Abstract
Aims
To investigate the therapeutic effects and acting mechanism of a combination of Chinese herb active components, i.e., a combination of baicalin, jasminoidin and cholic acid (CBJC) on Alzheimer’s disease (AD).
Methods
Male rats were intracerebroventricularly injected with ibotenic acid (IBO), and CBJC was orally administered. Therapeutic effect was evaluated with the Morris water maze test, FDG-PET examination, and histological examination, and the acting mechanism was studied with DNA microarrays and western blotting.
Results
CBJC treatment significantly attenuated IBO-induced abnormalities in cognition, brain functional images, and brain histological morphology. Additionally, the expression levels of 19 genes in the forebrain were significantly influenced by CBJC; approximately 60% of these genes were related to neuroprotection and neurogenesis, whereas others were related to anti-oxidation, protein degradation, cholesterol metabolism, stress response, angiogenesis, and apoptosis. Expression of these genes was increased, except for the gene related to apoptosis. Changes in expression for 5 of these genes were confirmed by western blotting.
Conclusion
CBJC can ameliorate the IBO-induced dementia in rats and may be significant in the treatment of AD. The therapeutic mechanism may be related to CBJC’s modulation of a number of processes, mainly through promotion of neuroprotection and neurogenesis, with additional promotion of anti-oxidation, protein degradation, etc.
Introduction
Alzheimer’s disease (AD) has a very high morbidity in the senile population. It causes progressive impairment of cognitive performance, which develops into severe difficulty with household management and basic self-caring in the late stage [1]. At present, there is no satisfactory therapy for AD. Although several drugs have shown moderate amelioration of symptoms, none of them have sufficient potency to stop or reverse the pathological progression of AD [1].
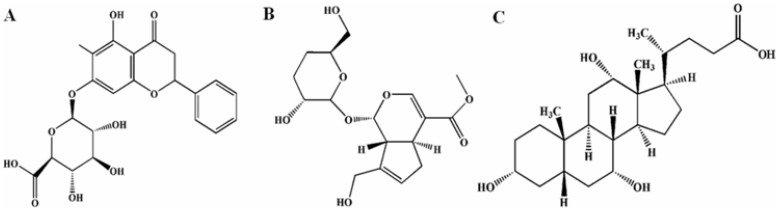
Baicalin, jasminoidin, and cholic acid (figure 1) are the main active components of Qingkailing (QKL), one of the most well-known Chinese herb preparations. QKL is an aqueous preparation containing extracts of 7 herbs. It has shown an outstanding therapeutic effect on a broad spectrum of diseases, including high fever, coma, and acute inflammation, especially on stroke [2], [3]. However, as with other herbal preparations, QKL includes numerous unidentified compounds, which makes elucidating the therapeutic mechanism and controlling the preparation quality difficult. Additionally, these unidentified compounds can even cause adverse effects such as allergies and side-effects. Together, these inherent defects hinder the acceptance of herbal preparations including QKL by the mainstream medicine. In recent years, the concept of “active component combinations” has arisen in Chinese herbal therapeutics; this concept is proposed to identify the main active compounds in a formula and use them in combination instead of their parent herbs, thus keeping the advantages of the herbal combination, avoiding problems of uncontrolled composition, and making Chinese herb preparations qualified to meet the modern standards [4].
Figure 1. The structures of baicalin, jasminoidin and cholic acid. A. baicalin; B. jasminoidin; C. cholic acid.
A series of pharmaceutical and pharmacodynamic studies have been conducted that identified more than 60 compounds in QKL and found 3 compounds, i.e. baicalin, jasminoidin, and cholic acid as the most active ones [4]. Baicalin, jasminoidin, and cholic acid are derived from 3 different herbs in QKL, Huangqin (the root of Scutellaria baicalensis), Zhizi (the fruit of Gardenia jasminoides Ellis), and Niuhuang (Calculus bovis), respectively; all of the 3 herbs have the function of “clearing heat and detoxifying”, according to Traditional Chinese Medicine theory. A combination of 3.75 mg baicalin, 18.75 mg jasminoidin and 5.25 mg cholic acid per kg of body weight was found to exert the greatest therapeutic effect in rat ischemic stroke models [5], [6].
Although this combination shows potential to replace QKL in stroke therapy, its effect on AD, another central nervous system disease with a number of similarities to stroke in its pathogenesis, has not yet been examined.
In the present study, the therapeutic potential and mechanism of action of this combination of baicalin, jasminoidin, and cholic acid (CBJC) on AD were evaluated in ibotenic acid (IBO)-injured rats. To our knowledge, this is the first report on the use of active component combinations of Chinese herbs in the treatment of AD, which may be significant in searching for effective drugs and improving Chinese herbal therapy for AD.
Materials and Methods
This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Review Board (IRB) of Beijing Normal University Animal Center approved the use of rats in this research project (January 1st, 2011–May 31st, 2011).
Drugs and Reagents
Ibotenic acid (IBO) was purchased from Sigma (Atlanta, USA). Baicalin, jasminoidin and cholic acid were provided by Beijing University of Traditional Chinese Medicine (Beijing, China). 2-deoxy-2-(F-18)fluoro-D-glucose (FDG) was provided by the General Hospital of the People’s Liberation Army (Beijing, China). The rat cDNA microarray chip with 15,000 spots was purchased from Shanghai Biochip Inc. (Shanghai, China). Polyclonal anti-CRBP1 antibody was purchased from Pierce Thermo Fisher Scientific Inc. (Rockford, USA); polyclonal anti-EGF, anti-TRH, and anti-CBR1 antibodies were purchased from Abcam Inc. (Cambridge, UK); and polyclonal anti-ACE, monoclonal anti-β-actin, and horseradish peroxidase-conjugated anti-IgG antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, USA). Amersham ECL Plus western blotting detection reagents were purchased from GE Healthcare UK Ltd. (London, UK).
Animal Surgery and Drug Administration
Male Sprague-Dawley rats weighing 250–300 g were purchased from the Experimental Animal Center of Beijing University. Animal treatment and care was conducted following NIH guidelines and was approved by the local animal care and use committee. The rats were randomly divided into 3 groups: control, IBO-model, and CBJC groups. Bilateral intracerebroventricular injection was performed under anesthesia induced by chloral hydrate (400 mg/kg, i.p.). The injection cannula was inserted into the lateral ventricle (3.0 mm posterior to the bregma, 2.0 mm lateral to the midline, and 2.8 mm below the dura), guided by a stereotaxic apparatus. A 1 µL injection of IBO solution (10 g/L in sterile water, for the IBO-model and CBJC groups) or sterile water (for the control group) was performed slowly and evenly over 5 minutes using a Hamilton syringe and a microinjection pump. The solution of CBJC (1.25 mg/mL baicalin, 6.25 mg/mL jasminoidin, and 1.75 mg/mL cholic acid) was prepared in sterilized water and administered intragastrically at a dose of 3 mL/kg once a day from the third day after surgery until the rats were sacrificed. The rats in the control and IBO-model groups received water instead of CBJC.
