Abstract
Percutaneous splenic biopsy and drainage are relatively safe and accurate procedures. The risk of major complication (1.3%) following percutaneous splenic biopsy does not exceed that of other solid intra-abdominal organ biopsies, and it has less morbidity and mortality than splenectomy. Both computed tomography and ultrasound can be used to provide image guidance for biopsy and drainage. The safety profile of fine-needle aspiration cytology is better than core needle biopsy, but core biopsy has superior diagnostic accuracy.
Keywords: spleen, biopsy, drainage, computed tomography, ultrasound, interventional radiology
Objectives: Upon completion of this article, the reader will be able to list the main indications and contraindications for splenic biopsy and drainage, describe the techniques used for image-guided splenic biopsy and drainage, and identify the advantages and limitations of percutaneous splenic biopsy and drainage and the possible complications that can occur.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Historically, radiologists have been reluctant to undertake percutaneous image-guided splenic interventions due to the perceived high risk of severe hemorrhage that could potentially lead to emergent splenectomy. This belief likely originated from high complication rates reported following percutaneous image-guided splenic biopsy using 14-gauge core biopsy needles. Splenic lesions, however, can be difficult to define on the basis of clinical and radiologic findings alone,1,2 and percutaneous core needle biopsy (CNB) of the spleen can provide important additional information. Recent use of smaller gauge needles for CNB (≤18 gauge) has a lower rate of major complications (1.9%). This is believed to be acceptable because it is comparable with major complication rates associated with other abdominal solid organ biopsies (0.5 to 3.3% for the liver and 0.7 to 6.3% for the kidney).3
Indications and Contraindications
The principal indications for splenic biopsy include the need to characterize a cystic or solid splenic lesion, or to elucidate the etiology of splenomegaly of unknown cause. Lymphoma, a strong clinical suspicion of lymphoma, and a splenic mass in the presence of an extrasplenic malignancy represent the most common clinical indications for CNB in practice.4,5 This is because imaging alone cannot characterize many splenic lesions; splenic involvement can occur in up to 40% of patients with either non-Hodgkin or Hodgkin lymphoma.4
Lymphomatous involvement of the spleen is of prognostic importance and has an impact on treatment planning1,2,6,7(Fig. 1). Common primary malignancies that metastasize to the spleen include breast, lung, ovary, melanoma, and colon2 (Fig. 2). However, isolated splenic metastases are rare, and if there is another site of metastatic disease, biopsy of these should be considered before a decision is made to biopsy the spleen.8 Another diagnostic challenge facing radiologists is single or multiple splenic lesions in the setting of known malignancy. Because many of these patients are on immunosuppressant treatment and the most likely differential diagnosis includes metastatic disease or infection with abscess or microabscess formation, the treatment of which entities is vastly different; thus there is a need for a definitive diagnosis4 (Fig. 3). Multiple splenic lesions are also a feature of many benign conditions including sarcoidosis and hamartomas (Figs. 4 and 5). Splenic biopsy is also indicated for the assessment of splenomegaly of uncertain etiology in the absence of a focal lesion that can be caused by lymphoma or extramedullary hematopoiesis (Fig. 6 and 7). Other indications for splenic CNB include assessment of pyrexia of unknown origin and the investigation of cystic or mixed solid-cystic splenic pathology (Fig. 8). Biopsy may also be performed in a combined focal-nonfocal manner for innumerable tiny splenic lesions, given the high likelihood that a random biopsy will sample abnormal tissue (Fig. 9).
Figure 1.

Splenic biopsy in a 75-year-old man with splenomegaly due to marginal zone lymphoma. (A) Ultrasound-guided fine-needle aspiration and core biopsy (arrow) demonstrated no malignancy. (B) Computed tomography performed 3 hours subsequent to biopsy due to pain showed no hematoma around the spleen (arrow). Subsequent elective splenectomy showed marginal zone lymphoma at pathology.
Figure 2.
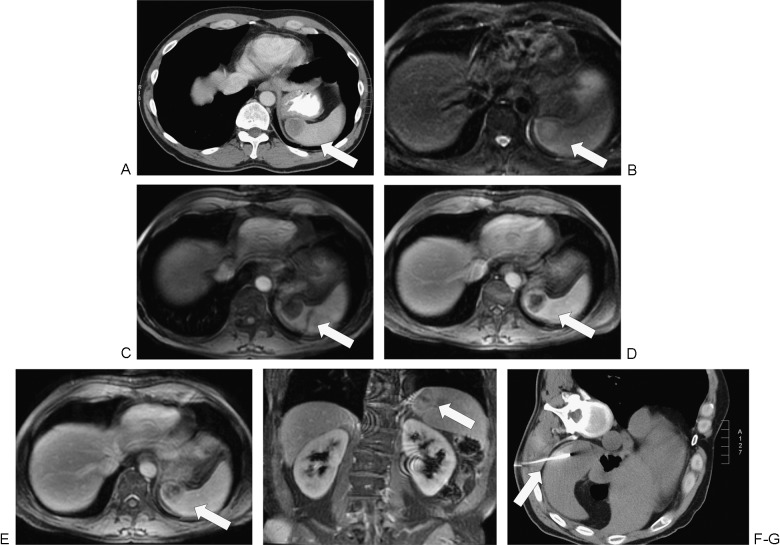
Splenic biopsy in a 45-year-old man with metastatic disease to the spleen from non–small cell lung cancer. (A) Staging computed tomography (CT) demonstrates a hypoattenuating lesion in the liver spleen (arrow) but no suspicion for other organ metastases. (B) T2 fat-saturated magnetic resonance imaging (MRI) demonstrates a hyperintense lesion in the spleen (arrow) that distorts the splenic contour. (C) The lesion is hypointense (arrow) on T1-weighted MRI. (D) Delayed (portal venous phase) post contrast imaging shows heterogenous enhancement of the mass (arrow). (E, F) More delayed imaging (180 seconds post contrast administration) in the axial and coronal planes demonstrates washout of the periphery of the lesion (arrow). (G) The lesion was suspicious for metastasis by imaging, and the spleen was the only site of metastatic disease. Splenic biopsy was performed under CT guidance (arrow), which confirmed the diagnosis of metastatic lung carcinoma.
