Abstract
The objective of percutaneous chest biopsy is to consistently reach the amount of tissue required to meet or exceed published diagnostic accuracy rates. Many recent publications have reevaluated the subject of chest biopsy to assess borderline or controversial indications such as very small lesions, ground-glass opacities, and cases with a past nondiagnostic percutaneous biopsy. In addition, publications have reviewed sample adequacy for the accurate determination of molecular markers. These new indications promise to expand the numbers of biopsy procedures performed by radiologists. This article discusses the current role of image-guided percutaneous lung biopsies in the management of patients with pulmonary malignancies.
Keywords: interventional radiology, percutaneous biopsy, lung malignancy, molecular markers
Objectives: Upon completion of this article, the reader will be able to identify the current role of percutaneous biopsy of lung lesions in the evaluation of pulmonary nodules, as well as describe the current technical advancements and limitations of the technique.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The objective of percutaneous chest biopsy is to consistently obtain the amount of tissue required to meet or exceed published diagnostic accuracy rates. Past publications have established standards for the histologic diagnosis of pulmonary nodules deemed large and dense enough to qualify for percutaneous sampling. Many recent publications have reevaluated the subject of chest biopsy to assess borderline or controversial indications such as very small lesions, ground-glass opacities, and cases with a past nondiagnostic percutaneous biopsy, particularly in the context of the perceived advantages of computed tomography (CT) fluoroscopy and on-site cytopathologists. In addition, publications have reviewed sample adequacy for the accurate determination of molecular markers. Molecular markers for both neoplastic and nonneoplastic disease processes show great promise in determining diagnosis, disease progression, and choice of therapy, and therefore the detection of a variety of markers in multiple organ systems is the subject of ongoing research. Genetic molecular markers determined from percutaneous biopsy specimens of lung adenocarcinoma have emerged as key components in the stratification of patients for the application of now-standard treatment strategies. Targeting one's individual disease type based on the findings of direct tissue analysis promises improved clinical outcomes via a more tailored treatment approach. These new indications promise to expand the numbers of biopsy procedures performed by radiologists.
Diagnostic Accuracy Rates: High Standards
Historically, diagnostic accuracy rates for imaging-guided percutaneous fine-needle aspiration (FNA) of pulmonary malignancy were reported to be as high as 94%.1 Over the years, a wide range of published diagnostic accuracy rates have emerged, likely secondary to variability in patient cohorts, definitions of lesions amenable to percutaneous sampling, operator experience, the presence of comorbidities, and variable cytopathology support. However, more recently, large quality studies have reinforced existing high standards for diagnostic accuracy in the routine performance of chest biopsy. In 2006, Gong et al published a large retrospective study comparing the diagnostic accuracy of FNA (n = 362) with core needle biopsy (n = 350) performed in the same patients.2 FNA was performed via a 20- or 22-gauge aspiration needle with two to three passes through an 18-gauge introducer needle, the number of passes depending on adequacy assessment of smears by an on-site cytopathologist. Core needle biopsy was performed using a 20-guage core biopsy gun with two to four passes through an introducer needle depending on adequacy assessment of touch preps by an on-site cytopathologist. This technique has become standard among many authors and institutions. Diagnostic accuracy rates for FNA and core needle biopsy were 85.1% and 86.7%, respectively, for malignant tumors; 86.4% and 85.2% for malignant epithelial neoplasms; 77% and 96% for malignant nonepithelial neoplasms; and 40% and 92% for benign-specific lesions. Combined FNA and core needle biopsy resulted in a 95.2% diagnostic accuracy. These results indicate the benefit of using combined FNA and core needle biopsy, particularly when malignancy is likely, as well as describing the benefit of core needle biopsy in establishing a specific diagnosis in benign cases. Large studies such as this set a high standard for procedural technique and diagnostic accuracy rates achievable by imaging-guided percutaneous sampling methods.