Morris Water Maze Test
The Morris water maze test began 1 month after the IBO injection. The water maze was a circular pool with a diameter of 120 cm and a height of 50 cm that was divided into four quadrants, filled with water and maintained at 24±1°C. Initially, a visible platform test was performed, which confirmed that there were no significant differences in sensory, motor or motivational activities between the groups. Then, hidden platform and reverse hidden platform tests were conducted in succession. For the hidden platform test, a round platform with a diameter of 9 cm was placed at the midpoint of the fourth quadrant, 2 cm below the water surface. A training trial was conducted once a day for 5 days. During each trial, the rats were placed in the water at a fixed position, opposite the platform and at the edge of the pool. The rats were allowed to swim freely until they escaped onto the platform. Swimming was recorded with a camera, and the escape latency time, swim distance, and swim speed were measured. The reverse hidden platform test was identical to the hidden platform test except the locations of the platform and the swim start were reversed; the reverse hidden platform trial lasted for 3 days.
FDG Position-emission Tomography (FDG-PET) Examination
FDG-PET examination was performed on the day after the Morris water maze test finished. Rats were intravenously injected with FDG (diluted in saline, 3 mCi/kg). Forty minutes after injection, the rats were anesthetized by inhaling 5% isoflurane-95% O2 mixed gas, placed in a prone position and maintained under anesthesia with 1.5% isoflurane-98.5% O2 mixed gas. An Eplus166 micro PET scanner (manufactured by the Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, China) was used to acquire the data. Images were reconstructed using the FORE plus OSEM method with a 128×128×63 matrix and saved in ANALYZE 7.5 format. Images were normalized, trimmed to remove data external to the brain, and smoothed with a 3.0×3.0×6.0 FWHM isotropic Gaussian kernel using a brain FDG-PET template created from the PET data of 24 normal rats and a statistical parametric mapping software (SPM2, Wellcome Department of Cognitive Neurology, London, UK). Finally, the images were subjected to voxel-based statistical analysis based on the general linear model.
Histological Examination
After FDG-PET examination, 3 rats in each group were randomly selected for histological examination; the remaining animals were left for DNA microarray and western blotting analysis. The rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and their brains were perfused with saline followed by 4% formaldehyde via the ascending aorta. The perfused brains were then removed, post-fixed in 4% formaldehyde, and embedded in paraffin. The paraffin-embedded brains were cut into 5 µm coronal sections and subjected to hematoxylin and eosin (HE) staining. The histological morphology of the hippocampal CA1 region was observed under a light microscope.
Tissue Preparation for DNA Microarray and Western Blotting
Rats were euthanized by decapitation, and their forebrains were rapidly removed, frozen in liquid nitrogen, and stored at −80°C until analysis. Each forebrain was divided into two parts by a vertical incision through the midline of the callosum; the left part was used for DNA microarray analysis, and the right part was used for western blotting.
DNA Microarray
Tissues were homogenized in TRIzol, and the total RNA in each sample was extracted with chloroform and isopropyl alcohol. After purification with 75% alcohol, the RNA samples were qualified and quantified by agarose electrophoresis and UV spectrometry. RNA samples were reverse transcribed into cDNAs labeled with Cy3 (for samples in the control and CBJC groups) or Cy5 (for samples in the IBO-model group). The cDNA samples in the control and CBJC groups were randomly paired with samples from the IBO-model group. The paired samples were pooled and analyzed on the cDNA chip; the fluorescence ratio of Cy3 to Cy5 in each spot was measured according to the operator’s manual. A significant difference in Gene expression between groups was determined to exist when the average of the Cy3/Cy5 ratio was >1.6 or <0.6 and the P value for the comparison between the Cy3/Cy5 ratio and the Cy5/Cy5 ratio was <0.05.
Western Blotting
The tissues were lysed in RIPA lysis buffer containing a cocktail of protease inhibitors (Roche Applied Science, Germany), and protein concentrations were determined using the BCA method. Aliquots containing 50 µg of protein in loading buffer (10% glycerol, 2% SDS, 60 mmol/L Tris-HCl, 0.01% bromophenol blue, and 100 mmol/L dithiothreitol, pH 6.8) were boiled for 5 minutes, subjected to SDS-PAGE and transferred to NC membranes. The levels of the proteins of interest and β-actin were detected using their corresponding primary antibodies and horseradish peroxidase-conjugated secondary antibodies at appropriate dilutions. Immunobands were lightened with Amersham ECL Plus western blotting detection reagents and imaged on X-ray film. The optical density (OD) of each protein band was quantified using the software Image J (NIH image, MD), and the OD value of each protein of interest was normalized to that of β-actin.
Statistical Analysis
The time effect and group differences in the Morris water maze test were analyzed by one-way ANOVA with repeated measures followed by LSD post hoc test. Group differences in FDG-PET examination and DNA microarray were analyzed by two-tailed t tests for independent samples. Group differences in western blotting were analyzed by one-way ANOVA followed by LSD post hoc test. A P value <0.05 was considered statistically significant.
Results
Morris Water Maze Test
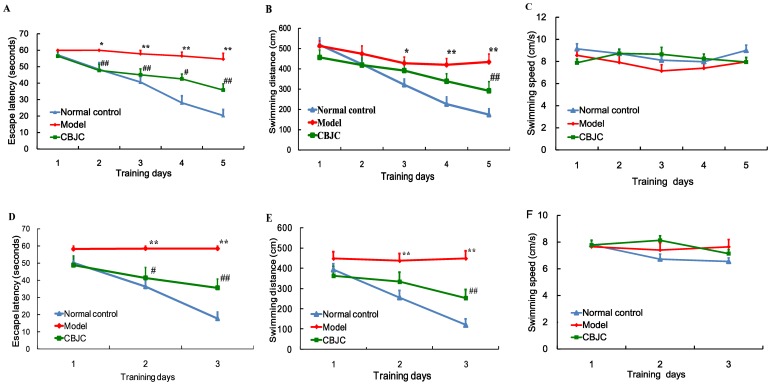
In the hidden platform test, the escape latency time was dependent on both the time effect (F4,108 = 18.178, P<0.001) and the group effect (F2,27 = 41.426, P<0.001); the control and CBJC groups escaped significantly faster than the IBO-model group (both P<0.001). A similar result was observed for the swim distance (F4,108 = 14.393 and P<0.001 for the time effect; F2,27 = 8.784 and P<0.001 for the group difference; and P<0.001 and P<0.05 for the comparisons of the control group and the CBJC group to the IBO-model group). Swim speed was not significantly different between the groups.
In the reverse hidden platform test, the time effect and differences between the groups were both significant factors in the escape latency time (F2,54 = 12.607, P<0.001; F2,27 = 23.013, P<0.001); compared with the IBO-model group, the escape latency times in the control group and the CBJC group were significantly shorter (both P<0.001). A similar result was observed for the swim distance (F2,54 = 12.886 and P<0.001 for the time effect; F2,27 = 12.268 and P<0.001 for the group difference; and P<0.001 for the comparisons of the control group and the CBJC group to the IBO-model group). No significant differences in the swim speed were observed between groups (figure 2).
Figure 2. Effects of CBJC on cognition evaluated with the Morris water maze test.
Escape latency time, swim distance and swim speed from the hidden platform test (A, B and C, respectively) and the reverse hidden platform test (D, E and F, respectively) are shown. The data are expressed as the means ± SEM (n = 10 in each group. Analysis was conducted by using one-way ANOVA with repeated measures followed by LSD post hoc test. * P<0.05, ** P<0.01, IBO-model group vs. control group; # P<0.05, ## P<0.01, CBJC group vs. IBO-model group).
