P R. Adjunctive intrapleural tissue plasminogen activator administered via chest tubes placed with imaging guidance: effectiveness and risk for hemorrhage. Radiology. 2008;246(3):956–963. doi: 10.1148/radiol.2463070235. [DOI] [PubMed] [Google Scholar]
- 31.Lang E V, Porter D H. Fairfax, VA: The Society of Cardiovascular and Interventional Radiology; 1999. Analgesia and sedation for interventional radiological procedures; pp. 65–90. [Google Scholar]
- 32.Le Frock J L, Molavi A. Transient bacteremia associated with diagnostic and therapeutic procedures. Compr Ther. 1982;8(2):65–71. [PubMed] [Google Scholar]
- 33.Funaki B. Embolization iatrogenic hemorrhage after paracentesis. Semin Intervent Radiol. 2008;25(3):329–333. doi: 10.1055/s-0028-1085934. [DOI] [PMC free article] [PubMed] [Google Scholar]