Small and Difficult-to-Reach Nodules: A Diagnostic Challenge
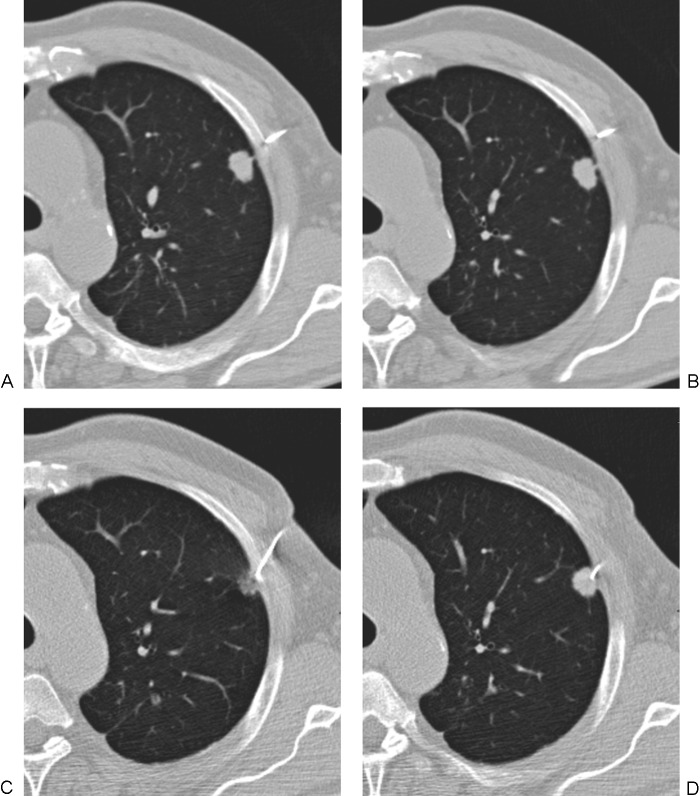
Small nodules still pose a difficult diagnostic challenge. The small size and the common additional finding of low-density or ground-glass components make positron emission tomography, bronchoscopy, and video-assisted thoracoscopic surgery frequently unsuccessful and imaging-assisted percutaneous sampling techniques challenging.3 In the past (and in many current practices), FNA was the sampling method of choice for small nodules because quality core samples were considered too difficult to obtain. Prior to 2000, nodules considered amenable to percutaneous core biopsy tended to be biased toward dense lesions measuring >1 cm in diameter, but more recent literature questions the validity of this arbitrary size threshold (Fig. 1). At minimum, these studies suggest that many subcentimeter lesions may be amenable to percutaneous biopsy or FNA as a competing strategy to surveillance with serial CT scans in patients with a higher likelihood of malignancy.
Figure 1.
Computed tomography (CT)-guided biopsy of small intraparenchymal nodules. (A) Axial CT lung windows showing multiple subcentimeter nodules suspicious for metastatic disease. (B) Despite the small size, both core biopsy samples and fine-needle aspirates were obtained, and the diagnosis of metastatic squamous cell carcinoma of the head and neck was made. High rates of diagnostic accuracy have recently been reported for subcentimeter nodules.
Options for the Small Nodule: Fluoroscopy, Computed Tomography Fluoroscopy or Cone-Beam Computed Tomography
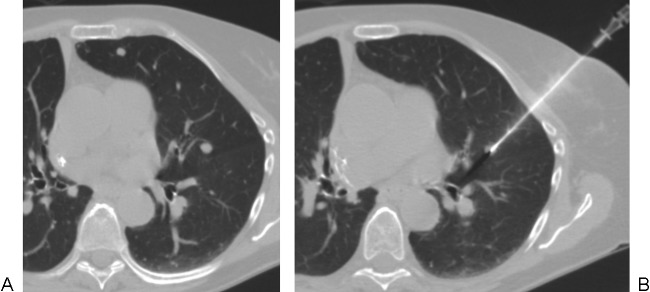
Fluoroscopic guidance of chest biopsy involving small nodules is avoided by some authors and encouraged by others. Fluoroscopy offers the advantages of real-time visualization of needle advancement—particularly advantageous for highly mobile lesions—and triangulation for verification of tip position, but a disadvantage is poor visualization of small nodules that are adjacent to large vessels and mediastinal structures (Fig. 2). Retrospective studies reviewing fluoroscopically guided chest biopsy of small nodules will likely be subject to significant selection bias because lesions accepted by radiologists will tend to be easy to visualize and access. That said, in 2012, Jae et al reviewed 42 cases of percutaneous core biopsy of small nodules measuring <10 mm in diameter. Cases were performed under fluoroscopic (n = 12) and CT (n = 30) guidance, which achieved accurate diagnosis rates of 86.7% and 91.7%, respectively.4
Figure 2.
