Abstract
With modern cross-sectional imaging techniques, cystic lesions are very common and usually incidental findings, especially if small. However, when cysts enlarge, become infected, bleed, or undergo torsion, they can be symptomatic, and percutaneous drainage can be effective in the management. When cysts recur after aspiration, which is often the case for hepatic and renal cysts, cyst sclerosis or surgical unroofing may be required. This article describes the indications for and technical aspects of percutaneous sclerotherapy of cystic lesions of multiple organ systems.
Keywords: interventional radiology, sclerotherapy, cysts, alcohol
Objectives: Upon completion of this article, the reader should be able to describe the patient population for whom sclerotherapy of cystic lesions is most appropriate, and the technique used during such procedures.
Accreditation: Tufts University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Cystic lesions can be characterized as either congenital or acquired. From a histologic perspective, cystic lesions are classified as true cysts, containing epithelium or endothelium, or pseudocysts. They can occur sporadically or as part of syndromes such as Von Hippel-Lindau or autosomal dominant polycystic kidney disease. Additionally, acquired cysts can result from trauma or infection. They can be found in essentially every solid organ in the body but most commonly affect the liver, pancreas, kidney, and ovary. Some authors extend the definition of cystic lesions to postoperative collections such as lymphoceles, hematomas, seromas, peritoneal inclusion cysts, urinomas, and bilomas.
With modern cross-sectional imaging techniques, cystic lesions are very common and usually incidental findings, especially if small. However, when cysts enlarge, become infected, bleed, or undergo torsion, they can be symptomatic, and percutaneous drainage can be effective in the management.1 When cysts recur after aspiration, which is often the case for hepatic and renal cysts,2 cyst sclerosis or surgical unroofing may be required.
General Principles of Sclerotherapy
Patients with symptomatic cysts should be referred for imaging. The chosen modality usually depends on the location of the cysts. Ultrasound is fast and readily available, and due to lack of radiation is usually used first. Ultrasound is also usually preferred in certain situations, such as in the evaluation of ovarian cysts or more superficial postoperative lymphoceles. Magnetic resonance imaging may be better at identifying the organ of origin for deep pelvic cysts. Simple cysts are typically dark on T1-weighed images and bright on T2-weighted images. However, these signals undergo changes if the cysts are proteinaceous or hemorrhagic. Contrast-enhanced computed tomography (CT) can detect solid organ cysts and cysts in other locations well. Simple cysts in general have a thin wall without mural thickening or nodularity.
Therapeutic options for cystic lesions include surgical resection or unroofing, percutaneous aspiration, percutaneous drainage, and sclerotherapy. Surgery, despite its higher morbidity and the added risk associated with anesthesia, is used in cases when there is no safe percutaneous window, when percutaneous treatment has failed, or in certain cysts located in the liver (surgical unroofing or laparoscopic fenestration) and pancreas (surgical marsupialization).
Percutaneous aspiration can be both therapeutic and diagnostic, in that complete aspiration results in resolution of symptoms and then the symptoms can be attributed to the cysts; this is confirmed especially when the symptoms return when the fluid reaccumulates. However, in most cases aspiration alone does not cure larger cysts (Fig. 1) and does not address the issue of recurring fluid accumulation, despite repeated attempts. Percutaneous drainage with drain placement uses small-bore catheters to continuously drain the cysts long term. The drain is left in place to prevent fluid reaccumulation, and when there is sufficiently decreased output, the drain is removed. The disadvantage to long-term drainage is the risk of infection and patient discomfort. Occasionally, suction drainage leads to a chronically collapsed cyst and the ultimate goal of cyst obliteration. In addition, when the cyst communicates with vital structures such as the biliary system, pancreatic duct, or renal collecting system, chronic drainage becomes a principal option because sclerotherapy is contraindicated.
Figure 1.