FDG-PET Examination
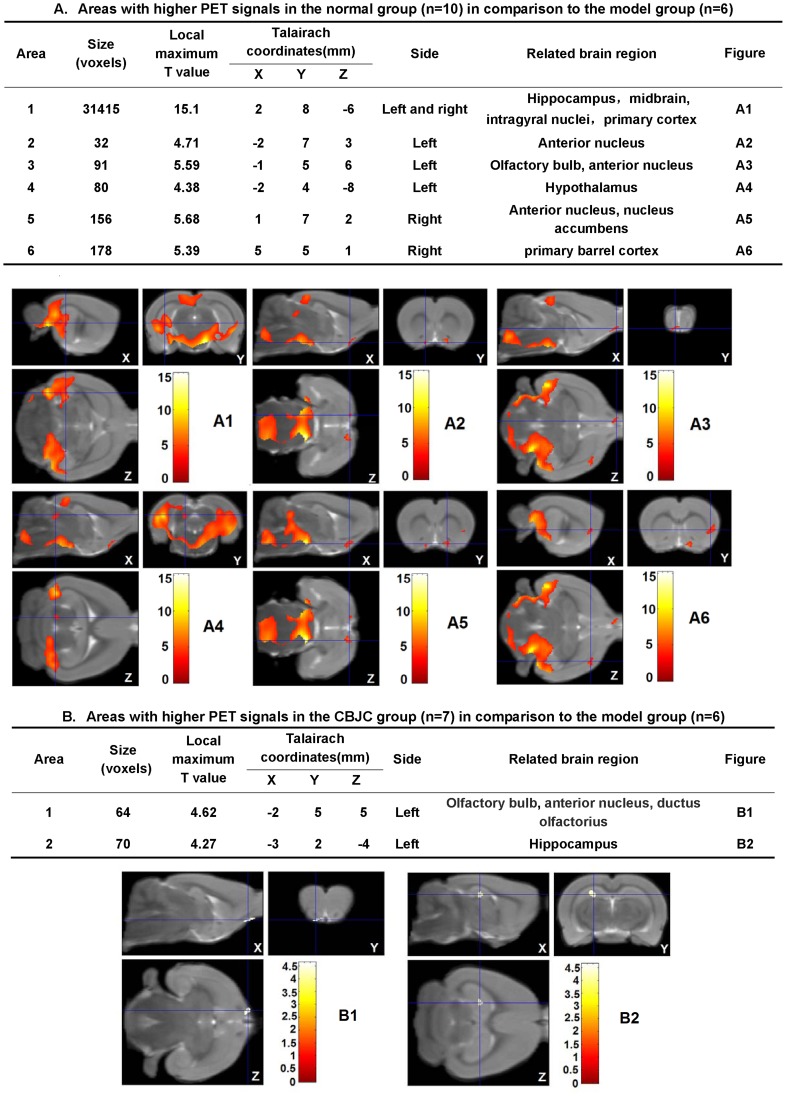
Compared with the control group, significant decreases in FDG-PET signals were observed in the IBO-model group for numerous brain regions. The affected regions included the anterior nucleus, the hippocampus, the midbrain, the intragyral nuclei and the primary cortex on both sides, the olfactory bulb and the hypothalamus on the left side, and the nucleus accumbens and the primary barrel cortex on the right side (P<0.05). Compared with the IBO-model group, in the CBJC group, the decrease in the left olfactory bulb and the left anterior nucleus was significantly attenuated; additionally, a significant increase in the FDG-PET signal was observed in the left hippocampus (P<0.05, figure 3).
Figure 3. Effects of CBJC on the glucose-uptake in the brain.
In the tables, the parameters of areas with significantly different FDG-PET signals between the normal group and the IBO-model group (A) or between the CBJC group and the IBO-model group (B) are shown (P<0.05). In the figures, the FDG-PET three-dimensional images of the areas listed in the tables are shown. In figure A3 and figure B1, the areas indicated by the intersection of blue lines are the common part of areas with decreased glucose-uptake in the IBO-model group in comparison to the normal group and that with increased glucose-uptake in the CBJC group in comparison to the IBO-model group. All the data were analyzed with 2-tailed t test for independent-samples.
Histological Examination of the Hippocampal CA1 Region
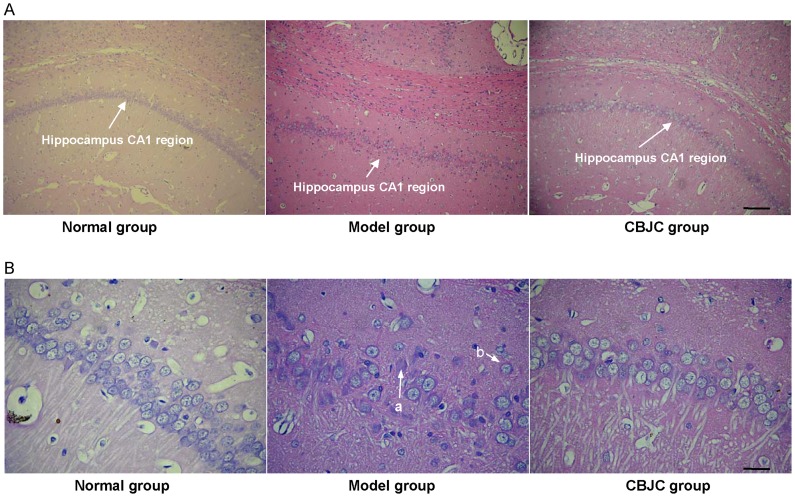
In the control group, neurons in the hippocampal CA1 region showed an orderly arrangement with no apparent abnormalities in cellular morphology. In the IBO-model group, neuron arrangement was disrupted with severe lesions in the nucleus and cytoplasm such as karyolysis and eosinophilia, and a neuronal cell loss was noted. In the CBJC group, these abnormalities were ameliorated (figure 4).
Figure 4. The histological morphology of the hippocampal CA1 region examined with HE staining.
A: photomicrographs under 100× magnification, scale bar = 40 µm; B: photomicrographs under 400× magnification, scale bar = 10 µm. In the IBO-model group, neuron arrangement is disrupted, severe lesions such as karyolysis (a) and eosinophilia (b) are observed in the nucleus and cytoplasm, and neuronal cell loss is noted.
Gene Expression in the Forebrain
Compared with the IBO-model group, in the CBJC group, the expression of 19 genes was significantly changed (P<0.05 or P<0.01). Of these 19 genes, 11 are related to neuroprotection and neurogenesis and the other 8 are related to anti-oxidation, protein degradation, cholesterol metabolism, angiogenesis, stress response, and apoptosis. Gene expression was increased in all cases except for the gene related to apoptosis (table 1).