Figure 3.
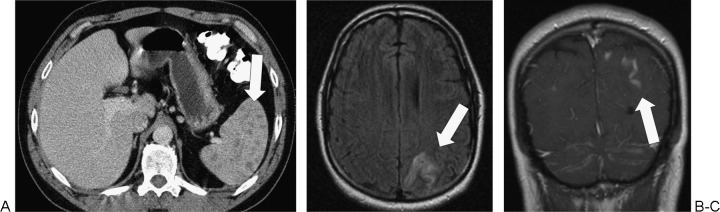
Cryptococcus of spleen and meninges in a 44-year-old man with CD4 lymphopenia (not related to human immunodeficiency virus). (A) Axial computed tomography (CT) of the abdomen demonstrates multiple tiny splenic hypodensities (arrow). (B) Axial fluid attenuated inversion recovery magnetic resonance imaging (MRI) of the brain demonstrates hyperintensity in the left posterior parietal cortex (arrow). (C) Coronal post intravenous contrast MRI of the brain demonstrates leptomeningeal enhancement compatible with cryptococcus infection(arrow).
Figure 4.
Splenic biopsy in a 35-year-old woman with multiple splenic lesions due to sarcoidosis. (A) Axial computed tomography demonstrates multiple small hypoenhancing splenic lesions (arrow). (B) The lesions are fluorodeoxyglucose (FDG) avid on positron emission tomography scan (arrow). There were no other areas of abnormal increased FDG uptake. (C) Ultrasound-guided splenic biopsy was performed (arrow), and the diagnosis of sarcoidosis was confirmed.
Figure 5.
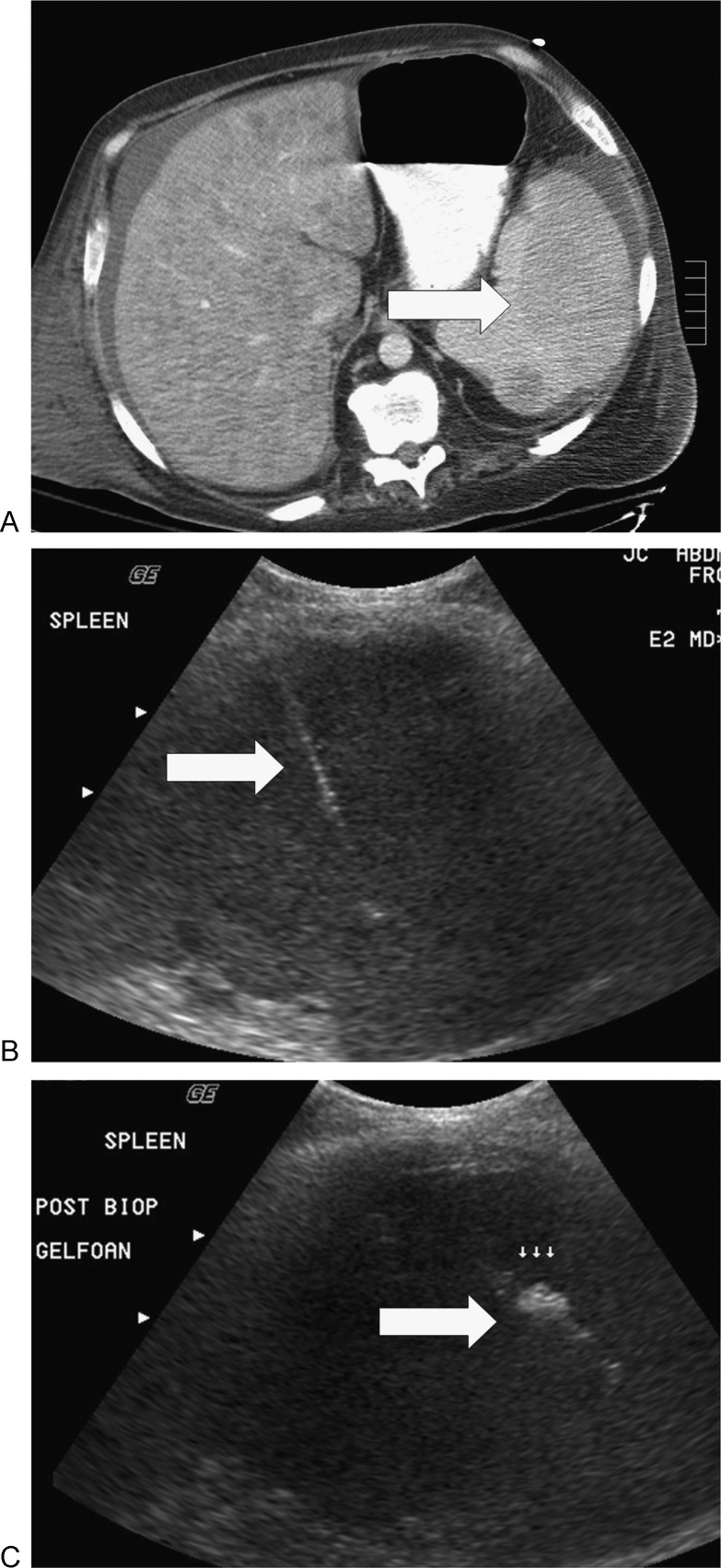
Biopsy of spleen in a man with a history of alcohol abuse. (A) He presented with a psoas abscess, pyrexia, and multiple splenic lesions (arrow). (B) Computed tomography guided fine-needle aspiration cytology and core biopsy (arrow) did not show malignancy or infection. (C) Gelfoam (arrow) was injected along the needle tract at the end of the procedure. The patient succumbed to multiorgan failure; multiple hamartomas were diagnosed at autopsy.
