Abstract
Fluid collections that are incompletely drained despite adequate catheter position, size, and number represent a minority of abscesses but a source of great frustration for patients, surgeons, and interventional radiologists. Drainage of such complex collections is known to be more effective with the adjunctive use of intracavitary fibrinolytic agents instilled via the drainage catheter. In this review article, we discuss the role of fibrinolytics specifically tissue plasminogen activator as explored by interventional radiologists in enhancing effective drainage of these complex abdominal and pelvic collections as well as complex pleural collections.
Keywords: abscess, empyema, percutaneous drainage, thrombolytic therapy, interventional radiology
Objectives: Upon completion of this article, the reader will be able to discuss the role of adjunctive fibrinolytic therapy in the percutaneous treatment of abdominal abscesses and empyemas.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Fluid collections that are incompletely drained despite adequate catheter position, size, and number represent a minority of abscesses but are a source of great frustration for patients, surgeons, and interventional radiologists. Drainage of such complex abdominopelvic and pleural collections is known to be more effective with the adjunctive use of intracavitary fibrinolytic agents instilled via the drainage catheter.1,2 Streptokinase and urokinase were the popular agents in the 1980s and 1990s.3,4 Streptokinase lost popularity because of potential antigenicity, and the US Food and Drug Administration removed urokinase from the US market in 1999. Ever since, interventional radiologists have focused their attention on tissue plasminogen activator (tPA) (alteplase). In this review article, we discuss the use of fibrinolytics in enhancing effective drainage in abdominal and pelvic collections as well as in complex pleural collections.
Role of Tissue Plasminogen Activator in Complex Pleural Collections
Background
Professor Désiré Collen, a Belgian physician and chemist, together with Alfons Billiau, discovered tissue plasminogen activator (tPA). Besides its extensive intravenous use for acute stroke, tPA has been used by interventional radiologists for intravascular thrombolysis and effectively explored as an adjunctive intrapleural fibrinolytic agent both in the pediatric5 and adult population6 since the early 2000s. More recently, Zuckerman et al2 demonstrated the effectiveness of the use of recombinant tPA in the treatment of loculated parapneumonic effusions. Finally, the second Multicentre Intrapleural Sepsis Trial, conducted in 2011, concluded that tPA in combination with DNase improves fluid drainage in patients with pleural infection compared to treatment with tPA alone.7
Indications and Contraindications
Review of the literature reveals more experience in treating parapneumonic loculated effusions and empyemas 2,5,6,7 with tPA compared with effusions secondary to other etiologies such as traumatic hemothorax and malignant pleural effusions. In their study Gervais et al8 included hemothorax (n = 8), postoperative effusions (n = 4), and malignant effusion (n = 1) in addition to parapneumonic effusions (n = 26) and empyemas (n = 27). The criteria to use tPA primarily includes extremely viscous contents with little or no drainage at immediate postdrainage imaging or macroscopically purulent fluid at initial drainage.7 Similarly, tPA may be used secondarily when follow-up imaging demonstrates a large residual collection with or without the presence of loculations on computed tomography (CT). The rationale for tPA is based on the mechanism of fibrinolysis in loculated effusions that involves degradation of fibrin strands promoting breakdown of septations, thus mobilizing additional fluid that can be drained via an indwelling catheter. In the early years of intracavitary fibrinolytic therapy, a clinical concern was the risk of hemorrhagic complications, but this is rare in the absence of systemic anticoagulation at therapeutic levels. Gervais et al8 demonstrated a statistically increased risk (p < 0.001) of intrapleural hemorrhage in patients concurrently receiving intracavitary fibrinolytic therapy and therapeutic doses of systemic anticoagulation, making this concomitant therapy a relative contraindication. In the same study, patients undergoing concurrent therapy with prophylactic systemic anticoagulation showed no additional risk of bleeding. Relative contraindications also include adverse reactions or sensitivity to tPA.