Table 1. Gene expression changes induced by CBJC in the forebrain.
| Function category | Gene | Ratio (M/C) | Ratio (CBJC/M) | Specific Function |
| Neuroprotection andneurogenesis | Cellular retinol binding protein 1 (CRBP1) | 1.05±0.45 | 2.71±1.18# | See discussion. |
| Epidermal growth factor (EGF) | 1.16±0.08* | 1.81±0.47# | See discussion. | |
| Fatty acid binding protein 7 (FABP7) | 0.74±0.11* | 1.84±0.21## | Promotes the up-take and storage of polyunsaturated fatty acids in neural cells. It is highly expressed in neuroepithelial cells and is essential for their maintenance during embryonic development. Up-regulated in proliferating neural progenitors after ischemia and in neural stem cells during their differentiation from embryonic stem cells [56]–[58]. | |
| Insulin-like growth factor binding protein 5 (IGFBP5) | 0.90±0.34 | 2.02±0.58# | Strongly enhances the activity of insulin-like growth factor which is a potent factor for neuron survival and genesis in neural tissue [59], [60]. | |
| Major vault protein (MVP) | 0.91±0.06* (n = 3) | 1.76±0.47# (n = 3) | An intracellular transport protein, highly expressed in developing neurons and possibly having the function of transporting substances including mRNA from the neuron soma to the synapse [61]. | |
| Midkine | 0.91±0.23 | 2.17±0.90# | A heparin-binding and retinoic acid inducible growth factor, promoting the growth of neural precursor cells, protecting neurons from NMDA agonist-induced injury, and ameliorating brain ischemic injury by promoting neuronal regeneration [62]–[64]. | |
| Potassium inwardly rectifying channel, subfamily J, member 13 (KCNJ13) | 0.77±0.29 | 4.38±2.55# | Mediates an intracellular potassium current, decreasing the membrane potential and inhibiting depolarization [65]. | |
| Retinoic acid induced 1 (RAI1) | 1.09±0.03** | 1.71±0.56# | A retinoic acid-inducible gene with an important role in the brain development, its loss results in defects in intelligence and locomotive activity, etc. [66]. | |
| Thyrotropin releasing hormone (TRH) | 0.79 (n = 1) | 3.70±2.87# | See discussion. | |
| Transgelin | 1.07±0.10 | 1.81±0.54# | An actin-binding protein, up-regulated in neuronal differentiation and regeneration [67], [68]. | |
| Vasoactive intestinal peptide receptor 2 (VIPR2) | 1.16±0.08* (n = 3) | 1.89±0.64# | Enhances the excitability of hippocampus CA1 neurons, protects neurons in excitotoxic injury, and promotes the proliferation of neural precursor cells [69]–[71]. | |
| Anti-oxidation | Carbonyl reductase 1 (CBR1) | 1.14±0.33 | 1.91±0.52# | See discussion. |
| Microsomal glutathione S-transferase 1 (MGST1) | 0.64±0.19* | 1.78±0.58# | Catalyzes the reduction of oxidants by glutathione and protects cells from multiple oxidant injuries [72]. | |
| Protein degradation | Angiotensin I converting enzyme (ACE) | 0.66±0.30 (n = 2) | 2.63±1.44# (n = 3) | See discussion. |
| RAD23 homolog B (S. cerevisiae) (RAD23B) | 1.59±0.37* | 2.69±0.64# | Transports proteins to the proteasome for degradation, possibly has a role in eliminating harmful proteins in neurodegenerative diseases [73]. | |
| Stress response | Cold inducible RNA binding protein (CIRBP) | 0.91±0.34 | 1.91±0.88# | A stress-responsible gene, induced by hypothermia, hypoxia, DNA damage, etc. [74]. Stabilizes specific transcripts required for cell survival and has a protective effect under various stresses [75], [76]. |
| Cholesterol metabolism | Lecithin cholesterol acyltransferase (LCAT) | 0.95±0.21 | 1.80±0.66# | Esterifies cholesterol and facilitates the efflux of cholesterol from the brain [77]. |
| Angiogenesis | Transmembrane 4 L six family member 5 (TM4SF5) | 1.06±0.15 | 1.63±0.27## | Facilitates angiogenesis [78]. |
| Apoptosis | Cyclin G1 | 1.72±0.71* (n = 3) | 0.57±0.18## | Facilitates neuronal apoptosis in AD [79]. |
Note: The table shows the expression data of the 19 genes that are significantly regulated by the CBJC treatment. The relative expression levels between the CBJC group and the IBO-model group, and between the IBO-model group and the control group of these 19 genes are shown (in the title line, M/C represents the ratio of the IBO-model group to the control group; CBJC/M represents the ratio of the CBJC group to the IBO-model group). The data are expressed as the means ± SD, n = 4 per group unless otherwise indicated. Analysis was conducted by using two-tailed t tests for independent-samples. *P<0.05, **P<0.01, IBO-model group vs. control group; # P<0.05, ## P<0.01, CBJC group vs. IBO-model group.
Verification for the Microarray Result by Western Blot
TRH, EGF, CRBP1, CBR1, and ACE were selected for verification by western blot because previous studies have shown that they are more important in preventing AD in comparison to the others in the 19 genes significantly regulated by CBJC. The results showed that the protein expression levels of these 5 genes were significantly increased in the CBJC group compared with the IBO-model group (P<0.05 or P<0.01, figure 5), which was consistent with the results of the DNA microarray.
Figure 5. Protein expression of CRBP1, EGF, TRH, CBR1, and ACE in the forebrain.
Representative western blots are shown on the left, the relative expression levels of the proteins of interest are shown on the right. The data are expressed as the means ± SD (n = 3 in each group. All the data were analyzed by one-way ANOVA followed by LSD post hoc test. *P<0.05, IBO-model group vs. control group; #P<0.05, ##P<0.01, CBJC group vs. model group).
Discussion
IBO is a potent NMDA receptor agonist that elicits severe injury and even death in neurons by inducing excessive calcium influx. Injecting IBO into the cerebroventricle leads to a direct exposure of the para-ventricular areas to this toxin; resulting in severe neuronal lesions in these regions, which may spread to the cerebral cortex. Intracerebral injection of IBO in animals such as rats and primates can elicit symptoms and pathological changes similar to those observed in human AD [7]–[9].
In the hidden platform and reverse platform tests, the escape latency time and the swim distance both significantly decreased as the training proceeded, indicating an ongoing spatial learning and memory in the rats; however, the extent of the decreases in the IBO-model group was significantly lower than that in the normal control and CBJC groups. As there was no significant difference in the swim speed among all the groups, this result indicates that the IBO injection elicited a significant impairment in spatial learning and memory and that this impairment was significantly ameliorated by the CBJC treatment. FDG-PET examination revealed a broad glucose-uptake decrease focused on the hippocampus, the basal nuclei, the cerebral cortex and the olfactory regions of the brain in the rats in the IBO-model group; this finding is indicative of hypofunction in the neurons in these regions, which is one of the most sensitive and characteristic features of AD [10]. CBJC significantly inhibited the decrease in the left olfactory brain and elicited a compensatory elevation in the left hippocampus. These two regions are both highly related to AD. The olfactory brain is the most sensitive region in AD, and it exhibits significant injuries even in very early stages of the disease [11]. The hippocampus is one of the pivotal regions of learning and memory, especially temporary memory, and its injury is recognized as a key procedure for AD [12]. Histological examination showed that the pathological changes induced by IBO in neurons in the hippocampus were inhibited by CBJC. These results indicate that CBJC is able to ameliorate the AD-like functional abnormalities and pathological changes in the IBO-injured rat model and therefore may be of use in retarding and even reversing the development of AD.
CRBP1 has a crucial role in maintaining retinoid contents; knockout of CRBP1 leads to an 80% decrease in retinoid contents in the body, while an up-regulation of CRBP1 results in a significant increase in body retinoid content [13], [14]. Interestingly, in the present study, besides CRBP1, the expressions of RAI1 and midkine were up-regulated by CBJC; they are both retinoid-inducible genes. These results strongly indicate that the content of retinoids in the forebrain might be significantly increased by the CBJC treatment, thus resulting in the activation of the retinoid pathway (the putative activation of CBJC on the retinoid pathway is shown in figure S1). The role of retinoids in AD is highlighted. Hypofunction of the retinoid pathway has been identified in the neocortex of AD patients and aged animals [15], [16]. The administration of retinoids shows a strong ameliorating effect on both transgenic and senile dementia mouse models [16], [17]. The anti-AD effect of retinoids is related to neuroprotection and neurogenesis. Retinol resists peroxidant injury in neurons and inhibits the formation and extension of Aβ in a cell-free system [18], [19]. More importantly, retinoids play a pivotal role in the modulation of neural precursor cells (NPCs). Retinoids are weak proliferating factors for NPCs; however, they are also strong neuronal differentiation-inducing factors [20], [21]. They elicit neuronal differentiation in a broad spectrum of naive cells including adult NPCs, neonatal neuroblasts, embryonic stem cells, and neural tumor cells [20]–[24]. In cultured NPCs, supplementation with retinoids can elevate the neuron differentiation ratio several times, even to nearly 100%, at the expense of glial differentiation [20]–[23].
