Abstract
Background
rs6943555 in AUTS2 has been shown to modulate ethanol consumption. We hypothesized that rs6943555 might be associated with completed suicide.
Methods
We genotyped rs6943555 in 625 completed suicides and 3861 controls using real-time TaqMan Allelic Discrimination Assay. All individuals were Polish Caucasians.
Results
We detected an association between suicide and rs6943555 A allele (OR = 1.17, P = 0.018 for allelic comparison, OR = 1.24, P = 0.013 for dominant, and OR = 1.18, P = 0.020 for co-dominant model of inheritance). The association remained significant after adjusting for age and gender (co-dominant: P = 0.002 and dominant model: P = 0.001). After stratifying suicides according to blood ethanol concentration (BAC≤ 20 mg/dl and BAC > 20 mg/dl) the association remained significant only for cases who committed suicide under influence of alcohol (co-dominant: OR = 1.37, P = 0.004 and dominant model: OR = 1.45, P = 0.006). To validate this finding we genotyped another cohort of 132 cases. We reproduced the association between rs6943555 A allele and suicide under influence of ethanol (allelic comparison: OR = 1.55, P = 0.023; co-dominant : OR = 1.54, P = 0.031; dominant model: OR = 1.84, P = 0.015). Analyzing pooled suicides with BAC >20 mg/dl (N = 300) we found the association of rs6943555 A allele not only vs. controls (allelic OR = 1.41, P = 0.00029) but also vs. cases with BAC ≤ 20 mg/dl (N = 449, allelic OR = 1.33, P = 0.019).
Conclusions
In our study rs6943555 A allele is associated with suicide committed after drinking ethanol shortly before death. The rs6943555 A allele may be linked to adverse emotional reaction to ethanol, which could explain the association with lower consumption in general population as well as the predisposition to suicide under influence of ethanol.
Introduction
Suicidal behavior encompasses a spectrum ranging from the suicidal thoughts to suicide attempts and completed suicide [1]. There is considerable evidence for contribution of genetic factors in suicidal behavior, both from twin [2], [3], family [4], [5] and molecular studies [6], [7]. However, the results from the genome-wide association scans suggest a role of multiple genes with small effects [8]–[11].
AUTS2 (autism susceptibility candidate 2) is located on 7q11.22 and encodes a nuclear protein expressed mainly in developing brain [12] and amygdala (http://biogps.org/#goto=genereport&id=26053). Although the function of AUTS2 is not known, it has been implicated in neurobehavioral disorders [13]. AUTS2 was first associated with autism by studies of monozygotic twin pairs with an identical balanced translocation [14], [15]. Subsequently, balanced and/or unbalanced chromosomal rearrangements including AUTS2 were found in other cases of autism [16]–[18] as well as mental retardation [19], epilepsy [20], dyslexia [13] and attention deficit hyperactivity disorder (ADHD) [21].
Recently rs6943555 in AUTS2 has been convincingly associated (P<< 10−6) with alcohol consumption based on a genome-wide study including 12 population samples of European ancestry comprising 26 316 individuals with replication genotyping in additional 21 185 subjects [22]. The direction of the observed effect was such that the minor (ancestral) A allele was associated with 5.5% lower alcohol consumption [22]. The role of AUTS2 in modulating alcohol consumption was further evidenced by correlations of its transcripts levels with voluntary alcohol consumption and alcohol sensitivity in mice and Drosophila models, respectively [22], whereas the significance of AUTS2 variation was shown by the genotype-specific expression of this locus in human prefrontal cortex [22].
There are numerous reports linking alcohol abuse and the risk for suicide [23]–[27]. Furthermore, there is also evidence for shared predisposition for suicide and autism spectrum disorders [28], [29], including failures with social problem solving [30]–[32], lower socialization [33], deficits in emotion recognition [29], [34] and executive functioning [35]–[39].
Given these data we hypothesized that there was an association between rs6943555 and completed suicide, which could possibly be modulated by exposure to ethanol shortly before death. The aim of out study was to test this hypothesis.
