Abstract
Cytochrome oxidase subunit 3 (Cox3) is a mitochondrion-encoded core membrane protein of complex IV of the mitochondrial respiratory chain, and consists of seven trans-membrane helices. Here we show that in diverse later-branching dinoflagellates, cox3 is consistently split into two exons in the mitochondrial genome between helices six and seven. Gene exons are transcribed as two discrete oligoadenylated precursor RNAs, and these are subsequently trans-spliced to form a complete coding mRNA. This trans-splicing is highly unusual in that some of the oligoadenylated tail is incorporated at the splice site, such that a short string of adenosines links the two coding exons. This feature is consistently represented in diverse dinoflagellates, however the number of adenosines added varies according to the size of the coding gap between the two exons. Thus we observed between zero (Amphidinium carterae) and 10 (Symbiodinium sp.) adenosines added in different taxa, but the final coding sequence length is identical with the reading frame maintained. Northern analyses show that precursor cox3 transcripts are approximately equally abundant as mature cox3 mRNAs, suggesting a slow or regulated maturation process. These data indicate that the splicing mechanism in dinoflagellate mitochondria is tolerant of variations in the length of the precursor coding sequence, and implicates the use of a splicing template, or guide molecule, during splicing that controls mature mRNA length.
Introduction
The expression pathway from gene to protein is not always a simple one. One of the most common elaborations on the gene→transcript→protein dogma is the presence of introns which break up otherwise contiguous coding sequences within a genome, and which must be removed by cis-splicing of the gene transcript [1]. A rarer form of gene interruption is when gene exons are more distantly separated on the genome and/or encoded on opposite strands, dictating that individual exons are separately transcribed. In these cases, re-constitution of complete coding mRNAs requires a process of trans-splicing of the transcript exons.
In organelles (plastids and mitochondria), the most prevalent form of RNA trans-splicing known occurs via discontinuous group I and II introns. These two intron families differ in the chemistry of their splicing reactions, but in both cases splicing involves the formation of a catalytic secondary structure by the intron sequence itself [2], [3]. Thus, for discontinuous introns inter-molecular base pairing of the partial group I or II intron sequences regenerates the required catalytic function, enabling the trans-splicing of exons. Most known organelle trans-splicing examples involve group II introns in the chloroplasts and mitochondria of plants and green algae, but discontinuous group I introns have also been reported from plants and early-branching animals (placazoans) [4]–[6]. Nucleus-encoded genes can also undergo trans-splicing, and two broad splicing categories can be defined: the joining of separate protein-coding exons via fragmented spliceosomal introns, and the splicing of a short UTR exon onto the 5′ end of gene transcripts (spliced-leader (SL) trans-splicing) [1]. The former type is found rarely, with examples from Drosophila [7] and the protist Giardia intestinalis [8], [9], whereas SL splicing is found broadly in eukaryotes including many metazoans, as well as protists such as dinoflagellates, diplonemids, and kinetoplastids [10]–[12]. Both nuclear trans-splicing types rely on elements of the same spliceosomal machinery involved in classical intron removal via cis-splicing [12]. In dinoflagellates, SL trans-splicing occurs throughout dinoflagellate diversity, including the basal species Hematodinium sp., Oxyrrhis marina and Perkinsus marinus [13]–[15]. The dinoflagellate SL transcript is ∼50–60 nucleotides long, and contains a 22-nucleotide exon at the 5′ end as well as downstream intron sequence. A conserved spliceosomal binding site occurs in the exon sequence, and the trans-splicing reaction apparently utilizes canonical GU-AG intron boundaries, with the GU donor dinucleotide encoded on the SL transcript intron. Dinoflagellate SL splicing is thought to be catalysed by components of the nuclear spliceosome [11]. Yet another type of trans-splicing occurs in the tRNA genes of Archaea, and involves reconstitution of introns characterised by a bulge-helix-bulge (B-H-B) motif at the intron-exon junctions; unlike the cases above, removal of B-H-B introns requires an endonuclease and a ligase [16].
Recently a further example of RNA trans-splicing has emerged, occurring in the mitochondrion of the dinoflagellate Karlodinium veneficum (synonym: K. micrum) [17], [18]. While dinoflagellate mitochondrial genomes are among the smallest known in terms of gene content, encoding a paltry three proteins, these genomes are otherwise highly complex. The genes occur in multiple copies including numerous and variously fragmented forms, suggesting a genome that is highly recombinatorial [18], [19]. For one of the K. veneficum mitochondrial genes, cox3, no intact gene remains on this genome. Despite this, complete transcripts of cox3 have been detected as oligoadenylated cDNAs, implying that the cox3 gene exons are transcribed and trans-spliced together to generate a complete mRNA [17]. Consistent with this, transcriptome data additionally reveal an oligoadenylated but truncated transcript encoding the first 85% (nucleotides 1–731) of this gene, corresponding to the largest cox3 gene fragment found in the genome. The remainder of cox3 occurs as a separate gene fragment (nucleotides 737–858), and a transcript of this fragment was presumed to complete the mRNA [17], [18]. Two features of this trans-splicing case are unusual: 1) no genomic sequence around the splice sites could be identified that could participate in a known splicing reaction such as group I/II intron fragments, or bulge-helix-bulge formation; and 2) five, non-encoded adenosine nucleotides bridge the gap in cox3 transcripts between the two gene exons (nts 1–731, 737–858), presumably donated from the oligoadenosine tail of the 731-nucleotide transcript [17]. In this report we describe an unusual partial conservation of this splicing reaction seen across diverse dinoflagellates that provides insight into the novelty of this splicing mechanism.