Figure 6.
Splenic biopsy in a 53-year-old man with anemia and splenomegaly, hepatitis C, metastatic testicular seminoma, and renal insufficiency with prior failed renal transplant. (A) There was uniformly mildly increased fluorodeoxyglucose uptake in the spleen (arrow). (B) Fine-needle aspiration cytology and core biopsy (arrow) demonstrated extramedullary hematopoiesis. (C) The patient had perisplenic hematoma (arrow) after biopsy that was successfully treated medically. The patient had a splenectomy 2 months later due to persistent concerns for lymphoma, but the diagnosis of extramedullary hematopoiesis was confirmed following surgical resection.
Figure 7.
Splenic biopsy in a 41-year-old man with splenomegaly and pancytopenia. (A) Axial computed tomography demonstrates a uniformly enlarged spleen (arrow) without focal mass. (B) Ultrasound-guided fine-needle aspiration cytology and needle core biopsy (arrow) was inconclusive. Chronic lymphocytic lymphoma was diagnosed following splenectomy.
Figure 8.
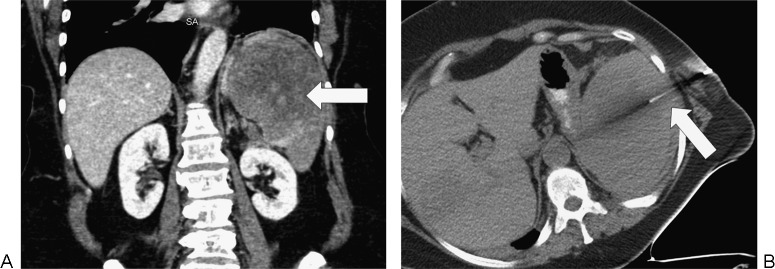
Splenic biopsy in a 66-year-old woman with splenomegaly due to lymphoma. (A) Axial computed tomography (CT) demonstrates splenomegaly and a hypoenhancing infiltrative process (arrow). (B) CT-guided fine-needle aspiration cytology and core biopsy of the spleen (arrow) showed fibrosis and necrosis. Subsequent splenectomy demonstrated lymphoma.
Figure 9.

Positron emission tomography (PET) scan and computed tomography (CT) of the abdomen in a 73-year-old woman with chronic lymphocytic lymphoma. (A) There is avid fluorodeoxyglucose uptake in the spleen on PET (arrow). (B) There are multiple tiny nodules in the spleen (arrow) on contrast-enhanced CT.
Splenic drainage is an uncommon procedure because the spleen is an uncommon site for infection (Fig. 10). Tuberculosis, fungal, and bacterial infections are all known to cause abscess formation within the spleen.2 Splenic abscess formation is more common in an immunocompromised patient. Percutaneous drainage of a splenic abscess has a reported success rate of 80 to 100%,8,9,10 although the presence of multiple collections or internal septations can limit the success of percutaneous drainage. Ultrasound (US) can be useful when considering the suitability of an abscess or fluid collection for percutaneous drainage because it is much superior to computed tomography (CT) for determining the internal architecture of a collection and for excluding the presence of loculation or septations that can have a severe impact on the likelihood of successful percutaneous drainage.
Figure 10.

Splenic drainage in a 71-year-old woman with splenic abscess following resection of a left lower lobe metastatic deposit from pancreatic cancer complicated by diaphragmatic injury. (A) Axial computed tomography (CT) demonstrates a collection in the spleen (arrow) and around the spleen (arrowhead). (B) Drainage catheter was inserted by tandem trochar technique under CT guidance.
There are few absolute contraindications to splenic biopsy or drainage, which are similar to those of any other solid abdominal organ biopsy. Uncorrectable coagulopathy, severe cardiopulmonary compromise, hemodynamic instability, lack of a safe biopsy/drainage pathway, and an uncooperative patient should prompt reconsideration.11
Patient Care
Patient preparation prior to splenic drainage closely resembles prebiopsy preparation for other organ biopsy. Patients should be fasting for 8 hours prior to administration of conscious sedation. Conscious sedation is typically administered using fentanyl and midazolam. Conscious sedation should be titrated in small increments, if required, while monitoring the patient's blood pressure, heart rate, and oxygen saturation level. Emergency resuscitation equipment should always be located nearby, and oxygen should be administered to the patient via nasal prongs. Recent blood and coagulation profiles should be available prior to the procedure. The international normalized ratio should be corrected if >1.5. Unfractionated heparin should be stopped at least 90 minutes prior to the procedure so that the activated partial thromboplastin time is <1.5 times normal, and the platelet count should be >50,000/μL. If possible, aspirin and clopidogrel should be stopped 5 days preprocedure, and fractionated heparin should be withheld for 24 hours.12 Post biopsy/drainage, the patient's vital signs and biopsy site should be checked every 15 minutes for the first hour and every 30 minutes for the next 3 hours. The patient should be closely monitored for 4 hours, and if there are no signs or symptoms suggestive of a complication, discharge home is possible after biopsy. Lucey et al reported that 92.5% of their patients had a biopsy undertaken on an outpatient basis.5 Most patients who require drain placement require overnight monitoring after the procedure.
