Abstract
Campylobacter concisus, a Gram-negative bacterium that colonizes the human oral cavity, has been shown to be associated with inflammatory bowel diseases (IBD). The effects of different C. concisus strains on intestinal epithelial expression of Toll like receptors (TLR) have not been investigated. This study examined the effects of C. concisus strains isolated from patients with IBD and controls on expression of TLR4, its co-receptor myeloid differentiation factor (MD)-2; TLR2, TLR5, cyclooxygenase-2 (COX-2) and interleukin (IL)-8 in HT-29 cells.
Fourteen oral and enteric C. concisus strains isolated from patients with IBD and healthy controls were co-incubated with HT-29 cells. Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells in response to C. concisus infection was examined by Western blot, flow cytometry analysis and immunofluorescent staining visualized by confocal microscope. Production of IL-8 was evaluated by enzyme-linked immunosorbent assay.
Both oral and enteric C. concisus strains upregulated expression of TLR4 in HT-29 cells. The levels of glycosylated TLR4 (Gly-TLR4) and surface TLR4 induced by C. concisus strains isolated from patients with IBD were significantly higher than those induced by C. concisus strains isolated from the healthy controls. Four C. concisus strains isolated from patients with IBD induced more than two-fold increase of surface expression of MD-2. C. concisus did not affect expression of TLR2 and TLR5. All C. concisus strains induced production of IL-8 and COX-2 in HT-29 cells.
This study shows that some C. concisus strains, most from patients with IBD, upregulate surface expression of TLR4 and MD-2 in HT-29 cells. These data suggest that a potential role of specific C. concisus strains in modulating the intestinal epithelial responses to bacterial LPS needs to be investigated.
Introduction
Campylobacter concisus is a Gram-negative bacterium commonly found in the human oral cavity [1], [2]. C. concisus is motile by means of a single polar flagellum and requires H2-enriched microaerobic conditions for growth [3].
C. concisus has been shown to be associated with inflammatory bowel disease (IBD) [4], [5], [6], [7], [8]. A significantly higher prevalence of C. concisus in intestinal biopsies and fecal samples of patients with IBD was detected as compared with controls [4], [5], [6], [7]. IBD is a chronic inflammatory disorder of the gastrointestinal tract; its most common incidence is in adolescents and young adults [9], [10]. The two major types of IBD are Crohn's disease (CD) and ulcerative colitis (UC). The aetiology of IBD is unknown. Multiple factors including intestinal microbiota, genetic factors, environmental factors and aberrant responses in the innate and adaptive immune system contribute to the development of IBD [9], [10].
The intestinal microbiota play a key role in the development of IBD. Studies from both human and animal models of IBD have demonstrated that colitis does not occur in the absence of intestinal microbiota [11], [12], [13], [14]. Furthermore, a breakdown in tolerance of the gut immune system to commensal intestinal bacteria in patients with IBD has been detected [15], [16]. Despite the strong evidence supporting the important role of intestinal microbiota in the development of IBD, a causative agent(s) of human IBD remains elusive.
C. concisus colonizing the oral cavity has been shown to be a source of C. concisus colonizing the intestinal tissues in some patients with IBD [17]. Recently C. concisus was detected in fecal and saliva samples of domestic dogs and cats [18], [19]. This bacterium has also been isolated from chicken and beef meat [20]. These data suggest that domestic pets, chicken and beef meat may also serve as a source of human intestinal colonization of C. concisus.
Despite its high prevalence in the intestinal tract of patients with IBD, whether C. concisus contributes to the pathogenesis of IBD is unknown. A number of studies have examined the effects of C. concisus on intestinal epithelial cells using in vitro cell culture models. Some oral and enteric C. concisus strains were shown to be invasive to Caco2 cells [5], [17]. Increased intestinal epithelial permeability and epithelial apoptosis were also observed following the incubation of Caco2 cells with both oral and enteric C. concisus strains [5], [21]. Enteric C. concisus strains were shown to induce the production of IL-8 in HT-29 cells [5], [22]. These data suggest that some C. concisus strains have a potential to cause enteric diseases.
The effects of C. concisus strains on intestinal epithelial expression of Toll like receptors (TLR) have not been investigated. A low level expression of TLR4 and its co-receptor myeloid differentiation factor (MD)-2 in intestinal epithelial cells under normal physiological conditions is a strategy of the intestinal immune system to avoid dysregulated inflammatory responses to bacterial lipopolysaccharide (LPS) [23]. In patients with IBD, increased levels of intestinal expression of TLR4 and other proinflammatory molecules such as cyclooxygenase-2 (COX-2) and interleukin (IL)-8 have been observed [24], [25]. Examination of the effects of different C. concisus strains on intestinal epithelial expression of TLRs and other proinflamatory molecules will further shed light on whether some C. concisus strains have the potential to contribute to the pathogenesis of human enteric diseases including IBD. Given that C. concisus is a Gram-negative flagellated bacterium, in this study, we examined the effects of both oral and enteric C. concisus strains isolated from patients with IBD and controls on intestinal epithelial expression of TLR4 and its co-receptor myeloid differentiation factor (MD)-2, which recognizes LPS found in Gram-negative bacteria, of TLR2, which recognizes bacterial lipoproteins, and of TLR5, which recognizes bacterial flagellin. Furthermore, the induction of COX-2 and IL-8 in HT-29 cells by both oral and enteric C. concisus strain was assessed.
Results
Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells induced by different C. concisus strains detected by Western blot
On Western blot, TLR4, MD-2 and TLR2 revealed two protein bands (glycosylated and non-glycosylated proteins); TLR5 and COX-2 revealed one protein band. The intensity of each protein band detected by Western blot was normalized to the intensity of α-Tubulin (internal control) of the same sample. The levels of TLR4, MD-2, TLR2, TLR5 and COX-2 in each sample were expressed as the fold change of the normalized band intensity relative to the normalized band intensity of the negative control (HT-29 cells without C. concisus infection), which are shown in Table 1. For TLR4 and TLR2, the glycosylated receptors (Gly-TLR4 and Gly-TLR2) and the non-glycosylated receptors (Non-Gly-TLR4 and Non-Gly-TLR2) were analyzed separately. For MD-2, the glycosylated MD-2 and the non-glycosylated MD-2 were analyzed together, due to the narrow distance of glycosylated MD-2 and the non-glycosylated MD-2 bands on Western blot that made it difficult to analyze the two bands separately. The representative Western blot patterns and the schematic levels of TLR4, MD-2, TLR2, TLR5 and COX-2 are shown in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 respectively.
Table 1. Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells induced by C. concisus strains detected by Western blot.
| Gly-TLR4 | Non-Gly-TLR4 | MD-2 | Gly-TLR2 | Non-Gly-TLR2 | TLR5 | COX-2 | |
| P1CDO2 | 5.7±1.3 | 3.2±0.3 | 1.6±0.2 | 0.87±0.06 | 1.11±0.08 | 1.3±0.1 | 3.4±0.7 |
| P1CDO3 | 8.5±0.8 | 3.1±0.5 | 1.6±0.2 | 0.83±0.09 | 0.95±0.03 | 1.4±0.04 | 2.7±0.02 |
| PACDO1 | 6.6±0.6 | 2.6±0.4 | 2.3±0.04 | 1.11±0.09 | 1.25±0.13 | 1.7±0.02 | 4.6±0.6 |
| P1CDB1 | 7.3±0.9 | 2.2±0.4 | 2.4±0.1 | 1.15±0.11 | 1.28±0.15 | 0.9±0.01 | 3.0±0.4 |
| PBUCO1 | 4.1±1.1 | 6.2±1.1 | 3.1±0.3 | ND | ND | 1.8±0.1 | 6.1±1.4 |
| P3UCO6 | 5.5±0.4 | 3.4±0.7 | 2.1±0.2 | 1.14±0.08 | 1.30±0.15 | 1.2±0.06 | 4.2±1.0 |
| P3UCB2 | 4.4±0.5 | 1.8±0.3 | 1.9±0.5 | 0.87±0.09 | 0.92±0.04 | 0.9±0.05 | 2.5±0.5 |
| P3UCLW2 | 4.3±0.6 | 1.7±0.2 | 2.3±0.6 | 0.74±0.13 | 0.91±0.09 | 0.6±0.02 | 2.3±0.4 |
| P2CDO4 | 1.6±0.2 | 1.3±0.08 | 1.2±0.02 | 1.06±0.23 | 0.95±0.06 | 1.0±0.05 | 1.3±0.08 |
| H2O1 | 2.6±0.4 | 3.0±0.7 | 1.2±0.05 | ND | ND | 1.1±0.1 | 1.2±0.07 |
| H3O1 | 3.8±0.8 | 5.2±1.0 | 1.5±0.1 | ND | ND | 1.4±0.1 | 1.9±0.3 |
| H4O1 | 5.0±1 | 4.5±0.5 | 1.8±0.05 | 1.02±0.17 | 0.97±0.02 | 1.3±0.08 | 1.8±0.1 |
| H5O1 | 2.5±0.4 | 6.3±0.5 | 2.0±0.3 | 0.88±0.10 | 0.93±0.07 | 1.2±0.1 | 1.9±0.04 |
| H6O1 | 1.6±0.1 | 2.3±0.03 | 1.6±0.2 | 1.06±0.16 | 0.85±0.18 | 1.1±0.1 | 1.5±0.05 |
The levels of molecules were expressed as the fold change of the band intensity of HT-29 cells infected with a C. concisus strain relative to the band intensity of the negative control (HT-29 cells without C. concisus infection), after normalization to the intensity of the internal control α-Tubulin of the same sample. Data were the average of triplicate experiments ± standard error. Gly: glycosylated. Non-Gly: non-glycosylated. Five C. concisus strains (H2O1-H6O1) were isolated from healthy controls; the remaining nine strains were from patients with IBD. P1CDB1, P3UCB2 and P3UCLW2 were enteric strains; the remaining 11 strains were oral strains.