Materials and Methods
Subjects
Suicide victims were consecutive cases from the Warsaw metropolitan area autopsied in the Department of Forensic Medicine at the Medical University of Warsaw, Poland. The information about clinical variables, such as age, gender, suicide method, blood ethanol concentration, psychiatric diagnosis and history of addiction was collected from the post-mortem medical and forensic examination protocols. Due to limited number of cases, for which definite negative history of addiction and information about psychiatric diagnosis could be obtained, we pooled those with the negative history with the cases, for which data were missing. Characteristics of suicides are shown in Table 1. We also studied a replication cohort of 134 suicides who were ascertained from after the analysis in the main cohort was completed. Characteristics of cases from the replication cohort are shown in Table 2.
Table 1. Characteristics of suicide subjects.
| Number of cases (%) | ||
| Gender * (data available for 608 cases) | Males | 494 (81.3) |
| Females | 114 (18.7) | |
| Age ** (data available for 542 cases) | mean = 43.71, SD = 16.82 | 542 |
| Blood ethanol concentration (data available for 625 cases) | Under influence of alcohol (ethanol concentration > 20 mg/dl), mean = 185.0, SD = 110.1 | 235 (37.6) |
| Without influence of alcohol (ethanol concentration ≤ 20 mg/dl) | 390 (62.4) | |
| Method of committing suicide (data available for 582 cases) | Hanging | 446 (76.6) |
| Jumping from a high place | 68 (11.7) | |
| Self-harm by sharp object | 17 (2.9) | |
| Shot with a firearm | 17 (2.9) | |
| Jumping or lying before moving object | 14 (2.4) | |
| Toxic effect from ingested substance | 11 (1.9) | |
| Other | 9 (1.6) | |
| Psychiatric disorders | No | 585 (93.6) |
| Yes: | 40 (6.4) | |
| Depression | 13 (32.5) | |
| Schizophrenia | 2 (5.0) | |
| Other or unknown | 25 (62.5) | |
| History of addiction | No | 602 (96.3) |
| Yes: | 23 (3.7) | |
| Alcoholism | 21 (91.3) | |
| Drug addiction | 1 (4.4) | |
| Alcoholism and drug addiction | 1 (4.4) |
We observed statistically significant difference regarding gender between cases and controls (47.11% males for controls, p<0.001 vs. suicides)
We observed statistically significant difference regarding age between cases and controls (mean = 45.70, SD = 14.91 for controls, p = 0.003 vs. suicides)
Table 2. Characteristics of suicide subjects from the replication cohort.
| Number of cases (%) | ||
| Gender (data available for 131 cases) | Males | 117 (89.3) |
| Females | 14 (10.7) | |
| Age (data available for 122 cases) | mean = 42.89, SD = 16.76 | 122 |
| Blood ethanol concentration (data available for 124 cases) | Under influence of alcohol (ethanol concentration > 20 mg/dl), mean = 157.5, SD = 86.5 | 65 (52.4) |
| Without influence of alcohol (ethanol concentration ≤ 20 mg/dl) | 59 (47.6) | |
| Method of committing suicide (data available for 127 cases) | Hanging | 101 (79.5) |
| Jumping from a high place | 14 (11.0) | |
| Self-harm by sharp object | 3 (2.4) | |
| Shot with a firearm | 2 (1.6) | |
| Jumping or lying before moving object | 5 (3.9) | |
| Toxic effect from ingested substance | 1 (0.8) | |
| Other | 1 (0.8) | |
| Psychiatric disorders | No | 111 (84.1) |
| Yes: | 21 (15.9) | |
| Depression | 11 (52.4) | |
| Schizophrenia | 2 (9.5) | |
| Other or unknown | 8 (38.1) | |
| History of addiction | No | 127 (96.2) |
| Yes: | 5 (3.8) | |
| Alcoholism | 5 (100) | |
| Drug addiction | 0 | |
| Alcoholism and drug addiction | 0 |
A special consideration was given to the use of postmortem samples from suicides from whom informed consent could not be obtained. The testing was performed using a part of blood sample routinely collected during all autopsies. All samples and phenotype data were anonimised immediately after obtaining. An approval of this protocol was obtained from the Warsaw Medical University Ethical Board (KB/185/2010).