Methods
Cell Culture, Nucleic acid Extraction, cRT-PCR
Karlodinium veneficum (strain CCMP415), Alexandrium catenella (strain ACPP01), Amphidinium carterae (strain CCMP121) and Symbiodinium sp. (strain Tc 13) were cultured in Guilard’s f2 media at 16°C (K. veneficum and A. carterae) or 25°C (Symbiodinium sp. and A. catenella) on a 12-h light/12-h dark cycle. Cells were harvested by centrifugation (10 min, 2,600 g), and total RNA was extracted using Trizol (Invitrogen). For each species, ∼750 ng of otherwise untreated total RNA was treated with DNase I (Invitrogen). Subsequently, each RNA sample was ligated head to tail using an RNA ligase (Promega), according to the manufacturer’s instructions in a total volume of 40 µL (∼10 µL DNAse-treated RNA sample, 20 µL PEG 8000, 4 µL T4 RNA ligase buffer, 1 µL RNasin® Ribonuclease Inhibitor, 1 µL/10 units T4 RNA ligase, 4 µL nuclease-free water, incubated at 37°C for 30 mins). First strand cDNA synthesis across the ligated mRNA ends was performed for cox3 using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions, using 10 µL of ligated RNA as template for each 20 µL reaction (primer for K. veneficum cox3H7: KVcox3H7rev (AACTCTTAAATTTAAAAACCAAAC); Symbiodinium sp. and A. catenella cox3H7: SspAcatcox3H7rev (GATTATAAAATAAATGAACTTCTGA); A. carterae cox3H7: Acarcox3H7rev (CAAGCAAAAAATAAATGTACTTCTG); K. veneficum, Symbiodinium sp. cox3H1-6: KVcox3H1-6rev (AGACAAAATGCACCTGATGC); A. catenella cox3H1-6: Acatcox3H1-6rev (AATCTGATGCAACTTCCAGATG); A. carterae cox3H1-6: Acarcox3H1-6rev (GCAAAATACATAGAATAAAACAGG). Subsequently, PCR was performed with Phusion ® High-Fidelity DNA polymerase (NEB) (2 µL cDNA template, initial denaturation 98°C 2 mins, then 35 cycles of 98°C 30 secs, 55°C 30 secs, 72°C 1 min ) using primers directed outward toward the gene termini (K. veneficum cox3H7: KVcox3H7rev and KVcox3H7for (AATCTTATGGTTATTTATCTTTC); Symbiodinium sp. and A. catenella cox3H7: SspAcatcox3H7rev and SspAcatcox3H7for (AATTTCTATTGGCATTTTCTTG) or Kvcox3H7for (for A. catenella only); K. veneficum, Symbiodinium sp. cox3H1-6: KVcox3H1-6rev and KVcox3H1-6for (TTTCTTTCATCTTGTCGTTGG); A. catenella coxH1-6: Acatcox3H1-6rev and KVcox3H1-6for; A. carterae cox3H1-6: Acarcox3H1-6rev and Acarcox3H1-6for (TTTCTTTCACCTTATTGTTGG); A. carterae cox3H7: Acarcox3H7rev and Acarcox3H1-6for (TTTATTGGCATTTTGTTGAGG). As primers to cox3 precursors also bound to full-length cox3 transcripts, gels of cRT-PCR products contained larger bands corresponding to head-to-tail ligated full-length cox3 molecules, with sequence spanning the splice site. For A. catenella and A. carterae these larger bands were cloned, whereas cDNAs for K. veneficum cox3 (strain CCMP415) were available from a previously constructed cDNA library [20]. PCR products were ligated into the pGEM T-easy vector (Promega), cloned, and fully sequenced.
Northern Blot Analysis
Hybridization probe templates for K. veneficum cox3H1-6 and cox3H7 were generated using PCR from a full-length cDNA cloned into pGEM-T Easy vector (cox3H1-6 primers: KvH1-6ProbeF (AGTATTCATCAGGAAGTTGC) and KvH1-6ProbeR (TTAGAAGAAGAAGACCAACGAC); cox3H7 primers: KvH7ProbeF (TTGGTTTTTAAATTTAAGAG) and KvH7ProbeR (ATAACGAGTAAAGGAATAGAAAG). PCR fragments were purified from gels and random hexamer-based probes were constructed using the Prime-a-gene labeling system (Promega) and 32P-labeled dATP, according to the manufacturer’s instructions. Total RNA (5µg per lane) was separated on a 4% polyacrylamide/urea gel (per 5 mL of gel solution: 0.5 mL 10X Tris/Borate/EDTA buffer, 3.5 mL 10M urea, 0.5 mL 40% 19∶1 Acrylamide/Bis solution, 50 µL 10% ammonium persulphate, 450 µL water, 5 µL TEMED) at 150V in 1X TBE running buffer (Mini-Protean® 3 Cell, Biorad). Separated RNA was transferred to Hybond N+ membrane (GE Healthcare) via electroblotting with 0.5X TBE transfer buffer, at 80 volts for 1 hour at 4°C, (Mini Trans-Blot® Electrophoretic Transfer Cell, Biorad), and RNA was cross-linked by UV irradiation. Membrane blocking was performed with modified Churches buffer (51 g Na2HPO4.2H20, 16.8 g anhydrous NaH2PO4, 4 ml of 0.5 M ethylenediaminetetraacetic acid, and 70 g SDS per liter) for 2 hours at 65°C. Probe hybridization was performed overnight at 65°C in modified Churches buffer. Following hybridization, membranes were washed twice for 5 min each in 4×SSC +0.5% SDS then with the following series: 2×SCC +0.5% SDS; 4×SSC +0.5% SDS; 2×SCC +0.5% SDS; 4×SSC +0.5% SDS. All wash steps were carried out for 1 h at 65°C. Membranes were visualized using X-ray film, (exposure time ∼1 hour).