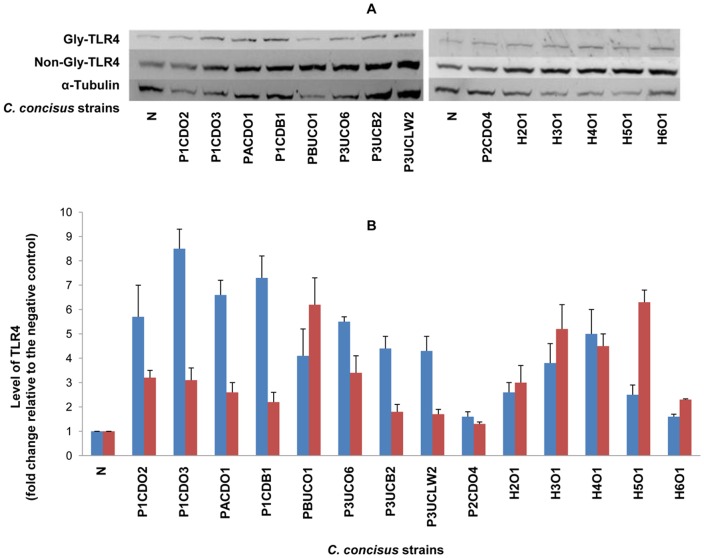
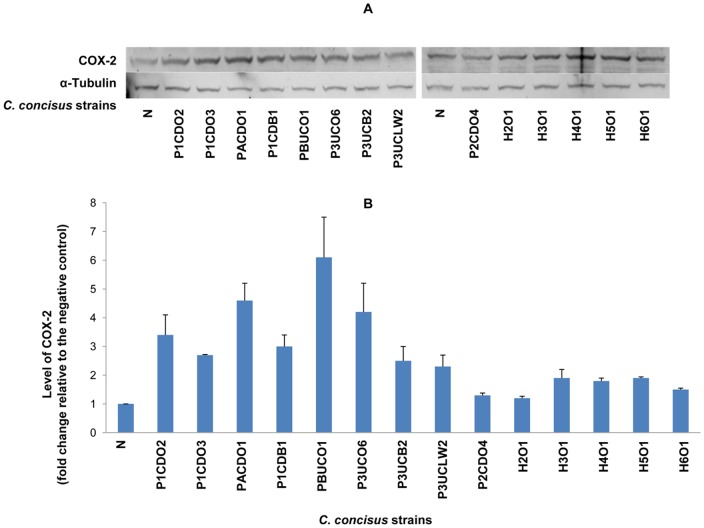
Figure 1. Detection of TLR4 by Western blot in HT-29 cells infected with C. concisus strains.
HT-29 cells were lysed following incubation with C. concisus strains for 24 hours. Expression of TLR4 in HT-29 cells was detected by Western blot. Two bands, the Glycosylated TLR 4 (Gly-TLR4) and non-glycosylated TLR4 (Non-Gly-TLR4), were revealed on Western blot. The levels of Gly-TLR4 and Non-Gly-TLR4 of each sample were expressed as the fold change of the band intensity relative to the band intensity of the negative control (HT-29 cells without C. concisus infection), after normalization to the intensity of the internal control α-Tubulin of the same sample. A: Representative Western blot of Gly-TLR4 (120 kD), Non-Gly-TLR4 (95 kD) and α-Tubulin (55 kD). B: Level of Gly-TLR4 (blue column) and Non-Gly-TLR4 (red column) induced by different C. concisus strains; data were the average of triplicate experiments ± standard error. N: negative control. H2O1-H6O1: C. concisus strains isolated from healthy controls. The remaining nine C. concisus strains were from patients with IBD. The average level of Gly-TLR4 induced by C. concisus strains from patients with IBD was significantly higher than that induced by C. concisus strains from healthy controls (P<0.05).
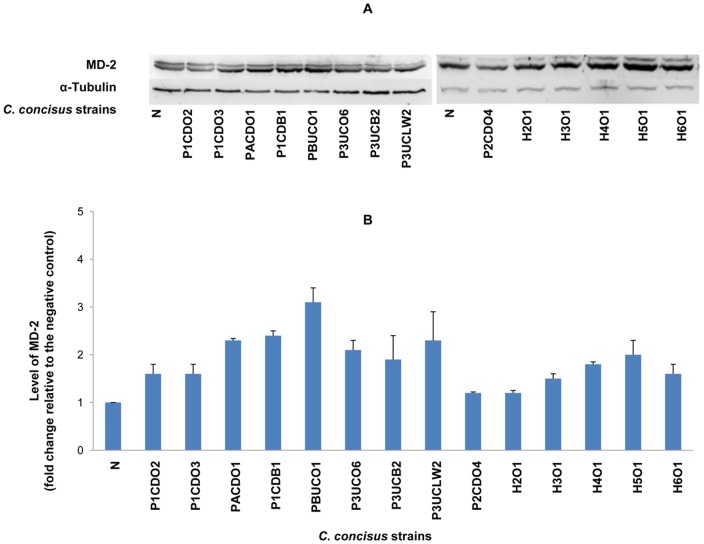
Figure 2. Detection of MD-2 by Western blot in HT-29 cells infected with C. concisus strains.
HT-29 cells were lysed following incubation with C. concisus strains for 24 hours. Expression of MD-2 in HT-29 cells was detected by Western blot. Two bands, the Glycosylated MD-2 and non-glycosylated MD-2, were revealed on Western blot. Giving the close distance of Glycosylated MD-2 and non-glycosylated MD-2 protein bands which made it difficult to analyze the bands separately, these two protein bands were analyzed together. The level of MD-2 was expressed as the fold change of the normalized band intensity of a sample relative to the normalized band intensity of the negative control (HT-29 cells without C. concisus infection), after normalization to the intensity of the internal control α-Tubulin of the same sample. A: Representative Western blot of MD-2 (23–25 kD) and α-Tubulin (55 kD). B: Level of total MD-2 induced by different C. concisus strains; data were the average of triplicate experiments ± standard error. N: negative control. H2O1-H6O1: C. concisus strains isolated from healthy controls. The remaining nine C. concisus strains were from patients with IBD. The average level of MD-2 induced by C. concisus strains from patients with IBD was not significantly higher than that induced by C. concisus strains from healthy controls (P>0.05).