The control group, which has been used in a previous study [40], [41] comprised subjects from the WOBASZ project - the Polish National Multicenter Health Survey – a cross-sectional study on the prevalence and control of risk factors for cardiovascular disease conducted in 2003–2005 by the Institute of Cardiology in Warsaw. The WOBASZ cohort includes a representative random sample of the Polish population aged 20–74 years (mean age = 45.72, standard deviation = 14.91). The control group consisted of 1819 males (47.11%) and 2042 females (52.89%). All subjects filled out the questionnaire (the response rate was 74.3% and 79.3% for men and women, respectively) including among others the Beck Depression Inventory (BDI). The following classification was adopted for interpreting BDI score: BDI score >19 - moderately/severely depressed, 11< BDI score ≤ 19 - mildly depressed, BDI score ≤ 11- not depressed [42], [43].
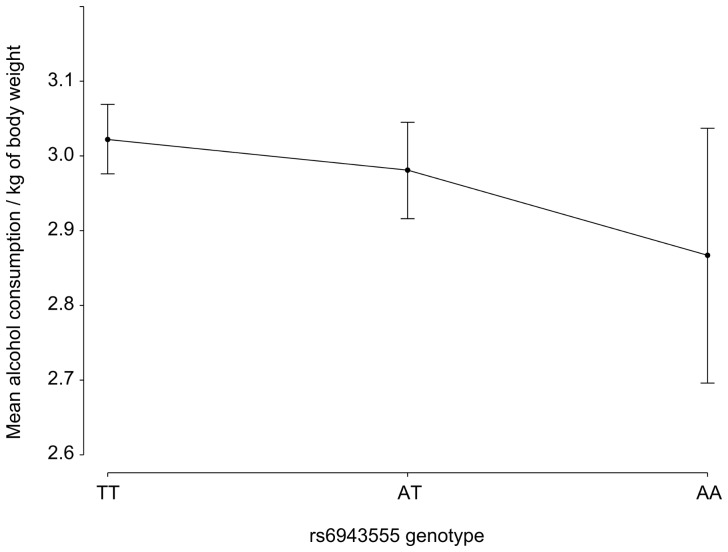
The alcohol drinking habits, including the size and frequency of drinks, were obtained from a questionnaire administered by a certified interviewer as described previously [40], [41]. From these data the mean amount of ethanol consumed daily was calculated assuming that distilled spirits, wine and beer contained 316 g, 94.81g and 31.6 g of ethanol in one liter, respectively. Among studied subjects the data on alcohol consumption were available for 3830 subjects of whom 17.15% (N = 657) were classified as non drinkers. The median alcohol consumption per day per 1 kg of body weight among those who reported drinking alcohol was 0.027 with quartile range from 0.008 to 0.089. After adjustment for sex and age, among the WOBASZ subjects there was an inverse trend (P = 0.076) for correlation between the rs6943555 genotype (encoded as the number of the A alleles) and alcohol consumption expressed as quintile of amount consumed per day per kg of body weight (Fig. 1), which is consistent with previously reported results on association between AUTS2 and alcohol consumption in a general population [22].
Figure 1. Age and sex adjusted mean quintile of alcohol consumption per day per kg of body weight vs. the rs6943555 genotype encoded as the number of the A alleles among 3830 WOBASZ subjects.
Vertical bars indicate 95% confidence intervals.
All cases and controls were Polish Caucasians. The study was approved by the Bioethical Committee of Medical University of Warsaw and all control subjects gave written consent for the anonymous use of their DNA for research.
Genotyping
Genomic DNA from cases and from controls was isolated from EDTA blood samples using salting out procedure described by Miller [44]. The rs6943555 polymorphism was genotyped using Real-time TaqMan Allelic Discrimination Assay using pre-designed primers obtained from Applied Biosystems (Pre-designed TaqMan SNP Genotyping Assays, 7500 Real time PCR System, Applied Biosystems, Assay ID C____240452_20) on ABI PRISM 9700 platform (Applied Biosystems). The results were analyzed using 7500 System SDS Software (Applied Biosystems).
We successfully genotyped 625 completed suicide subjects (+ 62 undetermined samples, 91% call rate) and 3861 controls (+ 157 undetermined genotypes, 96% call rate). Our study could detect with power of 0.8 (alpha = 0.05) an allelic association conferring OR = 1.17.
Statistical analysis
Distribution of rs6943555 genotypes among cases and controls was compared assuming dominant, co-dominant or recessive models of effect using Web-Assotest program (http://www.ekstroem.com/ assotest/assotest.html) [45]. The most likely model of inheritance was determined by P value for model fit (Pfit.). Pfit allows estimating whether given model is consistent with the distribution of genotypes among cases and controls (P fit<0.05 indicates that given model should be rejected).
