Abstract
With advances in imaging technology, there has been a significant increase in the number and range of interventional musculoskeletal image-guided procedures. One of the most commonly performed image-guided musculoskeletal interventions is the diagnostic and therapeutic percutaneous aspiration and drainage of multiple types of intra-articular, juxta-articular, and intramuscular pathologic fluid collections. These procedures may be performed under fluoroscopic, ultrasound, computed tomography, or even magnetic resonance guidance depending on the location to be accessed, type of pathology, patient characteristics, and operator preference. Musculoskeletal image-guided aspiration and drainage procedures are minimally invasive and generally very safe while offering valuable diagnostic information as well as therapeutic benefit. This article focuses on the appropriate indications, contraindications, and general technique for accessing the major joints via imaging guidance. For each joint, we discuss pertinent anatomy, appropriate imaging modalities, and preferred approaches to gaining intra-articular access. Additionally, the article discusses some of the more frequently encountered juxta-articular and intramuscular fluid collections that can be accessed and aspirated via percutaneous intervention, with mention of the importance of recognizing extremity sarcomas that can mimic these benign collections.
Keywords: Aspiration, interventional, musculoskeletal, arthrocentesis, imaging guided
Objectives: Upon completion of this article, the reader will be able to describe indications, contraindications, and the general procedure for accessing major joints for aspiration and drainage via imaging-guidance. The reader should also be able to identify how to access juxta-articular and extremity fluid collections.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
With advances in imaging technology, there has been an increase in the number and range of interventional musculoskeletal (MSK) image-guided procedures. One of the most commonly performed image-guided MSK interventions is percutaneous aspiration and drainage of intra-articular, juxta-articular, and intramuscular fluid collections. Image guidance allows for the safe access of nearly every major joint and bursa. In addition to the therapeutic benefits of evacuating or decompressing a fluid collection, aspiration helps guide medical therapy by providing fluid samples for analysis in microbiology and cytology laboratories, allowing for identification of the disease process and institution of the most appropriate treatment.1
By definition, aspiration refers to using a catheter or needle to evacuate a fluid collection with immediate postprocedural removal of the needle or catheter. Alternatively, drainage is defined as leaving a catheter in place in an attempt to completely empty and/or collapse a fluid collection with continuous drainage. Image guidance refers to the use of an imaging modality to identify the precise location of the abnormality to be sampled or treated and to direct placement of the needle or catheter. A wide range of imaging modalities are at the interventional radiologist's disposal including fluoroscopy, ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI). The selection of the ideal imaging modality to guide a procedure depends on multiple factors including the anatomical target, the patient's age and body habitus, the radiologist's preference and expertise, and the suspected disease entity. No matter which modality is ultimately selected, the interventionalist must document that the needle or catheter tip resides within the desired location.2
Arthrocentesis
General Indications
Arthrocentesis is defined as the puncture of a joint with subsequent aspiration of joint fluid. There are many indications for arthrocentesis, the most common of which is the diagnosis of septic arthritis or crystal-induced (e.g., monosodium urate, calcium pyrophosphate) arthropathies via synovial fluid analysis. Additional indications for arthrocentesis include diagnosis of bone or ligamentous injury by confirmation of blood within a joint, relief of pain via decompression of an acute effusion or hemarthrosis, and determination of whether or not a laceration or fistula communicates with a joint.3
Imaging Features of Septic Arthritis
Because of the morbidity and mortality associated with septic arthritis, any monoarticular arthritis should be considered infectious until proven otherwise. A prompt and accurate diagnosis is imperative because the outcome of septic arthritis depends on the length of time to diagnosis.4 Risk factors include preexisting joint damage, immunosuppression, and intravenous drug abuse. Joint contamination can occur due to hematogenous seeding of the synovial membrane, extension of infection from an adjacent soft tissue source, or direct inoculation by penetration. Patients may present with a “red-hot joint” and soft tissue swelling; a joint effusion is typical but difficult to detect on physical examination in deeper joints such as the hip and shoulder.
Radiographs may be normal initially. Soft tissue swelling and effusion represent the earliest findings. Effusions can usually be easily detected on radiographs of the knee, ankle, and elbow (Fig. 1). Hip joint effusions can be detected radiographically in children based on the “widened teardrop” distance, but this is not applicable to adults.5 Demineralization of the bones adjacent to the joint and marginal erosions become noticeable as synovial inflammation progresses (Fig. 2). Loss of the subchondral bone margin initially gives a dot-dash appearance, followed by more focal erosions. As the articular cartilage is destroyed, the joint narrows uniformly, and additional erosions appear. Advanced cases result in gross bone destruction and malalignment. When radiographs are nondiagnostic, MRI is a reasonable choice for further imaging and can reveal joint fluid and synovial thickening, as well as surrounding soft tissue edema, bone marrow edema, and erosions. US can be used to detect or exclude joint fluid and synovial thickening, and it is particularly well suited in the evaluation of the pediatric hip. The differential diagnosis of septic arthritis includes other inflammatory and crystalline-induced arthritides whose presence may be supported by imaging findings (e.g., chondrocalcinosis in the setting of calcium pyrophosphate deposition disease arthropathy); however, patients with joints damaged from other disease processes are also at risk for septic arthritis. In many cases, imaging alone cannot accurately distinguish infected from noninfected joints, and ultimately, aspiration and culture are necessary to confirm the presence of septic arthritis.
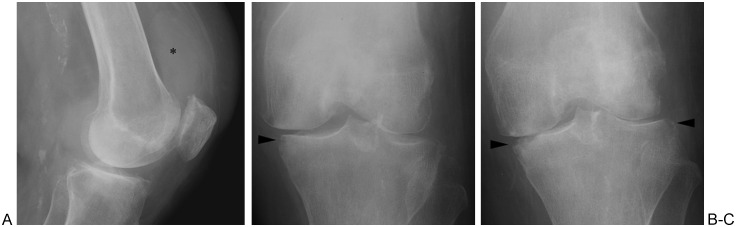
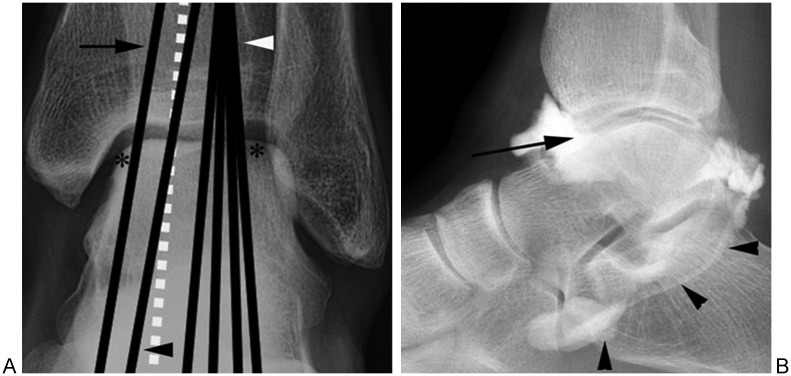
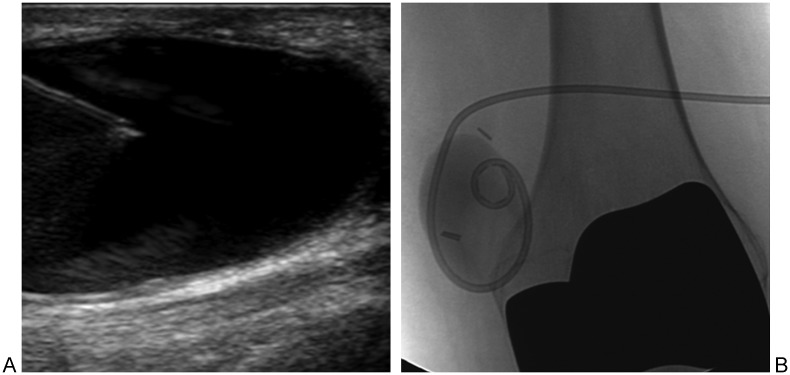
Figure 1.
Septic arthritis of the knee. (A) Lateral radiograph shows joint effusion (asterisk). (B) Anteroposterior (AP) radiograph shows marginal erosion of medial tibial plateau (arrowhead). (C) AP radiograph obtained 1 month later shows progression of cartilage damage (evidenced by joint narrowing) and progression of erosion (arrowheads).
Figure 2.
Septic hip in patient with sickle cell disease. Note narrowing of left hip joint and demineralization of adjacent bones relative to right hip. Margins of femoral head and acetabular dome are indistinct with erosions.
Contraindications to Arthrocentesis
Although arthrocentesis is classified as an invasive procedure, it is well tolerated by most patients with few true contraindications. An absolute contraindication is the inability to access the joint without traversing inflamed or infected superficial tissues because the needle could infect a previously sterile joint. Established bacteremia is considered a relative contraindication with a theoretical risk of infection spreading to the joint.3 There is little consensus regarding the handling of oral anticoagulant therapy before percutaneous interventions; however, studies have reported that arthrocentesis is safe in patients receiving chronic warfarin therapy at therapeutic levels.6
General Technique
Before beginning the procedure, informed consent must be obtained so the patient is made aware of the risks and benefits of arthrocentesis as well as alternative courses of action. Arthrocentesis is generally a safe procedure, but the risks of bleeding, infection, temporary nerve block (e.g., of the femoral nerve as a result of local analgesia), and inability to access the joint should be addressed. If performing the procedure under fluoroscopic or CT guidance, the risks and benefits of arthrography relative to radiation exposure should also be relayed to the patient. With the exception of pain at the injection site that may reflect synovitis caused by contrast injection, complications associated with arthrography are rare. A so-called allergic reaction to intra-articular contrast is controversial.7 Newberg et al polled multiple radiologists experienced in arthrography regarding complications.8 The respondents, who had performed or supervised >126,000 arthrograms collectively, reported no deaths, 61 cases of urticaria, 4 hypotensive episodes, and 1 case of vasomotor collapse and laryngeal edema. It is difficult to determine, however, which or how many of these complications were the result of contrast injection as opposed to other medications (i.e., skin cleansing agents, local analgesic agents).