EGF is a potent growth factor that is highly related to AD. The plasma content of EGF is significantly decreased in dementia patients, whereas supplementation with EGF strongly ameliorates cognitive defects in Aβ injured rats and senile rats [25]–[27]. One of the foundations of EGF’s anti-AD effect is its neuroprotective ability. EGF supports neuronal survival and outgrowth and protects neurons from multiple excitotoxic injuries [28]–[30]. Additionally, similarly to retinoids, EGF has an important role in the modulation of NPCs. The effects of EGF and retinoids are complementary. EGF is a potent proliferative factor for NPCs that strongly promote the mitogenesis of NPCs in vitro, in the normal adult brain, and in brains injured by Aβ, ischemia, and trauma [25], [31]–[35]; the differentiation-inducing effect of EGF is weak and exhibits a glial tendency [31], [32]. Interestingly, in the present study, the expression levels of EGF and CRBP1 were simultaneously increased by the CBJC treatment. This combined up-regulation might result in synergy in the re-genesis of neurons, with the up-regulation of EGF inducing more NPC proliferation and the up-regulation of CRBP1 increasing retinoid contents, which would lead to a higher neuronal differentiation ratio in the proliferated NPCs. The concurrent increase in the expression of EGF and CRBP1 might generate a greater rise in newborn neurons than could be achieved by any increase in the expression of either gene in isolation. The co-treatment of EGF and retinoids is a commonly used method for obtaining neurons from NPC [23], [33]; moreover, the combination of EGF and retinoids has been shown to have a synergic effect on neuron re-genesis in a neurodegenerative rat model [36].
TRH is a tripeptide that is distributed in neurons throughout the brain and acts as an endocrine hormone and a paracrine neuroregulator [37]. Administration of TRH to senescence-accelerated mice, fimbria-fornix lesioned rats, and AD patients was reported to ameliorate cognitive defects [38]–[40]. Moreover, the depletion of TRH in the culture medium of hippocampal neurons results in tau protein phosphorylation and neuron axonal retraction that is identical to the lesions observed in AD patients, indicating that the anti-AD effect of TRH is mainly related to its neuroregulatory role, which includes its ability to maintain neuron survival [41].
CBR1 strongly reduces the carbonyl in reactive lipid aldehydes, which are regarded as one of the most dangerous pathogenic factors in AD [42]. The overexpression of CBR1 prevents cells in culture from oxidant- and hypoxia-induced injuries [43], [44]; in Drosophila, overexpression of a human CBR1-resembled gene significantly attenuates neurodegeneration in the brain and the reduction in life span induced by oxidative stress, and prevents age-dependent defects in locomotor activity [45].
The effect of ACE on AD is to act as an Aβ-degrading protein, which is different from its traditional role in angiotensin metabolism. The genotype with a higher ACE expression was found at a significantly lower frequency in AD patients than in controls; in contrast, the genotype with a lower ACE expression was found at a significantly higher frequency [46]. Further studies found that Aβ deposition in the brain increased significantly when the activity of ACE was inhibited [47]. In vitro studies confirmed the ability of ACE to degrade Aβ and protect cells from Aβ-induced injury [48].
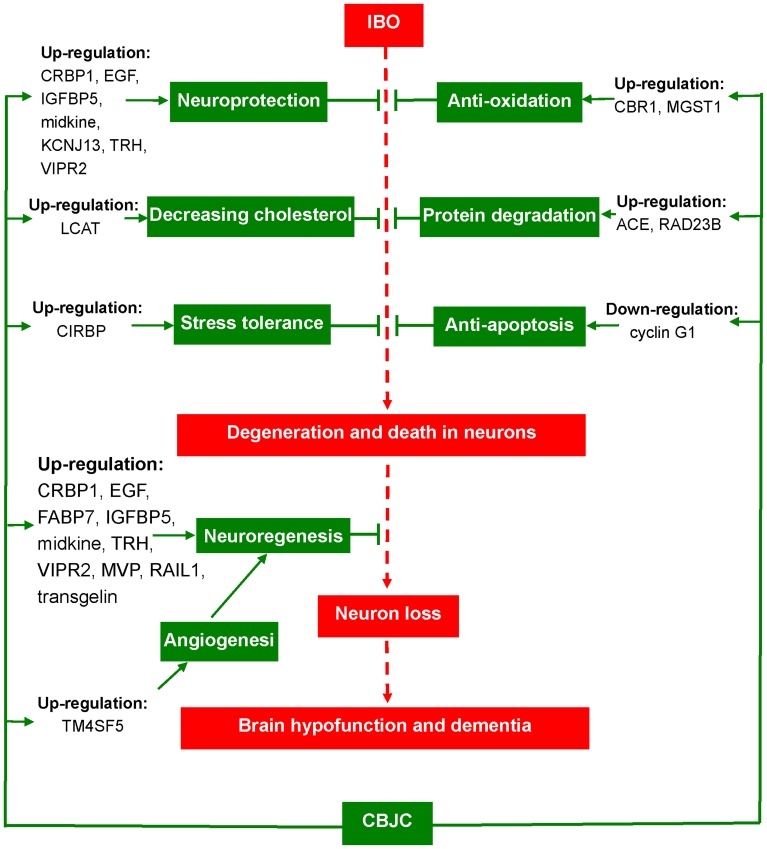
Generally, the gene expression profile showed that CBJC exerted its anti-AD effect by modulating a number of pathways in several AD-relevant areas, including neuroprotection and neurogenesis, oxidation, protein degradation, cholesterol metabolism, angiogenesis, stress, and apoptosis (the possible therapeutic mechanism of CBJC is summarized in figure 6). This multiple point-modulating capability is very suitable for treating complex diseases with multiple pathogenic factors, including AD. Pharmacodynamically, this capability is beneficial to achieve an amplified therapeutic effect through harmonious co-operation between the modulated points, such as the synergy of EGF and CRBP1 in the re-genesis of neurons.
Figure 6. An ideograph of the putative therapeutic mechanism of CBJC on the AD model induced by IBO.
Finally, the study raises the question of whether it is possible that CBJC can be further purified and replaced by one of its components. In our opinion, this outcome is unlikely as the 3 components of CBJC have distinct pharmacological characteristics. Baicalin has a strong anti-oxidation ability and a similar effect to retinoids in NPC modulation [49]–[51]; jasminoidin has a strong protective effect on neurons under a broad range of stresses [52]–[54]; and cholic acid strongly promotes the expression of growth factors in the brain [55]. Therefore, the combination could elicit all their effects together, acting like a poly-pill, and resulting in the multiple point-modulation that cannot be achieved by any single component treatment. CBJC has also shown a synergic effect on ischemic stroke [5].