Comparison of the genotype distribution after adjusting for age and gender was performed by multiple logistic regression analysis using SPSS software Release 11.5.0.
Search for genotype-phenotype correlation among suicides was performed by chi square test or Mann-Whitney U test using Statistica software version 10.0 (StatSoft, Inc., Tulsa, OK).
Results
Analysis of the association in the study population
The distribution of genotypes was in Hardy-Weinberg Equilibrium (HWE) among subjects and controls (Table 3). As shown in Table 3 we observed an association between suicide and rs6943555. The frequency of rs6943555 A allele among controls (21%) was consistent with frequency among populations of European ancestry (22%, http://www.ensembl.org/Homo_sapiens/Variation/Population?db=corer=7:69805523-69806523v=rs6943555vdb=variationvf=24793612) The frequency of rs6943555 A allele among cases (24%) was higher than among controls (21%, OR = 1.17, P = 0.018). The genotype distribution supported the co-dominant (OR = 1.18, P = 0.020, Pfit = 0.381) or dominant (OR = 1.24, P = 0.013, Pfit = 0.970), but not recessive model of inheritance (Pfit = 0.018, Table 3). As the mood disorders, particularly depression, are strongly associated with suicide [46], we also compared suicides to controls without signs of depression (BDI scores ≤ 11) confirming the association (OR = 1.19, P = 0.017; OR = 1.19, P = 0.019 and OR = 1.25, P = 0.013 for the allelic, co-dominant and dominant model respectively, Table 3).
Table 3. Distribution of genotypes and analysis of the association between rs6943555 and suicide.
| Cohort | rs6943555 genotypes | HWE | Model | |||||
| Allelic comparison | Recessive | Co−dominant | Dominant | |||||
| OR (CI), P | OR (CI) P, Pfit | OR (CI) P, Pfit | OR (CI) P, Pfit | |||||
| TT (%) | AT (%) | AA (%) | (AA vs. TT/AT) | (AA vs. AT vs. TT) | (AT/AA vs. TT) | |||
| Suicide victims I (N = 625) | 359 (57.4) | 232 (37.1) | 34 (5.4) | 0.661 | 1.171 (1.03; 1.37), 0.018 | 1.161 (0.79; 1.68), 0.456, 0.018 | 1.181 (1.03; 1.36), 0.020, 0.381 | 1.241 (1.05; 1.48), 0.013, 0.970 |
| 1.192 (1.03; 1.38), 0.017 | 1.172 (0.80; 1.73), 0.426, 0.019 | 1.192 (1.03; 1.38), 0.019, 0.416 | 1.252 (1.05; 1.49), 0.013, 0.924 | |||||
| Suicide victims I with blood ethanol concentration > 20 mg/dl (N = 235) | 126 (53.6) | 92 (39.1) | 17 (7.2) | 0.971 | 1.381 (1.11; 1.70), 0.003 | 1.571 (0.94; 2.62), 0.105, 0.017 | 1.371 (1.11; 1.69), 0.004, 0.770 | 1.451 (1.12; 1.89), 0.006, 0.396 |
| Suicide victims I with blood ethanol concentration ≤ 20 mg/dl (N = 390) | 233 (59.7) | 140 (35.9) | 17 (4.36) | 0.482 | 1.081 (0.90; 1.29) 0.405 | 0.921 (0.55; 1.52), 0.732, 0.200 | 1.081 (0.90; 1.28), 0.411, 0.298 | 1.131 (0.92; 1.40), 0.256, 0.493 |
| Suicide victims II (N = 132) | 73 (54.5) | 51 (38.1) | 8 (6.0) | 0.818 | 1.28 (0.96–1.69) 0.089 | 1.30 (0.62–2.69) 0.50, 0.12 | 1.27 (0.96–1.68) 0.099, 0.064 | 1.36 (0.96–1.92) 0.089, 0.85 |
| Suicide victims II with blood ethanol concentration > 20 mg/dl (N = 65) | 31 (47.7) | 30 (46.2) | 4 (6.2) | 0.352 | 1.551 (1.06–2.27) 0.023 | 1.321 (0.47–3.66) 0.61, 0.017 | 1.541 (1.05–2.25) 0.031, 0.26 | 1.841 (1.13–3.01) 0.015, 0.9 |
| Suicide victims II with blood ethanol concentration ≤ 20 mg/dl (N = 59) 3 | 39 (66.1) | 16 (27.1) | 4 (6.8) | 0.210 | 0.961 (0.61–1.51) 0.85 | 1.461 (0.52–4.08) 0.50, 0.42 | 1 (0.61–1.50) 0.86, 0.30 | 0.861 (0.50–1.48) 0.60, 0.36 |
| Controls (N = 3861) | 2420 (62.7) | 1258 (32.6) | 183 (4.7) | 0.236 | ||||
| Controls BDI ≤ 11 (N = 2676) | 1681 (62.8) | 870 (32.5) | 125 (4.7) | 0.362 | ||||
P values <0.05 were boldfaced 1 comparison with all controls; 2 comparison with controls with BDI ≤ 11, HWE - Hardy-Weinberg equilibrium, 3for 8 subjects from suicide cohort II there were no data on blood ethanol concentration
The association between suicide and rs6943555 remained significant also after adjusting for age and gender (OR = 1.29, P = 0.002 and OR = 1.39, P = 0.001 for co-dominant and dominant model of inheritance, respectively).