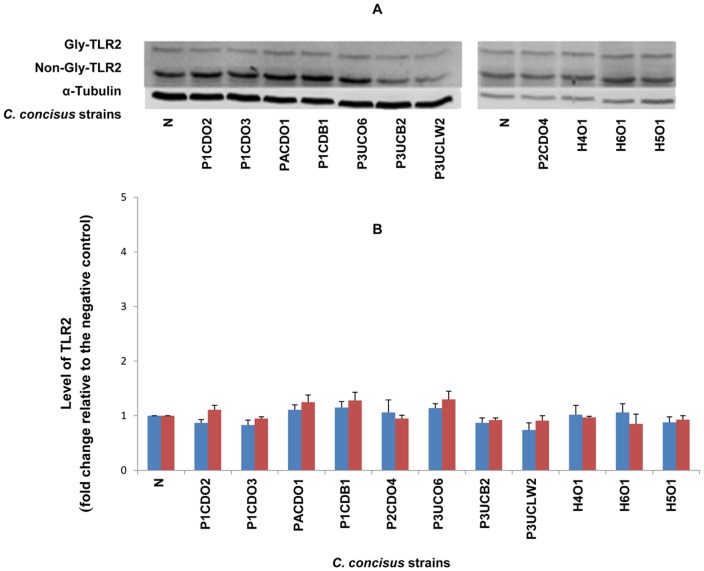
Figure 3. Detection of TLR2 by Western blot in HT-29 cells infected with C. concisus strains.
HT-29 cells were lysed following incubation with C. concisus strains for 24 hours. Expression of TLR2 in HT-29 cells was detected by Western blot. Two bands, the Glycosylated TLR 2 (Gly-TLR4) and non-glycosylated TLR2 (Non-Gly-TLR4), were revealed on Western blot. The levels of Gly-TLR2 and Non-Gly-TLR2 of each sample were expressed as the fold change of the band intensity relative to the band intensity of the negative control (HT-29 cells without C. concisus infection), after normalization to the intensity of the internal control α-Tubulin of the same sample. A: Representative Western blot of Gly-TLR2 (100 kD), Non-Gly-TLR2 (90 kD) and α-Tubulin (55 kD). B: Level of Gly-TLR2 (blue column) and Non-Gly-TLR2 (red column) induced by different C. concisus strains; data were the average of triplicate experiments ± standard error. N: negative control. H2O1-H6O1: C. concisus strains isolated from healthy controls. The remaining C. concisus strains were from patients with IBD. The average levels of Gly-TLR2 and Non-Gly-TLR2 induced by C. concisus strains from patients with IBD were not significantly different that induced by C. concisus strains from healthy controls (P>0.05).
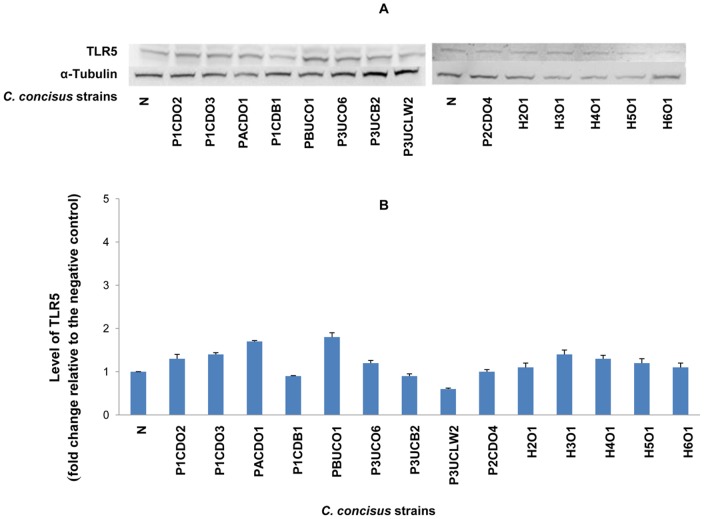
Figure 4. Detection of TLR5 by Western blot in HT-29 cells infected with C. concisus strains.
HT-29 cells were lysed following incubation with C. concisus strains for 24 hours. Expression of TLR5 in HT-29 cells was detected by Western blot. One TLR 5 band was revealed on Western blot. The intensity of TLR5 of each sample was normalized to the intensity of the internal control α-Tubulin of the same sample. The level of TLR5 was expressed as the fold change of the normalized band intensity of a sample relative to the normalized band intensity of the negative control (HT-29 cells without C. concisus). A: Representative Western blot of TLR5 (110 kD) and α-Tubulin (55 kD). B: Levels of TLR5 induced by different C. concisus strains; data were the average of triplicate experiments ± standard error. N: negative control. H2O1-H6O2: C. concisus strains isolated from healthy controls. The remaining nine C. concisus strains were from patients with IBD. The average level of TLR5 induced by C. concisus strains from patients with IBD was not significantly higher than that induced by C. concisus strains from healthy controls (P>0.05).
Figure 5. Detection of COX-2 by Western blot in HT-29 cells infected with C. concisus strains.
HT-cells 29 were lysed following incubation with C. concisus strains for 24 hours. Expression of COX-2 in HT-29 cells was detected by Western blot. The intensity of COX-2 band of each sample was normalized to the intensity of the internal control α-Tubulin of the same sample. The level of COX-2 was expressed as the fold change of the normalized band intensity of a sample relative to the normalized band intensity of the negative control (HT-29 cells without C. concisus infection). A: Representative Western blot of COX-2 (70 kD) and α-Tubulin (55 kD). B: Levels of COX-2 induced by different C. concisus strains; data were the average of triplicate experiments ± standard error. N: negative control. H2O1-H6O1: C. concisus strains isolated from healthy controls. The remaining nine C. concisus strains were from patients with IBD. The average level of COX-2 induced by C. concisus strains from patients with IBD was significantly higher than that induced by C. concisus strains from healthy controls (P<0.05).
C. concisus strains obtained from both patients with IBD and controls upregulated the expression of TLR4 in HT-29 cells. The levels of TLR4 induced by different C. concisus strains varied. Of the 14 C. concisus strains examined, 12 strains induced more than two-fold increase of expression of Gly-TLR4 and 11 strains induced more than two-fold increase of expression of Non-Gly-TLR4 (Figure 1 and Table 1). The average level of Gly-TLR4 induced by the nine C. concisus strains isolated from patients with IBD was significantly higher than that induced by the five C. concisus strains (H2O1-H6O1) isolated from healthy controls (5.3±0.7 vs 3.1±0.6, P<0.05). The average level of Non-Gly-TLR4, induced by C. concisus strains from patients with IBD was not statistically different from that induced by the C. concisus strains from the healthy controls (2.8±0.5 vs 4.3±0.7, P>0.05). The average levels of Gly-TLR4 and Non-GlyTLR4 induced by the five oral C. concisus strains isolated from patients with IBD were 5.33±2.33 and 3.31±1.61 respectively, which were not significantly different from that induced by the three enteric C. concisus strains isolated from patients with IBD (5.33±1.70 and 1.90±0.27 respectively) (P>0.05).
Six C. concisus strains induced more than two-fold increase of expression of MD-2; five of these strains were from patients with IBD (Figure 2 and Table 1). The average level of MD-2 induced by C. concisus strains isolated from patients with IBD was not statistically different from that induced by C. concisus strains isolated from healthy controls (1.92±0.15 vs 1.59±0.21, P>0.05). The average level of MD-2 induced by the oral C. concisus strains isolated from patients with IBD was not statistically different from that induced by the enteric C. concisus strains isolated from patients with IBD (1.77±0.20 vs 2.17±0.14, P>0.05).
C. concisus strains did not affect the expression of TLR2 and TLR5 in HT-29 cells. The changes of TLR2 and TLR5 expression in HT-29 cells infected with C. concisus strains were all below two-fold in comparison to the levels of these two proteins in HT-29 cells without C. concisus infection (Figure 3, Figure 4 and Table 1).
Of the 14 C. concisus strains examined, eight strains isolated from patients with IBD induced more than two fold increase of expression of COX-2 (Figure 5, Table 1). The average level of COX-2 induced by C. concisus strains isolated from patients with IBD was significantly higher than that induced by C. concisus strains isolated from healthy controls (3.34±1.43 vs 1.66±0.30, P<0.05) (Figure 5, Table 1).
Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells induced by different C. concisus strains detected by flow cytometry analysis
For flow cytometry analysis, the levels of surface TLR4, MD-2, TLR2 and TLR5 (non-permeabilized cells) and total TLR4, MD-2, TLR2, TLR5 and COX-2 (permeabilized cells) were expressed as the fold change of the mean channel fluorescence intensity (MFI) of HT-29 cells infected with a C. concisus strain relative to the MFI of the non-infected HT-29 cells (HT-29 cells without C. concisus infection).