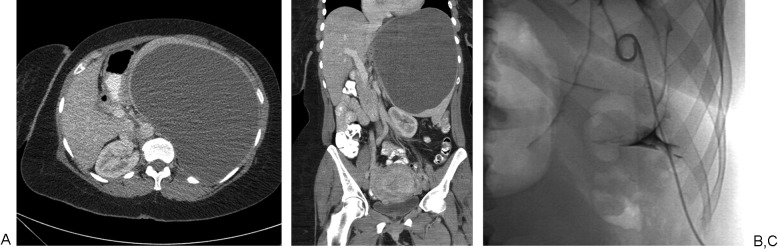
A 43-year-old woman with left upper abdominal pain. (A, B) Axial-reformatted and coronal-reformatted images from a contrast-enhanced computed tomography scan shows large thin-walled splenic cyst. (C)The cyst was aspirated with a 5F pigtail catheter, and a spot fluoroscopic image of the catheter after injection of diluted contrast demonstrates the contrast is entirely contained within the cyst, making it suitable for sclerosis.
Most symptomatic cysts are large and because of their fluid content, they are usually easily accessible by sonographic guidance. Typically, an 8 to 10F pigtail catheter is inserted into the cyst via the Seldinger or trocar technique, depending on the preference of the interventional radiologist performing the procedure. Conscious sedation is generally achieved with intravenous midazolam and fentanyl, under constant hemodynamic monitoring. Once a catheter is inserted into the cyst, all of the fluid is aspirated and a sample should be sent for Gram stain, culture, and cytology. A small amount of dilute contrast is injected through the catheter under fluoroscopic guidance, or a CT scan can be performed after injection to exclude leakage of fluid into the peritoneum or communication with blood vessels, the biliary system, or the renal collecting system. If any of these findings are present, sclerotherapy is contraindicated. Multiple agents for sclerotherapy have been used including bismuth,3 povidone-iodine,4,5 tetracycline,6 bleomycin,7 n-butyl cyanoacrylate with iodized oil,8 sodium tetradecyl,9 hypertonic saline,10 ethanolamine oleate,11 and acetic acid.12 However, the most commonly used sclerosant is ethanol because it is readily available, inexpensive, and generally well tolerated.
Sclerotherapy with Ethanol
Ethanol causes protein denaturation, cell death, and inflammatory fibrosis after contacting the cyst wall. Therefore, contact with the entire cyst wall during treatment is desirable. When leakage of contrast into vital structures has been excluded, ∼50% of the aspirated cyst volume is replaced with 95% ethanol (Fig. 2). The maximum volume of ethanol should be limited to 100 mL in adults to avoid alcohol toxicity from systemically absorbed alcohol. The patient's blood alcohol content will be elevated immediately after the procedure but will decrease within 1 hour.13 The patient should be monitored during this time for any signs of alcohol toxicity. After ethanol has been injected into the cyst, the patient should be rotated from prone to supine to right and left lateral decubitus positioning so the alcohol can contact the entire cyst wall. The patient should spend ∼5 to 10 minutes in each position, and the alcohol should be left in the cyst cavity for a minimum of 20 minutes. The alcohol should then be completely aspirated, the catheter should be placed to bulb suction, and sonography or CT should be performed to document complete evacuation before removal of the catheter. Follow-up imaging should be performed in 3 months; repeat sclerosis may be necessary, especially if the cyst is large. Complications with alcohol ablation include pain,13 which is usually secondary to leakage of alcohol into the peritoneum, as well as the standard risks of bleeding, infection, and injury to adjacent organs. If pain is encountered during injection, 10 to 15 mL of lidocaine can be instilled into the cyst cavity.14
Figure 2.