Biopsy Technique
After a review of the patient's clinical history and confirmation of the indications and the absence of contraindications, available previous imaging should be reviewed prior to CNB. This should help determine the appropriate method of image guidance and CNB trajectory. Where possible, a short direct needle path is desired; however, the colon, kidney, lung, and pleura should be avoided if possible. Although this is best achieved with a subcostal approach, traversal of the pleura is generally feasible and safe. The patient should be positioned based on the anticipated biopsy route, which is planned following review of preprocedure imaging. Placing the patient into a comfortable, stable position and administration of conscious sedation prior to commencing biopsy help ensure optimal patient cooperation. Conscious sedation reduces lung volumes and tends to raise the level of the hemidiaphragm and hence the spleen. Therefore, the choice of needle trajectory using CT and local anesthesia should be administered after conscious sedation has had time to take effect. We often prefer to position the patient in the left lateral decubitus position for many splenic biopsies because this maneuver compresses and displaces the ipsilateral lung away from the spleen and helps reduce diaphragmatic excursion and splenic movement.
Unlike splenic biopsy, pleural transgression should be avoided if possible for splenic collection drainage due to the risk of infecting another anatomical compartment (i.e., the pleural space). If there are multiple splenic lesions present, a peripheral lesion remote from the splenic hilum helps minimizes the amount of normal splenic parenchyma that is traversed and reduces bleeding risk.3,4,5,13 Ideally, some normal splenic tissue should be traversed prior to accessing a lesion to help tamponade bleeding from the lesion.
The choice of needle is generally based on the nature of the lesion and the clinical condition of the patient. Splenic sampling may be performed using cutting needles for core biopsy or fine-bore needles for aspiration cytology or microbiology. For a histologic diagnosis, a CNB is generally required in addition to fine-needle aspiration (FNA). Lower complication rates following splenic CNB are described using 18-gauge needles compared with 14-gauge needles3; 18-gauge needles have comparable diagnostic yield compared with 14-gauge needles. We prefer to use a coaxial system composed of an 18-gauge CNB and a 17-gauge guide needle because this allows multiple biopsies with a single needle pass through the spleen.
In situations where only a cytologic sample can be obtained, such as in the presence of coagulopathy, fine-needle aspiration cytology (FNAC) is performed using a 20- to 23-gauge Chiba needle (Cook Medical, Bloomington, IN). FNAC has a better safety profile than CNB but a lower diagnostic accuracy. This difference is particularly important in the assessment of lymphoma3,6; the diagnostic accuracy of FNAB is limited in lymphoma due to the difficulty in subtyping lymphoma, which is increasingly important with the development of targeted treatment for specific lymphoma subtypes.14,15,16 For the first FNAC specimen, we advocate the application of suction using an empty 10-mL syringe and needle excursion to shave cells from a lesion. If a large amount of blood is aspirated with the first specimen, subsequent FNAC samples are acquired without the application of suction because blood can hinder cytologic assessment. Our normal practice is to obtain both FNAC and CNB at the time of splenic sampling. FNA of a cystic lesion is often performed prior to consideration for drainage to assess the contents of a collection. If the contents are cystic, the lesion can be completely aspirated and a sample sent for microscopy, and culture and sensitivity. If a cystic lesion is large and requires drainage, aspiration prior to drain placement should be limited so as not to collapse the collection and to preserve the target.9,10 Aspiration of lesions containing solid and cystic elements is useful before obtaining CNB because removal of the cystic component can provide a more solid target for biopsy.
Typically two to three good quality core biopsies and two to three sets of slides prepared following FNA are taken at the time of sampling The presence of an on-site pathologist or cytopathologist can help minimize the amount of tissue required or prompt additional sampling because samples can be immediately assessed for adequacy. An advantage of requesting the help of a cytology colleague in this setting is that it frequently reduces the requirement for rebiopsy at a later date. It is also important to take additional samples for the purposes of flow cytometry if lymphoma is a consideration.
Drainage Technique
Drainage rather than aspiration is usually performed for collections >3 cm3 (Fig. 11). Abscess drainage can be performed under CT or US guidance, depending on operator preference. Both Seldinger and tandem trochar techniques are feasible (Fig. 12). Some authors favor the Seldinger technique for accurate drain placement; several series, however, report safe and accurate placement using a trochar technique.5,8,10 After the needle has been placed within the collection under imaging guidance, an aspirate should be taken to determine the consistency of the aspirated fluid, which can help in determining the size of catheter that will be needed. Normally, 8 to 12F pigtail catheters are selected (Fig. 13). When the catheter is in place it should be secured and left on gravity drainage. The catheter should be flushed with 10 mL of normal saline every 8 hours to maintain patency. The catheter can be removed after the patient's clinical status improves. The patient's white cell count should return to normal levels, pyrexia should resolve, and there should be <10 mL drainage from the catheter over the 24-hour period prior to catheter removal.8 The spleen should also be re-imaged prior to the removal of the catheter to ensure there is no residual collection; catheter injection under fluoroscopic guidance may also be useful to assess the presence of a fistula.