Case illustrating the limitations of fluoroscopically guided lung biopsy. (A) A small nodule adjacent to the heart in the lingula is poorly visualized radiographically. (B) Computed tomography–guided core biopsy of this lesion, which resides partly within the chest wall, allows careful placement of the needle tip via a direct approach. In this case, by avoiding the posterior approach, the operator avoided crossing the pulmonary fissure. An anterior approach was attempted in an effort to minimize risk to the heart but was not possible due to a rib overlying the nodule.
CT fluoroscopy may add the advantage of real-time needle advancement while still resolving the small nodule from adjacent vital structures. However, the benefit of CT fluoroscopy in minimizing nondiagnostic sampling for small and difficult lesions is a subject of some debate in the literature. For lesions >3 cm, diagnostic accuracy rates reported in studies specifically mentioning the use of CT fluoroscopy tend to produce results that are similar to those of past studies of cases performed without this technique. For example, Bladt et al reviewed 72 biopsies performed with CT fluoroscopic guidance and achieved diagnostic accuracy rates of only 78.5% for lesions 1 to 3 cm in size and 87% for lesions >3 cm.5 A more careful evaluation of chest biopsy cases of small nodules behind ribs or adjacent to vital structures with and without CT fluoroscopy might resolve the true benefit of this technique; no such prospective comparison has been published to date.
Yamauchi et al in 2011 emphasized the importance of both CT fluoroscopy as well as the practice of routinely obtaining core samples for lesions <1 cm in diameter,6 achieving a 94% diagnostic accuracy rate. One interesting finding was that diagnostic accuracy decreased with length of the needle path. Furthermore, Yamagami et al in 2004 reviewed 22 cases of percutaneous needle biopsy involving lesions <2 cm obstructed by ribs and used gantry tilt to facilitate CT fluoroscopic guidance of FNA and/or core biopsy to achieve a diagnostic accuracy rate of 95.4%.7
C-arm cone-beam CT (CBCT) offers a way to avoid time on the CT scanner while still combining real-time needle advancement under fluoroscopy with verification of needle-tip position by cross-sectional imaging. CBCT creates a cross-sectional image in the fluoroscopy suite, typically with a flat panel detector. Hwang et al in 2012 used a CBCT system to guide percutaneous biopsy of 27 small nodules (average: 1.3 cm). As in the CT fluoroscopy study by Yamaguchi et al, these authors obtained both 20-gauge cores and fine-needle aspirates, and they achieved similar results. Eighteen lesions were malignant, eight were benign, and one was indeterminate for a diagnostic accuracy rate of 90% for nodules <1 cm and 94% for nodules between 1 and 2 cm.8 CBCT resulted in reductions in procedure time compared with those reported with CT fluoroscopy.
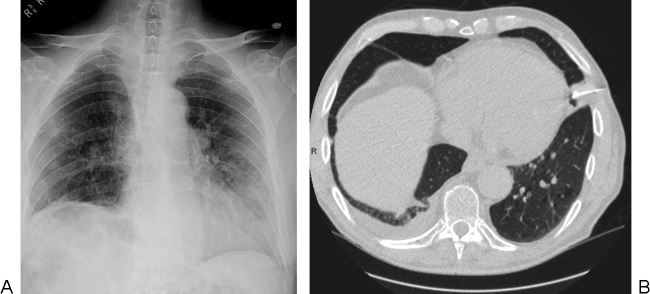
Practically speaking, both CT fluoroscopy and CBCT help to shorten procedure time9,10 and facilitate needle access into deep, mobile, or difficult-to-reach lesions (Fig. 3).