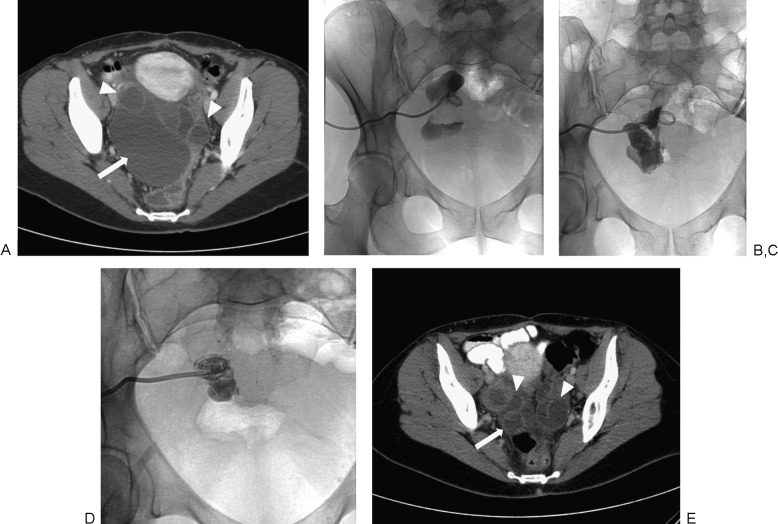
A 40-year-old woman with nausea, vomiting, and abdominal pain. (A) Axial-reformatted image from a contrast-enhanced computed tomography (CT) scan shows a large thin-walled cystic lesion in the pelvis (arrow), which was confirmed to be a peritoneal inclusion cyst. The dilated tubular structures in the pelvis are consistent with hydrosalpinx (arrowheads). (B) A spot fluoroscopic image after injection of diluted contrast shows the contrast is entirely contained within the cyst, making it suitable for sclerosis. The cyst was subsequently sclerosed with ethanol. (C) Contrast injection following ethanol sclerosis was performed, showing the cyst had decreased in size since the first treatment. (D) A final third ethanol sclerosis was performed, followed by a contrast injection demonstrating further decrease in the size of the cyst. (E) Axial-reformatted image from a contrast-enhanced CT scan after three ethanol sclerosis treatments shows a significant decrease in size of the peritoneal inclusion cyst (arrow). Hydrosalpinx persists (arrowheads).
Particularly in cases where sclerotherapy is being performed via transperitoneal access routes, care should be taken to be certain that 100% of the alcohol has been aspirated from the cavity prior to catheter removal. This maneuver helps to decrease the likelihood of the intraperitoneal spread of alcohol, which can cause very severe and difficult-to-control pain.
Finally, many commercially available drainage catheters are not compatible with ethanol, which can cause severe degeneration of the catheter. Confirmation of alcohol compatibility must be made prior to placing the drainage catheter.
Hepatic Cysts
Sonography and CT imaging detect simple hepatic cysts in ∼2.5 to 5% of the population. However, only ∼15% of these are symptomatic cysts, which more commonly occur in women >50 years of age.15 The treatment of simple hepatic cysts includes laparoscopic unroofing and percutaneous sclerotherapy. A review by Moorthy et al demonstrated percutaneous sclerotherapy with alcohol or minocycline to be as effective as laparoscopic unroofing; however, percutaneous therapy is associated with a lower incidence of complications when compared with laparoscopic surgery.15
Percutaneous sclerotherapy of simple hepatic cysts has been performed with ethanol, tetracycline, minocycline, and doxycyline.6,14 Several regimens of alcohol sclerotherapy have been reported in the literature varying from single-session techniques16,17,18 to multiple sessions.13,14 Larssen et al treated 10 hepatic cysts in a single session with alcohol dwell times of 10 minutes, resulting in a median reduction rate of 95% (range: 40 to 100%).17 Yang et al reported prolonged single-session alcohol sclerotherapy (2 or 4 hours) of large hepatic cysts that had comparable results to multiple sessions.18
Yoshida et al reported minocycline to be safe and effective in treating hepatic cysts with cyst regression and no recurrence in all patients treated. However, the number of patients treated in the study was small (n = 9), and the patients required daily injections for 7 to 8 days.6
Renal Cysts
Renal cysts are a ubiquitous finding, especially in the elderly. Autopsy series have shown that over half of the population >50 years of age have at least one renal cyst.19 Cysts can be categorized by the Bosniak classification system depending on a lesion's morphology and enhancing characteristics. The Bosniak classification system also provides management recommendations.20
Simple renal cysts (Bosniak category I lesions) measure water attenuation on CT and have a hairline thin wall.20 Most simple cysts are incidental imaging findings, but occasionally they can cause symptoms such as pain, hematuria, collecting system compression, hypertension, and secondary infection.21 Symptomatic renal cysts can be treated with percutaneous aspiration with or without sclerosis, or with laparoscopic or open surgical unroofing.