Technique
At present, no standardized optimal treatment protocols exist. Different reports demonstrate variability in the type of imaging guidance used for placement of chest tubes including either ultrasound, CT, or fluoroscopy. The technique of placement may be either trocar or Seldinger, the latter used often for the repositioning of previously placed tubes. The size of intrapleural catheters may also vary, ranging from 8.5 to 16F based on availability and operator preference. Overall, larger (16F) pigtail catheters (Cook, Bloomington, IN) were commonly used in one study 8 due to their stiffness and kink resistance. Other studies have used 10 to 24F catheters.2 Following placement, catheters are connected to a three-way stopcock and placed to continuous suction of 20 cm of water. Doses of fibrinolytics, frequency of administration, duration of a cycle (dwell time), and repetition of cycles if complete drainage is not achieved after a single cycle all vary among different studies.
The tPA dose is administered either through a single catheter or, in the presence of multiple catheters, divided evenly between the tubes. Doses vary from 4 to 6 mg tPA diluted in approximately ≤50 mL of 0.9% saline. Volume of the instillate should be ∼30 to 50% of the initial cavity volume. Doses are reduced to 1 to 4 mg in smaller pediatric patients.8 Zuckerman et al2 used 6 mg diluted in 50 mL of normal saline, and Rahman et al7 used a combination of 10 mg of tPA and 5 mg of DNase in unspecified dilution volumes. Following administration, the catheter is clamped for 30 minutes8 to 2 hours.2 Following this dwell time, wall suction is resumed. Doses are repeated twice daily for 3 days7,8 or for a total of 6 doses.2 CT chest or chest radiographs are repeated within 24 hours at the end of the entire cycle. At the authors' institution, a protocol currently in use is 4 to 6 mg tPA in 25 to 50 ml of 0.9% saline (depending on cavity size), administered twice a day for 3 days (six doses). Following this a repeat chest CT is obtained.
The catheter is removed after outputs decrease to physiologic range or less. For patients with incomplete drainage, an additional cycle of tPA may be administered if partial response to tPA is evidenced by volume of drainage during the cycle and imaging findings. Placement of additional catheters or larger catheters is sometimes needed, and the decision to proceed to surgery is made in conjunction with the surgical services.
Results of Thrombolysis
The major goal of adjuvant thrombolytic therapy in the treatment of complex pleural collections includes avoidance of surgery (drainage or decortication) with reduction of morbidity and mortality. To this end, in studies using chest CT as follow-up imaging, complete drainage avoiding surgery was noted in 86% of patients.8 In this cohort, of a total of 66 patients, 60 patients underwent a single tPA cycle with 52 complex effusions clearing without surgery (87% primary effectiveness rate). Six of the 66 patients required two tPA cycles with five of them clearing without requirement of surgical drainage. Skeete et al6 reported a success rate of 78% in 41 patients with 42 effusions; Zuckerman et al2 similarly reported a success rate of 72% in 25 patients.
In studies using chest radiographs for follow-up, resolution of pleural effusion was seen in 75% of patients.9 Wells and Havens10 reported an impressive 98% effectiveness rate in 45 children. Due to greater anatomical resolution, follow-up with chest CT is preferable to follow-up with chest radiographs. Figure 1 demonstrates the effectiveness of tPA in treating an empyema.