Figure 3.
Case illustrating the benefits of computed tomography (CT) fluoroscopy to biopsy small nodules behind ribs. (A, B) The nodule resides behind a rib on these two images obtained at two different points during the respiratory cycle. (C, D) Using CT fluoroscopy during suspended respirations, a 19-gauge introducer needle was quickly advanced around the rib and into the lesion.
Repeating the Nondiagnostic Biopsy?
Although biopsy samples deemed nondiagnostic often prompt more invasive “sampling” methods like surgical resection, limited data exist on the benefits of repeating the biopsy procedure (although this is a common request in clinical practice). In 2010, Lee et al demonstrated that the diagnostic accuracy rate can be reduced to a few percentage points in their patients when a single repeat procedure is performed for nondiagnostic cases.11 They reviewed procedures in 591 patients—20-gauge core biopsy performed in all patients and 20-gauge FNA in 96 patients—and reported a diagnostic accuracy rate of 96%. Repeat procedures were performed on all nondiagnostic cases, and 50%of these repeat procedures (12 of 24) yielded a diagnosis. Because this strategy further increased their rate of accurate diagnosis to 98%, repeating the biopsy of small or difficult-to-access lesions may be a superior option to proceeding with more invasive methods of tissue acquisition for repeat biopsies. One should consider altering the technique to increase the probability of achieving a diagnosis from the second biopsy, including altering the needle approach or patient positioning, altering the portion of the nodule biopsied, injecting saline into the pleura to move the nodule away from mediastinal structures, or altering the modality for imaging guidance.
On-Site Cytopathologists
Quality studies with high diagnostic accuracy rates2 often feature acquisition of samples in the presence of an on-site cytopathologist. However, no study has compared matched groups of needle aspiration and biopsy procedures with and without a cytopathologist. In 2009, Tsou et al reviewed the diagnostic accuracy of the core touch prep technique on the first and subsequent samples, each obtained after needle repositioning resulting from a previous question of sample adequacy by an on-site cytopathologist.12 A total of 432 biopsies were reviewed: 210 in the lungs and 222 in other locations. They found that cumulative diagnostic accuracy rates of the on-site cytopathologist for the first three samples were 80.6%, 85.9%, and 86.3%, respectively, and the corresponding final accuracies for biopsy were 88.2%, 93.8%, and 94.9%, respectively. The overall accuracy was 97.1% for lesions in the lungs. They found that the presence of the on-site cytopathologist was particularly helpful in improving the diagnostic accuracy for smaller lesions, but a benefit was noticed for all nodule sizes.
Ground-Glass Opacities: A Role for Percutaneous Chest Biopsy?
The role of percutaneous needle biopsy of ground-glass opacities (GGOs) is controversial and likely to remain quite limited, despite recent literature attempts to review diagnostic accuracy in the application of this technique. The principal problem is the intrinsic limitation associated with analyzing small quantities of tissue to distinguish the subtle and complex architectural features associated with the three types of GGO described in the recent classification scheme of the IASLC/ATS/ERS International Multidisciplinary Classification of Lung Adenocarcinoma. These three types of GGOs are classified as atypical adenomatous hyperplasia, adenocarcinoma in situ, or minimally invasive adenocarcinoma (MIA). Most experts recommend complete resection or surveillance by serial CT as the only options, although the former strategy is plagued by the absence of supportive data and the latter is plagued by long periods with barely perceptible increases in lesion size or density. Little or no agreement exists on follow-up protocols. Because a greater solid component makes MIA more likely, some recommend percutaneously sampling lesions with a solid component exceeding a certain size threshold (e.g., 5 mm), particularly in patients considered high risk for surgery or general anesthesia. No agreement exists regarding size threshold or technique or FNA versus core biopsy, but the previously mentioned studies by Jae et al and Yamauchi et al support the feasibility of core needle biopsy in lesions with small solid components.