Genotype-phenotype associations
Given the previously reported relationship between rs6943555 and alcohol consumption [22], we divided suicides into two groups: cases with blood ethanol concentration over 20 mg/dl and cases with blood ethanol concentration less or equal to 20 mg/dl. Interestingly, the observed association remained significant only for cases who committed suicide under influence of alcohol (OR = 1.38, P = 0.003; OR = 1.37, P = 0.004 and OR = 1.45, P = 0.006 for the allelic, co-dominant and dominant model respectively, Table 3).
We did not find statistically significant differences in the distribution of rs6943555 genotypes among suicide after stratifying for gender (P = 0.293), most prevalent methods of committing suicide (P = 0.112 for hanging vs. other methods, P = 0.136 for jumping from a high place vs. other methods), age (P = 0.985), history of addiction (P = 0.253) or psychiatric diagnosis (P = 0.739).
Analysis in a replication cohort
Relatively many samples from suicides (62 or 9%) had low DNA quality which precluded genotyping. This was most likely caused by low initial quality of some samples and their long storage (>5 years in some cases). Since high genotype failure rate may introduce a bias due to preferential genotyping of one allele we attempted a replication of our results using a second group of samples (N = 134). Since these samples were collected from recent autopsies we expected a higher rate of successful genotyping. In order to verify the DNA quality of these samples we typed them with a randomly selected real time SNP assay available in our lab (an assay for rs12936511). We obtained satisfactory results for 132 (>98.5%) samples. These 132 DNA samples were analyzed for rs6943555 and consistent with their good quality typing results were obtained in 100% of cases.
The distribution of rs6943555 genotypes in the whole second group of cases as well as after stratifying according to blood ethanol concentration is shown in Table 3 (suicide victims II). In the subset with blood ethanol concentration >20 mg/dl the frequency of the allele A was higher than among controls (OR = 1.55, P = 0.023). The genotype distribution supported the co-dominant (OR = 1.54, P = 0.031, Pfit = 0.261) or dominant (OR = 1.84, P = 0.015, Pfit = 0.9), but not recessive model of inheritance (Pfit = 0.017, Table 3). There were no such associations among those with blood ethanol concentration ≤ 20 mg/dl (Table 3).
When we pooled all suicides with blood ethanol concentration >20 mg/dl (N = 300) we found that the association with the rs6943555 A allele was statistically significant when assessed vs. controls (OR = 1.41, CI: 1.17-1.70, P = 0.00029; OR = 1.53, CI: 1.21-1.94, P = 0.00044; OR = 1.40, CI: 1.17-1.69, P = 0.00048, for allelic comparison and comparison assuming dominant, and co-dominant model, respectively) as well as vs. cases with blood ethanol concentration ≤ 20 mg/dl. (N = 449, OR = 1.33 CI:. 1.04-1.69, P = 0.019; OR = 1.40 CI: 1.04-1.88, P = 0.026; OR = 1.34 CI = 1.05-1.70, P = 0.019; for allelic comparison and comparison assuming dominant, and co-dominant model, respectively).