Next, the patient is properly positioned in accordance with the preferred imaging modality and the specific joint to be accessed (see later). Based on the review of the patient's previous imaging (if applicable) and focused physical examination, the safest needle entry site is marked on the skin and the skin is cleansed and draped using aseptic technique. Local analgesia is achieved by infiltrating both the subcutaneous and deep tissue beds, typically with lidocaine. The choice of needle used for joint access depends on the specific target, the patient's body habitus, and the consistency of the fluid to be aspirated. For deeper joints such as the hip and shoulder, 3.5-inch spinal needles with trocars are ideal for gaining intra-articular access because the inner stylet protects the needle core from being inadvertently filled with skin and subcutaneous tissue debris as the needle is advanced. Joints that are more superficial, such as the wrist and ankle, may be accessed via 1.5-inch needles. Although higher gauge (20 to 25G) needles are appropriate for injection of medications into joints, they may not facilitate easy aspiration of thick joint fluid, and 18-gauge or larger needles are frequently necessary. On occasion, we have had to use 14G needles to aspirate thick material, but generally we do not use a needle >18G unless necessary. Fluid samples are delivered to the microbiology laboratory and typically evaluated with Gram staining and culture, cell count and differential, and crystal analysis and/or additional tests requested by the referring service.9 Interventionalists should check with the laboratory at their institution regarding specific submission procedures. For Gram staining and culture, synovial fluid is often submitted in the syringe used for aspiration to minimize risk of contamination, but it may also be submitted in a sterile heparinized tube. For further microscopic studies, anticoagulant tubes treated with sodium heparin or liquid ethylenediaminetetraacetic acid (EDTA) are preferred; lithium heparin and powdered EDTA anticoagulants may form crystal artifacts that can be misleading during microscopic examination.10 When pus is encountered, the ordering service should be notified at once and a thorough attempt should be made to evacuate as much fluid as possible in an effort to decompress the joint. Although some authors argue that injection of contrast following aspiration of pus via fluoroscopy does not add diagnostic information and could theoretically lead to hematogenous propagation of infection, others maintain that arthrography is beneficial because it can reveal adjacent sinus tracks, abscesses, or bursae that may be infected as well as the joint with which they communicate.7,11 If no fluid can be aspirated, injection of iodinated contrast material is appropriate to confirm intra-articular needle tip location. Rotation of the bevel of the needle may also facilitate aspiration/injection. If this maneuver fails to yield fluid or opacify the joint, the needle may be slightly retracted and repositioned. If the needle is appropriately positioned within the joint, but no fluid can be aspirated, the joint should be gently lavaged with nonbacteriostatic saline, which is subsequently aspirated, placed in a labeled tube, and sent to the microbiology laboratory for culturing.
Although fluoroscopy is the most commonly used imaging modality for arthrocentesis, US and CT guidance may be preferable in certain cases. US-guided arthrocentesis may be used for a wide range of joints and spares ionizing radiation exposure, which is particularly appropriate when examining pediatric patients and pregnant women. Additional benefits of US include the identification of vasculature in real time using color Doppler, wide availability, relative ease of demonstrating fluid collections (including extra-articular collections such as abscesses and inflamed or infected bursae or tendon sheaths) and their aspiration in real time, and the ability to examine the contralateral side for symmetry. The primary drawback to US is that successful use of the modality depends on the user's skill, which may make standardized fluoroscopy a more attractive modality depending on the interventionalist's comfort level or the availability of US technologists.12 Additional drawbacks to US include difficulty performing arthrography as well as limited effectiveness in obese patients. CT is the preferred modality for accessing the sternoclavicular (SC) joint because it allows for visualization of vital mediastinal structures that are in close proximity to the SC joint and must be avoided during arthrocentesis. CT is also often the preferred modality for accessing the sacroiliac joint (SI) based on the complex orientation of the joint, which may be better appreciated on CT than fluoroscopy.11
Specific Joints
Hip
At our institution, the hip represents the joint for which image-guided synovial fluid aspiration is most commonly requested.
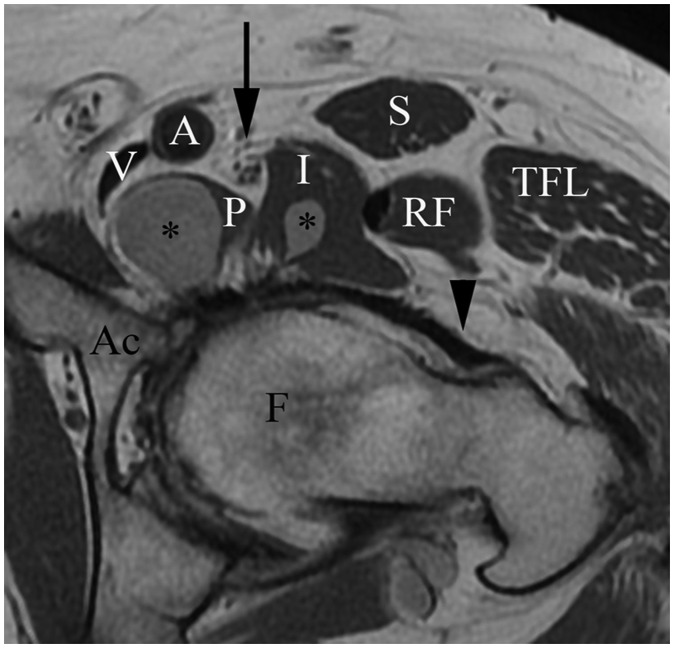
Anatomy: The hip is a ball-and-socket joint within a fibrous capsule lined by synovium. The capsule attaches proximally to the acetabular rim and extends distally to attach to the anterior femur along the intertrochanteric line proximal to the lesser trochanter.13,14 The capsule crosses the femur posteriorly, but there is no posterior osseous attachment. Circular capsular fibers surrounding the femoral neck form the zona orbicularis, which constricts the synovial sac. The center of the joint lies ∼1 to 2 cm caudal to the midinguinal ligament.15 The femoral nerve, artery, and vein (from lateral to medial) typically overlie the medial aspect of the joint. The iliopsoas muscle lies lateral to the femoral neurovascular structures and is separated from the underlying hip by the iliopsoas bursa (Fig. 3).
Figure 3.
Hip anatomy. Proton-density-weighted axial magnetic resonance image of left hip shows femoral head (F), acetabulum (Ac), iliofemoral ligament (arrowhead), components of fluid-filled iliopsoas bursa (asterisk), psoas (P), and iliacus (I) components of iliopsoas muscle, rectus femoris muscle (RF), tensor fascia lata muscle (TFL), sartorius muscle (S), femoral nerve (arrow), femoral artery (A), and femoral vein (V).
Approach: Numerous techniques for accessing the hip joint have been described, with an anterior approach under fluoroscopic guidance the most commonly used. The patient is placed supine, with the lower extremity in neutral or slight internal rotation to provide better visualization of the femoral neck; if necessary, abdominal pannus is repositioned away from the hip and secured by tape to facilitate the desired anterior approach into the joint. The femoral artery pulse is palpated, and the estimated course of the vessel can be marked on the skin. The skin entry point should be lateral (preferably by at least 2 cm) to the femoral artery pulsation and below the groin crease to avoid injury to the femoral vessels, peritoneum, and bowel, as well as inadvertent femoral nerve block; this entry point should be marked on the skin. The skin is subsequently prepared and local analgesia is administered. A 3.5-inch spinal needle is generally of sufficient length to access the joint; however, a longer needle may be needed for larger patients. The needle can be directed anterior to posterior toward either the lateral or medial femoral head-neck junction (Fig. 4). Lateral placement of the needle is appropriate for procedures in which aspiration is not the primary goal (i.e., steroid injection, contrast injection for MR or CT arthrography) because it avoids the femoral nerves and vessels; however, needle placement along the medial aspect of the femoral neck is often preferred for aspiration procedures because the capsule tends to be more redundant at this location allowing for easier acquisition of joint fluid.16 Targeting the middle of the femoral neck, particularly the region of the zona orbicularis, often results in an extra-articular injection.17 Some investigators have reported that an oblique rather than a perpendicular approach results in greater success rates.18
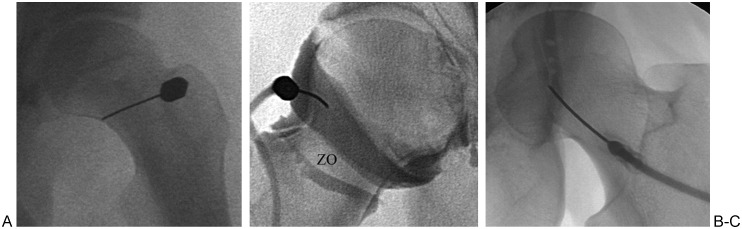
Figure 4.
Positioning of needle for hip joint aspiration/arthrography in three different patients. (A) Fluoroscopic image of left hip shows needle targeting medial aspect of femoral neck. (B) Fluoroscopic image of right hip shows needle targeting lateral aspect of femoral neck and normal distribution of contrast in joint. Note the relative lack of contrast opacification in region of zona orbicularis (ZO). (C) Fluoroscopic image of left hip shows partial opacification of the iliopsoas bursa.
Under intermittent fluoroscopy, the needle is advanced until it contacts the bone, at which point aspiration is attempted. If no fluid is aspirated, iodinated contrast can be injected; injection should reveal free flow of contrast along the femoral neck, often along its base, indicating intra-articular positioning of the needle tip. If the contrast extends in linear fashion along a superior-inferior direction, then the iliopsoas bursa, tendon, or muscle has been opacified instead of the joint (Fig. 4); communication between the iliopsoas bursa and the underlying hip joint is present in 15% of patients, however. Intentional access of the iliopsoas bursa may be accomplished under fluoroscopy by advancing the needle toward the anterior rim of the acetabulum as it courses over the superomedial quadrant of the femoral head; placing the patient in the opposite oblique position displaces the vessels medial to the needle trajectory.19 Upon contacting bone, retraction of the needle by ∼5 mm should result in an intrabursal needle tip location. Injection of a small amount of contrast should result in filling of an anterior elongated structure overlying the hip joint and iliac fossa that represents the iliopsoas bursa.
If the patient has a hip prosthesis, a skin entry point medial or lateral to the prosthesis avoids overlap that prohibits visualization of the needle fluoroscopically. The needle can be directed toward the medial or lateral aspect of the neck of the prosthesis until a characteristic “metal-on-metal” sensation is encountered. If aspiration is unsuccessful at this point, the needle can be advanced posteriorly to a more dependent portion of the pseudocapsule that surrounds the prosthesis.20 If a dry tap persists, nonbacteriostatic saline can be injected and aspirated in an attempt to obtain fluid. For patients treated with Girdlestone or resection arthroplasty, Swan et al found that directing the needle to the midpoint of the intertrochanteric line provided consistent and sufficient fluid return.21 Percutaneous catheter drainage of the hip has been described in the literature, but in our experience, further intervention, if necessary, is usually surgical.22
US has been increasingly used to access the hip joint and provides the advantage of avoiding radiation exposure. It is particularly useful for aspiration of the hip in children. Sonography is typically performed with a high-frequency linear probe positioned in the oblique sagittal plane along the axis of the femoral neck to examine the anterior recess of the joint.23 The acetabular labrum will have a hyperechoic triangular appearance just superior to the femoral head. Fluid within the joint will generally appear anechoic or hypoechoic in the anterior recess, displacing the capsule from the anterior aspect of the femoral neck; however, synovitis can have a similar appearance and may be difficult to distinguish from joint fluid. Comparison with the contralateral side is recommended to confirm any abnormality. If aspiration is desired, the needle can be advanced beneath and parallel to the face of the transducer, either in the sagittal or axial plane (Fig. 5).
