Abstract
Pancreatic fluid collections include a wide range of entities such as cystic neoplasms, both benign and malignant; vascular pathology such as pseudoaneurysms and hematomas; and a host of other entities such as pseudocysts, seromas, abscesses, and bilomas. The distinction between these entities requires correlating an often complex and overlapping clinical presentation with findings on imaging studies, typically computed tomography, magnetic resonance imaging, and ultrasound. As complex as the diagnostic work-up may be, the treatment of pancreatic collections poses its own set of challenges and often requires a multidisciplinary collaboration among interventional radiologists, surgeons, and gastroenterologists. The best treatment algorithm is determined by careful review of radiologic imaging studies combined with endoscopic retrograde cholangiopancreatography to apply therapies such as surgical resection; drainage or debridement; endoscopic ultrasound-guided drainage; aspiration or biopsy; and imaging-guided percutaneous drainage, aspiration, or biopsy. This article focuses on the diagnosis and multidisciplinary management of pancreatic fluid collections such as abscesses, pseudocysts, and necrosis.
Keywords: interventional radiology, pancreas, percutaneous drainage, abscess, pancreatitis
Objectives: Upon completion of this article, the reader will be able to describe the multidisciplinary roles of endoscopy, surgery, and interventional radiology in the management of pancreatic fluid collections.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Pancreatic fluid collections can be complex in presentation, diagnosis and treatment. The many and diverse etiologies of pancreatic entities addressed today often require a spectrum of imaging for initial workup. Even with clinical and imaging correlation, deciding on the most appropriate treatment approach can still be challenging. Currently, collaboration among interventional radiologists, surgeons, and gastroenterologists is often necessary to coordinate various imaging studies with therapies such as endoscopic retrograde cholangiopancreatography (ERCP), surgical resection, debridement, drainage or biopsy. The purpose of this article is the diagnosis and multidisciplinary management of pancreatic fluid collections.
Diagnosis
Evaluation of pancreatic fluid collections for appropriate therapeutic intervention should begin with a complete work-up to exclude neoplastic disease. Cystic neoplasms in the pancreas can mimic benign fluid collections. They vary greatly in prognosis, and commonly imaging alone is not sufficient for diagnosis. Depending on clinical presentation as well as the differential diagnosis and level of diagnostic confidence, the treatment algorithm may include surveillance with serial cross-sectional imaging, percutaneous biopsy, or surgical resection. The topic of cystic neoplasms of the pancreas is broad, and this article focuses primarily on the treatment of benign collections of the pancreas.
Common etiologies of benign pancreatic fluid collections include acute pancreatitis, trauma, or any insult to the pancreas that disrupts the normal barrier between stored enzymes and pancreatic parenchyma. Acute pancreatic fluid collections (APFCs), abscesses, and necrosis occur within the first few weeks of the initial insult. Most APFCs resolve with supportive care only,1 but abscesses and necrosis typically require complex drainage strategies. Pseudocysts are localized collections of pancreatic enzymes as well as necrotic and hemorrhagic tissue encased by a fibrous wall of granulation tissue, which according to the revised Atlanta Criteria develops 4 to 6 weeks after the initial insult.2 This wall indicates maturation of the pseudocyst and determines appropriateness and timing of treatment options. Around 10% of the cases of acute pancreatitis result in a pseudocyst, and most are asymptomatic and do not require interventional treatment. Symptoms result from biliary obstruction, painful or obstructive mass effect, or hemorrhage, and in such cases decompression is typically indicated.
Infected collections of the pancreas include pancreatic abscesses, infected pseudocysts, or superinfection of other entities such as pancreatic necrosis. Pancreatic abscesses typically occur within the first few weeks of the onset of pancreatitis or as a delayed complication related to progression of necrotic pancreatitis. Pancreatic necrosis also occurs early in most cases and results from compromise of the pancreatic arterial supply as a complication of pancreatitis. Careful attention should be given to distinguish an infected pancreatic pseudocyst from an abscess. Infected pancreatic pseudocysts occur after maturation at 4 to 6 weeks, whereas abscesses tend to occur earlier. Pancreatic abscesses tend to have lower internal CT attenuation than pseudocysts. Unlike cases of necrosis, abscesses are typically well circumscribed with an enhancing rim. All of these entities result in worsening clinical signs of infection such as high fevers and leukocytosis coupled with imaging findings like internal gas bubbles and surrounding fat stranding, and all require intervention—often multidisciplinary—for resolution.