Results and Discussion
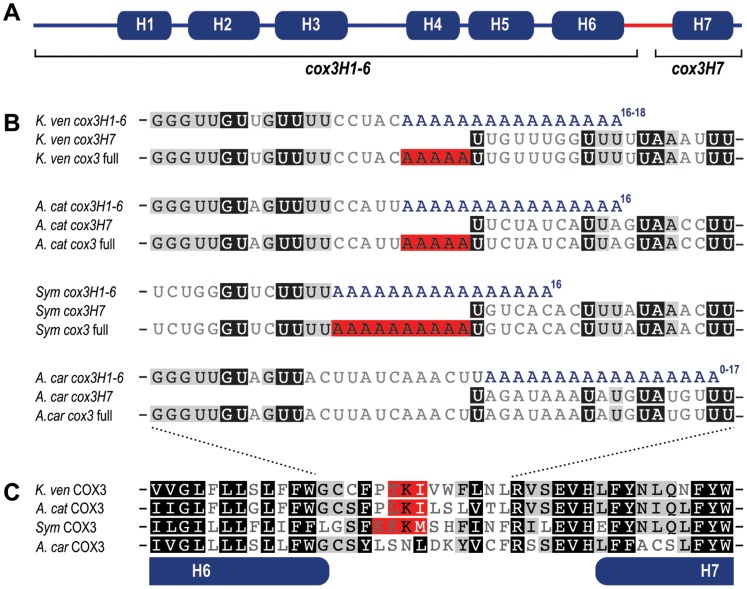
The cox3 gene codes for cytochrome oxidase subunit 3 (Cox3) of complex IV of the mitochondrial electron transport chain. The majority of this membrane protein is made up of seven trans-membrane spanning helices (Fig. 1A) [21]. The break in coding sequence in K. veneficum cox3 occurs between transmembrane helices six and seven, so we define the two gene exons as cox3H1-6 (helix 1 to 6), and cox3H7 (helix 7). To unambiguously characterise the length and sequence of precursor transcripts from these two genes, and the resultant full-length cox3 transcript, we used circular reverse transcription PCR (cRT-PCR) [22]. This technique uses RNA ligase to circularise RNA molecules harvested from cells, and then outward-orientated primers are used to RT-PCR amplify and sequence the joined ends. The presence of 3′ oligoadenylation enables the 3′-terminus of the transcript to be identified where it joins the 5′-terminus. Multiple, independent cRT-PCR generation of cox3H1-6, cox3H7, and cox3 transcripts confirmed that this technique faithfully identifies the mRNA ends (Data S1).
Figure 1. cox3 trans-splicing in diverse dinoflagellates.
A. Schematic of dinoflagellate Cox3 showing seven predicted trans-membrane helices encoded by fragmented cox3 coding sequences cox3H1-6 and cox3H7. B. Alignment of nucleotide sequence at the splice site of transcript precursors cox3H1-6 and cox3H7, and the splice product cox3. Corresponding sequences are shown for Karlodinium veneficum (K. ven), Alexandrium catenella (A. cat), Symbiodinium sp. (Sym) and Amphidinium carterae (A. car). The range of lengths observed for oligoadenylated tails on cox3H1-6 is shown in superscript. Red highlighting indicates A nucleotides from the oligoadenylated tail incorporated into the cox3 splice product. C. Dinoflagellate Cox3 amino acid sequence alignment at the splice site between helices 6 and 7. Amino acid codons determined by inclusion of oligoadenylation nucleotides are shown with red highlighting.
These cRT-PCR data revealed that precursor transcripts cox3H1-6 and cox3H7 correspond precisely to the respective sequence components of the complete cox3 transcript. The 5′ end of cox3H1-6 is exactly the same length as cox3, and the 5′ end of cox3H7 ends at the nucleotide 737, the exact position where it is subsequently joined to the cox3H1-6 transcript (Fig. 1B). The 3′ end of cox3H1-6 is oligoadenylated at position 731 (as previously described; Fig. 1B), and cRT-PCR shows that it receives between 16–18 A nucleotides. The 3′ end of cox3H7 matches the full-length cox3 end precisely in sequence and oligoadenylation site, and both bear 13–16 A nucleotides. These data suggest that the dominant precursor species contain only sequence that will be incorporated into the complete cox3 mRNA.