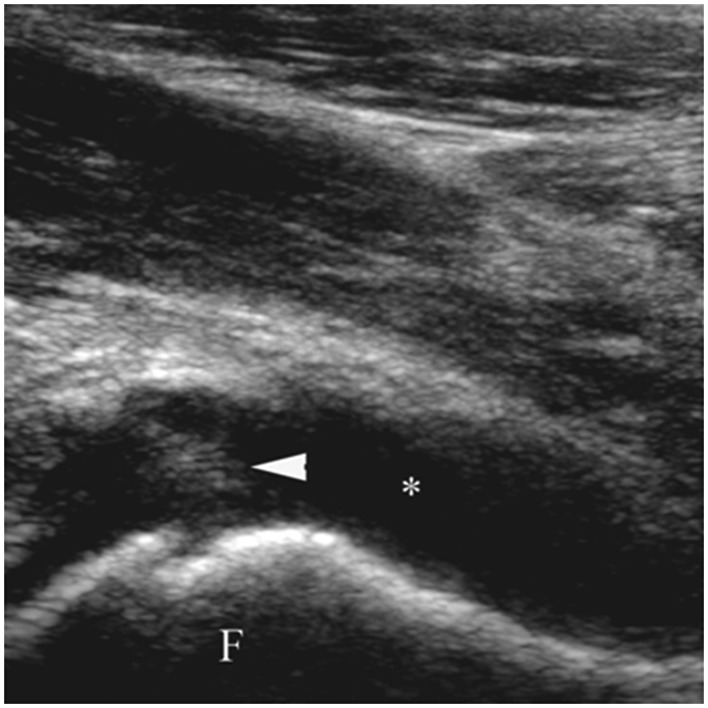
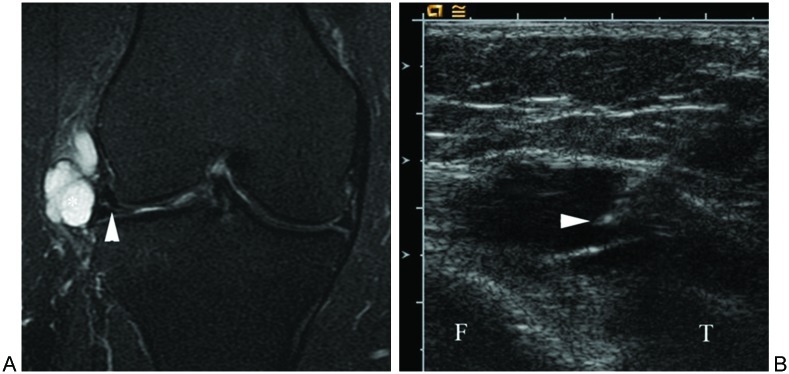
Figure 5.
Aspiration of hip joint effusion. Longitudinal sonogram image shows needle tip (arrowhead) in anechoic effusion (asterisk) along anterior aspect of femur (F).
Knee
The knee is the most commonly aspirated joint; however, unlike the hip joint, the knee joint can generally be aspirated without the need for image guidance, and hence requests for image-guided arthrocentesis of the knee are typically less common.24 Most such requests involve obese patients or a failure to obtain joint access without image guidance.
Anatomy: The knee is the largest joint in the body and consists of the tibiofemoral compartment (medial and lateral) and the patellofemoral compartment anteriorly. The patellofemoral compartment extends superiorly as the suprapatellar bursa, deep to the quadriceps tendon anteriorly. When distended with fluid, this bursa is typically easily identified on lateral knee radiographs; occasionally the bursa can be completely separated from the knee joint by a persistent suprapatellar plica.25 Fluid may also collect in the lateral synovial recess of the joint, which extends deep to the iliotibial band. Inferior to the patellofemoral compartment is the infrapatellar fat pad of Hoffa, situated posterior to the patellar tendon.
Approach: As with the hip joint, numerous techniques for accessing the knee joint have been described, with a medial or lateral patellofemoral approach the most common. The patient is placed supine. A tourniquet or compression wrap can be placed around the distal thigh above the patella in an effort to displace fluid from the suprapatellar pouch into the patellofemoral articulation. A foam wedge or towel can be placed beneath the knee to facilitate mild flexion of the joint. The patella is palpated, and its superior and inferior margins marked on the patient's skin. The patellofemoral articulation is then palpated and marked along its lateral or medial aspect; the medial approach is preferred by some who argue that the steeper angle of the medial patellar facet facilitates access to the joint; others, however, prefer the lateral approach. In either case, the skin entry point should lie along the inferior half of the patellofemoral joint to avoid inadvertently placing the needle in a fat pad that lies posterior to the superior half of the patella.26 The skin is subsequently prepared and local analgesia is applied. A 1.5-inch needle is generally of sufficient length to access the joint. The needle is advanced beneath the patella into the joint with one hand while the other hand displaces the patella away from the needle. If no fluid is aspirated, a small amount of iodinated contrast may be administered to confirm intra-articular location. In patients who are obese or have severe patellofemoral arthritis, one of several anterior approaches can be considered. Moser et al compared two routes, an anterior paramedian route and an anterolateral route, and concluded that the anterolateral route was better tolerated by patients.27 For this anterolateral route, the patient is placed supine, and the knee is flexed to 90°. The operator then palpates the anterolateral aspect of the lateral tibial plateau with his or her thumb, and the needle is introduced immediately superior to the thumb. The needle is directed posteriorly and slightly medially toward the articular surface of the lateral femoral condyle. Zurlo et al described yet another approach in which the patient is placed in a decubitus position with the symptomatic knee against the tabletop.28 The skin entry site is marked just medial to the edge of the patellar tendon, 1.5 cm distal to its patellar attachment. The needle is advanced cephalad toward the medial femoral condyle. Alternatively, the needle can be introduced into the medial aspect of the patellofemoral joint with the patient in a decubitus position. Regardless of the route, contrast should be injected if aspiration yields no fluid; if upon contrast injection there is stasis of contrast around the needle tip, the needle is extra-articular and should be repositioned.
The knee joint may also be accessed via sonography. Imaging the suprapatellar bursa in the longitudinal plane by placing the transducer over the quadriceps tendon is the easiest method for evaluating for a joint effusion; however, a transverse orientation of the transducer and needle generally allows easier access to the fluid, using either an anteromedial or anterolateral approach.29
Ankle
Anatomy: The ankle joint consists of the articulation of the talus, tibia, and fibula. The joint capsule is lined by synovium and has anterior and posterior recesses that, when distended with fluid, can be seen on lateral ankle radiographs. Smaller lateral and medial recesses extend slightly below the tips of the malleoli.13 Posteriorly, the tibiotalar joint can communicate with the sheath of the flexor hallucis longus tendon (Fig. 6). The extensor tendons (from medial to lateral: the tibialis anterior tendon, the extensor hallucis longus tendon, and the extensor digitorum longus tendon) course anterior to the joint. The tibialis anterior artery (as well as its continuation, the dorsalis pedis artery) and the deep peroneal nerve lie beneath the extensor retinaculum between the extensor hallucis longus and extensor digitorum longus tendons.
Figure 6.
Tibiotalar joint access and arthrography. (A) Anteroposterior projection of ankle shows approximate courses of tibialis anterior tendon (arrow), extensor hallucis longus tendon (black arrowhead), extensor digitorum longus tendons (white arrowhead), and anterior tibial / dorsalis pedis artery (white dashed line). Joint access may be accomplished medially or laterally, as designated by asterisks. Skin entry site should be marked just below projection of talar dome, with slight cranial angulation allowing joint access by avoiding anterior lip of distal tibia. (B) Lateral projection of ankle shows approximate trajectory of needle indicated by long arrow. Note contrast in tibiotalar joint extending into sheath of flexor hallucis longus tendon (arrowheads); such communication is not abnormal.
Approach: The dorsalis pedis artery can be readily palpated at the dorsal-most prominence of the navicular, and its pulsation is subsequently marked on the skin. With the patient supine, the skin entry point is marked along the anterior aspect of the ankle, at a safe distance (either medial or lateral) to the marked dorsalis pedis artery.30,31 The skin entry point should also lie slightly below the tibiotalar joint as seen on an anteroposterior fluoroscopic image of the ankle (Fig. 6); this allows one to avoid the anteroinferior lip of the distal tibia when advancing the needle. Once this site of entry is marked, the skin is subsequently prepared and local analgesia is administered. A 1.5-inch needle is generally of sufficient length to access the joint. The needle is advanced through the tissues of the anterior ankle, with cranial angulation allowing the needle to slide into the anterior recess of the joint without hitting the anterior lip of the tibial plafond; this may be performed with the patient supine or in the decubitus position.7 If aspiration is not successful, 1 to 2 mL of contrast material can be injected to confirm intra-articular needle placement; contrast should flow from the needle tip toward the capsular recesses.11
US may also be used to access the ankle joint, and it has the added advantage of being able to accurately identify additional infectious or inflammatory processes such as tenosynovitis.32 This is important for planning the desired joint approach because one can select a needle track that avoids inflamed structures, limiting the possibility of contaminating an aseptic joint.29 Correct intra-articular needle location is confirmed when the tip of the needle is identified adjacent to the hypoechoic cartilage of the tibiotalar joint.33
Shoulder
Anatomy: The primary joint of the shoulder is the ball-and-socket glenohumeral articulation, which is encased in a fibrous capsule with an inner synovial lining.13 The shoulder joint is supported by the rotator cuff, consisting of the subscapularis anteriorly, the supraspinatus superiorly (coursing between the top of the humeral head and the overlying acromion), and the infraspinatus and teres minor muscles posteriorly. The capsule of the glenohumeral joint extends inferiorly, between the subscapularis and teres minor muscles, as the axillary recess. Anteriorly, the joint capsule drapes over the subscapularis muscle and tendon, beneath the coracoid process, to form the subscapular recess. Inferior to the subscapular recess is the subcoracoid bursa, (located anterior to the subscapularis muscle and tendon), which generally does not communicate with the glenohumeral joint. The capsule also extends into the rotator interval between the supraspinatus and subscapularis tendons, forming a synovial-lined sheath for the tendon of the long head of the biceps muscle as it courses anteriorly within the bicipital groove of the humerus.34
Approach: The glenohumeral joint may be accessed in a variety of ways. For purposes of aspiration, the most frequently used technique of accessing the glenohumeral joint is an anterior approach with the patient positioned supine and the arm held in partial external rotation (e.g., with a sandbag placed on the patient's palm). This facilitates relocation of the tendon of the long head of the biceps lateral to the site of aspiration and to expose more of the joint.35 With true supine positioning (i.e., with the patient flat on his or her back), the anterior rim of the glenoid lies medial to the humeral head. Alternatively, a foam wedge may be placed under the contralateral shoulder to obtain a Grashey-like view of the symptomatic shoulder in which the glenohumeral joint is seen in tangent. Using either position, the skin entry site is superficial to the medial margin of the lower half of the humeral head. Once this site of entry is marked, the skin is subsequently prepared and local analgesia is administered. A 3.5-inch spinal needle is directed through the anterior soft tissue, the subscapularis tendon, and the joint capsule such that the tip of the needle lies a few millimeters lateral to the medial margin of the humeral head; this positioning avoids damage to the glenoid labrum (Fig. 7).16,36 Once the needle tip contacts the humeral head in this location, it is in the joint, and aspiration can be attempted. If the needle is too superficial, inadvertent aspiration or injection of the subcoracoid bursa may occur. Case reports of percutaneous catheter drainage of septic shoulder joints can be found in the literature.37
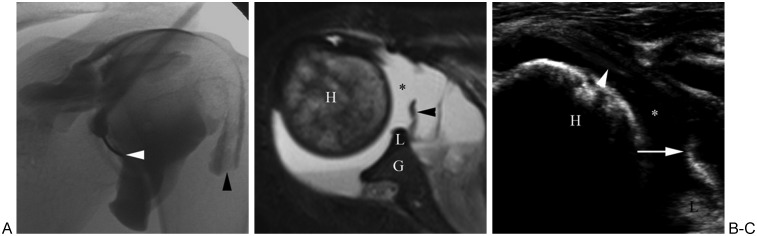
Figure 7.