Vascular pathology involving the arterial supply to the pancreas may result from pancreatitis, chronic and acute mesenteric ischemia, trauma, and iatrogenic causes. Resulting pathologies include pancreatic necrosis, hemorrhagic pseudocysts, and pseudoaneurysms. Imaging of “fluid collections” should include a careful attempt to rule out pseudoaneurysms to prevent catastrophic attempts at endoscopic, percutaneous, or surgical “drainage.” Findings include the classic “yin-yang” and “to-and-fro” appearance of color and pulsed-wave Doppler studies, respectively, as well as enhancement detected on CT, often indicated by subtle changes in attenuation between the nonenhanced study and the delayed contrast-enhanced study. Associated findings may include typical causes such as mesenteric arterial obstruction or pancreatitis. Because pseudoaneurysms of the mesenteric arteries are prone to rupture, treatment is indicated, and interventional radiology offers first-line options such as thrombin injection, embolization, and revascularization of obstructed mesenteric arteries.
Treatment Options
Much overlap exists in the various treatment options offered by interventional radiologists, gastroenterologists, and surgeons, and often a combined approach is needed. By considering some general guidelines, clinicians can offer the most appropriate therapeutic options.
A careful preliminary clinical and imaging evaluation of benign pancreatic fluid collections can avoid unnecessary intervention. For example, nearly all APFCs and most simple pseudocysts resolve without intervention. When intervention is required, the best application of all multidisciplinary options should be considered based on the initial imaging and clinical findings.
Image-guided percutaneous techniques by interventional radiologists are first-line options when the goal is to obtain tissue or fluid for the diagnosis of a suspected neoplasm, characterize sterile fluid as a seroma, biloma, or pseudocyst, or to culture fluid to rule out infection or hone antibiotic therapy. In such cases, percutaneous techniques are associated with high rates of success and low rates of complications. Success rates are buoyed by a wide range of possible access windows including transperitoneal, retroperitoneal, transgastric, transduodenal, or transhepatic approaches. However, when the goal is to place a drain in an abscess, pseudocyst, or focus of liquefactive necrosis, imaging-guided percutaneous intervention may be preferred in certain clinical contexts, and surgical or endoscopic techniques, or a combined approach, may be preferred in others. In the case of complicated pseudocysts, the American College of Radiology appropriateness criteria endorses the drainage of collections that are large (≥5 cm), rapidly enlarging, obstructing, and infected.3 Some authors further delineate pseudocysts into categories based on etiology, and they provide specific scenarios explaining which types of pseudocysts are likely to respond to each approved treatment.4 The most common methods of pseudocyst or abscess drainage are percutaneous external drainage (PED), endoscopic ultrasound-guided drainage, and surgical drainage. Both surgical and endoscopic approaches offer the advantage of cystgastrostomy or cystenterostomy creation. Marsupialization via cystgastrostomy or cystenterostomy is particularly advantageous when the pancreatic duct is obstructed or in cases involving chronic pancreatitis in which the duct is likely to be stenotic and scarred, precluding eventual spontaneous drainage. In such cases, PED would result in the undesirable outcome of protracted or indefinite external catheter placement. Each technique carries a different set of advantages and risks, and all depend on the availability of a skilled specialist.
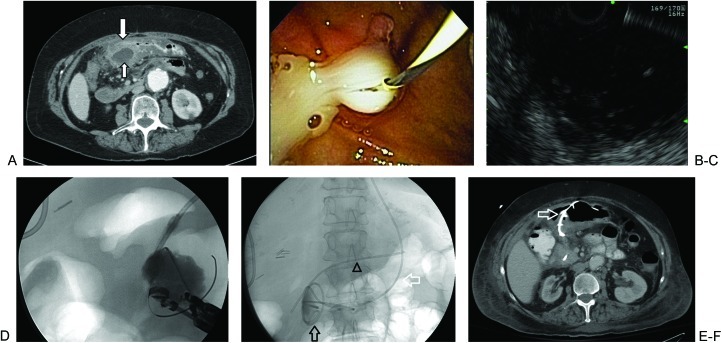
Endoscopic ultrasound-guided drainage avoids the need for an open incision or an external drainage catheter, and it is emerging as the preferred first-line therapeutic option in most cases (Fig. 1). Either conscious sedation or general anesthesia is induced prior to introducing the echoendoscope. The echoendoscope is used to locate the fluid collection and to verify that there are no overlying vessels or other structures to imperil the chosen approach. The endoscopist performs a transpapillary or transmural puncture into the pseudocyst, depending on the pseudocyst location and whether or not it communicates with the pancreatic ductal system. After obtaining wire access into the collection and performing dilation, one or several stents are placed and either connected to a nasocystic catheter for lavage and drainage, or to an endoprosthesis for continued drainage into the gastric or duodenal lumen.5 In one study of 92 patients who underwent endoscopic drainage, the technical success rate was reported to be 97%, with a mortality rate of 1%, serious complications requiring surgery in 9%, and overall clinical success at a mean follow-up of 43 months of 71%.6
Figure 1.