Figure 11.
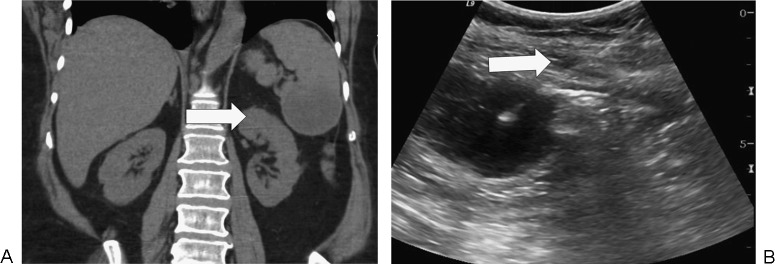
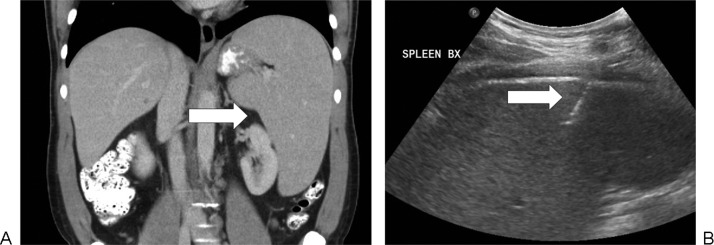
Splenic abscess drainage in a 66-year-old woman with small bowel obstruction due to a cecal mass. (A) There is a collection (arrow) in the lower spleen on coronal noncontrast computed tomography. (B) Drainage catheter (arrow) was inserted under ultrasound guidance.
Figure 12.
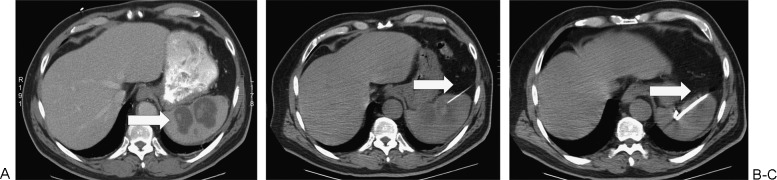
Splenic abscess drainage in a 67-year-old woman. (A) There is a bilobed collection (arrow) in the spleen on axial computed tomography (CT). (B) A 20-gauge Chiba needle (arrow) placed first under CT guidance. (C) Catheter (arrow) placed by tandem trochar technique under CT guidance.
Figure 13.

Splenic abscess drainage in a 46-year-old man who presented with abdominal pain due to splenic abscess, later attributed to infective endocarditis. (A) Drainage catheter (arrow) was inserted on the computed tomography (CT) table under ultrasound guidance. (B) CT confirmed catheter position (arrow) prior to aspiration.
Alcohol Ablation of Splenic Cysts
Alcohol ablation is widely used in the treatment of solid abdominal organ cysts including splenic cysts. Alcohol ablation works by causing protein denaturation, cell death, and fibrous scarring, and it should be considered in the setting of a large symptomatic splenic cyst. Most cysts requiring treatment with alcohol ablation are large and therefore easily accessible using US guidance. An 8 to 10F drainage catheter is initially placed, as described earlier; care should be taken to use a catheter that is alcohol compatible to prevent catheter breakdown. The contents of the cyst are drained dry and sent for analysis. Before alcohol is injected into the catheter, a small amount of dilute contrast material should be injected through the catheter under fluoroscopic or CT guidance to ensure the catheter tip is in the correct position, there is no peritoneal spill, and there is no communication with blood vessels, bowel, or the kidney. If there is any leakage of contrast material, alcohol ablation is contraindicated. Approximately 50% of the total drainage volume is then replaced with 95% ethyl alcohol. The amount of alcohol injected is at the discretion of the radiologist but should generally not exceed 100 mL in one setting. The alcohol is left within the cystic cavity for 20 to 30 minutes, and during this time the patient should rotate to ensure adequate coating of all the walls of the cyst. Alcohol ablation works on the cystic cavity lining, and thus adequate exposure of alcohol to the entire wall is required to treat the cyst and minimize recurrence. The alcohol should then be removed and US or CT performed postprocedure to ensure that all the contents have been removed before removal of the drainage catheter. Follow-up imaging should be performed 3 months postprocedure to ascertain if there is any recurrence.10,17,18,19,20,21,22 Some splenic cysts require repeated sclerotherapy, although initial relapse of a cyst may not represent true recurrence but a transient inflammatory/reactive response that will regress over time.23
Complications related to alcohol ablation are similar to those of catheter drainage including hemorrhage, damage to adjacent organs, and infection. Some patients experience severe pain due to leakage of alcohol into the peritoneal cavity; this can be treated with local anesthesia and/or intravenous analgesics (e.g., fentanyl).10,17
Imaging Guidance for Biopsy and Drainage of the Spleen
The method of image guidance is generally decided by operator preference and the ease with which a lesion can be identified. CT and US are both appropriate for guiding percutaneous splenic intervention. For lesions visible on both modalities, US has the added benefit of allowing real-time guidance in multiple planes without radiation exposure. Real-time guidance is also feasible using CT fluoroscopy, which may reduce procedural time compared with conventional CT.