Glenohumeral joint access and arthrography. (A) Fluoroscopic image of left shoulder arthrogram shows placement of needle along inferomedial aspect of humeral head (white arrowhead). Note normal pattern of joint opacification, as well as normal extension of contrast along tendon of long head of biceps (black arrowhead). (B) T2-weighted axial magnetic resonance image of shoulder of different patient shows large glenohumeral joint effusion (asterisk). Note edematous humeral head (H), as well as glenoid (G), labrum (L), and portion of middle glenohumeral ligament (arrowhead). (C) Sonogram image of same patient as in (B) shows needle (arrowhead) accessing glenohumeral joint fluid (asterisk). Humeral head (H), glenoid labrum (L), and middle glenohumeral ligament (arrow) are also visualized.
An alternative to the standard anterior approach is the rotator interval approach.38 The patient is positioned supine with the arm in external rotation. With this approach, the skin is marked superficial to the upper medial quadrant of the humeral head, such that the rotator interval above the subscapularis and lateral to the coracoid process is targeted. The needle is advanced straight down to the humeral head. The advantages of this technique are that it is easy to perform, can usually be accomplished with a shorter (1.5-inch) needle, and avoids the glenohumeral ligaments, labrum, and subcoracoid bursa. Although these advantages are desirable when injecting the joint for purposes of MR arthrography, it is not known if this particular approach is as effective for purposes of aspiration as the standard anterior approach. A final technique for accessing the shoulder is the posterior approach. With this approach, the patient is placed prone with the arm in internal rotation (i.e., palm down). A foam wedge is placed under the shoulder to obtain a Grashey-like view in which the glenohumeral joint is seen in tangent. The needle is advanced to the inferomedial aspect of the humeral head; once the needle tip contacts bone, continuous downward pressure is applied during the iodinated contrast test injection to find the potential space between the joint capsule and humerus. Without downward pressure, contrast from the test injection will likely end up within the posterior aspect of the rotator cuff. The posterior approach offers the advantage of traversing fewer important anterior stabilizing structures of the joint.39
The shoulder may also be accessed under US guidance, using either a posterior or anterior approach (Fig. 7). The anterior approach is similar to that used during fluoroscopy, with the needle tip directed toward the cartilage of the humeral head. The posterior approach is advocated by some due to the preferential accumulation of joint fluid in the posterior glenohumeral joint infraspinatus recess.40 With this approach, the patient is positioned sitting upright and the joint capsule is accessed along the medial aspect of the head of the humerus just lateral to the glenohumeral articulation. Given this patient positioning, close attention should be paid to any signs indicative of a vasovagal reaction.29
Elbow
Anatomy: The elbow joint consists of three articulations: humeroulnar, humeroradial, and proximal radioulnar. The distal humerus is divided into the capitellum and the trochlea that articulate, respectively, with the fovea on the head of the radius and the semilunar notch of the ulna.13 The proximal end of the ulna, the olecranon process, projects into the olecranon fossa on the posterior surface of the distal humerus when the elbow is extended. When the elbow is flexed, the radial head and the coronoid process of the ulna project, respectively, into the radial and coronoid fossae on the anterior surface of the distal humerus. Major ligaments include the collateral and annular ligaments. The annular ligament surrounds the radial head and attaches to the radial margin of the adjacent ulna. The lateral (radial) collateral ligament proper extends from the lateral epicondyle of the humerus to the annular ligament. The most important component of the ulnar (medial) collateral ligament, the anterior bundle, extends from the medial epicondyle of the humerus to the coronoid process of the ulna. Anteriorly, the elbow joint capsule extends from the distal humerus to the coronoid process, the annular ligament, and the radial head. Posteriorly, the capsule extends from the distal humerus to the olecranon process and annular ligament. The synovium lines the interior of the joint capsule; however, fat pads within the olecranon and coronoid/radial fossae lie between the synovium and the joint capsule. When the synovial sac is distended, these fat pads are displaced away from the humerus, allowing diagnosis of joint effusion on lateral elbow radiographs.
Approach: Several different fluoroscopically guided approaches have been described for accessing the elbow (Fig. 8). The lateral (radiocapitellar) approach is most common. A lateral view of the elbow is obtained by either placing the patient prone on the fluoroscopy table with the arm above the head in 90° of flexion or placing the patient in a chair adjacent to the table with the elbow on the table in the flexed position. The radiocapitellar joint is localized fluoroscopically, and the overlying skin is marked. After the skin is prepared and local analgesia is applied, a direct orthogonal trajectory with the needle will access the radiocapitellar articulation.11 A 1.5-inch needle is generally of sufficient length to access the joint space.
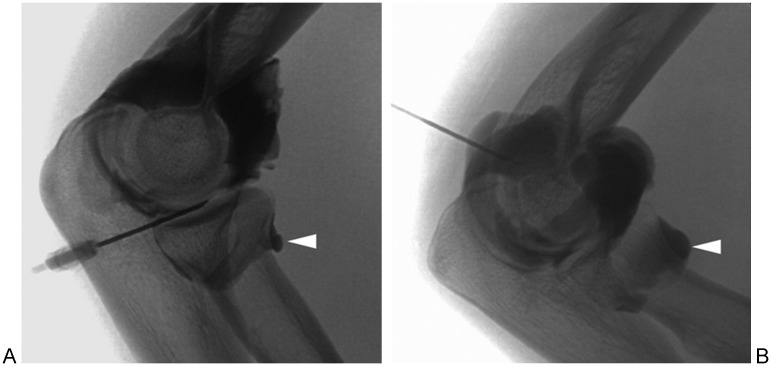
Figure 8.
Elbow joint access and arthrography. Arrowhead in both images denotes normal annular recess. (A) Lateral (radiocapitellar) approach. (B) Posterior approach.
Alternatively, a variety of posterior approaches may be utilized to access the flexed elbow joint as visualized on lateral radiography. Using the posterolateral approach, the skin entry site is located in the center of a triangle formed by palpating the olecranon, lateral epicondyle, and radial head. The needle is directed toward the olecranon fossa of the posterior humerus.11 Using a posteromedial approach, the needle is directed toward the space between the olecranon and medial epicondyle, avoiding the ulnar nerve by positioning the needle 1 cm lateral to the medial epicondyle.16,36 Finally, some investigators have advocated a trans-triceps approach, in which the needle entry site is equidistant between the medial and lateral epicondyles and just proximal to the olecranon.41 The needle should be advanced from this site, crossing the triceps and eventually entering the olecranon fossa, which is the desired needle tip location. These posterior approaches avoid damaging the lateral ligaments. The radiologist should keep in mind, however, that some patients have an olecranon foramen, rather than an olecranon fossa, in the distal humerus; in such patients, the needle could potentially be advanced through the distal humerus into the anterior aspect of the joint or beyond.
US may also be used for accessing the flexed elbow, with the preferred approach a posterior route with the target the posterior recess of the joint. The needle tip should be well visualized throughout the duration of the procedure.29
Wrist
Anatomy: Dividing the anatomy of the wrist into three major compartments simplifies the most complex of all articulations.42 These divisions include the distal radioulnar compartment, the radiocarpal compartment, and the midcarpal compartment. The radiocarpal compartment refers to the articulation between the distal radius and the proximal carpal row (scaphoid, lunate, and triquetrum). The midcarpal compartment lies between the previously mentioned proximal carpal row and the distal carpal row (trapezoid, trapezium, capitate, and hamate). These two compartments are separated by intrinsic ligaments connecting the scaphoid to the lunate and the lunate to the triquetrum. The radiocarpal joint is separated from the distal radioulnar joint by the triangular fibrocartilage, which extends from the ulnar styloid to the ulnar notch of the radius.
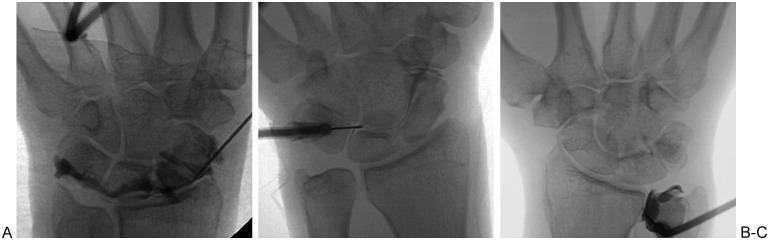
Approach: Fluoroscopically guided wrist aspiration may be performed with the patient lying prone on the fluoroscopy table with the arm outstretched above the head, or with the patient lying supine on (or sitting adjacent to) the fluoroscopy unit with the hand placed on the table. Regardless of the preferred patient position, the hand should be palm down on the fluoroscopy table. The radiocarpal joint is the most frequently accessed wrist articulation for aspiration of fluid. The key anatomical landmark is the radioscaphoid articulation on the fluoroscopic frontal view.16,36 Because the dorsal lip of the radius slightly overlaps the radiocarpal joint, the site of needle entry should be marked on the patient's skin a few millimeters distal to the articulation. After the skin is prepared and local analgesia is applied, the needle is advanced into the joint with ∼45° of proximal angulation, avoiding the dorsal lip of the radius and avoiding the scapholunate interval (Fig. 9). The wrist can be rotated into a lateral position to facilitate access into the joint if necessary. Alternatively, the radiocarpal joint can be accessed via an ulnar-sided injection that targets the proximal edge of the triquetrum.43
Figure 9.