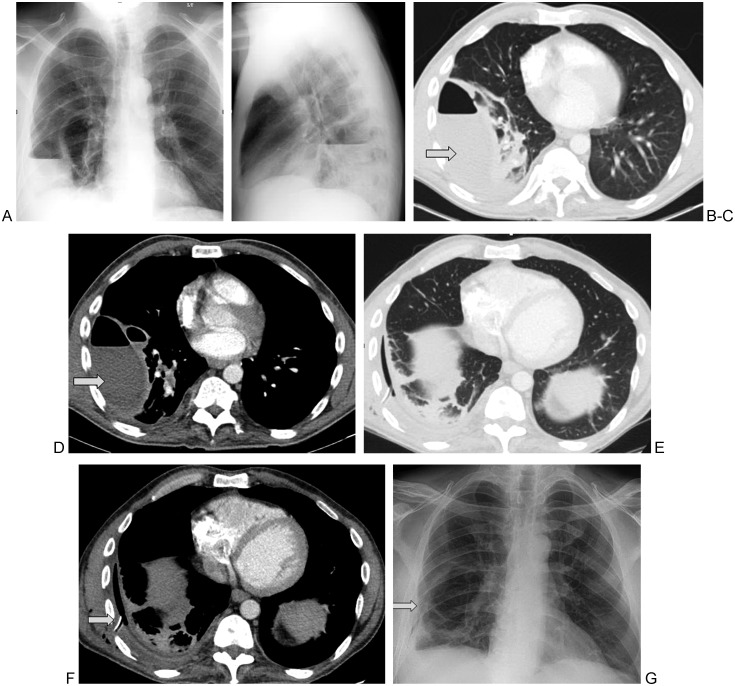
Figure 1.
45-year-old man with a history of intravenous drug abuse presented with right lower lobe pneumonia associated with a right-sided empyema. PA and lateral chest radiographs (A,B) and axial contrast enhanced computed tomography sections in lung (C) and soft tissue (D) windows reveal two septated loculated fluid collections containing air fluid levels (arrows). A 16F chest tube was placed into the larger collection under ultrasound guidance. The pleural fluid grew Streptococcus anginosis. Given the presence of thick septations, tissue plasminogen activator was administered via the chest tube with good results (E–G, arrows).
Complications
Hemorrhage is rare but has been reported in case reports or in cases of concurrent administration of systemic therapeutic anticoagulation. The existing literature includes a case report of life-threatening hemorrhage in an 8-month-old child receiving urokinase.11 In this case, however, imaging was limited to chest radiography, and no CT was performed to confirm chest tube positioning in the pleural space.
Defined criteria for tPA-related complications were proposed by the study conducted by Gervais et al8 where they described tPA related bleeding as a new hemorrhage appearing on CT scans after tPA administration or the chest catheter drainage becoming hemorrhagic after tPA administration with a concurrent hematocrit decrease. In their study of 66 patients, the rate of major hemorrhage was noted to be 6.9% overall. Most notably, all cases of major hemorrhage occurred in patients who were on therapeutic levels of anticoagulation. Specifically, there were four cases of hemorrhage in 12 patients (33%) simultaneously undergoing full-dose systemic anticoagulation. Despite this, only one of four patients who bled required surgery, and so the effectiveness was not significantly compromised in patients who bled. The authors propose weighing the risk-benefit ratio or possible interruption of systemic anticoagulation during the use of thrombolytic therapy with the larger goal of avoiding decortication. Also of note, in the study by Gervais et al,8 there were no cases of hemorrhage in 38 patients who were simultaneously on prophylactic levels of anticoagulation; thus tPA appears safe in this setting. This is a highly important finding given the frequent use of prophylactic anticoagulation in the inpatient setting. On a smaller scale, Gervais et al8 noted that 2 patients of 66 were on clopidogrel and did not bleed, and 12 patients were on aspirin (of whom 7 were also on therapeutic systemic anticoagulation, but only 2 of these 7 patients bled). Skeete et al6 reported a single patient undergoing dialysis in whom intrapleural tPA was used and who developed new hematuria. Most other studies did not encounter any major hemorrhage. Although unanswered questions with new anticoagulants remain, it appears safe to use intrapleural fibrinolytics in the setting of prophylactic anticoagulation and to weigh the risks, benefits, and alternatives in the setting of therapeutic anticoagulation.