Since the initial report by Bean,22 sclerotherapy of renal cysts has been most commonly performed with ethanol, and several techniques have since been described in the literature from single-session to multiple-session treatments. Positive results have been achieved with single-session treatments. Akinci et al reported a 93% reduction in average cyst volume at the end of the first year with a single-session treatment. Further, of the treated cysts, 17.5% resolved, 90% of patients reported improvement in flank pain, and ∼88% of hypertensive patients became normotensive.23 Lin et al reported a similar overall volume reduction rate of ∼98% after single-session sclerosis.24
Alternative sclerosing agents have been used including povidone-iodine,5 sodium tetradecyl,9 hypertonic saline,10 ethanolamine oleate,11 and acetic acid.12 Povidone-iodine has been advocated by some authors,5 but Madeb et al more recently showed it to be ineffective with a recurrence of cysts in 13 of 16 patients treated.25 Demir et al demonstrated ethanol and sodium tetradecyl to be similarly effective sclerosants with complete ablation in 82% and 76%, and partial regression in 9% and 6% of the ethanol and sodium tetradecyl groups, respectively. However, these authors preferred sodium tetradecyl because it causes less pain.26 Egilmez et al reported that 95% ethanol is more effective than 20% hypertonic saline, with a 94% complete regression ratio in the ethanol group versus 72% in the hypertonic saline group; however, patients undergoing sclerosis with hypertonic saline experienced less pain.10 Ethanolamine oleate was advocated as safe and effective in treating renal cysts by Yamamoto et al, but the number of patients treated was very small (n = 4).11 Yoo et al demonstrated acetic acid to be an effective alternative to ethanol with complete or partial regression of cysts in ∼98% of patients.12
Lymphoceles
Abnormal collections of lymphatic fluid commonly occurring after surgery were first described by Mori in 1955, using the term lymphocysts.27 They are acquired cavities lined by fibromembranous tissue with varying incidence rates; most are asymptomatic and spontaneously reabsorb over time (Fig. 3). Fluid analysis reveals a biochemical profile (protein, urea nitrogen, creatinine, bilirubin, and electrolytes) that parallels serum, thereby making differentiation from urinoma, hematoma, or abscess possible. Symptoms, if present, are usually due to secondary infection, hemorrhage, and/or, if large enough, compression of adjacent structures. For example, pelvic lymphoceles may compress the bladder or ureters and cause hydronephrosis, or compress the adjacent iliac veins and increase the risk for venous thrombosis.
Figure 3.
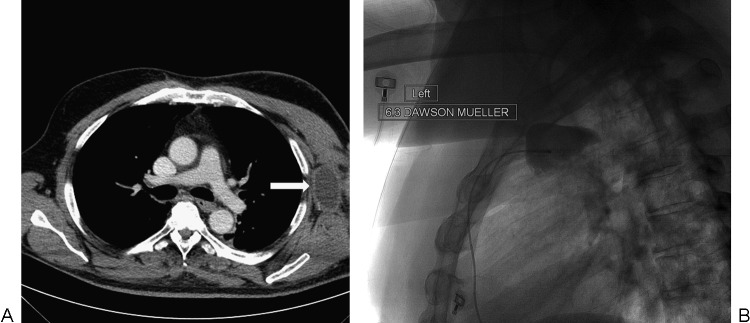
A 58-year-old man with a history of cutaneous apocrine adenocarcinoma of the left axilla status axillary lymph node dissection. (A) Axial-reformatted image from a contrast-enhanced computed tomography scan shows a loculated fluid collection in the left axilla consistent with a lymphocele (arrow). (B) Spot fluoroscopic image after contrast injection shows a 6.3F catheter in the lymphocele. The contrast is entirely contained within the lymphocele, making it suitable for sclerosis.