Endoscopic ultrasound-guided drainage of pseudocyst. (A) Contrast-enhanced computed tomography shows a pancreatic pseudocyst (small arrow) abutting the stomach (large arrow). Endoscopic drainage is ideal in this patient given the lack of a direct percutaneous window and the evidence of poor surgical candidacy (abdominal aortic aneurysm). (B) Endoscopy can establish the presence or absence of communication between the pseudocyst and pancreatic duct, and it can facilitate endoscopic ultrasound (EUS)-guided drainage. (C) Relatively anechoic pseudocyst demonstrated by EUS. (D) Wire access to the pseudocyst is achieved by a transgastric endoscopic approach, and a cystogram is obtained. (E) Final drainage image. Tubes include a pancreatic duct stent (arrowhead), two cystgastrostomy stents (black arrow), and a temporary nasocystic tube (white arrow). The nasocystic tube is placed to suction and eventually removed. (F) Contrast-enhanced computed tomography shows a collapsed pseudocyst as well as a cystgastrostomy stent (white arrow).
Percutaneous drainage of pseudocysts is performed under CT or ultrasound guidance using the Seldinger or trocar technique. The access route depends on the target location and may require a transperitoneal, retroperitoneal, transgastric, transduodenal, or transhepatic approach. Pancreatic duct anatomy plays an important role in determining the success of PED. In a study of 253 patients between 1985 and 2000, longer hospital stays and increased time required for drainage was associated with PED in patients who had main pancreatic duct communication with the pseudocyst cavity.7 In the same study, it was found that normal duct anatomy was the only ideal setting for percutaneous drainage, but that PED was acceptable if a stricture was present in an otherwise normal duct. In other words, if the pancreatic duct is patent, the pseudocyst should be allowed to drain spontaneously over time unless it becomes infected, in which case a temporary PED may be used in addition to antibiotics. Both ERCP and MRI are effective means to demonstrate the patency of the pancreatic duct and its communication with a pseudocyst (Fig. 2). Even in patients who have superimposed infection of an existing pseudocyst requiring immediate treatment, it is not unusual for drains to be left in place for a month or longer (Fig. 3), leading to decreased patient satisfaction regardless of ultimate treatment success.8
Figure 2.

Magnetic resonance cholangiopancreatography with secretin administration. The pseudocyst (large arrow) communicates with the pancreatic duct, and there is a mid-pancreatic duct stricture (small arrow).
Figure 3.

Noncontrast-enhanced computed tomography shows percutaneous drainage of a peripancreatic pseudocyst (arrow).
When patients have pseudocysts associated with chronic pancreatitis, pancreatic necrosis, a strictured and dilated pancreatic duct, a suspected neoplasm, or when complications such as perforation or hemorrhage arise, surgery is still a first-line treatment option.9 Whether laparoscopic or open, surgery allows for cystgastrostomy or cystenterostomy marsupialization, debridement, and resection, as indicated by the presenting pathology. Laparoscopic surgery decreases wound morbidity compared with the open approach and is associated with shorter hospital stays and less time needed for recovery.9 Furthermore, laparoscopic drainage may have a lower risk of complications associated with hemorrhage than endoscopic drainage, likely attributable to superior vascular visualization. Pancreatic necrosis is a classic surgical case because it involves nonliquefactive tissue that typically does not respond to drain placement and suction. Surgical options include open evacuation, laparoscopic evacuation, or endoscopic debridement through sheaths placed after access has been obtained by image-guided percutaneous techniques in interventional radiology.10 Foci of liquefactive necrosis are sometimes treated by PED during the course of the patient's management, particularly when infected.