Wrist joint access and arthrography. (A) Radiocarpal joint. Note distal-to-proximal trajectory of needle, facilitating joint access by avoiding dorsal lip of distal radius. (B) Midcarpal joint. Note positioning of needle tip in space between lunate, hamate, triquetrum, and capitate. (C) Distal radioulnar joint. Note positioning of needle along lateral aspect of ulnar cortex.
Access to the midcarpal joint is generally straightforward and involves marking the dorsal skin entry site directly superficial to the triquetrolunohamate space. The needle is advanced straight down into the joint (Fig. 9). Access to the distal radioulnar joint is obtained by placing the needle adjacent to the lateral aspect of the ulnar cortex, a few millimeters proximal to the distal ulnar surface (Fig. 9).44
Other Joints
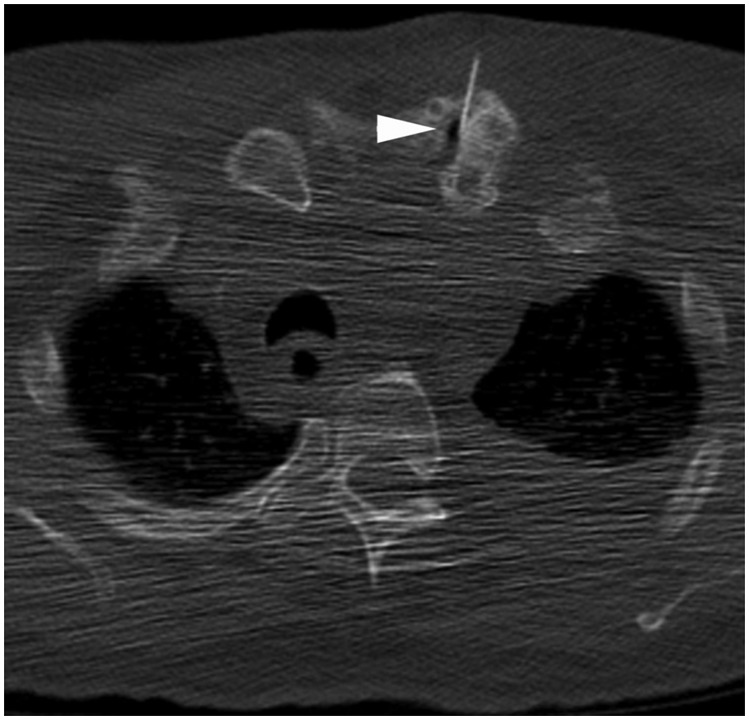
Although fluoroscopy and US are the most common methods of accessing the joints of the extremities, CT is often preferred for aspiration of the sternoclavicular and sacroiliac joints. The SC joint is a diarthrodial joint composed of the sternal end of the clavicle, lateral and upper part of the manubrium sterni, and the cartilage of the first rib. The SC joint serves as the only connection between the upper extremity and axial skeleton. The preferred method of accessing the SC joint is under CT guidance to avoid the nearby vascular structures in the underlying mediastinum. The skin entry site is marked with the understanding that slight lateral to medial needle angulation will be necessary to parallel the orientation of the SC joint. Following standard preparation and administration of local anesthesia, the needle is advanced toward the SC joint using intermittent CT guidance to confirm placement and avoid key mediastinal structures deep to the joint (Fig. 10). A small amount of iodinated contrast or air may be injected to confirm satisfactory intra-articular needle placement.45
Figure 10.
Sternoclavicular joint access. Axial computed tomography image through upper thorax shows needle entering left sternoclavicular joint (arrowhead). Air was injected to confirm placement.
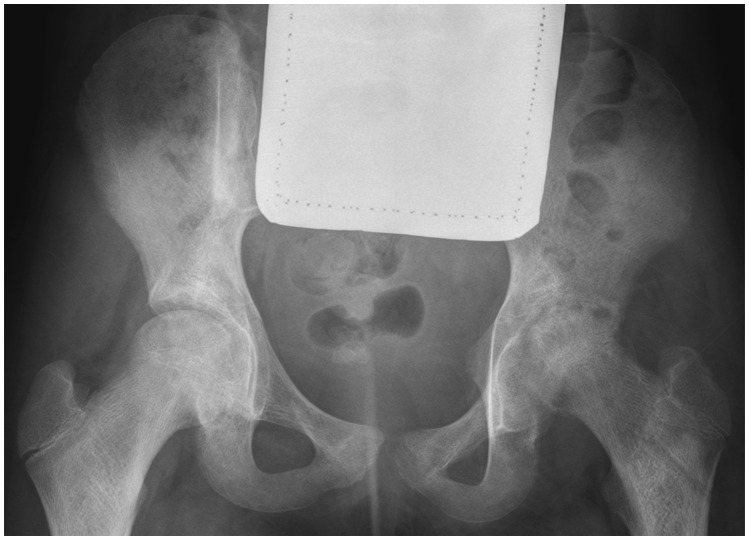
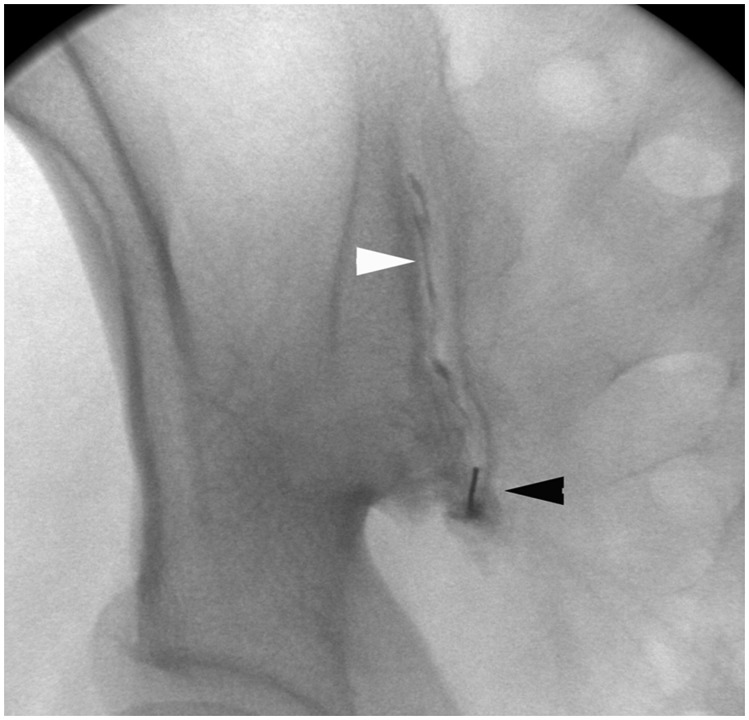
The SI joints are paired L-shaped or kidney bean–shaped joints that are formed by the articular surfaces of the ilium and sacrum. The SI joint is curved, resulting in the posterior portion of the joint being located more medially compared with the anterior portion. Furthermore, the inferior two thirds of the joint are synovial, whereas the superior one third is fibrous. A key structure to avoid while accessing the SI joint is the sciatic nerve, which is located immediately anterior to the piriformis muscle and at the same depth as the most inferior portion of the SI joint.46 Traditionally, the SI joint has been challenging to access fluoroscopically due to its complex composition, and therefore CT is often used to access the SI joint because it is believed to be more straightforward than using fluoroscopy. To access the SI joint, the patient is placed prone on the CT table. Using CT guidance, the SI joint is localized and, following standard preparation and administration of analgesia, the needle is advanced using an approach that targets the inferior third of the joint space. The ideal needle track mirrors the orientation of the SI joint and requires slight medial to lateral angulation to enter the intra-articular space.47 Using fluoroscopy, the patient is positioned in a prone oblique manner with the side to be accessed facing down. This position serves to create a vertical approach to the inferior aspect of the synovial joint. Some investigators also promote angling the tube 20 to 25° cephalad to allow easier access to the joint.46 A spinal needle may then be used to access the inferior aspect of the joint (Fig. 11). One potential pitfall of accessing the SI joint is irritation of the sciatic nerve; this may occur secondary to inadvertent needle placement into the nerve or overzealous administration of local anesthetic, resulting in lower extremity weakness that is transient. Due to this potential complication, it is standard practice to ensure another person is available to drive the patient home if the SI joints are accessed in an outpatient setting.46
Figure 11.
Sacroiliac joint access and arthrography. Fluoroscopic image of right sacroiliac joint injection shows needle (black arrowhead) in inferior portion of joint, with contrast (white arrowhead) extending superiorly within joint.
For aspiration of small joints such as those of the hand, US guidance using a high-frequency transducer with a small footprint is preferred. CT is generally used for access to the facet joints of the spine.
Aspiration of Juxta-Articular Collections
In addition to aspiration and drainage of intra-articular fluid, a host of juxta-articular collections may also be aspirated by the interventionalist using fluoroscopic, US, or CT imaging guidance. Such entities include inflamed or infected distended bursal collections (Fig. 12), juxta-articular cysts associated with underlying internal derangement of the joint (Fig. 13), and ganglia or fluid collections resulting in nerve compression or other symptoms (Fig. 14).48
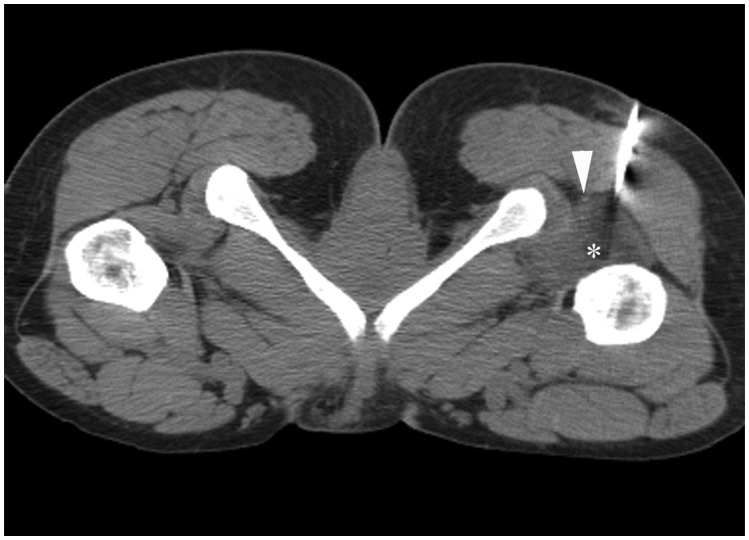
Figure 12.