Transcatheter sclerotherapy is an effective, minimally invasive treatment option for collections that do not resolve over time or for those that recur despite percutaneous aspiration and simple drainage. Ethanol remains the most cost-efficient sclerosing agent even though repeated sessions are often required to achieve sufficient inflammatory changes and scarring to obliterate the injured lymphatic channels; this is also the case for povidone-iodine.28,29 For example, Zuckerman and Yeager,30 as well as Sawhney et al,28 reported average drainage durations of 19 and 36 days, respectively, and success rates of 94% and 100%, respectively, with percutaneous drainage and alcohol sclerotherapy. Limited long-term data also support the use of these minimally invasive techniques. Tasar et al reported no late complications (up to a year of follow-up) from ethanol sclerosis in 18 patients with symptomatic lymphoceles following kidney transplantation.31 Doxycycline has also proved advantageous as a sclerosing agent due to its relatively low cost, lack of side effects, and effectiveness in a single dose.29
Lymphatic Malformations
Congenital lymphatic malformations (often called lymphangiomas) can occur anywhere in the body, including within organs such as the liver, spleen, and kidney. They are most commonly seen in the cervicofacial soft tissues with complete surgical excision the favored treatment modality. Nevertheless, intralesional sclerotherapy has become a safe, acceptable therapeutic approach, particularly in extensive cases where surgery would be difficult and/or lead to incomplete resection. Although various sclerosing agents, including ethanol, have been used, the most popular sclerosants are bleomycin, a chemotherapeutic agent, and OK-432, a biological preparation containing penicillin-killed lyophilized Streptococcus pyogenes that activates the immune system (although the latter is still not approved by the Food and Drug Administration for use in the United States). Rozman et al reported complete resolution upon intralesional injection of bleomycin in 15 of 24 children diagnosed with a lymphangioma; 5 of 24 children had a good response (i.e., >50% reduction in size), with poor response noted (<50% reduction in size) in the remaining 4 children (17%).32 Their analysis showed that poor response rates were related to a predominant microcystic component, with greater macrocystic components yielding better responses. Mathur et al also concluded bleomycin sclerotherapy was effective (>50% reduction in size) in the treatment of 7 of 10 pediatric cases of congenital malformations in the head and neck, with complete or near complete response in 3 of those 7 patients.33 Moreover, Watari et al described a case of safe intrauterine treatment of a fetal cystic hygroma with OK-432.34
Conclusion
Cystic lesions can involve almost every organ in the body. Most are incidental findings, but when large they can be symptomatic. Treatment options include surgery, percutaneous aspiration, percutaneous drainage, and sclerotherapy. In the appropriate setting, percutaneous sclerotherapy is effective in the management of symptomatic cystic lesions. Although many sclerosing agents are available, ethanol is the most commonly used because it is safe, readily available, and inexpensive.
References
- 1.Lucey B C, Kuligowska E. Radiologic management of cysts in the abdomen and pelvis. AJR Am J Roentgenol. 2006;186(2):562–573. doi: 10.2214/AJR.04.1051. [DOI] [PubMed] [Google Scholar]
- 2.Saini S, Mueller P R, Ferrucci J T Jr, Simeone J F, Wittenberg J, Butch R J. Percutaneous aspiration of hepatic cysts does not provide definitive therapy. AJR Am J Roentgenol. 1983;141(3):559–560. doi: 10.2214/ajr.141.3.559. [DOI] [PubMed] [Google Scholar]