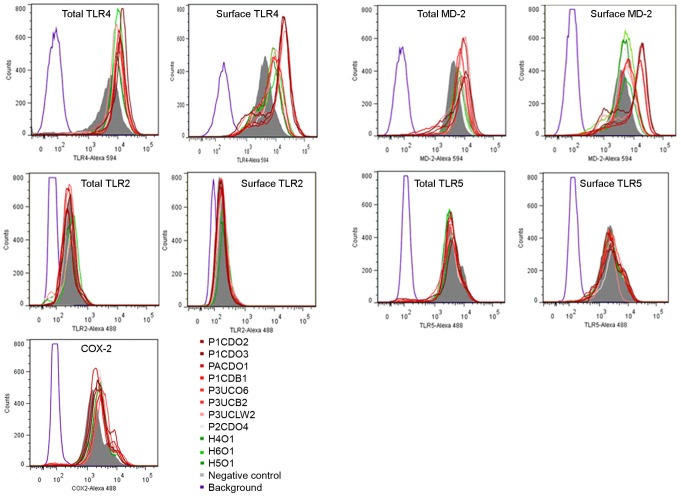
All 11 C. concisus strains examined upregulated both surface expression and total expression of TLR4 in HT-29 cells (Figure 6 and Table 2). Nine strains induced more than two fold increase of surface expression of TLR4 (Table 2). The average level of surface TLR4 induced by C. concisus strains isolated from patients with IBD was significantly higher than that induced by C. concisus strains isolated from healthy controls (3.70±0.46 vs 1.93±0.05, P<0.05). The average level of total TLR4 induced by C. concisus strains isolated from patients with IBD was not statistically different from that induced by the C. concisus strains from the healthy controls (1.81±0.08 vs 1.63±0.05, P>0.05).
Figure 6. Detection of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells by flow cytometry analysis.
Flow cytometry histogram showing the expression of surface and total TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells with and without infection of C. concisus strains. A surface expression was measured from non-permeabilized cells and a total expression was measured from permeabilized cells. Background (purple) was from HT-29 cells that were not exposed to antibodies. Negative control (grey): HT-29 cells without C. concisus infection. H4O1, H5O1 and H6O1 were strains isolated from healthy controls (green); the remaining eight C. concisus strains were from patients with IBD (red).
Table 2. Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells induced by C. concisus strains detected by flow cytometry.
| Strain | Surface TLR4 | Total TLR4 | Surface MD-2 | Total MD-2 | Surface TLR2 | Total TLR2 | Surface TLR5 | Total TLR5 | COX-2 |
| P1CDO2 | 4.18±0.56 | 2.10±0.19 | 2.46±0.32 | 1.87±0.07 | 1.11±0.16 | 0.99±0.02 | 1.11±0.06 | 1.16±0.11 | 1.43±0.08 |
| P1CDO3 | 5.03±0.67 | 1.92±0.18 | 2.96±0.39 | 1.86±0.07 | 0.99±0.07 | 0.87±0.05 | 0.99±0.03 | 1.07±0.05 | 1.38±0.08 |
| PACDO1 | 5.40±0.71 | 1.74±0.15 | 2.66±0.30 | 1.49±0.09 | 1.12±0.16 | 0.97±0.02 | 1.01±0.06 | 1.12±0.09 | 1.38±0.09 |
| P1CDB1 | 3.50±0.26 | 2.12±0.09 | 1.90±0.18 | 1.69±0.08 | 1.13±0.14 | 0.94±0.04 | 0.91±0.12 | 1.08±0.05 | 1.53±0.04 |
| P2CDO4 | 2.30±0.29 | 1.65±0.09 | 1.54±0.15 | 1.91±0.10 | 1.19±0.14 | 1.14±0.04 | 1.14±0.10 | 0.97±0.05 | 1.33±0.02 |
| P3UCO6 | 2.23±0.22 | 1.62±0.11 | 1.51±0.04 | 2.00±0.17 | 1.32±0.33 | 1.28±0.24 | 1.13±0.03 | 1.06±0.05 | 1.58±0.13 |
| P3UCB2 | 4.61±0.61 | 1.51±0.11 | 2.29±0.29 | 1.77±0.04 | 1.21±0.11 | 1.01±0.11 | 0.73±0.09 | 0.85±0.19 | 1.33±0.08 |
| P3UCLW2 | 2.30±0.23 | 1.82±0.07 | 1.46±0.03 | 1.61±0.06 | 1.24±0.12 | 1.10±0.05 | 1.10±0.13 | 1.08±0.05 | 1.44±0.06 |
| H4O1 | 1.84±0.20 | 1.64±0.08 | 1.36±0.06 | 1.68±0.10 | 1.20±0.19 | 0.95±0.04 | 1.02±0.06 | 0.97±0.04 | 1.45±0.03 |
| H5O1 | 1.92±0.21 | 1.54±0.05 | 1.40±0.07 | 1.76±0.10 | 1.36±0.13 | 1.05±0.06 | 1.00±0.03 | 1.24±0.10 | 1.27±0.05 |
| H6O1 | 2.02±0.23 | 1.71±0.09 | 1.32±0.05 | 1.40±0.07 | 1.14±0.11 | 1.17±0.04 | 1.02±0.05 | 0.96±0.04 | 1.35±0.07 |
The levels of molecules were expressed as the fold change of the mean channel fluorescence intensity (MFI) of HT-29 cells infected with a C. concisus strain relative to the MFI of the non-infected HT-29 cells (HT-29 cells without C. concisus infection). A surface expression was measured from non-permeabilized cells and a total expression was measured from permeabilized cells. Data were the average of triplicate experiments ± standard error. Three C. concisus strains (H4O1, H5O1 and H6O1) were isolated from healthy controls; the remaining eight strains were from patients with IBD. P1CDB1, P3UCB2 and P3UCLW2 were enteric strains; the remaining 11 strains were oral strains.
Four C. concisus strains isolated from patients with IBD, P1CDO2, P1CDO3, PACDO1 and P3UCB2, induced more than two-fold increase of surface expression of MD-2 (Figure 6 and Table 2). The average level of surface MD-2 induced by C. concisus strains isolated from patients with IBD was higher than that induced by C. concisus strains isolated from healthy controls (2.10±0.57 vs 1.36±0.04). However, this was not statistically significant (P = 0.059). The average level of total MD-2 induced by C. concisus strains isolated from patients with IBD was not statistically different from that induced by C. concisus strains isolated from healthy controls (1.78±0.17 vs 1.61±0.19, P>0.05).
C. concisus strains did not affect the expression of TLR2 and TLR5 (Figure 6 and Table 2) in HT-29 cells.
All C. concisus strains induced an increased expression of COX-2 (Figure 6). However, the increment levels were all below two-fold (Table 2).
Confocal microscopy observation of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells
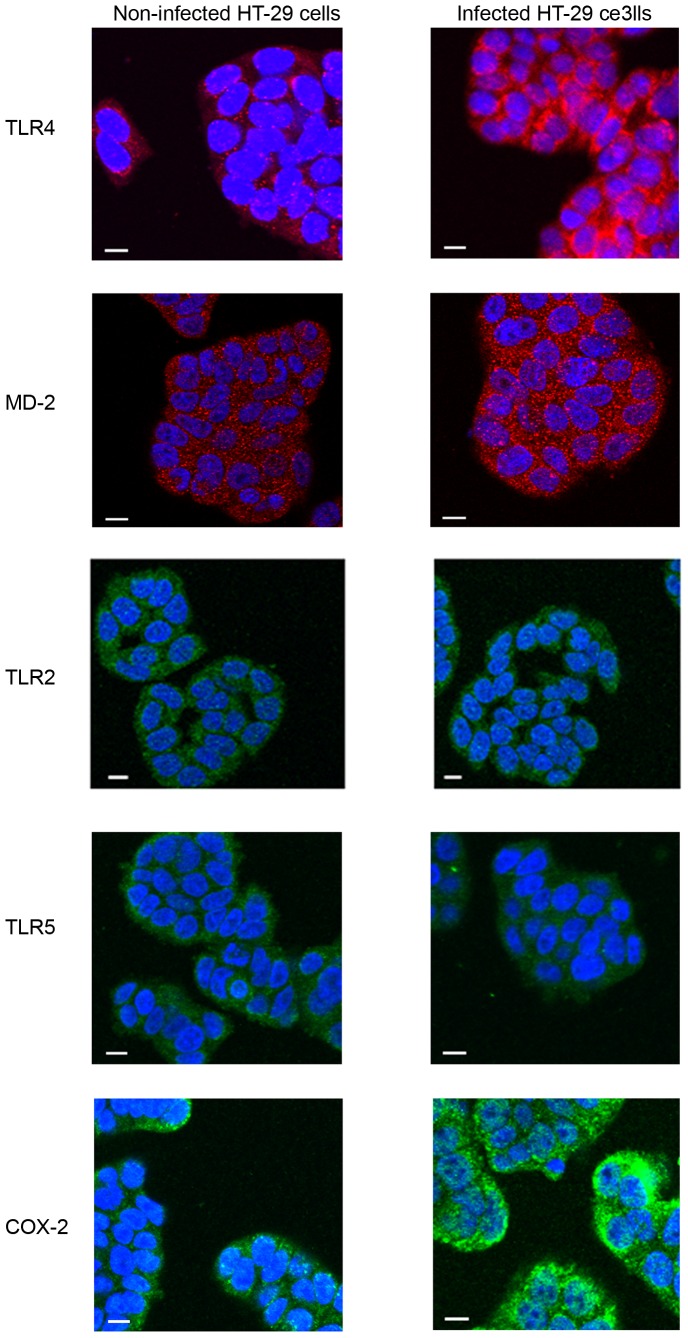
The effects of C. concisus strains on expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells were visualized by confocal microscopy analysis. Confocal microscopy images, which showed an increased expression of TLR4 and COX-2, a slightly increased expression of MD-2 and no change of TLR5 and TLR2 in HT-29 after coincubation with a representative C. concisus strain (P1CDB1) for 24 hours are shown in Figure 7.