Additionally, the result of DNA microarray offers a foundation and some clues for the future studies to further elucidate the mechanism of CBJC on AD. In these studies, the observations on the direct effects of CBJC on neurogenesis, neuronal oxidative stress, and Aβ degradation, etc. should be necessary.
In conclusion, the results of the present study suggest that CBJC is able to ameliorate the dementia induced by ibotenic acid in rats and may be of significant use in the treatment of AD. The therapeutic mechanism of CBJC may be related to its modulation of a number of pathways, principally involving the promotion of neuroprotection and neurogenesis, with additional involvement in anti-oxidation, protein degradation, etc.
Supporting Information
The activation of CBJC on the retinoid pathway inferred from the results of the present study. CBJC treatment leads to an increase in the expression of CRBP1, which next promotes the intracellular uptake of retinol through binding with CRBP1. The intracellular retinol is then metabolized into retinal and retinoic acid, and these three are called “retinoids” collectively. Retinoids can exert direct neuroprotective effects, such as anti-oxidation and inhibiting the extension Aβ. Retinoids can also form a complex with RAR (retinoic acid receptors) and/or RXR (retinoid X receptors), inducing the expressions of genes with the abilities of neuroprotection and neurogenesis, such as RAI1 and midkine, through interacting with DNA.
(TIF)
Funding Statement
This study was supported by the Fundamental Research Funds for the Central Universities (248-105102), Program for New Century Excellent Talents in University (NCET-10) and Project of Institute of Basic Clinical Medicine, China Academy of Chinese Medical Sciences (Z0175). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Klafki HW, Staufenbiel M, Kornhuber J, Wiltfang J (2006) Therapeutic approaches to Alzheimer’s disease. Brain 129: 2840–2855. [DOI] [PubMed] [Google Scholar]
- 2. Guo X, Lai S (2000) Meta-analysis of Qingkailing Injection for Treating Acute Stroke. Guang Zhou Zhong Yi Yao Da Xue Xue Bao 17: 9–14. [Google Scholar]
- 3. Lee K, Wang H, Itokawa H, Morris-Natschke SL (2000) Current perspectives on Chinese medicines and dietary supplements in China, Japan and the United States. Journal of Food and Drug Analysis 8: 219–228. [Google Scholar]
- 4. Luo GA, Liang QL, Zhang RL, Wang YM, Liu QF, et al. (2006) Study of chemieal matteromies and prescription of Traditional Chinese Medicine and an analysis of material foundation of compound prescription Qingkailing. Shi Jie Ke Xue Ji Shu – Zhong Yi Yao Xian Dai Hua 8: 6–15. [Google Scholar]
- 5.Zhang ZJ (2006) Research on pharmacodynamic characteristics analysis of genes’expression profile weighing the advantages and disadvantages of Chinese medicine prescription compatibility treating cerebral ischemia. Thesis, Beijing: Beijing University of Traditional Chinese Medicine 31–48.
- 6. Zhang ZJ, Wang Z, Li PT, Zhang WS, Li XY, et al. (2006) The pharmacodynamic evaluation of QKL combination herbs on focal cerebral ischemia-reperfusion injury. Zhong Guo Yao Li Xue Tong Bao 22: 964–967. [Google Scholar]
- 7. Ji C, Li Q, Aisa H, Yang N, Dong YL, et al. (2009) Gossypium herbaceam extracts attenuate ibotenic acid-induced excitotoxicity in rat hippocampus. J Alzheimer Dis 16: 331–339. [DOI] [PubMed] [Google Scholar]
- 8. Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, et al. (2000) Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci 20: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark RE, Zola SM, Squire LR (2000) Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20: 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordberg A, Jelic V, Arnáize E, Långström B, Almkvist O (2001) Brain functional imaging in early and preclinical Alzheimer’s disease. In: Iqbal K, Sisodia SS, Winblad B, editors. Alzheimer’s Disease: Advances in Etiology, Pathogenesis and Therapeutics. Chichester: John Wiley & Sons Ltd. 153–164.
- 11. Kovâcs T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. NeuroReport 12: 285–288. [DOI] [PubMed] [Google Scholar]
- 12. Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, et al. (2003) Amyloid deposition in the hippocampus and entorhinal cortex: Quantitative analysis of a transgenic mouse model. Proc Natl Acad Sci USA 100: 4837–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matt N, Schmidt CK, Dupé V, Dennefeld C, Nau H, et al. (2005) Contribution of cellular retinol-binding protein type 1 to retinol metabolism during mouse development. Dev Dyn 233: 167–176. [DOI] [PubMed] [Google Scholar]
- 14. Farias EF, Ong DE, Ghyselinck NB, Nakajo S, Kuppumbatti YS, et al. (2005) Cellular retinol-binding protein I, a regulator of breast epithelial retinoic acid receptor activity, cell differentiation, and tumorigenicity. J Natl Cancer Inst 97: 21–29. [DOI] [PubMed] [Google Scholar]
- 15. Corcoran JPT, So PL, Maden M (2004) Disruption of the retinoid signalling pathway causes a deposition of amyloid β in the adult rat brain. Eur J Neurosci 20: 896–902. [DOI] [PubMed] [Google Scholar]
- 16. Etchamendy N, Enderlin V, Marighetto A, Vouimba RM, Pallet V, et al. (2001) Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J Neurosci 21: 6423–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding Y, Qiao A, Wang ZQ, Goodwin JS, Lee ES, et al. (2008) Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer’s disease transgenic mouse model. J Neurosci 28: 11622–11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarang SS, Yoshida T, Cadet R, Valeras AS, Jensen RV, et al. (2002) Discovery of molecular mechanisms of neuroprotection using cell-based bioassays and oligonucleotide arrays. Physiol Genomics 11: 45–52. [DOI] [PubMed] [Google Scholar]
- 19. Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, et al. (2004) Vitamin A exhibits potent antiamyloidogenic and fibril-destabilizing effects in vitro. Exp Neurol 189: 380–392. [DOI] [PubMed] [Google Scholar]
- 20. Wang TW, Zhang H, Parent JM (2005) Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development 132: 2721–2732. [DOI] [PubMed] [Google Scholar]
- 21. Cui J, Yag W, Wang J, Li K, Wang J (2003) Influence of retinoic acid on proliferation and differentiation of neural stem cells from embryonic rat. Zhong Guo Zu Zhi Hua Xue Yu Xi Bao Hua Xue Za Zhi 12: 81–85. [Google Scholar]
- 22. Takahashi J, Palmer TD, Gage FH (1999) Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol 38: 65–81. [PubMed] [Google Scholar]
- 23. Christie VB, Maltmana DJ, Hendersona AP, Whiting A, Marder TB, et al. (2010) Retinoid supplementation of differentiating human neural progenitors and embryonic stem cells leads to enhanced neurogenesis in vitro. J Neurosci Meth 193: 239–245. [DOI] [PubMed] [Google Scholar]