- 3.Holmberg G, Hietala S O. Treatment of simple renal cysts by percutaneous puncture and instillation of bismuth-phosphate. Scand J Urol Nephrol. 1989;23(3):207–212. doi: 10.3109/00365598909180843. [DOI] [PubMed] [Google Scholar]
- 4.Montalvo B M, Yrizarry J M, Casillas V J. et al. Percutaneous sclerotherapy of lymphoceles related to renal transplantation. J Vasc Interv Radiol. 1996;7(1):117–123. doi: 10.1016/s1051-0443(96)70746-7. [DOI] [PubMed] [Google Scholar]
- 5.Phelan M, Zajko A, Hrebinko R L. Preliminary results of percutaneous treatment of renal cysts with povidone-iodine sclerosis. Urology. 1999;53(4):816–817. doi: 10.1016/s0090-4295(98)00557-3. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Onda M, Tajiri T. et al. Long-term results of multiple minocycline hydrochloride injections for the treatment of symptomatic hydrochloride injections for the treatment of symptomatic solitary hepatic cyst. J Gastroenterol Hepatol. 2003;18:595–598. doi: 10.1046/j.1440-1746.2003.03025.x. [DOI] [PubMed] [Google Scholar]
- 7.Kerlan R K Jr, LaBerge J M, Gordon R L, Ring E J. Bleomycin sclerosis of pelvic lymphoceles. J Vasc Interv Radiol. 1997;8(5):885–887. doi: 10.1016/s1051-0443(97)70678-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim S H, Moon M W, Lee H J, Sim J S, Kim S H, Ahn C. Renal cyst ablation with n-butyl cyanoacrylate and iodized oil in symptomatic patients with autosomal dominant polycystic kidney disease: preliminary report. Radiology. 2003;226(2):573–576. doi: 10.1148/radiol.2262011574. [DOI] [PubMed] [Google Scholar]
- 9.Demir E, Alan C, Kilciler M, Bedir S. Comparison of ethanol and sodium tetradecyl sulfate in the sclerotherapy of renal cyst. J Endourol. 2007;21(8):903–905. doi: 10.1089/end.2006.0462. [DOI] [PubMed] [Google Scholar]
- 10.Egilmez H, Gok V, Oztoprak I. et al. Comparison of CT-guided sclerotherapy with using 95% ethanol and 20% hypertonic saline for managing simple renal cyst. Korean J Radiol. 2007;8(6):512–519. doi: 10.3348/kjr.2007.8.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto K, Sakaguchi H, Anai H. et al. Sclerotherapy for simple cysts with use of ethanolamine oleate: preliminary experience. Cardiovasc Intervent Radiol. 2005;28(6):751–755. doi: 10.1007/s00270-004-0277-0. [DOI] [PubMed] [Google Scholar]
- 12.Yoo K H, Lee S J, Jeon S H. Simple renal cyst sclerotherapy with acetic acid: our 10-year experience. J Endourol. 2008;22(11):2559–2563. doi: 10.1089/end.2008.0110. [DOI] [PubMed] [Google Scholar]
- 13.Kairaluoma M I, Leinonen A, Ståhlberg M, Päivänsalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg. 1989;210(2):208–215. doi: 10.1097/00000658-198908000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.vanSonnenberg E, Wroblicka J T, D'Agostino H B. et al. Symptomatic hepatic cysts: percutaneous drainage and sclerosis. Radiology. 1994;190(2):387–392. doi: 10.1148/radiology.190.2.8284385. [DOI] [PubMed] [Google Scholar]
- 15.Moorthy K, Mihssin N, Houghton P WJ. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl. 2001;83(6):409–414. [PMC free article] [PubMed] [Google Scholar]
- 16.Tikkakoski T, Mäkelä J T, Leinonen S. et al. Treatment of symptomatic congenital hepatic cysts with single-session percutaneous drainage and ethanol sclerosis: technique and outcome. J Vasc Interv Radiol. 1996;7(2):235–239. doi: 10.1016/s1051-0443(96)70767-4. [DOI] [PubMed] [Google Scholar]
- 17.Larssen T B, Rosendahl K, Horn A, Jensen D K, Rørvik J. Single-session alcohol sclerotherapy in symptomatic benign hepatic cysts performed with a time of exposure to alcohol of 10 min: initial results. Eur Radiol. 2003;13(12):2627–2632. doi: 10.1007/s00330-003-1923-7. [DOI] [PubMed] [Google Scholar]