Figure 7. Confocal microscope image of TLR4, MD-2, TLR2, TLR5 and COX-2 expression in HT-29 cells with and without C. concisu infection.
HT-29 cells were incubated with a representative C. concisus strain (P1CDB1) for 24 hours. Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells with and without C. concisus infection were detected by immunostained using specific antibodies, followed by Alex Fluor conjugated secondary antibodies. The image was viewed using an Olympus FluoView FV1000 Confocal laser scanning microscope. The secondary antibodies used for detection of TLR4 and MD-2 were conjugated with Alexa Fluor 594 (emission colour red). The secondary antibodies used for detection of TLR2, TLR5 and COX-2 were conjugated with Alexa Fluor 488 (emission colour green). Scale Bar = 10 µm.
IL-8 production in HT-29 cells induced by oral and enteric C. concisus strains
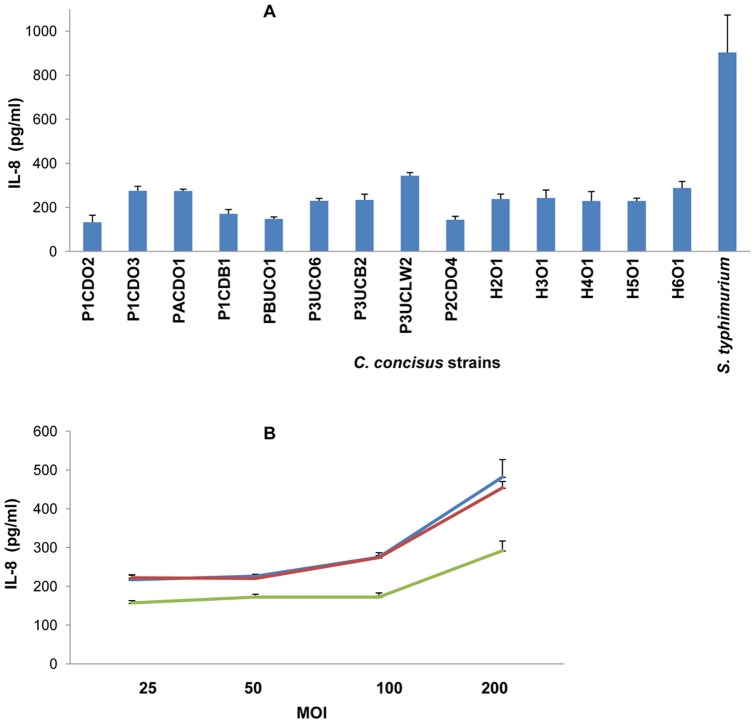
The concentrations of IL-8 in HT-29 cell culture supernatants were determined by enzyme linked immunosorbent assay (ELISA). The basal amount of IL-8 production by HT-29 cells (HT-29 cells without C. concisus infection) was subtracted from the concentration of IL-8 in each sample (HT-29 cells incubated with a C. concisus strain) and the results are shown in Figure 8A. The concentration of IL-8 induced by the positive control Salmonella enterica serovar Typhimurium LT2 (Salmonella typhimurium) was 903±130 pg/ml. Both oral and enteric C. concisus strains isolated from patients with IBD and controls induced the production of IL-8. The concentrations (pg/ml) of IL-8 induced by different C. concisus strains at a multiplicity of infection (MOI) of 100 were 133±31 for P1CDO2, 276±20 for P1CDO3, 274±9 for PACDO1, 171±19 for P1CDB1, 148±9 for PBUCO1, 234±26 for P3UCO6, 244±14 for P3UCB2, 344±16 for P3UCLW2, 130±11 for P2CDO4, 238±23 for H2O1, 243±36 for H3O1, 229±43 for H4O1, 229±13 for H5O1 and 288±30 for H6O1 (Figure 8 A). The average concentration of IL-8 induced by C. concisus strains from patients with IBD was not significantly different from that induced by C. concisus strains from healthy controls (217±24 vs 245±11, P>0.05).
Figure 8. Production of IL-8 by HT-29 cells induced by C. concisus strains.
A. HT-29 cells were incubated with C. concisus strains at a multiplicity of infection (MOI) of 100 for 24 hours. Concentrations of IL-8 in the cell culture supernatants were measured by ELISA. The basal production of IL-8 (HT-29 cells without C. concisus infection) has been subtracted from values shown in Figure 8A. Data were the average of triplicates ± standard error. The level of IL-8 induced by C. concisus strains from patients with IBD was not statistically different from that induced by C. concisus strains from the healthy controls (P>0.05). Five C. concisus strains (H2O1-H6O1) were isolated from healthy controls; the remaining nine strains were from patients with IBD. B. HT-29 cells were incubated with three C. concisus strains respectively at four different MOIs (25, 50, 100 and 200) for 24 hours. The three C. concisus strains used were P1CDO2 (blue), P1CDO3 (red) and P1CDB1 (green). Concentrations of IL-8 in the cell culture supernatants were measured by ELISA. The basal production of IL-8 (HT-29 cells without C. concisus infection) has been subtracted from values shown in Figure 8B. Data were the average of triplicates ± standard error.
The relationship between the dose of C. concisus and the production of IL-8 in HT-29 cells was assessed by measurement of IL-8 concentrations in cell culture supernatants of HT-29 cells infected with three C. concisus strains, P1CDO2, P1CDO3 and P1CDB1, at four different MOIs (25, 50, 100 and 200). Concentrations of IL-8 induced by P1CDO2 C. concisus strain at the four MOIs were 217±11, 226±1, 276±12 and 482±45 respectively. Concentrations of IL-8 induced by P1CDO3 C. concisus strain at the four MOIs were 222±8, 220±8, 274±5 and 453±16 respectively. Concentrations of IL-8 induced by P1CDB1 C. concisus strain at the four MOIs were 157±6, 172±7, 171±11 and 292±25 respectively (Figure 8B).
Discussion
This study examined the effects of oral and enteric C. concisus strains isolated from patients with IBD and controls on intestinal epithelial expression of TLR4, MD-2, TLR2, TLR5, COX-2 and IL-8 using an in vitro cell culture model (HT-29 cells).
Functional membrane LPS receptor is a multiple protein complex including at least three proteins, TLR4, MD-2 and CD14 [26]. In physiological conditions, intestinal epithelial cells express a low level of LPS receptor proteins [23], [24], [27], [28]. In patients with IBD, increased intestinal epithelial expression of TLR4 has been detected [24]. In our study, we found that all C. concisus strains upregulated the expression of TLR4 in HT-29 cells. Furthermore, we found that most C. concisus strains isolated from patients with IBD, rather than those strains isolated from healthy controls, predominately upregulated surface expression of TLR4 and Gly-TLR4 (Figures 1 and 6, Tables 1 and 2). These data suggest that some C. concisus strains may have the potential to enhance the intestinal epithelial inflammatory responses to LPS derived from other bacterial species such as those from intestinal commensal bacteria. Further studies are needed to investigate this issue.