- 24. Encinas M, Iglesias M, Liu Y, Wang HY, Muhaisen A, et al. (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 75: 991–1003. [DOI] [PubMed] [Google Scholar]
- 25. Zhao BQ, Guo YR, Li XL, Zang T, Qu HY, et al. (2011) Amelioration of dementia induced by Aβ22–35 through rectal delivery of undecapeptide-hEGF to mouse brain. Int J Pharm 405: 1–8. [DOI] [PubMed] [Google Scholar]
- 26. Chen-Plotkin AS, Hu WT, Siderowf A, Weintraub D, Gross RG, et al. (2011) Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol 69: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flore M, Triaca V, Amendola T, Tirassa P, Aloe L (2002) Brain NGF and EGF administration improves passive avoidance response and stimulates brain precursor cells in aged male mice. Physiol Behav 77: 437–443. [DOI] [PubMed] [Google Scholar]
- 28. Morrison RS, Keating RF, Moskal JR (1988) Basic fibroblast growth factor and epidermal growth factor exert differential trophic effects on CNS neurons. J Neurosci Res 21: 71–79. [DOI] [PubMed] [Google Scholar]
- 29. Abe K, Saito H (1992) Protective effect of epidermal growth factor on glutamate neurotoxicity in cultured cerebellar neurons. Neurosci Res 14: 117–123. [DOI] [PubMed] [Google Scholar]
- 30. Hicks D, Heidinger V, Mohand-Said S, Sahel J, Dreyfus H (1998) Growth factors and gangliosides as neuroprotective agents in excitotoxicity and ischemia. Gen Pharmacol 30: 265–273. [DOI] [PubMed] [Google Scholar]
- 31. Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH (1997) Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci 17: 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benoit BO, Savarese T, Joly M, Engstrom CM, Pang LZ, et al. (2001) Quesenberry, Neurotrophin channeling of neural progenitor cell differentiation. J Neurobiol 46: 265–280. [PubMed] [Google Scholar]
- 33. Marchal-Victorion S, Deleyrolle L, de Weille J, Saunier M, Dromard C, et al. (2003) The human NTERA2 neural cell line generates neurons on growth under neural stem cell conditions and exhibits characteristics of radial glial cells. Mol Cell Neurosci 24: 198–213. [DOI] [PubMed] [Google Scholar]
- 34. Yu J, Zeng J, Cheung RT, Xiong L, He MX, et al. (2009) Intracerebroventricular injection of epidermal growth factor reduces neurological deficit and infarct volume and enhances nestin expression following focal cerebral infarction in adult hypertensive rats. Clin Exp Pharmacol Physiol 36: 539–546. [DOI] [PubMed] [Google Scholar]
- 35. Laskowski A, Schmidt W, Dinkel K, Martinez-Sanchez M, Reymann KG (2005) bFGF and EGF modulate trauma-induced proliferation and neurogenesis in juvenile organotypic hippocampal slice cultures. Brain Res 1037: 78–89. [DOI] [PubMed] [Google Scholar]
- 36. Calzà L, Giuliani A, Fernandez M, Pirondi S, D’Intino G, et al. (2003) Neural stem cells and cholinergic neurons: Regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad Sci USA 100: 7325–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada M, Satoh T, Mori M (2003) Mice lacking the thyrotropin-releasing hormone gene: what do they tell us? Thyroid 13: 1111–1121. [DOI] [PubMed] [Google Scholar]
- 38. Mellow AM, Sunderland T, Cohen RM, Lawlor BA, Hill JL, et al. (1989) Acute effects of high-dose thyrotropin releasing hormone infusions in Alzheimer’s disease. Psychopharmacology 98: 403–407. [DOI] [PubMed] [Google Scholar]
- 39. Miyamoto M, Hirai K, Heya T, Nagaoka A (1994) Effects of a sustained release formulation of thyrotropin-releasing hormone on behavioral abnormalities in senescence-accelerated mice. Eur J Pharmacol 271: 357–366. [DOI] [PubMed] [Google Scholar]
- 40. Bennett GW, Ballard TM, Watson CD, Fone KC (1997) Effect of neuropeptides on cognitive function. Exp Gerontol 32: 451–469. [DOI] [PubMed] [Google Scholar]
- 41. Luo LG, Yano N, Mao QF, Jackson IMD, Stopa EG (2002) Thyrotropin releasing hormone (TRH) in the hippocampus of Alzheimer patients. J Alzheimers Dis 4: 97–103. [DOI] [PubMed] [Google Scholar]
- 42. Maser E (2006) Neuroprotective role for carbonyl reductase? Biochem Biophy Res Co 340: 1019–1022. [DOI] [PubMed] [Google Scholar]
- 43. Rashid MA, Lee S, Tak E, Lee J, Choi TG, et al. (2010) Carbonyl reductase 1 protects pancreatic β-cells against oxidative stress-induced apoptosis in glucotoxicity and glucolipotoxicity. Free Radical Bio Med 49: 1522–1533. [DOI] [PubMed] [Google Scholar]
- 44. Tak E, Lee S, Lee J, Rashid MA, Kim YW, et al. (2011) Human carbonyl reductase 1 upregulated by hypoxia renders resistance to apoptosis in hepatocellular carcinoma cells. J Hepatol 54: 328–339. [DOI] [PubMed] [Google Scholar]
- 45. Botella JA, Ulschmid JK, Gruenewald C, Moehle C, Kretzschmar D, et al. (2004) The Drosophila carbonyl reductase sniffer prevents oxidative stress-induced neurodegeneration. Curr Biol 14: 782–786. [DOI] [PubMed] [Google Scholar]
- 46. Kehoe PG, Russ C, McIlory S, Williams H, Holmans P, et al. (1999) Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer disease. Nat Genet 21: 71–72. [DOI] [PubMed] [Google Scholar]
- 47. Zou K, Yamaguchi H, Akatsu H, Sakamoto T, Ko M, et al. (2007) Angiotensin-converting enzyme converts amyloid β-protein 1–42 (Aβ1–42) to Aβ1–40, and its inhibition enhances brain Aβ deposition. J Neurosci 27: 8628–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu J, Igarashi A, Kamata M, Nakagawa H (2001) Angiotensin-converting enzyme degrades Alzheimer amyloid β-peptide (Aβ); retards Aβ aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem 276: 47863–47868. [DOI] [PubMed] [Google Scholar]
- 49. Kim DH, Kim HK, Park S, Kim JY, Zou Y, et al. (2006) Short-term feeding of baicalin inhibits age-associated NF-kB activation. Mech Ageing Dev 127: 719–725. [DOI] [PubMed] [Google Scholar]
- 50. Heo H, Shin Y, Cho W, Choi YS, Kim H, et al. (2009) Memory improvement in ibotenic acid induced model rats by extracts of Scutellaria baicalensis. J Eth Pharm 122: 20–27. [DOI] [PubMed] [Google Scholar]
- 51. Li M, Tsang KS, Choi ST, Li K, Shaw PC, et al. (2011) Neuronal differentiation of C17.2 neural stem cells induced by a natural flavonoid, baicalin. Chembiochem 12: 449–456. [DOI] [PubMed] [Google Scholar]
- 52. Tanaka M, Yamazaki M, Chiba K (2009) Neuroprotective action of genipin on tunicamycin-induced cytotoxicity in neuro2a cells. Biol Pharm Bull 32: 1220–1223. [DOI] [PubMed] [Google Scholar]
- 53. Yamazaki M, Chiba K, Yoshikawa C (2009) Genipin suppresses A23187-induced cytotoxicity in neuro2a cells. Biol Pharm Bull 32: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 54. Koriyama Y, Chiba K, Yamazaki M, Suzuki H, Muramoto K, et al. (2010) Long-acting genipin derivative protects retinal ganglion cells from oxidative stress models in vitro and in vivo through the Nrf2/antioxidant response element signaling pathway. J Neurochem 115: 79–91. [DOI] [PubMed] [Google Scholar]
- 55. Zhong XG, Li PT, Wang YY (2004) Influence of the effective fractions of Qingkailing on the levels of neurotrophic factors in the brain of the rat with cerebral ischemia. Bei Jing Zhong Yi Yao Da Xue Xue Bao 27: 21–24. [Google Scholar]