Ultrasound
US enables real-time multiplanar needle or catheter guidance; however, not all lesions are easily identifiable on US. An initial US using a 3.5-MHz curvilinear probe is therefore important to locate the lesion of interest and to choose the optimum skin entry site and needle trajectory. Color Doppler can be used to ensure there are no major vessels along the planned biopsy tract. We favor guiding needles and catheters along the longitudinal axis of a covered probe to allow optimum visualization of the needle tip at all times. Many authors favor needle advancement under suspended respiration. It is important to confirm that the biopsy needle tip lies within or just proximal to the lesion prior to sample acquisition. It is also advisable to confirm and document needle placement after a sample has been taken prior to removing the needle gun. This helps maximize sample adequacy and is also important at a later stage if biopsy fails to yield a diagnosis. We often perform drainage procedures using US on a CT table. This allows quick catheter placement and immediate confirmation of adequacy of drainage at a single visit. If US is not sufficient for image guidance, CT can be used for needle or catheter guidance with minimal disruption to the patient. One of the advantages of CT over US is that it lessens the risk of bowel transgression and also allows better visualization of deep-seated lesions or collections, thus reducing the risk of unintentional injury to intervening organs or vessels.
Computed Tomography Guidance
If CT is the modality of choice, a spiral noncontrast CT should be performed of the spleen with a radiopaque marker or grid over the area of interest to identify the shortest and safest route for biopsy. Once the route has been identified, the skin entry site can be marked using the CT gantry laser light and the radiopaque grid for the Z and X coordinates. Both conventional CT and CT fluoroscopy are feasible for needle and catheter insertion. It is important to confirm safe needle trajectory and needle placement during splenic procedures under CT. It is preferable to minimize the number of times the splenic capsule is traversed by the access or biopsy needle, and so the needle trajectory should be optimized prior to entering the spleen; in particular, pneumothorax and hematoma formation should be sought on all images.
Diagnostic Accuracy and Safety of Percutaneous Splenic Biopsy
The spleen is an uncommon site for solid organ metastases and rarely the only site of metastatic disease. Other conditions that affect the spleen include lymphoma, benign splenic tumors, infection (including tuberculosis and fungal infections), and infiltrative processes like sarcoidosis.1,2 As mentioned previously, with the increased use of immunosuppressants, patients with known extrasplenic malignancies are more susceptible to infection, which can be difficult to differentiate from metastasis on imaging and clinical history alone.4
One meta-analysis showed a sensitivity of 86.8% (95% confidence interval [CI], 78.2 to 92.4) and specificity of 96.8% (95% CI, 90.4 to 99) for CNB alone, and a sensitivity of 84.1% (95% CI, 77 to 89.3) and specificity of 92.5% (95% CI, 35.6 to 89.4) for FNAC. There was considerable heterogeneity in the specificity of the FNAC alone, which we felt was likely secondary to the need for a tissue sample for the diagnosis of lymphoma and inadequate cytologic sample at the time of FNAC.3 However, according to one large series, the greatest diagnostic accuracy for lymphoma is obtained in the setting of combined FNAB and CNB.24
In one study of US-guided CNB of the spleen, all conclusive results changed patient management.25 There are also several published case reports and series demonstrating prolonged patient survival post metastatectomy/splenectomy for a single splenic metastasis.26,27,28 These findings highlight the value of percutaneous splenic biopsy in the clinical setting of undefined splenic lesions, as an aid to future patient management and treatment planning.
Although diagnostic accuracy is likely higher with splenectomy, percutaneous image-guided splenic biopsy is less invasive and associated with a lower complication rate. Postsplenectomy patients have a high morbidity mainly due to the increased risk of a streptococcal infection.29,30 Laparoscopic splenic biopsy has similar diagnostic accuracy to PNB but is more invasive, time consuming, and expensive. The advantage of laparoscopic splenic biopsy over PNB is the ability to see the tract and any postbiopsy bleeding. This can be dealt with at the time of biopsy by cauterizing the tract.31,32 However, laparoscopic splenic biopsy is limited with regard to the visualization of intrasplenic lesions.6
The meta-analysis also showed an overall complication rate of 4.2% for splenic biopsy; however, with the removal of biopsies undertaken with a 14-gauge needle, the overall complication rate fell to 3.9%. The major complication rate was 1.9% for CNB and 1.3% for FNAC. This compares with a higher morbidity (8.6 to 37%) and mortality (0 to 2.9%) following splenectomy.3,29,30
Hemorrhage can be a major complication following splenic intervention. Small-volume perisplenic blood, which is asymptomatic postprocedure, is a minor complication. However, there can be significant blood loss postsplenic intervention that may require emergent splenectomy. In the meta-analysis by McInnes et al,3 9 of the 10 major complications were related to hemorrhage. There were two cases requiring splenectomy (including one postbiopsy with a 14-gauge needle). In the retrospective study by Lucey et al,5 there were 3 cases in 39 procedures (7.7%) where there was severe hemorrhage postbiopsy, and all of these cases required splenectomy. There were no reported deaths. Large-volume postbiopsy hemorrhage should be managed with fluid resuscitation and blood transfusion. If the patient is not responding to these measures, there should be consideration of transcatheter embolization or splenectomy.10
No randomized trials have been performed with regard to the effectiveness of embolization of the biopsy tract postprocedure. Singh et al reported in their institution that they inject a hemostatic gelatin sponge along the biopsy tract in most cases.10 A study performed on dogs showed a significant reduction in the amount of hemorrhage postbiopsy when postbiopsy bleeding reduction measures were taken. The study compared radiofrequency cauterization, splenic biopsy tract embolization using absorbable gelatin sponge and Histoacryl/Lipiodol mixture plugging to control CNB sites. However, on CT imaging performed 3 days later, there were no significant complications at any of the biopsy sites.33 Multiple other studies published using both CNB and FNAB do not mention the use of biopsy tract embolization and report acceptable postprocedure complication rates.4,5,6,8,13,34,35 As a result, biopsy tract embolization or cauterization should be at the operator's discretion and considered for high-risk cases.