TLR4 glycosylation has been shown to be essential in forming the functional LPS receptor [26]. Upregulation of gastric epithelial expression of Gly-TLR4 by the gastric mucosal associated Gram-negative bacterium Helicobacter pylori was previously observed. In a study examining the effects of H. pylori LC11 and LC20 strains on expression of TLR4 in MKN45 gastric epithelial cells, Su et al found that H. pylori LC11 strain upregulated the expression of Gly-TLR4 at a much greater level than that induced by the LC20 strain [29]. The difference between these two strains is that LC11 contains the pathogenicity island [30]. The mechanisms by which some C. concisus strains more effectively upregulate Gly-TLR4 in HT-29 cells are currently unknown, which remains to be investigated.
Mucosal-associated bacterial species have different effects on TLR4 expression in HT-29 cells. In a study by Furrie et al, mRNA levels of TLR4 in HT-29 cells in response to six bacterial species isolated from intestinal biopsies, Enterococcus faecalis, Escherichia coli, Peptostreptococcus anaerobium, Bifidobacterium longum, Bifidobacterium bifidum, and Bacteroides fragilis, were assessed. This study showed that E. coli significantly reduced mRNA level of TLR4 and E. faecalis significantly increased the mRNA of TLR4 in HT-29 cells. The remaining four bacterial species did not significantly affect the expression of TLR4 in HT-29 cells [31].
MD-2 is a protein that is associated with the extracellular domain of TLR4, which enables TLR4 to function as the LPS receptor [32], [33], [34]. Increased intestinal epithelial expression and sera MD-2 activity in patients with IBD have been reported [35], [36]. In our study, increased MD-2 expression in HT-29 cells following C. concisus infection was detected by both Western blot and flow cytometry analysis. However, the levels of increment of MD-2 induced by C. concisus were not as great as the increment of TLR4. Some C. concisus strains from patients with IBD induced more than two-fold increase of surface expression of MD-2 (Figure 6 and Table 2). Similar to TLR4 glycosylation, MD-2 glycosylation was also shown to be essential in maintaining the functional integrity of LPS receptor [26]. However, in our study we could not assess the levels of glycosylated MD-2 and non-glycosylated MD-2 separately due to the proximity of these two protein bands on Western blot.
TLR5 recognizes a conserved site on bacterial flagellin [37]. The flagellin of S. typhimurium is the major epithelial proinflammatory determinant; activating intestinal epithelial expression of TLR5 [38], [39]. However, the flagellin of members of ε-Proteobacteria, which include genera of Helicobacter, Campylobacter and Wollinella, is able to evade recognition by TLR5, owing to altered amino acids at the TLR5 recognition site [40]. In our study C. concisus strains showed no or minimal effects on TLR5 expression in HT-29 cells (Figures 4 and 6). Previously we showed that C. concisus attached to Caco2 cells using their flagella [41]. The evasion of TLR5 would allow C. concisus to attach to the intestinal epithelial cells using flagellum without the notice of the innate immune system, providing the opportunity for this bacterium to modulate the gut innate immune system as discussed above. TLR2 recognizes bacterial lipoproteins. In our study, we found that C. concisus did not affect the expression of TLR2 in HT-29 cells.
COX-2 is an inducible enzyme responsible for producing prostaglandins and other important inflammatory mediators [42], [43]. Singer et al showed that COX-2 was not detected in normal colonic epithelial cells but was induced in patients with IBD [25]. COX-2 has also been shown to be associated with intestinal epithelial high fluid secretion induced by enteric pathogens [44], [45]. In addition to its involvement in inflammation, COX-2 has been linked to several malignancies including colorectal cancer [46]. In this study, we found that C. concisus strains isolated from patients with IBD induced a significantly higher level of COX-2 in HT-29 cells in comparison to C. concisus strains isolated from healthy controls by Western blot. However through flow cytometry analysis, a low level of COX-2 increase induced by these C. concisus strains was detected. Furthermore, the increased levels of COX-2 induced by C. concisus strains isolated from patients with IBD and controls were not significantly different by flow cytometry analysis. As there may be discrepancies in the sensitivities of Western blot and flow cytometry techniques in the detection of low abundance proteins, it is unclear whether this may have an effect on the differing levels of COX-2 detected. Whether the increment of COX-2 induced by C. concisus has a biological impact remains to be examined.
The production of IL-8 induced by different C. concisus strains in HT-29 cells was investigated. In this study, we did not observe a correlation between the increased expression levels of TLR4 induced by different C. concisus strains and the production of IL-8 in HT-29 cells. The reason for this is not clear. One explanation is that in this study, whole bacteria were used in the induction of IL-8 in HT-29 cells. Given that IL-8 is induced by multiple bacterial components through multiple receptors and pathways, it is therefore difficult to correlate its expression levels to a single receptor. The second possibility is that different C. concisus strains may have different types of LPS that vary in their abilities to induce proinflammatory mediators, which further complicates the matter of assessing the correlation between the levels of TLR4 and IL-8 between different C. concisus strains. This view is supported by many previous studies, as they showed that C. concisus is a bacterial species with great diversity [17], [47], [48], [49], [50]. Future studies are required to investigate whether the upregulation of TLR4 by C. concisus enhances the intestinal epithelial inflammatory responses to LPS originating from intestinal commensal bacteria, enteric pathogens as well as varying C. concisus strains.
Examination of the relationship between C. concisus dose and the production of IL-8 in HT-29 cells showed that at lower MOIs (MOI 25–100), an increase in bacterial dose did not affect the production of IL-8. At higher MOIs (MOI 100–200), a bacterial dose dependent production of IL-8 in HT-29 cells was observed (Figure 8B). Interestingly, upregulation of TLR4 in HT-29 cells by C. concisus strains does not require a high dose of C. concisus. For example, we found that P1CDB1 strain upregulated TLR4 in HT-29 cells at a MOI of 5 and that the upregulation of TLR4 by P1CDB1 at a MOI of 100 was not as efficient as the upregulation of TLR4 by the same strain at a MOI of 25 (data not shown).
In summary, in this study we found that both oral and enteric C. concisus strains upregulated expression of TLR4 and MD-2 in HT-29 cells. Some C. concisus strains, most of them from patients with IBD, were more effective in regulating surface expression of TLR4 and MD-2, as well as Gly-TLR4. C. concisus infection in HT-29 cells did not affect the expression of TLR2 and TLR5. All C. concisus strains induced production of IL-8 and COX-2 in HT-29 cells. These data suggest that a potential role of specific C. concisus strains in modulating the intestinal epithelial responses to bacterial LPS needs to be investigated.
Materials and Methods
Ethics statement
C. concisus strains used in this study were isolated in our previous studies [2], [4], [7]. Ethics approvals were granted by the Ethics Committees of the University of New South Wales and the South East Sydney Area Health Service (Ethics Nos: HREC 09237/SESIAHS 09/078, HREC08335/SESIAHS(CHN)07/48 and HREC 06233/SESAHS (ES) 06/164). Written informed consent was obtained from the subjects or the parents/guardians of the minors.
C. concisus strains and cultivation conditions
Fourteen oral (isolated from saliva) and enteric (isolated from intestinal biopsies and feces) C. concisus strains we previously isolated were included in this study [2], [4], [7]. Details of the C. concisus strains used in this study were listed in Table 3.
Table 3. C. concisus strains used in this study.
| Strain ID | Source | Clinical conditions |
| P1CDO2 | Saliva | Crohn's disease |
| P1CDO3 | Saliva | Crohn's disease |
| P1CDB1 | Intestinal biopsy | Crohn's disease |
| PACDO1 | Saliva | Crohn's disease |
| P3UCO6 | Saliva | Ulcerative colitis |
| P3UCB2 | Intestinal biopsy | Ulcerative colitis |
| P3UCLW2 | Luminal washout | Ulcerative colitis |
| PBUCO1 | Saliva | Ulcerative colitis |
| P2CDO4 | Saliva | Crohn's disease |
| H2O1 | Saliva | Healthy |
| H3O1 | Saliva | Healthy |
| H4O1 | Saliva | Healthy |
| H5O1 | Saliva | Healthy |
| H6O1 | Saliva | Healthy |
These C. concisus strains were isolated from previous studies [2], [4], [7]. P1CDO2, P1CDO3 and P1CDB1 were isolated from a patient with CD. P3UCO6, P3UCB2 and P3UCLW2 were isolated from a patient with UC. The remaining strains were isolated from individual patients with IBD and healthy controls. P1CDB1 was named as UNSWCD and P1CDB1(UNSWCD) in previous studies [17], [41].