Recent reports describing percutaneous FNA or core biopsy of GGOs tends to report favorable rates of diagnostic accuracy, but those rates may not prove to be reproducible when strict imaging stratification is applied prior to biopsy, and strict histologic stratification is applied to biopsy specimens under the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) classification scheme. For example, Hur et al in 2009 used CT fluoroscopy to guide needle aspiration of 28 patients with GGOs of various sizes and densities.13 Only three lesions were nondiagnostic (later confirmed as benign), indicating a diagnostic accuracy rate of 89%. Interestingly, the size of the lesion (including 10 lesions that were ≤10 mm) and the length of the needle path did not influence diagnostic accuracy. Yamauchi et al in 2011 achieved a similar diagnostic accuracy of 88% for small (<10 mm) GGOs, but their series of 67 cases with variably sized GGOs yielded an overall diagnostic accuracy rate of 97%.14 Both groups described CT fluoroscopy as particularly beneficial during the performance of percutaneous sampling procedures for GGOs because directional adjustments of subsequent needle passes could be easily made. The presence of dense foci increased the likelihood of an accurate diagnosis, probably due to an associated increased likelihood of MIA. Again, the length of the needle path did not influence the results. A specific application for percutaneous biopsy in the diagnosis of GGOs may require a prospective study comparing specimens from surgical resection and percutaneous biopsy, following the new classification criteria.
Molecular Markers
In the past, lung cancers were classified as either small cell or non–small cell (NSCLC) tumors. Published techniques for percutaneous FNA and core needle biopsy focused mainly on maximizing the diagnostic accuracy rate. More recently, research has led to the discovery of mutations in two genes, KRAS (Kirsten ras viral oncogene homolog) and EGFR (epidermal growth factor receptor), which represent the most commonly mutated genes in NSCLC adenocarcinoma patients. EGFR mutations occur more frequently in NSCLC patients who have never smoked,15 but the relationship of smoking to KRAS mutations is a subject of continuing debate in the literature. The use of these molecular markers has become standard clinical practice because their detection predicts survival rate16 and the potential for response to chemotherapy agents as well as a new class of drugs known as EGFR tyrosine kinase inhibitors (e.g., erlotinib and gefitinib). Generally, EGFR positivity predicts a remarkable response potential, nearly doubling the duration of survival, and KRAS positivity may predict a lower response potential and a lower survival rate,17 potentially even worsened by the addition of EGFR tyrosine kinase inhibitors in the management of KRAS-positive patients.18 Hence, for lung cancer, the importance of aspiration and biopsy has increased, and the number of cases referred to radiologists is likely to rise.
How much tissue is necessary for successful molecular analysis of lung adenocarcinoma is open to debate. In 2010, Solomon et al reviewed a series of 18 patients who underwent core biopsy for gefitinib eligibility, and they compared the results of molecular analysis of these cores with molecular analysis of the resected tumors.19 They showed that an average of 1.8 needle passes with 18- or 20-gauge core biopsy guns was sufficient to perform EGFR and KRAS mutational analysis to correctly apply gefitinib therapy in 16 of 18 patients (88.9%). Cheung et al retrospectively reviewed 47 percutaneous core biopsy procedures using 18- and 20-gauge core biopsy guns and demonstrated successful DNA mutational analysis in all patients.20 They noted that sample weight and DNA content was greater in the 18-gauge cores, but that smaller samples were still sufficient for analysis.