Discussion
The main novel finding from our study is the association between the A allele of rs6943555 in the AUTS2 gene and completed suicide. We observed overrepresentation of the A alleles, AA and AT genotypes in the suicide group compared to controls. After dividing cases into those who committed suicide under influence of alcohol and those without such influence, the association was observed only among cases with blood ethanol concentration over 20 mg/dl. The association between the A allele of rs6943555 and suicide under influence of alcohol was subsequently replicated in a second cohort of cases.
The direction of the observed association of the rs6943555 A allele with suicide is opposite to the association with alcohol consumption reported previously [22]. We hypothesize that rs6943555 A allele may be linked to an adverse reaction to ethanol which would explain its association with lower alcohol consumption in general population (ref. [22] and the trend observed by us among the WOBASZ subjects) as well as the predisposition to suicide under influence of ethanol observed by us. Schumann et al. found increased expression of AUTS2 in human prefrontal cortex among the A vs. T allele carriers indicating that the excessive activity of the AUTS2 protein may be responsible for these effects [22]. Furthermore, inactivation or down-regulation of the AUTS2 homolog tay gene in Drosophila melanogaster caused reduced sensitivity to ethanol despite similar internal ethanol concentrations after ethanol exposure in mutant and wild type flies [22]. In the context of our observations this suggests a scenario where the carriers of the A allele have higher expression of AUTS2 and are more sensitive to ethanol induced negative emotions leading to suicide. Such processes could involve impulsivity, impaired decision making and emotion dysregulation after consumption [47].
Although we show that rs6943555 is associated with suicide it is not clear whether this is a primary association or an effect of marker(s) in linkage disequilibrium. It is also possible that multiple variants in AUTS2 have independent effects of suicide risk. Since we tested a single marker our data cannot answer this question. However, it should be emphasized that the association between rs6943555 and alcohol consumption which prompted our work emerged from an extensive genome-wide study in which ∼2,5 mln. SNPs were analyzed either directly or by imputation [22]. In particular Affymetrix 500 K which was the most frequently used chip among studies selected for meta-analysis by Schumann et al. allows to type 184 SNPs within AUTS2 and this number is further increased by imputation procedures used [22]. Whereas the Schumann et al. do not provide a detailed list of SNPs within AUTS2 which were analyzed Fig. S3A from the article [22] indicate that in addition to rs6943555 a substantial number of other SNPS in AUTS2 was covered. Furthermore, in addition to mapping the primary association via known SNPs Schuman et al. also performed a screen for nonsynonymous genetic variants in the exons most proximal to rs6943555 in 200 individuals [22]. Thus, although there is clearly a need for comprehensive evaluation of all AUTS2 variants vs. suicide risk it is possible that rs6943555 or a very closely linked SNP will remain the primary candidate.
It is increasingly recognized that pathophysiology of mental disorders is heterogenic and multifactorial [48]. Shared behavioral characteristics of many of them may be associated with shared underlying molecular mechanisms. Provided they are replicated in other populations our results should lead to a better understanding of the genetic risk factors, which play a role in the pathogenesis such psychiatric phenotypes as suicide, alcohol sensitivity and autism.
Acknowledgments
The authors would like to thank Waldemar Szelenberger, MD, PhD for his support for this study.