Isolated pancreatic abscesses may be treated by PED or endoscopic techniques. Surgical evacuation is still necessary in a minority of refractory cases. In one series of pancreatic abscesses with no coexisting pseudocysts, necrosis, or APFCs, 59 patients underwent PED and antibiotic therapy.11 A total of 51 patients were cured and discharged from the hospital without the need for surgery. Eight patients (14%) treated with PED failed initial therapy; five of those later required surgery for cure. The reported mean duration with a catheter in place was 33 days. Other authors cited variable success in using PED as primary therapy for pancreatic abscess, and they report problems such as drains clogged by necrotic tissue.12 Lee et al described 30 patients who were initially given PED, of which 16 later required surgery, and the mean duration with catheter in place was 5 weeks.13 Successful drainage of pancreatic abscesses by endoscopic ultrasound-guided techniques has been described with clinical success rates of 80 to 90% in some reports, and this option remains the principal first-line alternative to PED for these cases.14 It is understood that successful PED for pancreatic abscess requires close monitoring throughout the often protracted duration of therapy, appropriate antibiotics according to culture and sensitivity, and often catheter manipulation or replacement.
Conclusion
Patients presenting with pancreatitis are at risk for several associated morbidities. Although close monitoring and supportive care are often enough to allow for uncomplicated resolution, certain cases require a more invasive approach. Although laparoscopy and surgery have been shown to provide more desirable outcomes than interventional techniques for specific scenarios, percutaneous techniques in interventional radiology have been shown to be an important part of a combined approach and can be used to augment other therapeutic choices. With careful patient evaluation, clinicians should be able to offer the most safe and efficient options for the treatment of complications arising from pancreatitis.
References
- 1.Memi A, Parildar M. Intervent radiological treatment in complications of pancreatitis. Eur J Radiol. 2002;43(3):577–582. doi: 10.1016/s0720-048x(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 2.Thoeni R F. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262(3):751–764. doi: 10.1148/radiol.11110947. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz J M, Funaki B S, Ray C E Jr. et al. ACR Appropriateness Criteria on percutaneous catheter drainage of infected fluid collections. J Am Coll Radiol. 2009;6(12):837–843. doi: 10.1016/j.jacr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 4.D'Egidio A, Schein M. Pancreatic pseudocysts: a proposed classification and its management implications. Br J Surg. 1991;78(8):981–984. doi: 10.1002/bjs.1800780829. [DOI] [PubMed] [Google Scholar]
- 5.Vila J J, Carral D, Fernández-Urien I. Pancreatic pseudocyst drainage guided by endoscopic ultrasound. World J Gastrointest Endosc. 2010;2(6):193–197. doi: 10.4253/wjge.v2.i6.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahen D, Rauws E, Fockens P, Weverling G, Huibregtse K, Bruno M. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy. 2005;37(10):977–983. doi: 10.1055/s-2005-870336. [DOI] [PubMed] [Google Scholar]
- 7.Nealon W H, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage) Ann Surg. 2002;235(6):751–758. doi: 10.1097/00000658-200206000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantasdemir M, Kara B, Kantarci F, Mihmanli I, Numan F, Erguney S. Percutaneous drainage for treatment of infected pancreatic pseudocysts. South Med J. 2003;96(2):136–140. doi: 10.1097/01.SMJ.0000050682.65270.38. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Cruz L, Sáenz A, Astudillo E, Pantoja J P, Uzcátegui E, Navarro S. Laparoscopic pancreatic surgery in patients with chronic pancreatitis. Surg Endosc. 2002;16(6):996–1003. doi: 10.1007/s00464-001-9065-y. [DOI] [PubMed] [Google Scholar]
- 10.Tang L J, Wang T, Cui J F. et al. Percutaneous catheter drainage in combination with choledochoscope-guided debridement in treatment of peripancreatic infection. World J Gastroenterol. 2010;16(4):513–517. doi: 10.3748/wjg.v16.i4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.vanSonnenberg E, Wittich G R, Chon K S. et al. Percutaneous radiologic drainage of pancreatic abscesses. AJR Am J Roentgenol. 1997;168(4):979–984. doi: 10.2214/ajr.168.4.9124154. [DOI] [PubMed] [Google Scholar]
- 12.Mithöfer K, Mueller P R, Warshaw A L. Interventional and surgical treatment of pancreatic abscess. World J Surg. 1997;21(2):162–168. doi: 10.1007/s002689900209. [DOI] [PubMed] [Google Scholar]
- 13.Lee M J, Rattner D W, Legemate D A. et al. Acute complicated pancreatitis: redefining the role of interventional radiology. Radiology. 1992;183(1):171–174. doi: 10.1148/radiology.183.1.1549667. [DOI] [PubMed] [Google Scholar]
- 14.Seewald S, Ang T L, Teng K C, Soehendra N. EUS-guided drainage of pancreatic pseudocysts, abscesses and infected necrosis. Dig Endosc. 2009;21 01:S61–S65. doi: 10.1111/j.1443-1661.2009.00860.x. [DOI] [PubMed] [Google Scholar]