Other major complications include pneumothorax, colonic injury, and renal injury. Although these are reported less frequently in the literature,3 minor complications after percutaneous splenic interventions include local pain, small subcapsular hematoma, and small-volume hemoperitoneum.3
Conclusion
Percutaneous image-guided splenic intervention is a safe diagnostic procedure with complication rates similar to those of other solid abdominal organs. It has a specificity of 96.4% and sensitivity of 87%3 and a better morbidity and mortality profile than splenectomy. It should be considered as a safer and first-line option in the evaluation of splenic lesions. Although choice of image guidance and needle type should follow operator preference, we recommend dual sampling with FNAC and CNB using a coaxial system to maximize diagnostic yield. Percutaneous image-guided drainage of splenic abscesses and catheter placement are also safe and efficacious treatments that can reduce/negate the need for splenectomy.
References
- 1.Bhatia K, Sahdev A, Reznek R H. Lymphoma of the spleen. Semin Ultrasound CT MR. 2007;28(1):12–20. doi: 10.1053/j.sult.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Kamaya A, Weinstein S, Desser T S. Multiple lesions of the spleen: differential diagnosis of cystic and solid lesions. Semin Ultrasound CT MR. 2006;27(5):389–403. doi: 10.1053/j.sult.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.McInnes M D, Kielar A Z, Macdonald D B. Percutaneous image-guided biopsy of the spleen: systematic review and meta-analysis of the complication rate and diagnostic accuracy. Radiology. 2011;260(3):699–708. doi: 10.1148/radiol.11110333. [DOI] [PubMed] [Google Scholar]
- 4.Tam A, Krishnamurthy S, Pillsbury E P. et al. Percutaneous image-guided splenic biopsy in the oncology patient: an audit of 156 consecutive cases. J Vasc Interv Radiol. 2008;19(1):80–87. doi: 10.1016/j.jvir.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Lucey B C, Boland G W, Maher M M, Hahn P F, Gervais D A, Mueller P R. Percutaneous nonvascular splenic intervention: a 10-year review. AJR Am J Roentgenol. 2002;179(6):1591–1596. doi: 10.2214/ajr.179.6.1791591. [DOI] [PubMed] [Google Scholar]
- 6.Cavanna L, Civardi G, Fornari F. et al. Ultrasonically guided percutaneous splenic tissue core biopsy in patients with malignant lymphomas. Cancer. 1992;69(12):2932–2936. doi: 10.1002/1097-0142(19920615)69:12<2932::aid-cncr2820691211>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Cavanna L, Lazzaro A, Vallisa D, Civardi G, Artioli F. Role of image-guided fine-needle aspiration biopsy in the management of patients with splenic metastasis. World J Surg Oncol. 2007;5:13. doi: 10.1186/1477-7819-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang M, Kalra N, Gulati M, Lal A, Kochhar R, Rajwanshi A. Image guided percutaneous splenic interventions. Eur J Radiol. 2007;64(1):140–146. doi: 10.1016/j.ejrad.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Thanos L, Dailiana T, Papaioannou G, Nikita A, Koutrouvelis H, Kelekis D A. Percutaneous CT-guided drainage of splenic abscess. AJR Am J Roentgenol. 2002;179(3):629–632. doi: 10.2214/ajr.179.3.1790629. [DOI] [PubMed] [Google Scholar]
- 10.Singh A K, Shankar S, Gervais D A, Hahn P F, Mueller P R. Image-guided percutaneous splenic interventions. Radiographics. 2012;32(2):523–534. doi: 10.1148/rg.322115135. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Wallace M J, Cardella J F, Kundu S, Miller D L, Rose S C. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21(7):969–975. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Malloy P C, Grassi C J, Kundu S. et al. Standards of Practice Committee with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20(7, suppl):S240–S249. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Keogan M T, Freed K S, Paulson E K, Nelson R C, Dodd L G. Imaging-guided percutaneous biopsy of focal splenic lesions: update on safety and effectiveness. AJR Am J Roentgenol. 1999;172(4):933–937. doi: 10.2214/ajr.172.4.10587123. [DOI] [PubMed] [Google Scholar]
- 14.Caraway N P, Fanning C V. Use of fine-needle aspiration biopsy in the evaluation of splenic lesions in a cancer center. Diagn Cytopathol. 1997;16(4):312–316. doi: 10.1002/(sici)1097-0339(199704)16:4<312::aid-dc2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Lindgren P G, Hagberg H, Eriksson B, Glimelius B, Magnusson A, Sundström C. Excision biopsy of the spleen by ultrasonic guidance. Br J Radiol. 1985;58(693):853–857. doi: 10.1259/0007-1285-58-693-853. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman S, Libson E, Maly B, Lebensart P, Ben-Yehuda D, Bloom A I. Imaging-guided percutaneous splenic biopsy using a 20- or 22-gauge cutting-edge core biopsy needle for the diagnosis of malignant lymphoma. AJR Am J Roentgenol. 2003;181(4):1025–1027. doi: 10.2214/ajr.181.4.1811025. [DOI] [PubMed] [Google Scholar]