To explore the novelty of this trans-splicing process seen in K. veneficum, we have examined transcripts of cox3 in three further dinoflagellate taxa - Alexandrium catenella, Symbiodinium sp., and Amphidinium carterae - that represent a broad range of dinoflagellate diversity. cRT-PCR was used to recover transcripts of cox3 sequence and to characterise their lengths and transcript termini (Fig. 1B). Similar to K. veneficum, all new taxa show evidence of trans-splicing by the presence of truncated transcripts equivalent to cox3H1-6 and cox3H7, as well as a full-length cox3. The 5′ end of cox3H7 is conserved in length in all four taxa, despite sequence variation in the first eight nucleotides (Fig. 1B, Data S1). In all cases splicing occurs directly onto the first nucleotide of this transcript, which is a U in every case. The 3′ boundary of cox3H1-6, however, is variable. While A. catenella cox3H1-6 is oligoadenylated at precisely the same position as K. veneficum, Symbiodinium sp. is oligoadenylated at a position five nucleotides earlier, and A. carterae six nucleotides later (Fig. 1B). This variation, however, does not affect the mature cox3 length. The five nucleotide coding gap in A. catenella is filled with five A nucleotides exactly as for K. veneficum, presumably from the oligoadenosine tail. In Symbiodinium sp. the gap of 10 nucleotides is filled with 10 A nucleotides. In A. carterae, where no coding gap exists, splicing occurs one nucleotide upstream of the oligoadenosine tail so no non-coded A nucleotides are included (Fig. 1B). The length of oligoadenylation observed for all taxa and all cox3 products is similar, typically ranging from 12–19 nucleotides. For cox3H1-6 this is sufficient to span the respective coding gaps between exons.
The sequence termini of cox3 precursor transcripts and positions of oligoadenylation seen in the cRT-PCR data are corroborated by available transcriptome data. For example, the cox3H1-6 oligoadenylation sites (Fig. 1B) are identical in K. veneficum EST sequences [17], and from Symbiodinium sp. eight ESTs precisely match the cox3H7 5′ sequence (accessions; FE537727, FE537728, FE537811, FE537812, FE537869, FE537870, FE538147, FE538148). We did, however, recover some cRT-PCR data that showed some termini variation (Data S1). In K. veneficum cox3H7, two of six independent cRT-PCR products bore an additional 15 nucleotides at the 5′ terminus (UUCCAAGAAAAGCCU). This extra tag lacks any complementarity with cox3 coding sequence, BLAST searches did not recover matches to K. veneficum mitochondrial genomic sequence [17], and RT-PCR could not reproduce a cox3H7 fragment linked to this extension. Similarly, in Symbiodinium sp., one of seven cox3H7 amplicons is 5′ truncated by 10 nucleotides, relative to the other six sequences. These data are consistent with previous evidence of dinoflagellate mitochondrial transcripts occasionally occurring either fused to unrelated sequence, or truncated [23], and likely represent non-functional transcript species. In A. carterae, of three cox3H1-6 cRT-PCR amplicons, one lacks an oligo-A tail, and another is oligoadenylated one nucleotide earlier (c.f. Fig. 1B). Neither of these two variations would directly affect the sequence of complete cox3 as they occur downstream of the splice site, and therefore such variation in A. carterae might be tolerated.
Post-transcriptional RNA end capping has been described in some dinoflagellate organelles, but we observe no evidence of such modification to any of the cox3 transcripts. In the deep-branching dinoflagellate Oxyrrhis marinus 5′ capping by addition of 8–9 U nucleotides to mitochondrial protein-encoding transcripts has been reported, and in dinoflagellate plastids mRNAs gain 3′ polyuridine tracts of up to 40 nucleotides after transcription [24]–[26]. Both of these additions are detectible by cRT-PCR [24], [26], but were not observed in cox3 transcripts for any of the taxa examined. Further capping reactions that modify the 5′-phosphate group on RNA molecules, such as the modified guanine nucleotide added to the 5′ end of most eukaryotic nuclear transcripts [27], would prevent RNA ligation and detection by cRT-PCR. While such capping is not known from either bacteria or mitochondria, it remains possible that further cox3 transcript species might exist in addition to those detected by cRT-PCR and transcriptomics approaches.