Funding Statement
Research was supported by the Medical University of Warsaw Grant 1WY/WB1/10 and by the Fogarty International Center/NIAAA Grant 5 D43 TW007569. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, et al. (2009) Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry 65: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedersen NL, Fiske A (2010) Genetic influences on suicide and nonfatal suicidal behavior: twin study findings. Eur Psychiatry 25: 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voracek M, Loibl LM (2007) Genetics of suicide: a systematic review of twin studies. Wien Klin Wochenschr 119: 463–475. [DOI] [PubMed] [Google Scholar]
- 4. Brent DA, Mann JJ (2005) Family genetic studies, suicide, and suicidal behavior. Am J Med Genet C Semin Med Genet 133C: 13–24. [DOI] [PubMed] [Google Scholar]
- 5. Tidemalm D, Runeson B, Waern M, Frisell T, Carlstrom E, et al. (2011) Familial clustering of suicide risk: a total population study of 11.4 million individuals. Psychol Med 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim S, Choi KH, Baykiz AF, Gershenfeld HK (2007) Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics 8: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai SJ, Hong CJ, Liou YJ (2011) Recent molecular genetic studies and methodological issues in suicide research. Prog Neuropsychopharmacol Biol Psychiatry 35: 809–817. [DOI] [PubMed] [Google Scholar]
- 8. Butler AW, Breen G, Tozzi F, Craddock N, Gill M, et al. (2010) A genomewide linkage study on suicidality in major depressive disorder confirms evidence for linkage to 2p12. Am J Med Genet B Neuropsychiatr Genet 153B: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 9. Perlis RH, Huang J, Purcell S, Fava M, Rush AJ, et al. (2010) Genome-wide association study of suicide attempts in mood disorder patients. Am J Psychiatry 167: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schosser A, Butler AW, Ising M, Perroud N, Uher R, et al. (2011) Genomewide association scan of suicidal thoughts and behaviour in major depression. PLoS One 6: e20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Willour VL, Seifuddin F, Mahon PB, Jancic D, Pirooznia M, et al. (2012) A genome-wide association study of attempted suicide. Mol Psychiatry 17: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bedogni F, Hodge RD, Nelson BR, Frederick EA, Shiba N, et al. (2010) Autism susceptibility candidate 2 (Auts2) encodes a nuclear protein expressed in developing brain regions implicated in autism neuropathology. Gene Expr Patterns 10: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Girirajan S, Brkanac Z, Coe BP, Baker C, Vives L, et al. (2011) Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet 7: e1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de la Barra F, Skoknic V, Alliende A, Raimann E, Cortes F, et al. (1986) Twins with autism and mental retardation associated with balanced (7;20) chromosomal translocation. Rev Chil Pediatr 57: 549–554. [PubMed] [Google Scholar]
- 15. Sultana R, Yu CE, Yu J, Munson J, Chen D, et al. (2002) Identification of a novel gene on chromosome 7q11.2 interrupted by a translocation breakpoint in a pair of autistic twins. Genomics 80: 129–134. [DOI] [PubMed] [Google Scholar]
- 16. Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, et al. (2008) Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet 82: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ben David E, Granot-Hershkovitz E, Monderer-Rothkoff G, Lerer E, Levi S, et al. (2011) Identification of a functional rare variant in autism using genome-wide screen for monoallelic expression. Hum Mol Genet 20: 3632–3641. [DOI] [PubMed] [Google Scholar]
- 18. Huang XL, Zou YS, Maher TA, Newton S, Milunsky JM (2010) A de novo balanced translocation breakpoint truncating the autism susceptibility candidate 2 (AUTS2) gene in a patient with autism. Am J Med Genet A 152A: 2112–2114. [DOI] [PubMed] [Google Scholar]
- 19. Kalscheuer VM, FitzPatrick D, Tommerup N, Bugge M, Niebuhr E, et al. (2007) Mutations in autism susceptibility candidate 2 (AUTS2) in patients with mental retardation. Hum Genet 121: 501–509. [DOI] [PubMed] [Google Scholar]
- 20. Mefford HC, Muhle H, Ostertag P, von Spiczak S, Buysse K, et al. (2010) Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet 6: e1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elia J, Gai X, Xie HM, Perin JC, Geiger E, et al. (2010) Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry 15: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, et al. (2011) Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci U S A 108: 7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Branas CC, Richmond TS, Ten Have TR, Wiebe DJ (2011) Acute alcohol consumption, alcohol outlets, and gun suicide. Subst Use Misuse 46: 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stickley A, Jukkala T, Norstrom T (2011) Alcohol and suicide in Russia, 1870–1894 and 1956–2005: evidence for the continuation of a harmful drinking culture across time? J Stud Alcohol Drugs 72: 341–347. [DOI] [PubMed] [Google Scholar]