- 56. Arai Y, Funatsu N, Numayama-Tsuruta K, Nomura T, Nakamura S, et al. (2005) Role of Fabp7, a downstream gene of Pax6, in the maintenance of neuroepithelial cells during early embryonic development of the rat cortex. The Journal of neuroscience 25: 9752–9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boneva NB, Kaplamadzhiev DB, Sahara S, Kikuchi H, Pyko IV, et al. (2011) Expression of fatty acid-binding proteins in adult hippocampal neurogenic niche of postischemic monkeys. Hippocampus 21: 162–171. [DOI] [PubMed] [Google Scholar]
- 58. Akama K, Horikoshi T, Nakayama T, Otsu M, Imaizumi N, et al. (2011) Proteomic identification of differentially expressed genes in neural stem cells and neurons differentiated from embryonic stem cells of cynomolgus monkey (Macaca fascicularis) in vitro. Biochimica et Biophysica Acta (BBA)-Proteins & Proteomics 1814: 265–276. [DOI] [PubMed] [Google Scholar]
- 59. Beattie J, Allan GJ, Lochrie JD, Flint DJ (2006) Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochemical Journal 395: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hauck SM, Gloeckner CJ, Harley ME, Schoeffmann S, Boldt K, et al. (2008) Identification of paracrine neuroprotective candidate proteins by a functional assay-driven proteomics approach. Molecular & Cellular Proteomics 7: 1349–1361. [DOI] [PubMed] [Google Scholar]
- 61. Paspalas CD, Perley CC, Venkitaramani DV, Goebel-Goody SM, Zhang YF, et al. (2009) Major Vault Protein is Expressed along the Nucleus–Neurite Axis and Associates with mRNAs in Cortical Neurons. Cerebral Cortex 19: 1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salama RHM, Muramatsu H, Zou P, Okayama M, Muramatsu T (2006) Midkine, a heparin-binding growth factor, produced by the host enhances metastasis of Lewis lung carcinoma cells. Cancer letters 233: 16–20. [DOI] [PubMed] [Google Scholar]
- 63. Kim YB, Ryu JK, Lee HJ, Lim IJ, Park D, et al. (2010) Midkine, heparin-binding growth factor, blocks kainic acid-induced seizure and neuronal cell death in mouse hippocampus. BMC neuroscience 11: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ishikawa E, Ooboshi H, Kumai Y, Takada J, Nakamura K, et al. (2009) Midkine gene transfer protects against focal brain ischemia and augments neurogenesis. Journal of the neurological sciences 285: 78–84. [DOI] [PubMed] [Google Scholar]
- 65. Pattnaik BR, Hughes BA (2009) Regulation of Kir channels in bovine retinal pigment epithelial cells by phosphatidylinositol 4, 5-bisphosphate. American Journal of Physiology-Cell Physiology 297: C1001–C1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bi W, Yan J, Shi X, Yuva-Paylor LA, Antalffy BA, et al. (2007) Rai1 deficiency in mice causes learning impairment and motor dysfunction, whereas Rai1 heterozygous mice display minimal behavioral phenotypes. Human molecular genetics 16: 1802–1813. [DOI] [PubMed] [Google Scholar]
- 67. Hoffrogge R, Mikkat S, Scharf C, Beyer S, Christoph H, et al. (2006) 2-DE proteome analysis of a proliferating and differentiating human neuronal stem cell line (ReNcell VM). Proteomics 6: 1833–1847. [DOI] [PubMed] [Google Scholar]
- 68. Xiao L, Ma Z, Li X, Lin Q, Que H, et al. (2005) cDNA microarray analysis of spinal cord injury and regeneration related genes in rat. ACTA PHYSIOLOGICA SINICA-CHINESE EDITION- 57: 705. [PubMed] [Google Scholar]
- 69. Cunha-Reis D, Ribeiro JA, Sebastião AM (2006) VPAC2 receptor activation mediates VIP enhancement of population spikes in the CA1 area of the hippocampus. Annals of the New York Academy of Sciences 1070: 210–214. [DOI] [PubMed] [Google Scholar]
- 70. Rangon CM, Goursaud S, Medja F, Lelièvre V, Mounien L, et al. (2005) VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice. Journal of Pharmacology and Experimental Therapeutics 314: 745–752. [DOI] [PubMed] [Google Scholar]
- 71. Zaben M, John Sheward W, Shtaya A, Abbosh C, Harmar AJ, et al. (2009) The neurotransmitter VIP expands the pool of symmetrically dividing postnatal dentate gyrus precursors via VPAC2 receptors or directs them toward a neuronal fate via VPAC1 receptors. Stem Cells 27: 2539–2551. [DOI] [PubMed] [Google Scholar]
- 72. Johansson K, Järvliden J, Gogvadze V, Morgenstern R (2010) Multiple roles of microsomal glutathione transferase 1 in cellular protection: a mechanistic study. Free Radical Biology and Medicine 49: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 73. Bergink S, Severijnen LA, Wijgers N, Sugasawa K, Yousaf H, et al. (2006) The DNA repair-ubiquitin-associated HR23 proteins are constituents of neuronal inclusions in specific neurodegenerative disorders without hampering DNA repair. Neurobiology of disease 23: 708–716. [DOI] [PubMed] [Google Scholar]
- 74. Fujita J (1999) Cold shock response in mammalian cells. J Mol Microbiol Biotechnol 1: 243–255. [PubMed] [Google Scholar]
- 75. Wellmann S, Bührer C, Moderegger E, Zelmer A, Kirschner R, et al. (2004) Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. Journal of cell science 117: 1785–1794. [DOI] [PubMed] [Google Scholar]
- 76. Sakurai T, Itoh K, Higashitsuji H, Nonoguchi K, Liu Y, et al. (2006) Cirp protects against tumor necrosis factor-α-induced apoptosis via activation of extracellular signal-regulated kinase. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1763: 290–295. [DOI] [PubMed] [Google Scholar]
- 77. Zannis VI, Koukos G, Drosatos K, Vezeridis A, Zanni EE, et al. (2008) Discrete roles of apoA-I and apoE in the biogenesis of HDL species: lessons learned from gene transfer studies in different mouse models. Annals of medicine 40: 14–28. [DOI] [PubMed] [Google Scholar]
- 78.Lee JW, Choi S, Lee S, Kwak TK, Kim HJ, et al. (2009) Cooperation between integrin alpha5 and tetraspan TM4SF5 regulates VEGF-mediated angiogenic activity. [DOI] [PubMed]
- 79. Sultana R, Butterfield DA (2007) Regional expression of key cell cycle proteins in brain from subjects with amnestic mild cognitive impairment. Neurochemical research 32: 655–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The activation of CBJC on the retinoid pathway inferred from the results of the present study. CBJC treatment leads to an increase in the expression of CRBP1, which next promotes the intracellular uptake of retinol through binding with CRBP1. The intracellular retinol is then metabolized into retinal and retinoic acid, and these three are called “retinoids” collectively. Retinoids can exert direct neuroprotective effects, such as anti-oxidation and inhibiting the extension Aβ. Retinoids can also form a complex with RAR (retinoic acid receptors) and/or RXR (retinoid X receptors), inducing the expressions of genes with the abilities of neuroprotection and neurogenesis, such as RAI1 and midkine, through interacting with DNA.
(TIF)