To examine the relative abundance of cox3H1-6 and cox3H7 transcripts in comparison to full-length cox3 in dinoflagellate mitochondria, we performed Northern blot analysis of K. veneficum total RNA. Probes were made corresponding to either cox3H1-6 or cox3H7. Each would therefore detect the respective precursor and also the full-length cox3 transcript, enabling relative steady-state quantitation of these species before and after splicing. Indeed, two bands were detected in Northern blots for each probe, and in each case these bands corresponded in size to the respective precursor and full-length cox3 (Fig. 2, arrowheads). The two bands detected by cox3H1-6 are of approximately equal abundance, whereas the cox3H7 precursor band is even more abundant than the full-length band detected by this probe. Together, these Northern blots indicate that rather than precursor transcripts being very minor components of the total RNA pool, they are present in similar amounts to full length cox3 mRNA. The high relative abundance of precursors suggests either a slow rate of trans-splicing, or a regulated process that maintains a large pool of precursors. We tested to see if compounds that are known to perturb mitochondrial electron transport (antimycin A and Salicylhydroxamic Acid (SHAM) [28]), would lead to changes in the relative abundances of cox3 precursors, but found no evidence of such regulation in these experiments (not shown). A further result of the Northern blots was lack of evidence of additional cox3 size species as prevalent transcripts. Polycistronic transcript sequence has previously been detected in dinoflagellate mitochondria [17], [23], [29], [30], and generation of large transcripts from few promoters is quite common in mtDNAs where large precursor RNA molecules are processed to generate individual gene transcripts [31]. If cox3 precursor transcripts are similarly generated by processing large polycistronic transcripts, then processing to the final precise lengths must be fast enough that little intermediate is evident by Northern blot detection or sequencing methods described above. Alternatively, it is possible that the cox3 precursors could be transcribed as their final lengths; we presently have no data that can discern between these scenarios.
Figure 2. Northern blot analysis of K. veneficum cox3H1-6, cox3H7 and full-length cox3 transcripts.
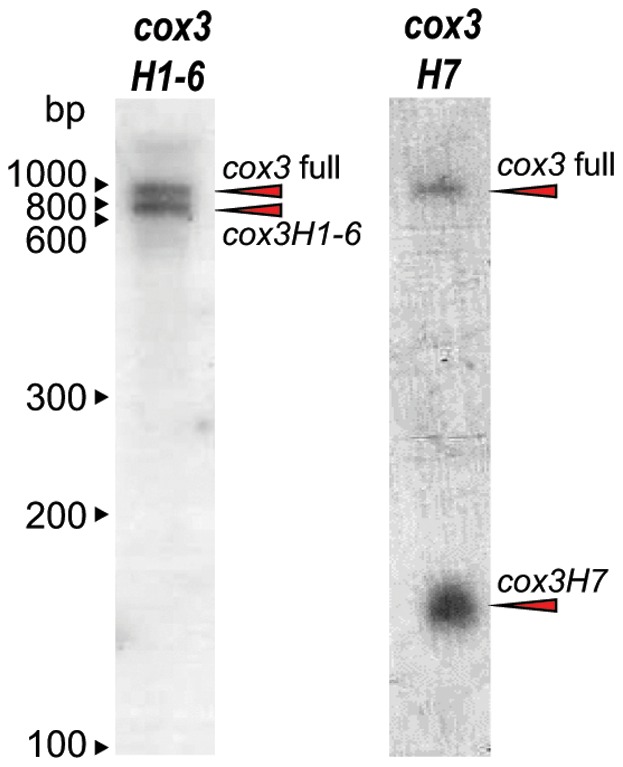
Total K. veneficum RNA was hybridized with either a probe corresponding to the cox3H1-6 or cox3H7 sequence. Bands observed correspond in size to the precursor molecules cox3H1-6 (∼745 nt) and cox3H7 (∼136 nt), along with full length cox3 (∼872 nt) (note: predicted RNA lengths include oligoadenylation tails).
A consequence of abundant precursor transcripts is that these would need to be excluded from the downstream expression machinery, namely translation. However, we detected no obvious differentiation of precursor versus complete transcript, such as post-transcriptional modifications or oligoadenylated tail length differences, that might distinguish precursors from mature transcripts ready for translation. The function of oligo-adenylation in dinoflagellate mitochondria is unknown (other than its inclusion in cox3 splice products), but it is consistently present in mitochondrial transcripts of both dinoflagellates and apicomplexans suggesting it does not serve as a cue for mRNA degradation as for some other organelle systems [18], [29], [32]–[34]. While RNA editing is a necessary process of mRNA maturation in dinoflagellate mitochondria [35], [36], we have previously shown that K. veneficum cox3H1-6 precursors are fully edited [17]. Instances of minor incomplete editing were observed in some of these cox3H1-6 transcripts, however this was also seen for cob transcripts (which are not trans-spliced), and appears to be a general feature of RNA editing [17]. It is, therefore, unclear how the abundant presence of these immature transcripts is managed. One possibility is that the precursor transcripts might be translated into partial Cox3 proteins that either function autonomously or are subsequently joined as proteins. Dinoflagellate mitochondria are known to be able to use alternative initiator and terminator translation signals [18], [19], so the lack of conventional open reading frames in the cox3H1-6 and cox3H7 transcripts might not be a barrier to translation (we have attempted to characterize Cox3 protein species by mass spectrometry but without success). However, if such novel routes to Cox3 function were viable, independent evolution of the trans-splicing process would be unnecessary. Thus we find such a scenario of partial Cox3 synthesis unlikely, although how it is avoided remains a conundrum.