Ischiofemoral bursitis. Computed tomography image through lower hip region shows needle entering low-density collection representing ischiofemoral bursitis (asterisk) situated between left ischium and left proximal femur. Bursitis was irritating the sciatic nerve (arrowhead), resulting in pain. Patient's symptoms improved following aspiration of bursal fluid.
Figure 13.
Parameniscal cyst. (A) Fat-suppressed T2-weighted coronal magnetic resonance image of the right knee demonstrates a hyperintense parameniscal cyst arising from the lateral meniscus. Note associated lateral meniscal tear (arrowhead). (B) Sonogram image obtained during aspiration of cyst shows needle tip (arrowhead) within cyst. Steroid was subsequently injected, and patient has done well since procedure.
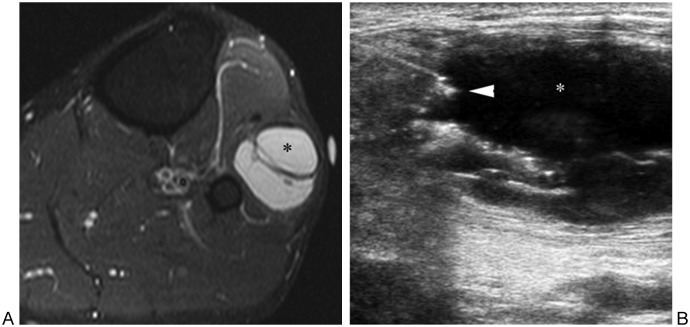
Figure 14.
Ganglion near proximal tibiofibular joint associated with peroneal nerve palsy and foot drop. (A) Fat-suppressed T2-weighted transverse magnetic resonance image of leg shows ganglion (asterisk) lateral to fibular neck. (B) Sonogram image shows needle tip (arrowhead) entering ganglion (asterisk). Subsequent aspiration of ganglion contents and steroid injection provided relief to patient.
Diagnosis of an inflamed bursa requires knowledge of bursal anatomy regardless of the imaging modality used. Inflamed bursae may be seen about a wide array of joints with some of the most commonly symptomatic including the prepatellar, infrapatellar, iliopsoas, trochanteric, olecranon, and subacromial bursae. These bursae may be accessed under US, fluoroscopic, or CT guidance for aspiration (e.g., to rule out septic bursitis) and/or for administration of corticosteroids (e.g., into the iliopsoas bursa of a patient with a clinical diagnosis of anterior snapping hip syndrome).49,50 Due to many of the advantages already discussed, US is the most frequently used imaging modality for accessing bursae and other juxta-articular fluid collections. The principles of accessing a juxta-articular collection under US guidance are similar to those of intra-articular access. One should be familiar with the pertinent anatomy based on the location of the lesion and take care to always visualize the needle tip throughout the duration of the procedure. One of the advantages US provides is the ability to observe decompression or the collapse of a cystic lesion or bursa in real time, which provides confirmation of appropriate needle tip placement (Fig. 15).
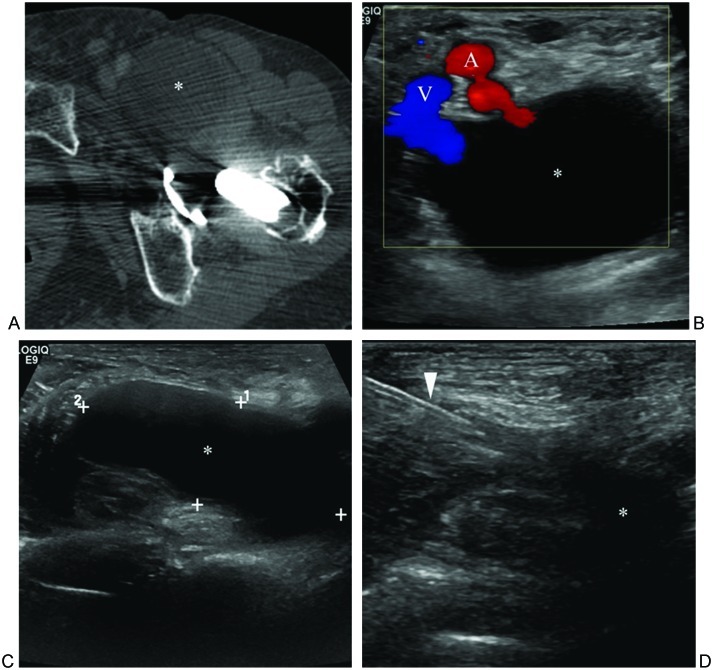
Figure 15.
Distended iliopsoas bursa. (A) Axial computed tomography image through left hip shows distended iliopsoas bursa (asterisk) in patient with total hip arthroplasty. (B) Transverse sonogram image shows fluid-filled bursa (asterisk) lateral to femoral artery (A) and vein (V). (C) Longitudinal sonogram image shows craniocaudal extent of distended bursa. (D) Magnified longitudinal sonogram image shows needle (arrowhead) within bursa following aspiration of contents, with only small residua of fluid remaining.
Juxta-articular cysts and ganglia generally appear as homogeneous masses with contents resembling fluid, although a hypoechoic rather than anechoic appearance may be encountered sonographically. The masses are frequently multilobulated and multiloculated with thin peripheral and sometimes septal enhancement seen on CT or MRI following contrast administration. Aspiration of juxta-articular lesions provides a therapeutic benefit via decompression as well as diagnostic information, especially if infection is suspected, and aspirated fluid should be sent to the microbiology laboratory in a manner similar to the handling of intra-articular fluid specimens. Additionally, the injection of corticosteroids into a noninfected juxta-articular cystic collection may offer an alternative for symptomatic relief in patients who are unwilling or poor surgical candidates.40
In the setting of meniscal tears, parameniscal cysts are not infrequently encountered, presenting focally as pain or a palpable lesion at the joint space. These lesions classically have been treated with either arthroscopic resection and debridement or open surgical excision. However, with advances in high-resolution US, US-guided cyst aspiration/injection has become a viable, minimally invasive therapeutic option in many cases (Fig. 13).51,52 Paralabral cysts of the shoulder and hip are similar lesions, often associated with underlying labral tears, and they may be painful or result in entrapment neuropathy.53 These too can be aspirated and injected with steroids using imaging guidance.
Ganglia are nonneoplastic cystlike masses of unknown etiology that typically arise in the vicinity of joints but seldom demonstrate a direct communication with the joint. They may become symptomatic due to compression or involvement of adjacent nerves resulting in pain, paresthesias, and eventual muscle denervation with associated weakness and atrophy (Fig. 14). High-frequency US-guided aspiration, using no smaller than 18 to 20-gauge spinal needles due to viscous contents, is often effective in relieving symptoms.54
Aspiration of Soft Tissue Collections
Extremity fluid collections, including abscesses (Fig. 16), lymphoceles (Fig. 17), and hematomas, can be successfully treated with image-guided percutaneous drainage. The increasing use of interventional MSK procedures has been driven by advances in minimally invasive techniques, allowing not only for the precise placement of catheters in locations that were previously inaccessible, but also the ability to drain complex collections via the instillation of pharmaceutical agents that may speed collection dissolution.55 A growing body of literature states that image-guided drainage of percutaneous fluid collections in the extremities, whether by US, CT, or fluoroscopy, is often as effective as surgical management. This is an attractive alternative to surgery due to minimal invasiveness and feasibility in some patient populations.40
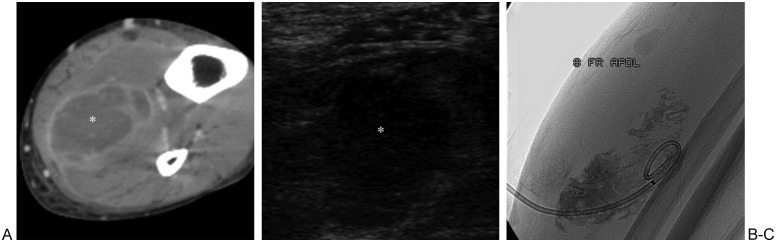
Figure 16.
Soleus abscess. (A) Axial computed tomography image of leg shows hypodense collection (asterisk) with rim enhancement within soleus muscle. (B) Sonogram image shows hypoechoic collection (asterisk) in soleus muscle prior to access via needle. (C) Fluoroscopic image shows 8F drain placed into collection, with contrast outlining inner aspect of abscess cavity.
Figure 17.
Lymphocele. (A) Sonogram image shows needle accessing hypoechoic lymphocele. (B) Fluoroscopic image shows pigtail catheter and contrast within lymphocele. Sclerosing agent (95% alcohol) was instilled through catheter and allowed to remain in cavity for 3 hours. Subsequent aspiration resulted in complete collapse of lymphocele.
Before accessing a soft tissue fluid collection, the interventionalist should review all available preprocedural imaging studies to characterize the collection as simple versus complex, determine the size and depth of the collection, and plan the safest/shortest needle trajectory. As with juxta-articular fluid collections, US is typically used for identification of intramuscular collections. After preparation of the skin, application of local analgesia (and initiation of conscious sedation, if necessary), a needle may be slowly advanced with imaging guidance into the targeted collection. Aspiration should be performed to decrease intracavitary pressure and reduce the risk of bacteremia. The aspirated contents should be sent for further laboratory analysis to identify specific pathogens and streamline antibiotic therapy. A small amount of contrast may be injected under fluoroscopy to confirm proper needle location within the targeted collection. Using the Seldinger technique, a guidewire is then inserted, the tract is serially dilated, and a pigtail drainage catheter, ranging in size from 8 to 16F, is placed within the fluid collection (Fig. 16).56
Immediately after catheter placement, a syringe should be attached to aspirate fluid until none is returned. If an insufficient amount of fluid is obtained, saline may be instilled and then aspirated in a manner similar to the previously discussed “dry tap.” Gentle irrigation of the fluid collection should be performed using 10-mL aliquots of saline until the returned aspirate is clear. The catheter is placed to gravity drainage, and postdrainage images are obtained to demonstrate the efficacy of the intervention. Catheter management consists of 5-mL saline flushes every 8 hours and recording of output volumes. End points for catheter removal include resolution of fluid collection on postprocedural imaging, outputs <10 to 20 mL per day, and clinical improvement such as defervescence and decreasing leukocytosis.57
Sources have cited clinical success rates (defined as no surgical intervention necessary) of 65 to 85% for imaging guided percutaneous drainage of fluid collections in the extremities.56,57 Success rates vary with the anatomical location of the fluid collection, with drainage in the hip/groin region the least successful. Additional factors associated with diminished success include patients on chemotherapy as well as the presence of concomitant skeletal infection including osteomyelitis, epidural abscess, and discitis. Even in patients who ultimately fail to avoid surgery, percutaneous drainage proves an extremely effective temporizing measure in controlling symptoms and optimizing clinical status prior to surgical intervention.