In 2011, Zhuang et al reported that even FNA may be sufficient for mutational analysis. They performed FNA in 43 patients using 18- to 20-gauge needles and obtained 0.5- to 1.5-cm core specimens that proved adequate to perform EGFR gene mutational analysis in all cases.21 Finally, even the wash fluid obtained from the chest biopsy needle can provide sufficient nucleic acid content to perform molecular analysis in most cases. In 2008, Otani et al evaluated the wash fluid of 26 chest biopsy needles used in the biopsy of NSCLCs for which pathologic correlation with a resected specimen was available.22 The EGFR mutational status determined in wash fluid correlated with the status determined by analysis of the resected specimen in all 26 cases. In summary, the standard technique of acquiring a few quality 20-gauge core samples and additional supplemental FNA samples appears to be sufficient for accurate histologic diagnosis and mutational analysis.
A Word of Caution: Standard of Care Versus Research Protocols
Inevitably with the demand for greater tissue characterization comes the demand for greater amounts of tissue. The unexpected request for “10 core specimens” should be met with caution and skepticism by the operating radiologist, particularly when little or no information regarding a standard clinical indication is provided to support this request. In the past, standard therapies were based on percutaneous biopsy results using standard techniques as well as standards for tissue quality and quantity. For example, as described here, literature studies demonstrate high diagnostic yields when two core specimens and fine-needle aspirates are obtained during percutaneous lung biopsy, and more needle passes may become necessary only if the tissue obtained from the first biopsies appears insufficient. Interdepartmental, and indeed ethical and legal, conflicts may arise when radiologists are asked to obtain multiple additional specimens, particularly in high-risk cases with emphysema, coagulopathy, or poor cardiopulmonary reserve to withstand complications of pneumothorax or hemorrhage. Suffice it to say, when a tension pneumothorax or hemothorax occurs after the tenth core biopsy, a medicolegal review of the case is likely, and the indications for the tissue quantity had better fall under the (understandably subjective) definition of standard of care or fall within an active institutional review board (IRB) protocol. Three important considerations may apply in these instances:
Multiple additional samples beyond those required for the accurate application of standard therapeutic options may breach the standard of care in some circumstances, and such acquisitions may require performance under the surveillance of an appropriate IRB protocol.
In all cases, the risks and indications associated with additional samples should be explained to the patient during the informed consent process. When the biopsy procedure falls under an IRB protocol, strict adherence of the approved consent form should be followed, including the information that additional specimens may add additional risk and are part of an experimental study.
A common requirement of IRB protocols is the written description and identification of all medical personnel and staff that will come in contact with the subjects of the study. This requirement is particularly true for radiologists performing invasive procedures such as percutaneous biopsy.
References
- 1.Boiselle P M, Shepard J A, Mark E J. et al. Routine addition of an automated biopsy device to fine-needle aspiration of the lung: a prospective assessment. AJR Am J Roentgenol. 1997;169(3):661–666. doi: 10.2214/ajr.169.3.9275873. [DOI] [PubMed] [Google Scholar]
- 2.Gong Y, Sneige N, Guo M, Hicks M E, Moran C A. Transthoracic fine-needle aspiration vs concurrent core needle biopsy in diagnosis of intrathoracic lesions: a retrospective comparison of diagnostic accuracy. Am J Clin Pathol. 2006;125(3):438–444. [PubMed] [Google Scholar]
- 3.Libby D M, Smith J P, Altorki N K, Pasmantier M W, Yankelevitz D, Henschke C I. Managing the small pulmonary nodule discovered by CT. Chest. 2004;125(4):1522–1529. doi: 10.1378/chest.125.4.1522. [DOI] [PubMed] [Google Scholar]