The presence of a conserved splice site across diverse dinoflagellates suggests that this trait was acquired relatively early in dinoflagellate radiation, although after divergence of deep-branching taxa such as Hematodinium sp. and Oxyrrhis which lack cox3 splicing [23], [24]. Further, from these data we can draw some conclusions about the mechanism of splicing. The lack of any flanking non-coding sequence in the cox3 transcript precursors (other than the oligoadenosine tails) argues against flanking split group I/II introns mediating the splicing events, as occurs in other organelle trans-splicing systems [1]. There is also no evidence of likely RNA helix formation between the cox3H1-6 3′ end, and the cox3H7 5′ end, that could potentially mediate bulge-helix-bulge splicing as seen in some archaeal tRNAs [16]. This absence of any putative self-splicing components suggests that splicing is directed by some additional guide molecule or complex. Such a guide must: 1) identify the two component molecules (cox3H1-6 and cox3H7); 2) define the correct length of final spliced product, allowing sufficient A nucleotides from the oligoadenylated tail to close any gap; and 3) direct the splicing reaction onto the 5′ end of cox3H7. Such a guide could consist of a protein (or proteins), or could be a further RNA molecule similar to RNA guides employed in editing of trypanosomatid mitochondria RNAs [37]. Extensive searching for evidence of any putative RNAs with limited complementarity to both cox3 precursors has failed to detect any candidates. A lack of conservation seen across taxa of either the position of oligoadenylation of cox3H1-6, or the sequence identity of the two ends to be joined, suggests that the guide molecule is tolerant of change in this region, and might interact with sequence regions more distal to the splice site (Fig. 3). The only conserved nucleotide within the immediate splicing region is a uracil found at the 5′ splice site of cox3H7 in all four taxa surveyed, and this nucleotide may reflect a conserved feature of the splicing reaction.
Figure 3. Model of cox3 trans-splicing mechanism.

Putative splicing mechanism employing a guide molecule that unites the two cox3 precursor transcripts, and determines the length of the final splice product by inclusion of the necessary number of A nucleotides from the oligoadenylated tail of cox3H1-6.
A consequence of the trans-splicing mechanism in dinoflagellate cox3, and the inclusion of part of the cox3H1-6 oligoadenosine tail in the spliced product, is that a variable number of A nucleotides occur at the join region. This results in one or more lysines (codon: AAA) encoded in the complete transcript (Fig. 1C). In a poly-topic membrane protein inclusion of charged residues might be expected to cause problems for membrane topology, with potential implications for protein function. However, the location of the splice site in cox3 is between the coding regions of two membrane helices, and presumably these charged residues (and variability in protein sequence) are tolerated at this site.
Overall, these new insights into trans-splicing of dinoflagellate mitochondrial cox3 show that it is an unusual process on multiple scores. Unlike discontinuous group I/II intron mediated trans-splicing, there is no evidence for the precursor transcripts directly contributing to the process of splicing. Thus evolution of this trans-splicing process is more likely to have developed by the introduction of a splicing capability into these mitochondria, rather than gradual corruption of an existing splicing function such as organelle intron removal. Deep-branching dinoflagellates (e.g. Oxyrrhis and Hematodinium sp.) lack trans-splicing, although they share the same very reduced set of mitochondrial genes, so there is no evidence of existing splicing capacity in mitochondria early in this lineage [23], [24]. Also unusual is that the splicing process in dinoflagellate mitochondria is imperfect. It does not always produce a seamless join between two complete gene exons, but leaves a footprint of multiple A nucleotides that has varied in length during divergence of different dinoflagellate taxa. While this is apparently tolerated in at least one position in the Cox3 gene, presumable this would not be viable in many other locations within the three proteins encoded in dinoflagellate mitochondria. Thus development of further trans-splicing events in this system might be constrained by the imperfect nature of this process.
Only one other known system displays a comparably unusual form of RNA trans-splicing - the mitochondria of diplonemid protists that belong to the supergroup Euglenozoa [38], [39]. Here, fragmented genes (up to nine pieces in the case of cox1) are transcribed as separate RNAs, trimmed down to only the coding sequences, and spliced together to form complete coding transcripts. A lack of flanking non-coding RNA suggests that splicing also relies on guide molecules, although in diplonemids these too are uncharacterized. Further, at one splice junction in cox1 a non-coded run of six uracils occurs in the mature transcript, although in this case RNA insertional editing is thought to be the mechanism, as occurs in trypanosomatid relatives of diplonemids [37], [40]. While superficially similar to the case of dinoflagellate trans-splicing, the mechanism of diplonemid trans-splicing is likely to be different to dinoflagellates, and these two groups are very distantly related to one another [41]. It is interesting to note, however, that both mitochondrial trans-splicing processes have developed in lineages that undergo trans-splicing of SLs onto their nucleus-encoded mRNAs, and also possess mitochondrial RNA editing machineries that are both presumed to entail RNA cleavage and re-ligation [18], [42]. This raises the question of whether these novel forms of RNA trans-splicing might have developed under the influence of any of this existing machinery. In dinoflagellates, SL trans-splicing involves a SL transcript containing an exon/intron GU boundary, and a corresponding AG intron/exon boundary in the nascent protein mRNA. The splicing reaction is presumed to utilize the nuclear splicesomal complex [11]. The cox3H1-6 and cox3H7 transcripts lack flanking intron sequences, suggesting it is unlikely to be a substrate for this complex (Fig. 1). Moreover, of 72 known genes whose products comprise the spliceosome, 66 were recently identified from the Symbiodinium transcriptome [40]. Importing such a complex into organelles is unprecedented, and would represent a considerable evolutionary challenge. The biochemistry of RNA editing in dinoflagellate mitochondria is currently entirely unknown, so it is difficult to speculate on whether this process could have serendipitously contributed to the novel trans-splicing process found in cox3.