Anytime a fluid collection is identified in an extremity, the interventionalist must consider the possibility of a malignant tumor such as a sarcoma masquerading as benign pathology. There have been multiple case reports of various sarcomas misdiagnosed as abscesses or hematomas.58,59 Myxoid tumors in particular may appear deceptively cystic on MRI; sequences following administration of intravenous gadolinium may assist in differentiating myxoid neoplasm from a cyst or ganglion. Hematoma may be difficult to distinguish from hemorrhagic neoplasm on imaging studies. A lack of trauma history in the setting of an intramuscular hematoma should raise suspicion for an underlying tumor; however, sarcomas mimicking hematomas have been identified serendipitously in patients after trauma, and therefore regardless of history, suspicious collections should be handled in consultation with an orthopedic oncologist. Certain MRI features may aid in diagnosis (Fig. 18); nodular or masslike enhancement associated with a fluid collection is suggestive of neoplasm.60 A “penumbra sign” of high signal intensity surrounding the collection on unenhanced T1-weighted images has been suggested as a specific feature of infection.61 MRI features of hematoma have been well described in the imaging literature (e.g., high signal intensity of subacute blood products on T1-weighted images, low signal intensity hemosiderin rim on T2-weighted images), and diffusion-weighted MRI shows the potential for distinguishing soft tissue sarcoma from abscess and hematoma.62,63 However, ultimately aspiration and/or biopsy may be necessary for definitive diagnosis. If soft tissue sarcoma is a consideration, aspiration should be performed in close consultation with the orthopedic surgeon who would remove the sarcoma because the needle track will need to be resected along with the tumor and hence may affect the surgeon's approach. Aspiration may be effective for clinical triage of extremity soft tissue masses, but as a general rule core biopsy should accompany aspiration if there is a question of neoplasm.64,65
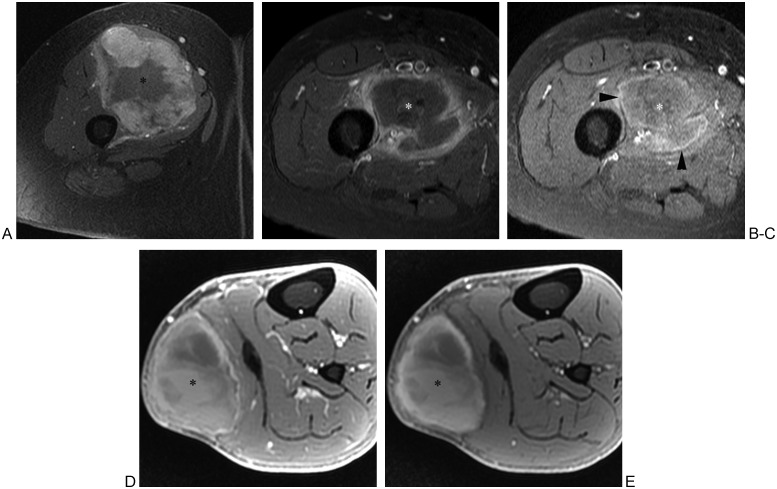
Figure 18.
Magnetic resonance (MR) images of soft tissue sarcoma, abscess, and hematoma in three different patients. (A) Fat-suppressed T1-weighted transverse MR image of thigh following intravenous administration of gadolinium chelate shows sarcoma (asterisk) in quadriceps musculature. Note the nodular peripheral enhancement, which should raise suspicion for tumor. (B) In another patient, fat-suppressed T1-weighted transverse MR image of thigh following intravenous administration of gadolinium chelate shows intramuscular abscess (asterisk) with peripheral enhancement of relatively uniform thickness. (C) Precontrast fat-suppressed T1-weighted MR image of same patient as in (B) shows high signal intensity “penumbra sign” (arrowheads) surrounding low signal intensity purulent fluid (asterisk), which has reportedly high specificity for musculoskeletal infection. (D) In another patient, fat-suppressed T1-weighted axial MR image of calf following intravenous administration of gadolinium chelate shows heterogeneous signal intensity of hematoma (asterisk) including high signal intensity components. (E) Precontrast fat-suppressed T1-weighted MR image of same patient as in (D) shows similar high signal intensity components, compatible with subacute blood products of hematoma. Follow-up MRI obtained 10 weeks later showed decrease in size of hematoma, arguing against underlying tumor.
Conclusion
With advances in imaging technology, there has been a significant increase in the number and range of interventional MSK image-guided procedures. One of the most commonly performed image-guided MSK interventions is the diagnostic and therapeutic percutaneous aspiration and drainage of multiple types of intra-articular, juxta-articular, and intramuscular pathologic fluid collections. These procedures may be performed under fluoroscopic, US, CT, or even MR guidance depending on the location to be accessed, type of pathology, patient characteristics, and physician preferences. MSK image-guided aspiration and drainage procedures are minimally invasive and generally very safe while offering valuable diagnostic information as well as therapeutic benefit.
References
- 1.Gibbon W W. Interventional radiology techniques in musculoskeletal disease. Baillieres Clin Rheumatol. 1996;10(4):711–727. doi: 10.1016/s0950-3579(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 2.Silverman S G ACR-SIR practice guideline for specifications and performance of image guided percutaneous drainage/aspiration of abscesses and fluid collections (PDAFC) in adultsACR Practice Guideline Revised. Available at: http://www.acr.org/~/media/ACR/Document/PGTS/guidelines/PDAFC.pdfAccessed 2008
- 3.Parillo S J Morrison D S Panacek E A Arthrocentesis 5th ed. Philadelphia, PA: Saunders; 2009 Chapter 53 [Google Scholar]
- 4.Mohana-Borges A VR, Chung C B, Resnick D. Monoarticular arthritis. Radiol Clin North Am. 2004;42(1):135–149. doi: 10.1016/S0033-8389(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 5.Learch T J. Imaging of infectious arthritis. Semin Musculoskelet Radiol. 2003;7(2):137–142. doi: 10.1055/s-2003-41347. [DOI] [PubMed] [Google Scholar]
- 6.Thumboo J, O'Duffy J D. A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium. Arthritis Rheum. 1998;41(4):736–739. doi: 10.1002/1529-0131(199804)41:4<736::AID-ART23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Hodler J. Technical errors in MR arthrography. Skeletal Radiol. 2008;37(1):9–18. doi: 10.1007/s00256-007-0329-z. [DOI] [PubMed] [Google Scholar]
- 8.Newberg A H, Munn C S, Robbins A H. Complications of arthrography. Radiology. 1985;155(3):605–606. doi: 10.1148/radiology.155.3.4001360. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen T W, Shen S, Shaffer R W, Setnik G S. Videos in clinical medicine. Arthrocentesis of the knee. N Engl J Med. 2006;354(19):e19. doi: 10.1056/NEJMvcm051914. [DOI] [PubMed] [Google Scholar]
- 10.McPherson R A Pincus M R, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods. 21st ed Philadelphia, PA: Saunders Elsevier; 2007437 [Google Scholar]
- 11.Lin H M, Learch T J, White E A, Gottsegen C J. Emergency joint aspiration: a guide for radiologists on call. Radiographics. 2009;29(4):1139–1158. doi: 10.1148/rg.294085032. [DOI] [PubMed] [Google Scholar]
- 12.Sofka C M, Collins A J, Adler R S. Use of ultrasonographic guidance in interventional musculoskeletal procedures: a review from a single institution. J Ultrasound Med. 2001;20(1):21–26. doi: 10.7863/jum.2001.20.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Arndt R D, Horns J W, Gold R H. Baltimore, MD; Williams and Wilkins; 1985. Clinical Arthrography. 2nd ed. [Google Scholar]
- 14.Wagner F V, Negrão J R, Campos J. et al. Capsular ligaments of the hip: anatomic, histologic, and positional study in cadaveric specimens with MR arthrography. Radiology. 2012;263(1):189–198. doi: 10.1148/radiol.12111320. [DOI] [PubMed] [Google Scholar]
- 15.Aliabadi P, Baker N D, Jaramillo D. Hip arthrography, aspiration, block, and bursography. Radiol Clin North Am. 1998;36(4):673–690. doi: 10.1016/s0033-8389(05)70055-5. [DOI] [PubMed] [Google Scholar]
- 16.Malfair D Therapeutic and diagnostic joint injections Radiol Clin North Am 2008463439–453., v [DOI] [PubMed] [Google Scholar]
- 17.Duc S R, Hodler J, Schmid M R. et al. Prospective evaluation of two different injection techniques for MR arthrography of the hip. Eur Radiol. 2006;16(2):473–478. doi: 10.1007/s00330-005-2865-z. [DOI] [PubMed] [Google Scholar]
- 18.Llopis E, Fernandez E, Cerezal L MR. MR and CT arthrography of the hip. Semin Musculoskelet Radiol. 2012;16(1):42–56. doi: 10.1055/s-0032-1304300. [DOI] [PubMed] [Google Scholar]
- 19.Vaccaro J P, Sauser D D, Beals R K. Iliopsoas bursa imaging: efficacy in depicting abnormal iliopsoas tendon motion in patients with internal snapping hip syndrome. Radiology. 1995;197(3):853–856. doi: 10.1148/radiology.197.3.7480768. [DOI] [PubMed] [Google Scholar]
- 20.Brandser E A, El-Khoury G Y, FitzRandolph R L. Modified technique for fluid aspiration from the hip in patients with prosthetic hips. Radiology. 1997;204(2):580–582. doi: 10.1148/radiology.204.2.9240558. [DOI] [PubMed] [Google Scholar]
- 21.Swan J S, Braunstein E M, Capello W. Aspiration of the hip in patients treated with Girdlestone arthroplasty. AJR Am J Roentgenol. 1991;156(3):545–546. doi: 10.2214/ajr.156.3.1899754. [DOI] [PubMed] [Google Scholar]
- 22.Renner J B, Agee M W. Treatment of suppurative arthritis by percutaneous catheter drainage. AJR Am J Roentgenol. 1990;154(1):135–138. doi: 10.2214/ajr.154.1.2104697. [DOI] [PubMed] [Google Scholar]
- 23.Rowbotham E L, Grainger A J. Ultrasound-guided intervention around the hip joint. AJR. 2011;197:122–127. doi: 10.2214/AJR.10.6344. [DOI] [PubMed] [Google Scholar]