Role of Tissue Plasminogen Activator in Abdominal and Pelvic Abscesses
Background
Although not as widely studied or reported as intrapleural fibrinolytics, the desire to avoid surgery has motivated the adjunctive use of fibrinolytics to promote more effective percutaneous abscess drainage in the abdomen and pelvis. In 1993, Lahorra et al12 demonstrated the safety of intracavitary urokinase following percutaneous abscess drainage. Since urokinase was removed from the American market, tPA has replaced it successfully.
Technique
As in the pleural space, there exist no universally accepted protocols for adjuvant intracavitary tPA in assisting effective percutaneous abscess drainage. Various studies have used catheter sizes ranging from 8.5 o 14F1,13 based on the initial aspirate from the guiding needle and/or collection size. The general experience is that catheter size does not play a pivotal role in determining the success of tPA use. However, no randomized trials exist that confirm this.
In one of the largest published studies,1 the tPA dose was standardized to 4 to 6 mg of tPA diluted in 25 mL of 0.9% saline, administered entirely through a single catheter or divided equally through multiple catheters. The catheter was clamped for 30 minutes after which it was opened to gravity drainage without aspiration. Each cycle lasted 2 to 3 days with two to six doses administered in each cycle. If required, a second cycle was performed within 14 days of completion of the first lasting for 2 to 3 days. Unlike in the chest, CT was the only imaging modality used for follow-up and was performed within 24 hours of completion of the last dose.
Results of Thrombolysis
Drainage success was defined as complete or near complete resolution of the abscess at CT follow-up coupled with clinical improvement. Beland et al1 demonstrated that 89% of abscess cavities (41 of 46) refractory to simple catheter drainage were successfully drained with adjunctive intracavitary fibrinolytic therapy. Partial success was determined by incomplete drainage with the need for surgical intervention. The drainage was deemed a failure if there were persistent signs of infection with a residual cavity on follow-up imaging necessitating surgical drainage. Failure was seen in 10.9% of patients (5 of 46), although 60% of these (3 of 5) had a documented fistula. Additionally, the authors postulated that administering intracavitary tPA earlier in the drainage therapy may improve the likelihood of drainage success. Figures 2 and 3 demonstrate effectiveness of tPA in treating complex abdomino-pelvic fluid collections.
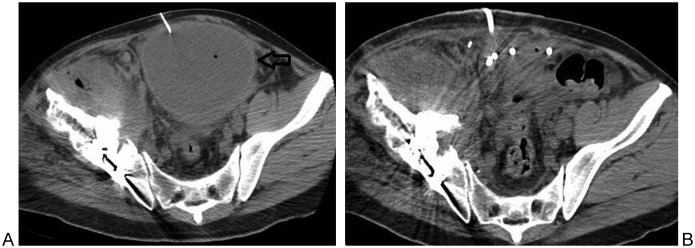
Figure 2.
(A) 53-year-old man with history of right pelvic chondrosarcoma presented with a new pelvic collections 1 month after excision and reconstructive surgery (arrow). CT guided aspiration revealed presence of a hematoma as shown on this non contrast enhanced axial CT image of the pelvis (A). A 12F biliary drainage catheter (used as a drainage catheter due to its multiple side holes) was placed followed by a single cycle of tPA. Follow-up CT on day 4 demonstrates near complete drainage of the hematoma as is shown in this axial CT image (B).