Supporting Information
cRT-PCR amplicon nucleotide sequences. Primer binding locations are underlined. Oligoadenylated tails are shown in blue. Dashes indicates gaps between outwards-facing primer pairs (unsequenced regions of the transcripts). The 15 base 5′ tag present on two of the six K. veneficum cox3H7 amplicons is italicised.
(RTF)
Funding Statement
This work was supported by an Australian Research Council Discovery grant (DP0663590), and C.J.J. was supported by the University of Melbourne Science Faculty Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moreira S, Breton S, Burger G (2012) Unscrambling genetic information at the RNA level. WIREs RNA 3: 213–228. [DOI] [PubMed] [Google Scholar]
- 2. Bonen L, Vogel J (2001) The ins and outs of group II introns. Trends Genet 17: 322–331. [DOI] [PubMed] [Google Scholar]
- 3. Haugen P, Simon DM, Bhattacharya D (2005) The natural history of group I introns. Trends Genet 21: 111–119. [DOI] [PubMed] [Google Scholar]
- 4. Bonen L (2008) Cis- and trans-splicing of group II introns in plant mitochondria. Mitochondrion 8: 26–34. [DOI] [PubMed] [Google Scholar]
- 5. Burger G, Yan Y, Javadi P, Lang BF (2009) Group I-intron trans-splicing and mRNA editing in the mitochondria of placozoan animals. Trends Genet 25: 381–386. [DOI] [PubMed] [Google Scholar]
- 6. Grewe F, Veihoever P, Weisshaar B, Knoop V (2009) A trans-splicing group I intron and tRNA-hyperediting in the mitochondrial genome of the lycophyte Isoetes engelmannii . Nucleic Acids Res 37: 5093–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorn R, Reuter G, Loewendorf A (2001) Transgene analysis proves mRNA trans-splicing at the complex mod(mdg4) locus in Drosophila . Proc Natl Acad Sci U S A 98: 9724–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamikawa R, Inagaki Y, Tokoro M, Roger AJ, Hashimoto T (2011) Split introns in the genome of Giardia intestinalis are excised by spliceosome-mediated trans-splicing. Curr Biol 21: 311–315. [DOI] [PubMed] [Google Scholar]
- 9. Roy SW, Hudson AJ, Joseph J, Yee J, Russell AG (2011) Numerous fragmented spliceosomal introns, AT-AC splicing, and an unusual dynein gene expression pathway in Giardia lamblia . Mol Biol Evol 29: 43–49. [DOI] [PubMed] [Google Scholar]
- 10. Hastings KE (2005) SL trans-splicing: easy come or easy go? Trends Genet 21: 240–247. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Hou Y, Miranda L, Campbell DA, Sturm NR, et al. (2007) Spliced leader RNA trans-splicing in dinoflagellates. Proc Natl Acad Sci U S A 104: 4618–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasda EL, Blumenthal T (2011) Trans-splicing. WIREs RNA 2: 417–434. [DOI] [PubMed] [Google Scholar]
- 13. Zhang H, Lin S (2008) mRNA editing and spliced-leader RNA trans-splicing groups Oxyrrhis, Noctiluca, Heterocapsa, and Amphidinium as basal lineages of dinoflagellates. J Phycol 44: 703–711. [DOI] [PubMed] [Google Scholar]
- 14. Zhang H, Dungan CF, Lin S (2011) Introns, alternative splicing, spliced leader trans-splicing and differential expression of pcna and cyclin in Perkinsus marinus . Protist 162: 154–167. [DOI] [PubMed] [Google Scholar]
- 15.Gornik SG, Ford KL, Mulhern TD, Bacic A, McFadden GI, et al.. (2012) Loss of Nucleosomal DNA Condensation Coincides with Appearance of a Novel Nuclear Protein in Dinoflagellates. Curr Biol: (in press). [DOI] [PubMed]
- 16. Randau L, Calvin K, Hall M, Yuan J, Podar M, et al. (2005) The heteromeric Nanoarchaeum equitans splicing endonuclease cleaves noncanonical bulge-helix-bulge motifs of joined tRNA halves. Proc Natl Acad Sci U S A 102: 17934–17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson CJ, Norman JE, Schnare MN, Gray MW, Keeling PJ, et al. (2007) Broad genomic and transcriptional analysis reveals a highly derived genome in dinoflagellate mitochondria. BMC Biol 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waller RF, Jackson CJ (2009) Dinoflagellate mitochondrial genomes: stretching the rules of molecular biology. Bioessays 31: 237–245. [DOI] [PubMed] [Google Scholar]
- 19. Nash EA, Nisbet RE, Barbrook AC, Howe CJ (2008) Dinoflagellates: a mitochondrial genome all at sea. Trends Genet 24: 328–335. [DOI] [PubMed] [Google Scholar]
- 20. Patron NJ, Waller RF, Keeling PJ (2006) A tertiary plastid uses genes from two endosymbionts. J Mol Biol 357: 1373–1382. [DOI] [PubMed] [Google Scholar]
- 21. Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, et al. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 272: 1136–1144. [DOI] [PubMed] [Google Scholar]