- 25. Allen J, Levintova M, Mohatt G (2011) Suicide and alcohol-related disorders in the U.S. Arctic: boosting research to address a primary determinant of health disparities. Int J Circumpolar Health 70: 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orui M, Kawakami N, Iwata N, Takeshima T, Fukao A (2011) Lifetime prevalence of mental disorders and its relationship to suicidal ideation in a Japanese rural community with high suicide and alcohol consumption rates. Environ Health Prev Med 16: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Conner KR, Phillips MR (2012) Case-control study in China of risk factors for suicide in men with alcohol use disorders. J Stud Alcohol Drugs 73: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raja M, Azzoni A, Frustaci A (2011) Autism Spectrum Disorders and Suicidality. Clin Pract Epidemiol Ment Health 7: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szanto K, Dombrovski AY, Sahakian BJ, Mulsant BH, Houck PR, et al. (2012) Social emotion recognition, social functioning, and attempted suicide in late-life depression. Am J Geriatr Psychiatry 20: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Embregts P, van Nieuwenhuijzen M (2009) Social information processing in boys with autistic spectrum disorder and mild to borderline intellectual disabilities. J Intellect Disabil Res 53: 922–931. [DOI] [PubMed] [Google Scholar]
- 31. Gibbs LM, Dombrovski AY, Morse J, Siegle GJ, Houck PR, et al. (2009) When the solution is part of the problem: problem solving in elderly suicide attempters. Int J Geriatr Psychiatry 24: 1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pollock LR, Williams JM (1998) Problem solving and suicidal behavior. Suicide Life Threat Behav 28: 375–387. [PubMed] [Google Scholar]
- 33. Duberstein PR, Conwell Y, Conner KR, Eberly S, Evinger JS, et al. (2004) Poor social integration and suicide: fact or artifact? A case-control study. Psychol Med 34: 1331–1337. [DOI] [PubMed] [Google Scholar]
- 34. Harms MB, Martin A, Wallace GL (2010) Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev 20: 290–322. [DOI] [PubMed] [Google Scholar]
- 35. Burton CZ, Vella L, Weller JA, Twamley EW (2011) Differential effects of executive functioning on suicide attempts. J Neuropsychiatry Clin Neurosci 23: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christ SE, Kanne SM, Reiersen AM (2010) Executive function in individuals with subthreshold autism traits. Neuropsychology 24: 590–598. [DOI] [PubMed] [Google Scholar]
- 37. Courtet P, Gottesman II, Jollant F, Gould TD (2011) The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies? Transl Psychiatry 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pellicano E (2010) Individual differences in executive function and central coherence predict developmental changes in theory of mind in autism. Dev Psychol 46: 530–544. [DOI] [PubMed] [Google Scholar]
- 39. Westheide J, Quednow BB, Kuhn KU, Hoppe C, Cooper-Mahkorn D, et al. (2008) Executive performance of depressed suicide attempters: the role of suicidal ideation. Eur Arch Psychiatry Clin Neurosci 258: 414–421. [DOI] [PubMed] [Google Scholar]
- 40. Broda G, Rywik S (2005) All-Polish Health Survey - WOBASZ Project. Defining the problem and aims of the study. Polish PopulationReview 27: 29–36. [Google Scholar]
- 41. Rywik S, Kupsc W, Piotrowski W, Broda G (2005) Multi-Center All-Polish Health Survey - WOBASZ Project. Methodological Assumptions and Logistics. Polish Population Review 27: 37–50. [Google Scholar]
- 42. Bolling-Sternevald E, Lauritsen K, Aalykke C, Havelund T, Knudsen T, et al. (2002) Effect of profound acid suppression in functional dyspepsia: a double-blind, randomized, placebo-controlled trial. Scand J Gastroenterol 37: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 43. Parnowski P, Jernajczyk W (1977) Inwentarz depresji Beck'a w ocenie nastroju osób zdrowych i chorych na choroby afektywne. Psychiatria Polska 11: 417–421. [PubMed] [Google Scholar]
- 44. Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hansen SK, Gjesing AP, Rasmussen SK, Glumer C, Urhammer SA, et al. (2004) Large-scale studies of the insulin gene variable-number-of-tandem-repeats polymorphism in relation to Type 2 diabetes mellitus and insulin release. Diabetologia 47: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 46. Kohli MA, Salyakina D, Pfennig A, Lucae S, Horstmann S, et al. (2010) Association of genetic variants in the neurotrophic receptor-encoding gene NTRK2 and a lifetime history of suicide attempts in depressed patients. Arch Gen Psychiatry 67: 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mitchell DG (2011) The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behav Brain Res 217: 215–231. [DOI] [PubMed] [Google Scholar]
- 48. Porteri C (2012) Genetics and psychiatry: a proposal for the application of the precautionary principle. Med Health Care Philos [Epub ahead of print] [DOI] [PubMed] [Google Scholar]