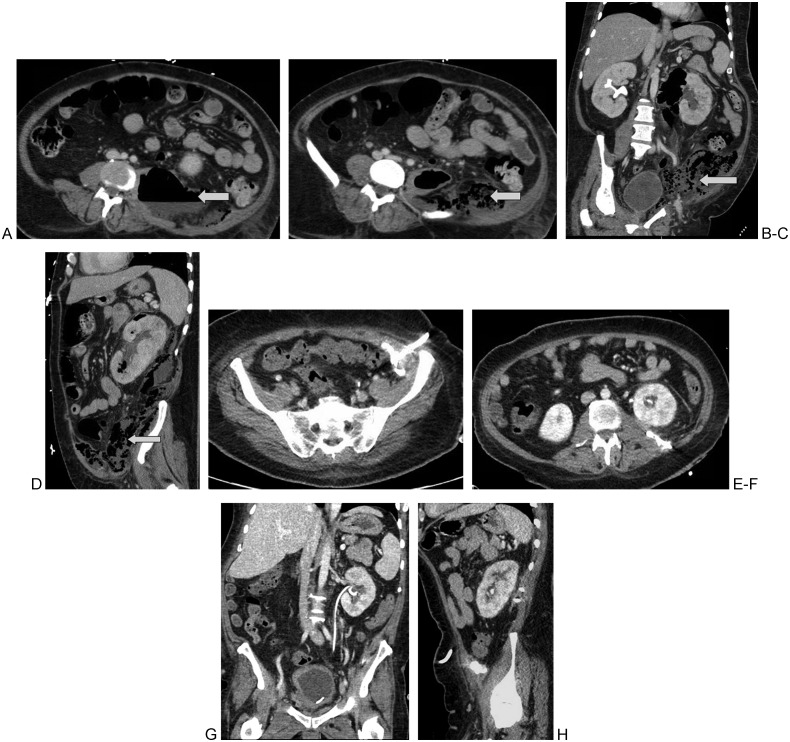
Figure 3.
41-year-old man, newly diagnosed with diabetes, presented with left-sided emphysematous pyelonephritis. Contrast enhanced CT of the abdomen demonstrated a large air containing left perinephric abscess tracking along the left psoas muscle as shown on these axial (A, B) coronal (C) and sagital (D) CT images. Two catheters, a 12 and 14 French multi sidehole catheters were placed into the perinephric and the left flank collection respectively. 6 doses of tPA were administered equally divided between both these catheters with excellent results (E–H).
Complications
In all the studies done so far, adjunctive tPA in abdominopelvic abscess cavities has been shown to be safe even in the setting of patients receiving concurrent systemic prophylactic or therapeutic anticoagulation. For example, in the era of urokinase use, Lahorra et al12 found no associated bleeding complications or changes in systemic coagulation parameters following the use of intracavitary urokinase in 31 abscesses. Similarly, Beland et al1 found no bleeding complications directly attributable to tPA; 24 of 46 patients in their study (52%) were undergoing prophylactic and 8.7% (4 of 46) were undergoing full therapeutic systemic anticoagulation at the time of the study. For the single patient in their study who required arterial embolization due to arterial injury, the complication was related to catheter placement and not directly to tPA administration. Thus, unlike in the pleural space, tPA use in patients on systemic therapeutic anticoagulation does not appear to be associated with an increased risk of bleeding. If there is a risk, it would appear to be much smaller than the 33% risk seen in patients receiving pleural tPA.
Conclusion
Adjunctive use of tPA in complex intrapleural and abdominopelvic collections has been shown to be effective in aiding percutaneous catheter drainage and in decreasing the need for surgical intervention. Table 1 summarizes the current strategies commonly used in the use of adjunctive intracavitary fibrinolytic therapy. Most of the currently reported studies have been retrospective, have included one or two multiday cycles of tPA, and are not standardized to control for variables such as catheter diameter, timing of commencement of tPA, or tPA dose. In complex pleural effusions, but not in abdominal or pelvic abscesses, therapeutic levels of anticoagulation result in an increased risk of pleural hemorrhage at the site of administration. In contrast, this does not appear to be true for prophylactic anticoagulation.
Table 1. Use of adjunctive fibrinolytics to ensure effective percutaneous catheter drainage.
| Location of collections | Pleural | Abdominopelvic |
|---|---|---|
| Types of collections amenable to treatment | Parapneumonic Hemothorax Empyema Postoperative Malignant effusion (rarely) |
Abscess Postoperative Hematomas |
| Safety of tPA use with concurrent systemic therapeutic anticoagulation | Increased risk of hemorrhage | No increased risk of hemorrhage |
| Safety of tPA use with concurrent systemic prophylactic anticoagulation | Safe | Safe |
| Follow-up imaging | Chest X-ray or CT of chest | Low-dose CT of abdomen |
CT, computed tomography; tPA, tissue plasminogen activator.