- 22.Mandl CW, Heinz FX, Puchhammer-Stockl E, Kunz C (1991) Sequencing the termini of capped viral RNA by 5′-3′ ligation and PCR. Biotechniques 10: 484, 486. [PubMed]
- 23. Jackson CJ, Gornik SG, Waller RF (2012) The mitochondrial genome and transcriptome of the basal dinoflagellate Hematodinium sp.: character evolution within the highly derived mitochondrial genomes of dinoflagellates. Genome Biol Evol 4: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slamovits CH, Saldarriaga JF, Larocque A, Keeling PJ (2007) The highly reduced and fragmented mitochondrial genome of the early-branching dinoflagellate Oxyrrhis marina shares characteristics with both apicomplexan and dinoflagellate mitochondrial genomes. J Mol Biol 372: 356–368. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Morse D (2006) Rampant polyuridylylation of plastid gene transcripts in the dinoflagellate Lingulodinium . Nucleic Acids Res 34: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dorrell RG, Howe CJ (2012) Functional remodeling of RNA processing in replacement chloroplasts by pathways retained from their predecessors. Proc Natl Acad Sci U S A 109: 18879–18884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shuman S (2001) Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol 66: 1–40. [DOI] [PubMed] [Google Scholar]
- 28. Escobar Galvis ML, Allen JF, Hakansson G (1998) Protein synthesis by isolated pea mitochondria is dependent on the activity of respiratory complex II. Curr Genet 33: 320–329. [DOI] [PubMed] [Google Scholar]
- 29. Chaput H, Wang Y, Morse D (2002) Polyadenylated transcripts containing random gene fragments are expressed in dinoflagellate mitochondria. Protist 153: 111–122. [DOI] [PubMed] [Google Scholar]
- 30. Imanian B, Keeling PJ (2007) The dinoflagellates Durinskia baltica and Kryptoperidinium foliaceum retain functionally overlapping mitochondria from two evolutionarily distinct lineages. BMC Evol Biol 7: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barbrook AC, Howe CJ, Kurniawan DP, Tarr SJ (2010) Organization and expression of organellar genomes. Philos Trans R Soc Lond B Biol Sci 365: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillespie DE, Salazar NA, Rehkopf DH, Feagin JE (1999) The fragmented mitochondrial ribosomal RNAs of Plasmodium falciparum have short A tails. Nucleic Acids Res 27: 2416–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rehkopf DH, Gillespie DE, Harrell MI, Feagin JE (2000) Transcriptional mapping and RNA processing of the Plasmodium falciparum mitochondrial mRNAs. Mol Biochem Parasitol 105: 91–103. [DOI] [PubMed] [Google Scholar]
- 34. Gagliardi D, Stepien PP, Temperley RJ, Lightowlers RN, Chrzanowska-Lightowlers ZM (2004) Messenger RNA stability in mitochondria: different means to an end. Trends Genet 20: 260–267. [DOI] [PubMed] [Google Scholar]
- 35. Lin S, Zhang H, Spencer DF, Norman JE, Gray MW (2002) Widespread and extensive editing of mitochondrial mRNAS in dinoflagellates. J Mol Biol 320: 727–739. [DOI] [PubMed] [Google Scholar]
- 36.Lin S, Zhang H, Gray MW (2008) RNA Editing in Dinoflagellates and its Implications for the Evolutionary History of the Editing Machinery. In: Smith HC, editor. RNA and DNA Editing: Molecular Mechanisms and Their Intergration into Biological Systems John Wiley and Sons, Inc. 280–309.
- 37. Shlomai J (2004) The structure and replication of kinetoplast DNA. Curr Mol Med 4: 623–647. [DOI] [PubMed] [Google Scholar]
- 38. Marande W, Burger G (2007) Mitochondrial DNA as a genomic jigsaw puzzle. Science 318: 415. [DOI] [PubMed] [Google Scholar]
- 39. Vlcek C, Marande W, Teijeiro S, Lukes J, Burger G (2011) Systematically fragmented genes in a multipartite mitochondrial genome. Nucleic Acids Res 39: 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiethega G, Turcotte M, Burger G (2011) Evolutionarily conserved cox1 trans-splicing without cis-motifs. Mol Biol Evol 28: 2425–2428. [DOI] [PubMed] [Google Scholar]
- 41. Keeling PJ, Burger G, Durnford DG, Lang BF, Lee RW, et al. (2005) The tree of eukaryotes. Trends Ecol Evol 20: 670–676. [DOI] [PubMed] [Google Scholar]
- 42. Stuart KD, Schnaufer A, Ernst NL, Panigrahi AK (2005) Complex management: RNA editing in trypanosomes. Trends Biochem Sci 30: 97–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
cRT-PCR amplicon nucleotide sequences. Primer binding locations are underlined. Oligoadenylated tails are shown in blue. Dashes indicates gaps between outwards-facing primer pairs (unsequenced regions of the transcripts). The 15 base 5′ tag present on two of the six K. veneficum cox3H7 amplicons is italicised.
(RTF)