All C. concisus isolates were grown in heart infusion broth (Oxoid, Hampshire, UK) containing 2.5% fetal blood serum (FBS) (Bovogen Biologicals, East Keilor, Australia) at 37°C with continuous agitation (120 rpm) under microaerobic condition. The microaerobic condition was generated using Campylobacter Gas Generating Kit (Oxoid).
Cultivation of HT-29 cells
Human intestinal epithelial cell line HT-29 cells (ATCC No. HTB-38), were maintained in McCoy's 5A medium (Invitrogen, California, USA) supplemented with 10% FBS (Bovogen Biologicals), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen). The cells were grown at 37°C in a humidified incubator containing 5% CO2.
Antibodies used in this study
All antibodies used in this study were purchased from Santa Cruz Biotechnology Inc (Santa Cruz biotechnology Inc, California, USA). Primary antibodies used were polyclonal anti-TLR4 (sc-10741), anti-MD-2 (sc-20668), anti-TLR2 (sc-166900), anti-TLR5 (sc-16243), anti-Cox-2 (sc-1746), and anti-α tubulin (sc-31779). Secondary antibodies conjugated with horseradish peroxidase (HRP) used for Western blot were bovine anti-goat IgG (sc-2352), goat anti-mouse IgG (sc-2031) and goat anti-rabbit IgG (sc-2054). Secondary antibodies used for flow cytometry analysis and immunostaining were Alexa Fluor® 488 donkey anti-goat IgG (A11055), Alexa Fluor® 488 goat anti-mouse IgG (A11029), and Alexa Fluor® 594 goat anti-rabbit IgG (A11037) (Invitrogen).
Western blot
Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in response to C. concisus infection in HT-29 cells was examined by Western blot. HT-29 cells at a concentration of 5×105 cell/ml were cultured in 6-well cell culture plates (3 ml cell suspension/well). The cells were grown for 48 hours. HT-29 cells were washed five times using Dulbecco's Phosphate-Buffered Saline (D-PBS) and then infected with C. concisus at a MOI of 25 in McCoy's 5A medium supplemented with 10% FBS without antibiotics. The cells were further incubated for 24 hours. HT-29 cells without C. concisus infection were used as the negative control.
HT-29 cells were harvested from culture plates by scraping and washed with pre-cooled D-PBS. The cells were lysed using RIPA Buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.1% SDS) containing a mixture of protease inhibitors (Sigma-Aldrich, Castle Hill Australia). Whole cell lysates were centrifuged twice at 14000 relative centrifugal force (RCF) for 20 minutes at 4°C. Supernatant was collected and stored at −80°C till use. Protein concentrations were determined using Pierce® BCA Protein Assay Kit (Thermo Fisher Scientific, Scoresby, Australia).
C. concisus whole cell proteins (30 µg) were separated on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel under reducing conditions and transferred onto polyvinylidine difluoride (PVDF) membranes for two hour at 100 Volt in a cold room (4°C). PVDF Membrane were immersed in absolute methanol for five seconds then equilibrated in the transfer buffer for 10–20 minutes prior to be used for protein transfer. The transfer buffer consists of 0.3% (w/v) Tris base, 1.44% (w/v) glycine and 20% methanol (v/v). The transfer buffer was pre-chilled on ice prior to use. Following protein transfer, PVDF membranes were blocked with 5% skim milk in washing buffer (0.05% Tween-20 in phosphate buffered saline (PBS)) for two hours then probed with primary antibodies (1∶350) overnight at 4°C, followed by secondary antibodies conjugated with HRP (1∶2500) for 90 minutes at room temperature (RT). Antibodies were diluted using blocking solution. The HRP-labeled antibodies were detected using Immun-Star™ WesterC™ Chemiluminescence Kits (Bio-Rad laboratory, Gladesville, Australia) and a LAS-3000 imaging system (Fujifilm). The intensities of protein bands were analyzed using Image J software (National institute of health, US). Western blot experiments were repeated three times. PVDF membranes were incubated with antibodies for the molecules examined (TLR4, MD-2, TLR2, TLR5 and COX-2) first, then stripped and re-incubacted with anti-alpha-tubulin antibody. One PVDF membrane was used for detection of one of the molecules examined.
Flow cytometry analysis
Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 cells was detected by flow cytometry analysis. HT-29 cells (10 ml at 5×105 cell/ml) were seeded onto T-25 tissue culture flasks (Nunc, Roskilde, Denmark) for 24 hours, then infected with C. concisus at a MOI of 25 and further incubated for 24 hours. HT-29 cells without C. concisus infection were used as the negative control.
Following infection with C. concisus, HT-29 cells were washed twice with D-PBS and detached from culture flasks by incubating with 0.25% trypsin (Invitrogen) for five minutes (1 ml each flask), followed by deactivation of trypsin using McCoy's 5A medium supplemented with 10% FBS without antibiotics. The cells were washed twice with pre-cooled D-PBS by centrifugation at 300 RCF for 5 minutes at 4°C.
HT-29 cells were fixed for 12 minutes in 3.7% paraformaldehyde in PBS, then permeabilized for 10 minutes with 0.1% Triton X-100 in PBS when required to analyse the total expression of the target protein in cells. HT-29 cells that were not permeabilized were used to analyse the surface expression of the target proteins. Cells were then washed twice using blocking solution (1% Bovine Serum albumin in PBS), and incubated with the blocking solution for 30 minutes at RT, HT-29 cells were then sequentially incubated at RT with a primary antibody (1∶40) and an Alexa Fluor conjugated secondary antibody (5 µg/ml) for one hour and 45 minutes respectively. Primary and secondary antibodies were diluted by blocking solution. Cells were washed three times with the blocking solution between incubations for 10 minutes each time. Cells were collected after each wash by centrifugation at 300 RCF for 5 minutes at 4°C. HT-29 cells that were not exposed to antibodies were used to assess the background signal. Data were acquired by BD LSRFortessa™ SORP cell analyser (BD Biosciences, San Jose, USA) and analysed in Flow Jo software (Tree star inc, OR, US). Flow cytometry experiments were in triplicate and repeated at least twice.
Immunofluorescence staining and confocal microscopy
Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in responses to C. concisus infection in HT-29 was visualized using immunofluorescence staining and confocal microscopy
HT-29 cells were seeded onto sterile cover-slips placed in 6-well cell culture plates (3 ml of cells at a concentration of 1×105 cell/ml) and allowed to grow for 48 hours. HT-29 cells were then infected with C. concisus at a MOI of 25 and incubated for further 24 hours. HT-29 cells without infection of C. concisus were used as negative control.
HT-29 cells grown on cover-slips were fixed in 3.7% paraformaldehyde (was diluted by PBS) for 17 minute at RT, permeabilized with 0.1% Triton X-100 in PBS for 10 minutes at RT. The cover-slips carrying HT-29 cells were blocked with 1% bovine serum albumin (Invitrogen) in PBS for one hour at RT. HT-29 cells were then sequentially incubated with a primary antibody (1∶40) and an Alexa Fluor conjugated secondary antibody (5 µg/ml) for 75 minutes respectively, followed by staining with Hoechst 33342 (1.5 µg/ml) (Invitrogen) diluted in PBS for 12 minutes at RT. Both primary and secondary antibodies were diluted in blocking solution. The incubations with the secondary antibodies and the Hoechst 33342 were carried out in the dark at RT.
The cover slips were washed three times with PBS between incubations for 10 minutes at RT. The cover slips were mounted using AF1 antifadent (Citifluor Ltd, London, UK) and HT-29 cells were observed using an Olympus FluoView FV1000 Confocal laser scanning microscope.
Measurement of IL-8 in HT-29 cell culture supernatant by ELISA
To examine the production of IL-8 by HT-29 cells induced by C. concisus, HT-29 cells (5×105cell/ml) were seeded in 24-well cell culture plates (1 ml/each well) and incubated for 48 hours. HT-29 cells were then infected with C. concisus and further incubated for 24 hours. Supernatants were collected and centrifuged twice for 2 minutes at 10,000 g. IL-8 secreted in the supernatants was measured by ELISA using Human IL-8 CytoSet™ (Invitrogen) according to manufacturer's instructions.
Supernatant collected from HT-29 cells co-incubated with S. typhimurium (UNSW culture collection) and supernatant from HT-29 cells without bacterial infection were used as the positive and negative control respectively.
Statistic analysis
Data were analyzed by means of unpaired t test using GraphPad Prism version 5.1 (San Diego, CA). P-values below 0.05 (two tailed, 95% confidence interval) were considered significant.