Acknowledgement
Diane A. Levis, PA. Abdominal Imaging and Intervention, Department of Radiology, Massachusetts General Hospital.
References
- 1.Beland M D, Gervais D A, Levis D A, Hahn P F, Arellano R S, Mueller P R. Complex abdominal and pelvic abscesses: efficacy of adjunctive tissue-type plasminogen activator for drainage. Radiology. 2008;247(2):567–573. doi: 10.1148/radiol.2472070761. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerman D A, Reed M F, Howington J A, Moulton J S. Efficacy of intrapleural tissue-type plasminogen activator in the treatment of loculated parapneumonic effusions. J Vasc Interv Radiol. 2009;20(8):1066–1069. doi: 10.1016/j.jvir.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 3.Tillett W S, Sherry S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent and sanguinous pleural exudations. J Clin Invest. 1949;28(1):173–190. doi: 10.1172/JCI102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulton J S, Moore P T, Mencini R A. Treatment of loculated pleural effusions with transcatheter intracavitary urokinase. AJR Am J Roentgenol. 1989;153(5):941–945. doi: 10.2214/ajr.153.5.941. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein M, Restrepo R, Chait P G, Connolly B, Temple M, Macarthur C. Effectiveness and safety of tissue plasminogen activator in the management of complicated parapneumonic effusions. Pediatrics. 2004;113(3 Pt 1):e182–e185. doi: 10.1542/peds.113.3.e182. [DOI] [PubMed] [Google Scholar]
- 6.Skeete D A, Rutherford E J, Schlidt S A, Abrams J E, Parker L A, Rich P B. Intrapleural tissue plasminogen activator for complicated pleural effusions. J Trauma. 2004;57(6):1178–1183. doi: 10.1097/01.ta.0000141879.67441.52. [DOI] [PubMed] [Google Scholar]
- 7.Rahman N M, Maskell N A, West A. et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365(6):518–526. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 8.Gervais D A, Levis D A, Hahn P F, Uppot R N, Arellano R S, Mueller P R. Adjunctive intrapleural tissue plasminogen activator administered via chest tubes placed with imaging guidance: effectiveness and risk for hemorrhage. Radiology. 2008;246(3):956–963. doi: 10.1148/radiol.2463070235. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto K, Kee S T, Semba C P, Razavi M K, Sze D Y, Dake M D. Safety and efficacy of tissue plasminogen activator to treat loculated pleural effusions. J Vasc Interv Radiol. 2001;12(Suppl):S107. [Google Scholar]
- 10.Wells R G, Havens P L. Intrapleural fibrinolysis for parapneumonic effusion and empyema in children. Radiology. 2003;228(2):370–378. doi: 10.1148/radiol.2282020486. [DOI] [PubMed] [Google Scholar]
- 11.Blom D, Aalderen W M van, Alders J M, Hoekstra M O. Life-threatening hemothorax in a child following intrapleural administration of urokinase [letter] Pediatr Pulmonol. 2000;30(6):493. doi: 10.1002/1099-0496(200012)30:6<493::aid-ppul10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Lahorra J M, Haaga J R, Stellato T, Flanigan T, Graham R. Safety of intracavitary urokinase with percutaneous abscess drainage. AJR Am J Roentgenol. 1993;160(1):171–174. doi: 10.2214/ajr.160.1.8416619. [DOI] [PubMed] [Google Scholar]
- 13.Diamond I R, Wales P W, Connolly B, Gerstle T. Tissue plasminogen activator for the treatment of intraabdominal abscesses in a neonate. J Pediatr Surg. 2003;38(8):1234–1236. doi: 10.1016/s0022-3468(03)00275-6. [DOI] [PubMed] [Google Scholar]