- 18.Yang C F, Liang H L, Pan H B. et al. Single-session prolonged alcohol-retention sclerotherapy for large hepatic cysts. AJR Am J Roentgenol. 2006;187(4):940–943. doi: 10.2214/AJR.05.0621. [DOI] [PubMed] [Google Scholar]
- 19.Kissane J M. The morphology of renal cystic disease. Perspect Nephrol Hypertens. 1976;4:31–63. [PubMed] [Google Scholar]
- 20.Israel G M, Bosniak M A. An update of the Bosniak renal cyst classification system. Urology. 2005;66(3):484–488. doi: 10.1016/j.urology.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Darcy M. Renal cysts and urinomas. Semin Intervent Radiol. 2011;28(4):380–391. doi: 10.1055/s-0031-1296080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bean W J. Renal cysts: treatment with alcohol. Radiology. 1981;138(2):329–331. doi: 10.1148/radiology.138.2.7455112. [DOI] [PubMed] [Google Scholar]
- 23.Akinci D, Akhan O, Ozmen M. et al. Long-term results of single-session percutaneous drainage and ethanol sclerotherapy in simple renal cysts. Eur J Radiol. 2005;54(2):298–302. doi: 10.1016/j.ejrad.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y H, Pan H B, Liang H L. et al. Single-session alcohol-retention sclerotherapy for simple renal cysts: comparison of 2- and 4-hr retention techniques. AJR Am J Roentgenol. 2005;185(4):860–866. doi: 10.2214/AJR.04.1219. [DOI] [PubMed] [Google Scholar]
- 25.Madeb R, Feldman P A, Knopf J, Rub R, Erturk E, Yachia D. Povidone-iodine sclerotherapy is ineffective in the treatment of symptomatic renal cysts. J Endourol. 2006;20(6):402–404. doi: 10.1089/end.2006.20.402. [DOI] [PubMed] [Google Scholar]
- 26.Demir E, Alan C, Kilciler M, Bedir S. Comparison of ethanol and sodium tetradecyl sulfate in the sclerotherapy of renal cyst. J Endourol. 2007;21(8):903–905. doi: 10.1089/end.2006.0462. [DOI] [PubMed] [Google Scholar]
- 27.Mori N. Clinical and experimental studies on the so-called lymphocyst which develops after radical hysterectomy in cancer of the uterine cervix. J Jpn Obstet Gynecol Soc. 1955;2(2):178–203. [PubMed] [Google Scholar]
- 28.Sawhney R, D'Agostino H B, Zinck S. et al. Treatment of postoperative lymphoceles with percutaneous drainage and alcohol sclerotherapy. J Vasc Interv Radiol. 1996;7(2):241–245. doi: 10.1016/s1051-0443(96)70769-8. [DOI] [PubMed] [Google Scholar]
- 29.Caliendo M V, Lee D E, Queiroz R, Waldman D L. Sclerotherapy with use of doxycycline after percutaneous drainage of postoperative lymphoceles. J Vasc Interv Radiol. 2001;12(1):73–77. doi: 10.1016/s1051-0443(07)61407-9. [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman D A, Yeager T D. Percutaneous ethanol sclerotherapy of postoperative lymphoceles. AJR Am J Roentgenol. 1997;169(2):433–437. doi: 10.2214/ajr.169.2.9242748. [DOI] [PubMed] [Google Scholar]
- 31.Tasar M, Gulec B, Saglam M, Yavuz I, Bozlar U, Ugurel S. Posttransplant symptomatic lymphocele treatment with percutaneous drainage and ethanol sclerosis: long-term follow-up. Clin Imaging. 2005;29(2):109–116. doi: 10.1016/j.clinimag.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Rozman Z, Thambidorai R R, Zaleha A M, Zakaria Z, Zulfiqar M A. Lymphangioma: is intralesional bleomycin sclerotherapy effective? Biomed Imaging Interv J. 2011;7(3):e18. doi: 10.2349/biij.7.3.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur N N, Rana I, Bothra R, Dhawan R, Kathuria G, Pradhan T. Bleomycin sclerotherapy in congenital lymphatic and vascular malformations of head and neck. Int J Pediatr Otorhinolaryngol. 2005;69(1):75–80. doi: 10.1016/j.ijporl.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Watari H, Yamada H, Fujino T. et al. A case of intrauterine medical treatment for cystic hygroma. Eur J Obstet Gynecol Reprod Biol. 1996;70(2):201–203. doi: 10.1016/s0301-2115(95)02547-2. [DOI] [PubMed] [Google Scholar]