Funding Statement
This work was supported by the Broad Medical Research Program of the Broad Foundation (Grant no: IBD0273-R) and a faculty research grant from the University of New South Wales. Yazan Ismail is supported by a PhD scholarship from Al-Balaq' Applied University, Jordan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tanner ACR, Badger S, Lai CH, Listgarten MA, Visconti RA, et al. (1981) Wolinella gen-nov, Wolinella-succinogenes (Vibrio-succinogenes-wolin et-al) comb-nov, and description of bacteroides-gracilis sp-nov, Wolinella-recta sp-nov, Campylobacter-concisus sp-nov, and Eikenella-corrodens from humans with periodontal-disease. Int J Syst Bacteriol 31: 432–445. [Google Scholar]
- 2. Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA, et al. (2010) Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol 48: 2965–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandamme P, Dewhirst FE, Paster BJ, On SLW (2005) Genus I. Campylobacter. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey's Manual of Syst Bacteriol. 2 ed. New York: Springer. pp. 1147–1160.
- 4. Zhang L, Man SM, Day AS, Leach ST, Lemberg DA, et al. (2009) Detection and isolation of Campylobacter species other than C. jejuni from children with Crohn's disease. J Clin Microbiol 47: 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Man SM, Zhang L, Day AS, Leach ST, Lemberg DA, et al. (2010) Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn's disease. Inflamm Bowel Dis 16: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 6. Mukhopadhya I, Thomson JM, Hansen R, Berry SH, El-Omar EM, et al. (2011) Detection of Campylobacter concisus and other Campylobacter species in colonic biopsies from adults with ulcerative colitis. Plos One 6: e:21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahendran V, Riordan S, Grimm M, Tran T, Major J, et al. (2011) Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. Plos One 6: e25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lastovica AJ (2009) Clinical relevance of Campylobacter concisus isolated from pediatric patients. J Clin Microbiol 47: 2360–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sartor RB (2008) Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594. [DOI] [PubMed] [Google Scholar]
- 10.Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory Bowel Disease. In: Paul WE, Littman DR, Yokoyama WM, editors. Ann Rev Immunol. pp. 573–621. [DOI] [PMC free article] [PubMed]
- 11. Taurog JDRJ, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE (1994) The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 180: 2359–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Haens GR, Geboes K, Peeters M, Baert F, Penninickx F, et al. (1998) Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 114: 262–267. [DOI] [PubMed] [Google Scholar]
- 13. Rutgeerts P, Goboes K, Peeters M, Hiele M, Penninckx F, et al. (1991) Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 338: 771–774. [DOI] [PubMed] [Google Scholar]
- 14. Veltkamp C, Tonkonogy SL, De Jong YP, Albright C, Grenther WB, et al. (2001) Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg is an element of 26 mice. Gastroenterology 120: 900–913. [DOI] [PubMed] [Google Scholar]
- 15. Macpherson A, Khoo UY, Forgacs I, PhilpottHoward J, Bjarnason I (1996) Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, et al. (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 102: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ismail Y, Mahendran V, Octavia S, Day AS, Riordan SM, et al. (2012) Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains Isolated from patients with inflammatory bowel disease. Plos One 7: e38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaban B, Ngeleka M, Hill JE (2010) Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol 10: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen RF, Harrington CS, Kortegaard HE, On SLW (2007) A PCR-DGGE method for detection and identification of Campylobacter, Helicobacter, Arcobacter and related Epsilobacteria and its application to saliva samples from humans and domestic pets. J Appl Microbiol 103: 2601–2615. [DOI] [PubMed] [Google Scholar]
- 20. Lynch OA, Cagney C, McDowell DA, Duffy G (2011) Occurrence of fastidious Campylobacter spp. in fresh meat and poultry using an adapted cultural protocol. Int J Food Microbiol 150: 171–177. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Gunzel D, et al. (2011) Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/b6 intestinal epithelial cells. Plos One 6: e23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalischuk LD, Inglis GD (2011) Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. Bmc Microbiol 11: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, et al. (2001) Decreased expression of toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 167: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 24. Cario E, Podolsky DK (2000) Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68: 7010–7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, et al. (1998) Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 115: 297–306. [DOI] [PubMed] [Google Scholar]
- 26. Correia JD, Ulevitch RJ (2002) MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 277: 1845–1854. [DOI] [PubMed] [Google Scholar]
- 27. Grimm MC, Pavli P, Vandepol E, Doe WF (1995) Evidence for a CD14(+) population of monocytes in inflammatory bowel-disease mucosa-implications for pathogenesis. Clin Exp Immunol 100: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA (2001) Evidence for an innate immune response in the immature human intestine: Toll-like receptors on fetal enterocytes. Pediatr Res 49: 589–593. [DOI] [PubMed] [Google Scholar]
- 29. Su B, Ceponis PJM, Lebel S, Huynh H, Sherman PM (2003) Helicobacter pylori activates toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun 71: 3496–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones NL, Day AS, Jennings HA, Sherman PM (1999) Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun 67: 4237–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Furrie E, Macfarlane S, Thomson G, Macfarlane GT (2005) Toll-like receptors-2,-3 and-4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunol 115: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, et al. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189: 1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, et al. (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 3: 667–672. [DOI] [PubMed] [Google Scholar]
- 34. Ohnishi T, Muroi M, Tanamoto K (2003) MD-2 is necessary for the Toll-like receptor 4 protein to undergo glycosylation essential for its translocation to the cell surface. Clin Diag Lab Immunol 10: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vamadevan AS, Fukata M, Arnold ET, Thomas LS, Hsu D, et al. (2010) Regulation of Toll-like receptor 4-associated MD-2 in intestinal epithelial cells: a comprehensive analysis. Innate Immun 16: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seksik P, Sokol H, Grondin V, Adrie C, Duboc H, et al. (2010) Sera from patients with Crohn's disease break bacterial lipopolysaccharide tolerance of human intestinal epithelial cells via MD-2 activity. Innate Immun 16: 381–390. [DOI] [PubMed] [Google Scholar]
- 37. Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, et al. (2003) Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 4: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 38. Zeng H, Carlson AQ, Guo YW, Yu YM, Collier-Hyams LS, et al. (2003) Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol 171: 3668–3674. [DOI] [PubMed] [Google Scholar]
- 39. Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL (2001) Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167: 1882–1885. [DOI] [PubMed] [Google Scholar]
- 40. Andersen-Nissen E, Smith KD, Strobe KL, Barrett SLR, Cookson BT, et al. (2005) Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA 102: 9247–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Man SM, Kaakoush NO, Leach ST, Nahidi L, Lu HK, et al. (2010) Host attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter jejuni Campylobacter species. J Infect Dis 202: 1855–1865. [DOI] [PubMed] [Google Scholar]
- 42. Williams CS, Mann M, DuBois RN (1999) The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18: 7908–7916. [DOI] [PubMed] [Google Scholar]
- 43. Fukata M, Chen AL, Klepper A, Krishnareddy S, Vamadevan AS, et al. (2006) Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology 131: 862–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JM, Lee JY, Yoon YM, Oh YK, Kang JS, et al. (2006) Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-kappa B activation. Eur J Immunol 36: 2446–2456. [DOI] [PubMed] [Google Scholar]
- 45. Bertelsen LS, Paesold G, Eckmann L, Barrett KE (2003) Salmonella infection induces a hypersecretory phenotype in human intestinal xenografts by inducing cyclooxygenase 2. Infect Immun 71: 2102–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thun MJ, Henley SJ, Patrono C (2002) Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst 94: 252–266. [DOI] [PubMed] [Google Scholar]
- 47. Aabenhus R, On SLW, Siemer BL, Permin H, Andersen LP (2005) Delineation of Campylobacter concisus genomospecies by amplified fragment length polymorphism analysis and correlation of results with clinical data. J of Clin Microbiol 43: 5091–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vandamme P, Falsen E, Pot B, Hoste B, Kersters K, et al. (1989) Identification of EF group-22 Campylobacters from gastroenteritis cases as Campylobacter concisus . J Clin Microbiol 27: 1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matsheka MI, Lastovica AJ, Elisha BG (2001) Molecular identification of Campylobacter concisus . J Clin Microbiol 39: 3684–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bastyns K, Chapelle S, Vandamme P, Goossens H, Dewachter R (1995) Specific detection of Campylobacter concisus by PCR amplification of 23S rDNA areas. Mol Cell Probes 9: 247–250. [DOI] [PubMed] [Google Scholar]