- 17.Lucey B C, Kuligowska E. Radiologic management of cysts in the abdomen and pelvis. AJR Am J Roentgenol. 2006;186(2):562–573. doi: 10.2214/AJR.04.1051. [DOI] [PubMed] [Google Scholar]
- 18.Simonetti G, Profili S, Sergiacomi G L, Meloni G B, Orlacchio A. Percutaneous treatment of hepatic cysts by aspiration and sclerotherapy. Cardiovasc Intervent Radiol. 1993;16(2):81–84. doi: 10.1007/BF02602983. [DOI] [PubMed] [Google Scholar]
- 19.Sawhney R, D'Agostino H B, Zinck S. et al. Treatment of postoperative lymphoceles with percutaneous drainage and alcohol sclerotherapy. J Vasc Interv Radiol. 1996;7(2):241–245. doi: 10.1016/s1051-0443(96)70769-8. [DOI] [PubMed] [Google Scholar]
- 20.Tikkakoski T, Mäkelä J T, Leinonen S. et al. Treatment of symptomatic congenital hepatic cysts with single-session percutaneous drainage and ethanol sclerosis: technique and outcome. J Vasc Interv Radiol. 1996;7(2):235–239. doi: 10.1016/s1051-0443(96)70767-4. [DOI] [PubMed] [Google Scholar]
- 21.Ustünsöz B, Akhan O, Kamiloğlu M A, Somuncu I, Uğurel M S, Cetiner S. Percutaneous treatment of hydatid cysts of the liver: long-term results. AJR Am J Roentgenol. 1999;172(1):91–96. doi: 10.2214/ajr.172.1.9888746. [DOI] [PubMed] [Google Scholar]
- 22.Kairaluoma M I, Leinonen A, Ståhlberg M, Päivänsalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg. 1989;210(2):208–215. doi: 10.1097/00000658-198908000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn S T, Han S Y, Yun E H. et al. Recurrence after percutaneous ethanol ablation of simple hepatic, renal, and splenic cysts: is it true recurrence requiring an additional treatment? Acta Radiol. 2008;49(9):982–986. doi: 10.1080/02841850802325974. [DOI] [PubMed] [Google Scholar]
- 24.Civardi G, Vallisa D, Bertè R. et al. Ultrasound-guided fine needle biopsy of the spleen: high clinical efficacy and low risk in a multicenter Italian study. Am J Hematol. 2001;67(2):93–99. doi: 10.1002/ajh.1085. [DOI] [PubMed] [Google Scholar]
- 25.Muraca S, Chait P G, Connolly B L, Baskin K M, Temple M J. US-guided core biopsy of the spleen in children. Radiology. 2001;218(1):200–206. doi: 10.1148/radiology.218.1.r01ja16200. [DOI] [PubMed] [Google Scholar]
- 26.Lauro S, Trasatti L, Capalbo C. et al. Solitary splenic recurrence of epithelial ovarian cancer: a case report and review. Anticancer Res. 2002;22(6B):3643–3645. [PubMed] [Google Scholar]
- 27.Trindade M RM, Blaya R, Trindade E N. Melanoma metastasis to the spleen: laparoscopic approach. J Minim Access Surg. 2009;5(1):17–19. doi: 10.4103/0972-9941.51316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Showalter S L, Hager E, Yeo C J. Metastatic disease to the pancreas and spleen. Semin Oncol. 2008;35(2):160–171. doi: 10.1053/j.seminoncol.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Cadili A, de Gara C. Complications of splenectomy. Am J Med. 2008;121(5):371–375. doi: 10.1016/j.amjmed.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Lehne G, Hannisdal E, Langholm R, Nome O. A 10-year experience with splenectomy in patients with malignant non-Hodgkin's lymphoma at the Norwegian Radium Hospital. Cancer. 1994;74(3):933–939. doi: 10.1002/1097-0142(19940801)74:3<933::aid-cncr2820740322>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Dagnini G, Cladironi M W, Marin G, Patella M. Laparoscopic splenic biopsy. Endoscopy. 1984;16(2):55–58. doi: 10.1055/s-2007-1018533. [DOI] [PubMed] [Google Scholar]
- 32.Werner T, Koch J, Frenzel C, Lohse A W, Denzer U W. Effectiveness and safety of minilaparoscopy-guided spleen biopsy: a retrospective series of 57 cases. Surg Endosc. 2012;26(9):2416–2422. doi: 10.1007/s00464-012-2190-y. [DOI] [PubMed] [Google Scholar]
- 33.Choi S H, Lee J M, Lee K H. et al. Postbiopsy splenic bleeding in a dog model: comparison of cauterization, embolization, and plugging of the needle tract. AJR Am J Roentgenol. 2005;185(4):878–884. doi: 10.2214/AJR.04.1395. [DOI] [PubMed] [Google Scholar]
- 34.Gómez-Rubio M, López-Cano A, Rendón P. et al. Safety and diagnostic accuracy of percutaneous ultrasound-guided biopsy of the spleen: a multicenter study. J Clin Ultrasound. 2009;37(8):445–450. doi: 10.1002/jcu.20608. [DOI] [PubMed] [Google Scholar]
- 35.Liang P, Gao Y, Wang Y, Yu X, Yu D, Dong B. US-guided percutaneous needle biopsy of the spleen using 18-gauge versus 21-gauge needles. J Clin Ultrasound. 2007;35(9):477–482. doi: 10.1002/jcu.20390. [DOI] [PubMed] [Google Scholar]