- 4.Jae L I June I H Miyeon Y Kwanseop L Yul L Hoon B S Percutaneous core needle biopsy for small (≤10 mm) lung nodules: accurate diagnosis and complication rates Diagn Interv Radiol 2012; June 5 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 5.Bladt O, De Wever W. Additional value of CT-fluoroscopic biopsy of pulmonary lesions: a retrospective study of 69 patients. JBR-BTR. 2006;89(6):298–302. [PubMed] [Google Scholar]
- 6.Yamauchi Y, Izumi Y, Nakatsuka S. et al. Diagnostic performance of percutaneous core-needle lung biopsy under CT scan fluoroscopic guidance for pulmonary lesions measuring ≤10 mm. Chest. 2011;140(6):1669–1670. doi: 10.1378/chest.11-1821. [DOI] [PubMed] [Google Scholar]
- 7.Yamagami T, Kato T, Iida S, Hirota T, Nishimura T. Percutaneous needle biopsy for small lung nodules beneath the rib under CT scan fluoroscopic guidance with gantry tilt. Chest. 2004;126(3):744–747. doi: 10.1378/chest.126.3.744. [DOI] [PubMed] [Google Scholar]
- 8.Hwang H S, Chung M J, Lee J W, Shin S W, Lee K S. Percutaneous transthoracic biopsy: usefulness in evaluation of small pulmonary nodules. AJR Am J Roentgenol. 2010;195(6):W400–7. doi: 10.2214/AJR.09.3963. [DOI] [PubMed] [Google Scholar]
- 9.Gianfelice D, Lepanto L, Perreault P, Chartrand-Lefebvre C, Milette P C. Value of CT fluoroscopy for percutaneous biopsy procedures. J Vasc Interv Radiol. 2000;11(7):879–884. doi: 10.1016/s1051-0443(07)61805-3. [DOI] [PubMed] [Google Scholar]
- 10.Froelich J J, Ishaque N, Regn J, Saar B, Walthers E M, Klose K J. Guidance of percutaneous pulmonary biopsies with real-time CT fluoroscopy. Eur J Radiol. 2002;42(1):74–79. doi: 10.1016/s0720-048x(01)00391-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee I J, Bae Y A, Kim D G. et al. Percutaneous needle aspiration biopsy (PCNAB) of lung lesions: 5 years results with focusing on repeat PCNAB. Eur J Radiol. 2010;73(3):551–554. doi: 10.1016/j.ejrad.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Tsou M H, Tsai S F, Chan K Y. et al. CT-guided needle biopsy: value of on-site cytopathologic evaluation of core specimen touch preparations. J Vasc Interv Radiol. 2009;20(1):71–76. doi: 10.1016/j.jvir.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Hur J, Lee H J, Nam J E. et al. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol. 2009;192(3):629–634. doi: 10.2214/AJR.08.1366. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi Y, Izumi Y, Nakatsuka S. et al. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol. 2011;79(2):e85–e89. doi: 10.1016/j.ejrad.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 15.Pham D, Kris M G, Riely G J. et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol. 2006;24(11):1700–1704. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]
- 16.Johnson M L Sima C S Chaft J et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas Cancer 2012; July 18 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascaux C, Iannino N, Martin B. et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92(1):131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhard D A, Johnson B E, Amler L C. et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23(25):5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 19.Solomon S B, Zakowski M F, Pao W. et al. Core needle lung biopsy specimens: adequacy for EGFR and KRAS mutational analysis. AJR Am J Roentgenol. 2010;194(1):266–269. doi: 10.2214/AJR.09.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung Y C, Chang J W, Hsieh J J, Lin G, Tsai Y H. Adequacy and complications of computed tomography-guided core needle biopsy on non-small cell lung cancers for epidermal growth factor receptor mutations demonstration: 18-gauge or 20-gauge biopsy needle. Lung Cancer. 2010;67(2):166–169. doi: 10.1016/j.lungcan.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang Y P, Wang H Y, Shi M Q, Zhang J, Feng Y. Use of CT-guided fine needle aspiration biopsy in epidermal growth factor mutation analysis in patients with lung cancer. Acta Radiol. 2011;52(10):1083–1087. doi: 10.1258/ar.2011.110150. [DOI] [PubMed] [Google Scholar]
- 22.Otani H, Toyooka S, Soh J. et al. Detection of EGFR gene mutations using the wash fluid of CT-guided biopsy needle in NSCLC patients. J Thorac Oncol. 2008;3(5):472–476. doi: 10.1097/JTO.0b013e31816de2cd. [DOI] [PubMed] [Google Scholar]