- 24.Courtney P, Doherty M. Joint aspiration and injection and synovial fluid analysis. Best Pract Res Clin Rheumatol. 2009;23(2):161–192. doi: 10.1016/j.berh.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Morrison J L, Kaplan P A. Water on the knee: cysts, bursae, and recesses. Magn Reson Imaging Clin N Am. 2000;8(2):349–370. [PubMed] [Google Scholar]
- 26.Manaster B J, Disler D G, May D A. Philadelphia, PA: Mosby; 2007. The Requisites: Musculoskeletal Imaging. 3rd ed; p. 662. [Google Scholar]
- 27.Moser T, Moussaoui A, Dupuis M, Douzal V, Dosch J C. Anterior approach for knee arthrography: tolerance evaluation and comparison of two routes. Radiology. 2008;246(1):193–197. doi: 10.1148/radiol.2461070045. [DOI] [PubMed] [Google Scholar]
- 28.Zurlo J V, Towers J D, Golla S. Anterior approach for knee arthrography. Skeletal Radiol. 2001;30(6):354–356. doi: 10.1007/s002560100363. [DOI] [PubMed] [Google Scholar]
- 29.Fessell D P, Jacobson J A, Craig J. et al. Using sonography to reveal and aspirate joint effusions. AJR Am J Roentgenol. 2000;174(5):1353–1362. doi: 10.2214/ajr.174.5.1741353. [DOI] [PubMed] [Google Scholar]
- 30.Cerezal L Llopis E Canga A Rolón A MR arthrography of the ankle: indications and technique Radiol Clin North Am 2008466973–994., v [DOI] [PubMed] [Google Scholar]
- 31.Cerezal L Abascal F García-Valtuille R Canga A Ankle MR arthrography: how, why, when Radiol Clin North Am 2005434693–707., viii [DOI] [PubMed] [Google Scholar]
- 32.Bureau N J, Chhem R K, Cardinal E. Musculoskeletal infections: US manifestations. Radiographics. 1999;19(6):1585–1592. doi: 10.1148/radiographics.19.6.g99no061585. [DOI] [PubMed] [Google Scholar]
- 33.Sofka C M, Adler R S. Ultrasound-guided interventions in the foot and ankle. Semin Musculoskelet Radiol. 2002;6(2):163–168. doi: 10.1055/s-2002-32362. [DOI] [PubMed] [Google Scholar]
- 34.Helms C A, Major N M, Anderson M W, Kaplan P A, Dussault R. Philadelphia, PA; Saunders; 2009. Musculoskeletal MRI. 2nd ed; pp. 177–182. [Google Scholar]
- 35.Jacobson J A Lin J Jamadar D A Hayes C W Aids to successful shoulder arthrography performed with a fluoroscopically guided anterior approach Radiographics 2003232373–378.; discussion 379 [DOI] [PubMed] [Google Scholar]
- 36.Masala S, Fiori R, Bartolucci D A. et al. Diagnostic and therapeutic joint injections. Semin Intervent Radiol. 2010;27(2):160–171. doi: 10.1055/s-0030-1253514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders T R, Staple T W. Percutaneous catheter drainage of septic shoulder joint. Radiology. 1983;147(1):270–271. doi: 10.1148/radiology.147.1.6828746. [DOI] [PubMed] [Google Scholar]
- 38.Dépelteau H, Bureau N J, Cardinal E, Aubin B, Brassard P. Arthrography of the shoulder: a simple fluoroscopically guided approach for targeting the rotator cuff interval. AJR Am J Roentgenol. 2004;182(2):329–332. doi: 10.2214/ajr.182.2.1820329. [DOI] [PubMed] [Google Scholar]
- 39.Catalano O A, Manfredi R, Vanzulli A. et al. MR arthrography of the glenohumeral joint: modified posterior approach without imaging guidance. Radiology. 2007;242(2):550–554. doi: 10.1148/radiol.2422051964. [DOI] [PubMed] [Google Scholar]
- 40.Cardinal E, Chhem R K, Beauregard C G. Ultrasound-guided interventional procedures in the musculoskeletal system. Radiol Clin North Am. 1998;36(3):597–604. doi: 10.1016/s0033-8389(05)70048-8. [DOI] [PubMed] [Google Scholar]
- 41.Lohman M, Borrero C, Casagranda B, Rafiee B, Towers J. The posterior transtriceps approach for elbow arthrography: a forgotten technique? Skeletal Radiol. 2009;38(5):513–516. doi: 10.1007/s00256-008-0634-1. [DOI] [PubMed] [Google Scholar]
- 42.Moser T Dosch J C Moussaoui A Buy X Gangi A Dietemann J L Multidetector CT arthrography of the wrist joint: how to do it Radiographics 2008283787–800.; quiz 911 [DOI] [PubMed] [Google Scholar]
- 43.Cerezal L Abascal F García-Valtuille R Del Piñal F Wrist MR arthrography: how, why, when Radiol Clin North Am 2005434709–731., viii [DOI] [PubMed] [Google Scholar]
- 44.Tirman R M, Weber E R, Snyder L L, Koonce T W. Midcarpal wrist arthrography for detection of tears of the scapholunate and lunotriquetral ligaments. AJR Am J Roentgenol. 1985;144(1):107–108. doi: 10.2214/ajr.144.1.107. [DOI] [PubMed] [Google Scholar]
- 45.Peterson C K, Saupe N, Buck F, Pfirrmann C WA, Zanetti M, Hodler J. CT-guided sternoclavicular joint injections: description of the procedure, reliability of imaging diagnosis, and short-term patient response. AJR. 2010;195:435–439. doi: 10.2214/AJR.10.4501. [DOI] [PubMed] [Google Scholar]
- 46.Dussault R G, Kaplan P A, Anderson M W. Fluoroscopy-guided sacroiliac joint injections. Radiology. 2000;214(1):273–277. doi: 10.1148/radiology.214.1.r00ja28273. [DOI] [PubMed] [Google Scholar]
- 47.Silbergleit R Mehta B A Sanders W P Talati S J Imaging-guided injection techniques with fluoroscopy and CT for spinal pain management Radiographics 2001214927–939.; discussion 940–942 [DOI] [PubMed] [Google Scholar]
- 48.Weidner S, Kellner W, Kellner H. Interventional radiology and the musculoskeletal system. Best Pract Res Clin Rheumatol. 2004;18(6):945–956. doi: 10.1016/j.berh.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Dyke J A Van, Holley H C, Anderson S D. Review of iliopsoas anatomy and pathology. Radiographics. 1987;7(1):53–84. doi: 10.1148/radiographics.7.1.3448631. [DOI] [PubMed] [Google Scholar]
- 50.Sartoris D J, Danzig L, Gilula L, Greenway G, Resnick D. Synovial cysts of the hip joint and iliopsoas bursitis: a spectrum of imaging abnormalities. Skeletal Radiol. 1985;14(2):85–94. doi: 10.1007/BF00349741. [DOI] [PubMed] [Google Scholar]
- 51.Chang A. Imaging-guided treatment of meniscal cysts. HSS J. 2009;5(1):58–60. doi: 10.1007/s11420-008-9098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macmahon P J, Brennan D D, Duke D, Forde S, Eustace S J. Ultrasound-guided percutaneous drainage of meniscal cysts: preliminary clinical experience. Clin Radiol. 2007;62(7):683–687. doi: 10.1016/j.crad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Tung G A, Entzian D, Stern J B, Green A. MR imaging and MR arthrography of paraglenoid labral cysts. AJR Am J Roentgenol. 2000;174(6):1707–1715. doi: 10.2214/ajr.174.6.1741707. [DOI] [PubMed] [Google Scholar]
- 54.Jose J, Fourzali R, Lesniak B, Kaplan L. Ultrasound-guided aspiration of symptomatic intraneural ganglion cyst within the tibial nerve. Skeletal Radiol. 2011;40(11):1473–1478. doi: 10.1007/s00256-011-1209-0. [DOI] [PubMed] [Google Scholar]
- 55.Beland M D, Gervais D A, Levis D A, Hahn P F, Arellano R S, Mueller P R. Complex abdominal and pelvic abscesses: efficacy of adjunctive tissue-type plasminogen activator for drainage. Radiology. 2008;247(2):567–573. doi: 10.1148/radiol.2472070761. [DOI] [PubMed] [Google Scholar]
- 56.Wu H P, Vesely T M. Percutaneous drainage of fluid collections in the extremities. Radiology. 1998;208(1):159–165. doi: 10.1148/radiology.208.1.9646808. [DOI] [PubMed] [Google Scholar]
- 57.Cronin C G, Gervais D A, Hahn P F, Arellano R, Guimaraes A R, Mueller P R. Treatment of deep intramuscular and musculoskeletal abscess: experience with 99 CT-guided percutaneous catheter drainage procedures. AJR Am J Roentgenol. 2011;196(5):1182–1188. doi: 10.2214/AJR.09.4082. [DOI] [PubMed] [Google Scholar]
- 58.Gomez P, Morcuende J. High-grade sarcomas mimicking traumatic intramuscular hematomas: a report of three cases. Iowa Orthop J. 2004;24:106–110. [PMC free article] [PubMed] [Google Scholar]
- 59.Dion E, Forest M, Brasseur J L, Amoura Z, Grenier P. Epithelioid sarcoma mimicking abscess: review of the MRI appearances. Skeletal Radiol. 2001;30(3):173–177. doi: 10.1007/s002560000312. [DOI] [PubMed] [Google Scholar]
- 60.Kransdorf M J, Murphey M D. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575–587. doi: 10.2214/ajr.175.3.1750575. [DOI] [PubMed] [Google Scholar]
- 61.McGuinness B, Wilson N, Doyle A J. The “penumbra sign” on T1-weighted MRI for differentiating musculoskeletal infection from tumour. Skeletal Radiol. 2007;36(5):417–421. doi: 10.1007/s00256-006-0267-1. [DOI] [PubMed] [Google Scholar]
- 62.Bush C H. The magnetic resonance imaging of musculoskeletal hemorrhage. Skeletal Radiol. 2000;29(1):1–9. doi: 10.1007/s002560050001. [DOI] [PubMed] [Google Scholar]
- 63.Costa F M Canella C Gasparetto E Advanced magnetic resonance imaging techniques in the evaluation of musculoskeletal tumors Radiol Clin North Am 20114961325–1358., vii–viii [DOI] [PubMed] [Google Scholar]
- 64.Ng V Y, Thomas K, Crist M, Wakely P E Jr, Mayerson J. Fine needle aspiration for clinical triage of extremity soft tissue masses. Clin Orthop Relat Res. 2010;468(4):1120–1128. doi: 10.1007/s11999-009-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasraeian S, Allison D C, Ahlmann E R, Fedenko A N, Menendez L R. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res. 2010;468(11):2992–3002. doi: 10.1007/s11999-010-1401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]